Abstract
Clinical evidence links the inhibition of VEGF to hypertension. However, the mechanisms by which VEGF affects the pathogenesis of hypertension remain in question. We determined 1) whether administration of VEGF receptor inhibitor SU5416 enhances dietary salt-induced hypertension in Sprague-Dawley (SD) rats, and 2) whether VEGF or SU5416 directly affects proliferation of cultured human renal proximal tubular epithelial cells (HRPTEC) and endothelial nitric oxide synthase (eNOS) expression in cultured human glomerular microvessel endothelial cells (HGMEC). Ten 10-wk-old male SD rats received a high sodium diet (HS; 8%) and the other 10 SD rats received a normal sodium diet (NS; 0.5%) for 4 wks. After 2 wks of the dietary program, five rats were administered with SU5416 at 10 mg·kg−1·day−1 ip or DMSO (vehicle) for 14 days in HS and NS groups. Mean arterial pressure was significantly higher in rats treated with SU5416, as opposed to those treated with DMSO and fed with HS for 4 wk (157.6 ± 3.9 vs. 125.9 ± 4.3 mmHg, P < 0.01). Increased proteinuria and albuminuria were associated with marked renal histological abnormalities in HS group with SU5416 administration, compared with those in the vehicle HS group. 3H-thymidine incorporation assay showed that SU5416 blocked the actions of both exogenous and endogenous VEGF on the proliferation of HRPTEC. VEGF (10 ng/ml) significantly increased eNOS protein levels by 29% in cultured HGMEC, but its action was completely abolished by SU5416. These results suggest that VEGF receptor inhibition enhances dietary salt-induced hypertension and kidney injury, possibly by direct damage on renal cells and decreasing NO production by eNOS.
Keywords: VEGF, SU5416, high-salt diet, renal injury, renal proximal tubular epithelial cells, glomerular microvessel endothelial cells, eNOS
clinical evidence is emerging as the inhibition of VEGF is being linked to hypertension. Significantly increased levels of circulating soluble fms-like tyrosine kinase-1 (sFlt-1), an endogenous VEGF inhibitor, contribute to hypertension in patients with preeclampsia (17, 24). Hypertension and proteinurina were the common adverse effects in patients with lung cancer who were treated with anti-VEGF antibody (22). In clinical trials of ZD6474, an inhibitor of VEGF receptors, 24% of the cancer patients developed hypertension (11). Five cases of new onset hypertension emerged from among 25 multiple myeloma patients who received SU5416, an inhibitor of VEGF receptors (35). Shortly after the therapy their hypertension reversed completely. Small-molecule inhibitors of VEGF signaling (PTK787 and SU11248) were associated with similar proportions of patients who developed hypertension during the therapy (5, 28). However, there is no study available for examining whether administration of VEGF inhibitor causes hypertension and renal injury in an animal model. The mechanisms underlying VEGF inhibition-induced hypertension are unclear.
VEGF is a key regulator of angiogenesis in both physiological and pathological conditions (21). It has been shown to regulate vascular permeability, endothelial cell migration, proliferation, survival, and nitric oxide (NO) availability (21). The dysregulation of the VEGF signaling system has been identified in a wide variety of renal diseases such as diabetic nephropathy, puromycin animonucleoside nephrosis, and glomerulo-nephritides (3, 12, 32). Peritubular capillary loss, cortical tubular atrophy, and interstitial fibrosis are also correlated with the downregulation of VEGF in a mouse folic acid nephropathy model (34). Treatment with VEGF showed the protective effects in animals with cyclosporine-mediated nephropathy or a remnant kidney (14–15). Homozygous deletion of VEGF-A in the glomeruli resulted in perinatal lethality, while heterozygous deletion of renal VEGF-A caused end-stage kidney failure by 9–12 wk of age in mice (1). We have recently reported that a long-term (8 wk), high-salt (HS) diet induces renal injury and hypertension, which are associated with decreased renal VEGF expression in Sprague-Dawley (SD) rats (7). These findings suggest that the role of VEGF in maintaining kidney structure and function is mediated by its actions on renal endothelial and renal tubular cells. Renal proximal tubular epithelial cells, glomerular microvascular endothelial cells, and endothelial NO synthase (eNOS) are very critical for normal kidney functions, such as salt retention and excretion. Based on the fact that the kidneys play a key role in long-term regulation of blood pressure and that VEGF is very critical in maintaining renal structure and function, we hypothesize that attenuated response to VEGF in the kidneys contributes to hypertension and renal injury.
The present study examines 1) whether administration of SU5416 enhances dietary salt-induced hypertension and renal injury in SD rats, 2) whether VEGF or SU5416 directly affects proliferation of cultured human renal proximal tubular epithelial cells (HRPTEC) compared with human umbilical vein endothelial cells (HUVEC) and human aortic smooth muscle cells (HASMC), and 3) whether VEGF or SU5416 directly affects the expression of eNOS in cultured human glomerular microvascular endothelial cells (HGMEC).
METHODS
Animal protocol and SU5416 administration.
The protocols were carried out according to the guidelines for the care and use of laboratory animals implemented by the National Institutes of Health and the Guidelines of the Animal Welfare Act and were also approved by the University of Mississippi Medical Center's Institutional Animal Care and Use Committee. Male SD rats were received at 9 wk of age and were allowed to acclimate for 1 wk with standard rat diet (Teklad; Harlan Sprague-Dawley, Indianapolis, IN) and tap water before beginning the experiment. Rats were placed in a temperature-controlled room with a 12:12-h light-dark cycle. During the 4-wk experimental period, the normal sodium diet (NS) group (n = 10) received an NS (0.5%) diet (Teklad; Harlan Sprague-Dawley) and the HS diet group (n = 10) received an HS (8%) diet (Teklad; Harlan Sprague-Dawley). After 2 wks of the dietary program, five rats were administered with SU5416 at 10 mg·kg−1·day−1 ip (SU5416 dissolved in 100 μl of DMSO) or DMSO (vehicle) for 14 days in the HS and NS groups, respectively. During the last week of the experimental period, the rats were placed in metabolic cages, and water intake and urinary volume output were measured daily. Twenty-four-hour urine samples were collected daily, and urine sodium concentration was determined by flame photometry. Urine protein concentration was determined using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Urine albumin concentration was measured using a Nephrat ELISA Kit (Exocell, Philadelphia, PA).
Measurements of blood pressure and heart rate.
At the end of the experimental period, rats were anesthetized by isoflurane inhalation using a gas vaporizer (Harvard Apparatus, Holliston, MA). Using aseptic techniques, a micro-catheter was placed into the carotid artery and routed under the skin to the back of the neck and secured. Two days after recovering from the surgery, the catheter was connected to a PowerLab computerized monitoring system (AD Instruments, Castle Hill, Australia) to continuously record mean arterial pressure (MAP) and heart rate in the conscious rats 4 h/day for 2 days. The measurements of blood pressure and heart rate in each rat were made simultaneously, and all the values for each rat represented the average of the 4-h per day for 2 days of measurements. At the end of experiment, blood samples were collected through the catheters, and again the rats were anesthetized with isoflurane and the kidneys were removed for various analyses.
ELISA for VEGF, sFlt-1, and eNOS.
Protein levels of VEGF and sFlt-1 in the plasma and urine of different groups of SD rats were determined using appropriate ELISA kits (R&D Systems, Minneapolis, MN) according the manufacturer's instructions. The total urinary protein excretion of VEGF or sFlt-1 was calculated by the concentration multiplied by the total volume over 24 h and expressed as nanograms per day. The protein levels of eNOS in cultured HGMEC were determined using an ELISA kit (R&D Systems) according the manufacturer's instructions, in the absence (vehicle control) and presence of VEGF (10 ng/ml) or VEGF plus SU5416 (10 μmol/l) for 18 h. The total protein concentration of cultured HGMEC was determined using a protein assay (Bio-Rad Laboratories). The protein levels of eNOS were normalized and expressed as picograms per milligram total cellular protein.
Cell cultures and proliferation assay.
The cell lines of HRPTEC, HUVEC, and HASMC were obtained from the American Type Culture Collection (Rockville, MD). The HGMEC were ordered from Angio-Proteomie (Boston, MA). The cells were seeded into T-75 flasks using M199 media (GIBCO) supplemented with 10% FBS (HyClone), 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B, and incubated at 37°C in a humidified 5% CO2/air-injected atmosphere. When the monolayer reached ∼80% confluence, the cells were washed with PBS and incubated with fresh M199 media with 10% FBS in the absence (vehicle control) and presence of VEGF (10 ng/ml), VEGF plus SU5416 (10 μmol/l), or SU5416 (1, 5, 10, and 20 μmol/l) for 18 h. 3H-thymidine incorporation assay was used to determine the cell proliferation during the last 6 h of incubation as previously described (6).
Histological analysis by light microscopy.
Mid-coronal sections of the left kidneys collected from the rats with NS- and HS-fed rats in the absence or presence of SU5416 administration were fixed in buffered formalin and embedded in paraffin for histological studies as previously described (8, 25). Samples were cut into 3-μm sections and stained with periodic acid-Schiff reagent (PAS), followed by hematoxylin counterstaining. All sections were analyzed by semiquantitative histological grading (0 = absent, 1+ = mild, 2+ = moderate, and 3+ = severe) for the severity of tubulointerstitial infiltration with inflammatory cells, focal segmental glomerulosclerosis, tubulointerstitial injury, and extracellular matrix expansion. The average glomerular area in the PAS-stained sections was determined from 30 to 40 glomeruli in each kidney on the computer screen using Image-Pro Plus Software (Media Cybernetics, Silver Spring, MD). In addition, the glomerulosclerosis index was determined in 20 microscope-fields per kidney, and lesions were graded as follows: grade 0, no changes; grade 1, lesion involving < 10%; grade 2, lesion affecting 10–20%; and grade 3, lesion involving > 20% of the field, in which the glomerulosclerosis lesion also included the acute glomerular injury, such as fibrinoid necrosis in some glomeruli. The resulting index in each animal was expressed as mean value of all scores obtained. All injury parameters were assessed independently by two investigators in a blinded manner.
Statistical analyses.
All determinations were performed in duplicated sets. Where indicated, data is presented as means ± SE. Statistically significant differences in mean values between two groups were tested by an unpaired Student's t-test. ANOVA was used to analyze the differences between two groups with multiple comparisons. A value of P < 0.05 was considered statistically significant. All statistical calculations were performed using SPSS (Chicago, IL) software.
RESULTS
Effects of SU5416 administration on blood pressure and heart rate.
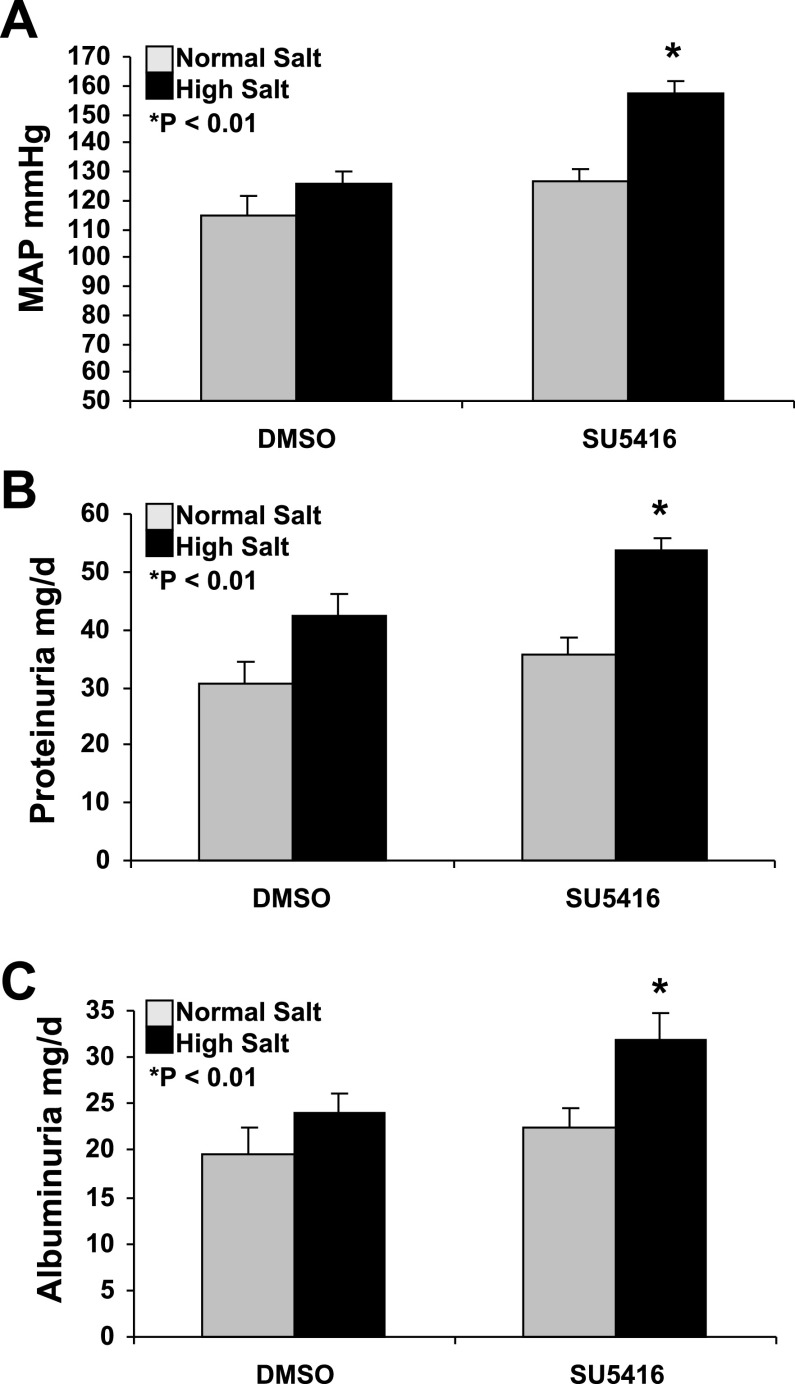
Figure 1A demonstrates that 2 wk of SU5416 (VEGF receptor inhibitor) administration significantly increases MAP in conscious SD rats on an HS diet, compared with those treated with DMSO (vehicle) and fed with HS for 4 wks (157.6 ± 3.9 vs. 125.9 ± 4.3 mmHg, P < 0.01). There were no significant changes in MAP between SU5416 and DMSO-treated rats fed with NS diet (126.4 ± 4.1 vs. 115.1 ± 6.3 mmHg; P = 0.213). In DMSO-treated rats, 4 wk of HS diet did not significantly increase MAP, compared with the rats fed with NS diet (125.9 ± 4.3 vs. 115.1 ± 6.3 mmHg; P = 0.233). There was no significant difference in the heart rate between SU5416 and DMSO groups fed with an HS diet (482 ± 32 vs. 495 ± 61 beats/min; P = 0.574). There was also no significant difference in the heart rate between SU5416 and DMSO groups fed with a NS diet (501 ± 41 vs. 523 ± 56 beats/min; P = 0.612).
Fig. 1.
Increased mean arterial pressure (MAP), proteinuria, and albuminuria in SU5416-treated Sprague-Dawley (SD) rats on a high-salt (HS) diet. A: 2 wk of SU5416 (VEGF receptor inhibitor) administration significantly increased MAP in conscious SD rats on an HS diet, compared with those treated with DMSO (vehicle) and fed with HS for 4 wks (157.6 ± 3.9 vs. 125.9 ± 4.3 mmHg, n = 5, P < 0.01). B: SU5416 treatment significantly increased the total urinary protein excretion in SD rats fed with HS, compared with the DMSO HS group (53.7 ± 2.1 vs. 42.5 ± 4.6 mg/day; P < 0.01). C: SU5416 treatment significantly increased albuminuria in SD rats fed with HS, compared with the SMDO HS group (31.8 ± 3.1 vs. 24.1 ± 2.2 mg/day; P < 0.01).
Effects of SU5416 administration on urine protein and albumin excretion.
We also examined the effect of chronic administration of SU5416 on renal injury assessed by proteinuria and albuminuria. Figure 1B indicated that SU5416 treatment significantly increased the total urinary protein excretion in SD rats fed with HS, compared with the vehicle (DMSO) HS group (53.7 ± 2.1 vs. 42.5 ± 4.6 mg/day; P < 0.01). There was no significant difference in the total urinary protein excretion between SU5416 and DMSO groups fed with NS (35.5 ± 3.1 vs. 30.5 ± 3.6 mg/day; P > 0.05). In DMSO-treated animals, there was a marginal increase in the total urinary protein secretion in the HS group, compared with the NS group (42.5 ± 4.6 vs. 30.5 ± 3.6 mg/day; P < 0.05). Consistent with the total urinary protein, Fig. 1C showed that SU5416 treatment significantly increased albuminuria in SD rats fed with HS, compared with the vehicle HS group (31.8 ± 3.1 vs. 24.1 ± 2.2 mg/day; P < 0.01).
Effect of SU5416 administration on the pressure-natriuresis curve.
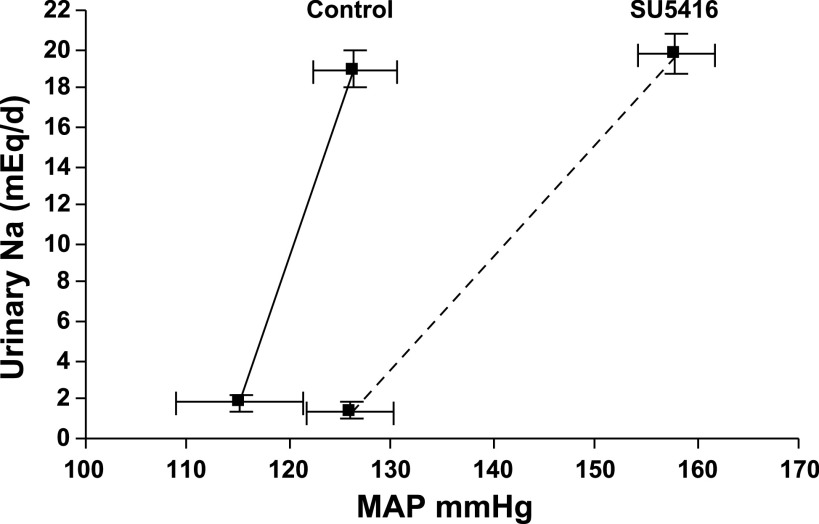
Based on the measurements of urinary sodium output and MAP, we examined whether chronic administration of SU5416 impaired renal-pressure natriuresis. Figure 2 indicated that SU5416 caused a significant decrease in slope of the pressure-natriuresis mechanism, which was characterized by a significantly decreased slope and a slightly increased intercept of the pressure-natriuresis mechanism in SD rats (P < 0.01). The slope of the pressure-natriuresis mechanisms has been widely used as a measure of salt sensitivity. This suggests that VEGF inhibition may contribute to salt-sensitive hypertension in humans.
Fig. 2.
SU5416 caused a right shift and rotation of the pressure-natriuresis curve in SD rats (n = 5, P < 0.01), which was characterized by a significantly decreased slope and a slightly increased intercept of the pressure-natriuresis mechanism. This suggests that VEGF inhibition may cause salt-sensitive hypertension in humans.
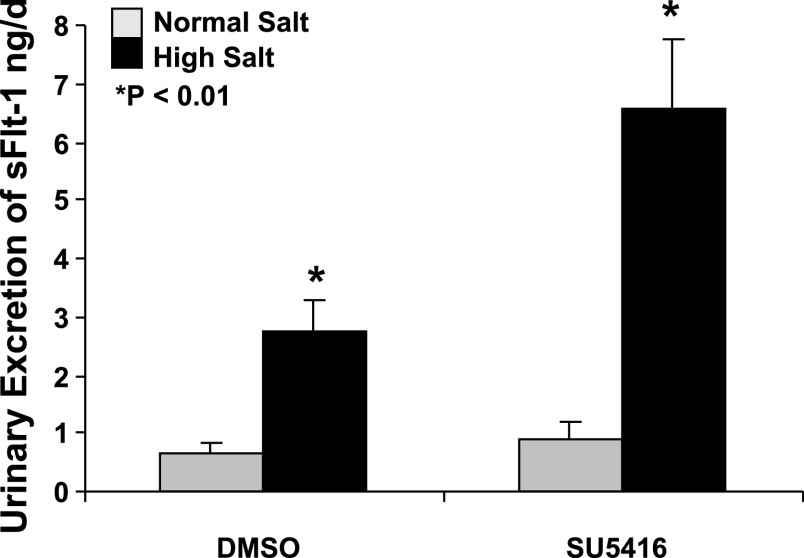
SU5416 administration increased total urinary excretion of sFlt-1 in SD rats on the HS diet.
sFlt-1 is an endogenous VEGF inhibitor. Figure 3 indicated that SU5416 treatment significantly increased renal excretion of sFlt-1 in SD rats on the HS diet, compared with the DMSO (vehicle)-treated animals fed with HS (6.57 ± 1.19 vs. 2.76 ± 0.49 ng/day; P < 0.01). There was no significant difference in renal excretion of sFlt-1 between SU5416- and DMSO-treated animals fed with NS (0.92 ± 0.31 vs. 0.64 ± 0.19 ng/day; P > 0.05). In the vehicle (DMSO) groups, an HS diet also significantly increased renal excretion of sFlt-1, compared with those with an NS diet (2.76 ± 0.49 vs. 0.64 ± 0.19 ng/day; P < 0.01). ELISA indicated that there were no significant changes in urinary excretion of VEGF between HS and NS groups (1.42 ± 0.26 vs. 1.45 ± 0.29 ng/day; P > 0.05) and that SU5416 administration did not change urinary excretion of VEGF in both groups fed with NS and HS, respectively. There were no significant changes in plasma levels of VEGF (6.9 ± 2.4 pg/ml) and sFlt-1 (710 ± 47 pg/ml) in SD rats among the four groups.
Fig. 3.
SU5416 treatment significantly increased renal excretion of fms-like tyrosine kinase-1 (sFlt-1) in SD rats on an HS diet, compared with the DMSO-treated animals fed with HS (6.57 ± 1.19 vs. 2.76 ± 0.49 ng/day; n = 5, P < 0.01). In the DMSO groups, an HS diet also significantly increased renal excretion of sFlt-1, compared with those with a normal salt (NS) diet (2.76 ± 0.49 vs. 0.64 ± 0.19 ng/day; P < 0.01).
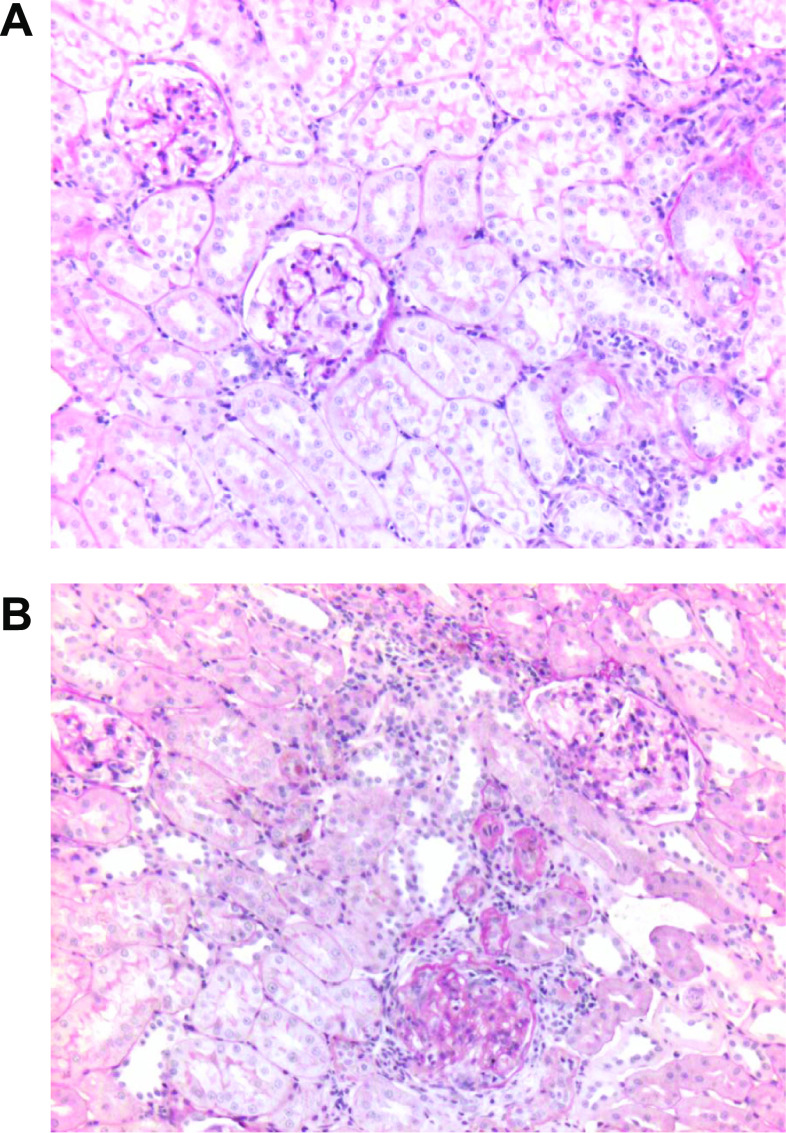
Renal histological changes due to SU5416.
Histological analysis by light microscopy of PAS-stained sections of the kidneys showed that there were no marked renal abnormalities in SD rats with NS diet in the absence or presence of SU5416 administration or SD rats with 4 wk of HS diet in absence of SU5416 administration (Figs. 4A). In contrast (4B), SD rats with 4 wk of HS diet in the presence of SU5416 administration developed moderate glomerulosclerosis, extracellular matrix expansion, tubulointerstitial injury, and tubulointerstitial infiltration with inflammatory cells. Fibrinoid necrosis in some glomeruli were also observed in HS group with SU5416 (Fig. 4B). The statistical analysis also indicated that the glomerulosclerosis index was significantly increased in the HS group with SU5416 administration, compared with the HS group without SU5416 administration (1.69 ± 0.08 vs. 0.83 ± 0.07 mean grade scores; n = 5; P < 0.01). The average glomerular area in the PAS-stained sections was increased by 33% in the HS group with SU5416 administration, compared with the HS group without SU5416 administration (22,145 ± 3,864 vs. 16,651 ± 3,476 μm2; n = 5; P < 0.01). The increased glomerular area in the HS group with SU5416 administration indicated that glomerular hypertrophy, hyperfiltration, and volume increased. These renal pathological changes further demonstrated that VEGF receptor inhibition caused kidney injury in SD rats with 4 wk of an HS diet.
Fig. 4.
Representative pathological changes in the kidneys of SD rats with 4-wk HS diet in the absence (A) and presence of SU5416 administration (B). Kidneys were removed after 4 wk of the dietary program. Periodic acid-Schiff reagent (PAS) stain was used. A: normal glomeruli, arterioles, and tubules. B: glomerulosclerosis, tubulointerstitial injury, inflammatory cells infiltration, extracellular matrix expansion, and increased glomerular area. Fibrinoid necrosis in some glomeruli were also observed in the HS group with SU5416 (B). Statistical analysis also indicated that the glomerulosclerosis index was significantly increased in the HS group with SU5416 administration, compared with the HS group without SU5416 administration (1.69 ± 0.08 vs. 0.83 ± 0.07 mean grade scores; n = 5; P < 0.01). Magnification, ×100.
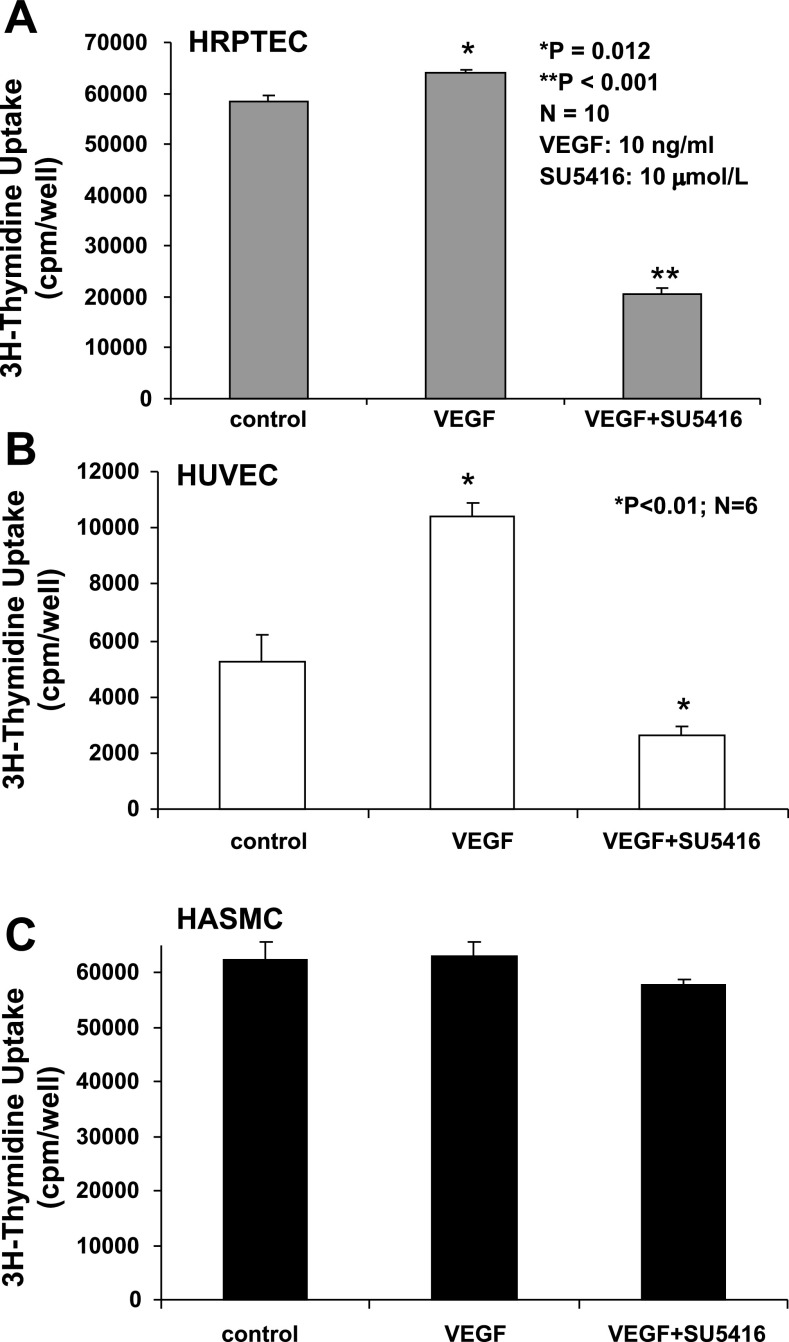
Effects of VEGF or VEGF plus SU5416 on proliferation of HRPTEC, HUVEC, and HASMC.
A 3H-thymidine incorporation assay indicated that VEGF (10 ng/ml) slightly but significantly increased the proliferation of cultured HRPTEC (Fig. 5A), compared with the control group (64,076 ± 629 vs. 58,454 ± 1,132 cpm/well; P = 0.012). There was a significant decrease by 65% in the proliferation of HRPTEC treated with VEGF plus SU5416 (10 μmol/l), compared with the control (P < 0.01). Clearly, SU5416 blocked the actions of both exogenous and endogenous VEGF on the proliferation of HRPTEC. We also found that SU5416 at 1, 5, 10, or 20 μmol/l caused a dose-related inhibition on the proliferation of HRPTEC, by 25, 47, 60, and 58%, respectively. As shown, 20 μmol/l of SU5416 did not further inhibit the proliferation of HRPTEC, and this might suggest that other signaling systems are involved in the regulation of HRPTEC proliferation. In Fig. 5B, VEGF caused a twofold increase in the proliferation of HUVEC, compared with the control (P < 0.01), but its action was completely abolished by SU5416 (10 μmol/l). As shown in Fig. 5C, neither VEGF (10 ng/ml) nor SU5416 (10 μmol/l) exerted any effect on the proliferation of cultured HASMC that did not express VEGF receptors. In addition, in the absence of VEGF (10 ng/ml) in the media, SU5416 (10 μmol/l) caused a 47% decrease in the proliferation of HRPTEC (P < 0.01), a 15% decrease in the proliferation of HUVEC (P < 0.05), and no significant changes in the proliferation of HASMC (P > 0.05), compared with the control group, respectively.
Fig. 5.
Effects of VEGF or VEGF + SU5416 on proliferation of cultured human renal proximal tubular epithelial cells (HRPTEC), human umbilical vein endothelial cells (HUVEC), and human aortic smooth muscle cells (HASMC). VEGF (10 ng/ml) slightly, but significantly, increased proliferation of cultured HRPTEC (A), compared with the control group (64,076 ± 629 vs. 58,454 ± 1,132 cpm/well; n = 10; P = 0.012). There was a significant decrease by 65% in the proliferation of HRPTEC treated with VEGF + SU5416 (10 μmol/l) compared with the control (P < 0.01). VEGF caused a twofold increase in the proliferation of HUVEC, compared with the control (P < 0.01), but its action was completely abolished by SU5416 (B). B: neither VEGF nor SU5416 exerted any effect on the proliferation of cultured HASMC (C).
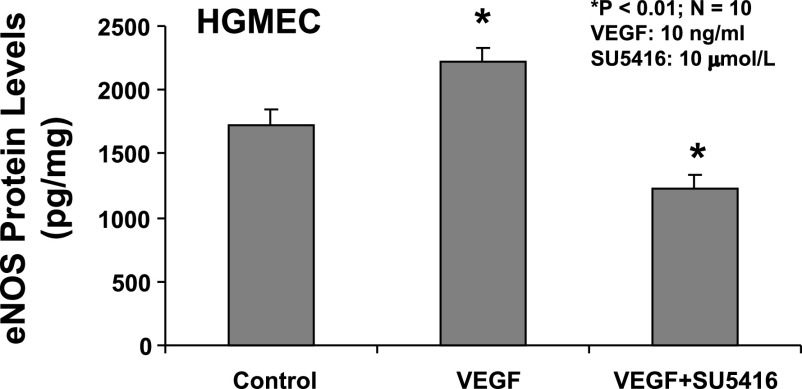
Effects of VEGF or VEGF plus SU5416 on eNOS expression in cultured HGMEC.
The expression of eNOS in cultured HGMEC was determined by ELISA in the presence and absence of VEGF and VEGF receptor inhibitor, SU5416. Figure 6 indicated that VEGF (10 ng/ml) significantly increased eNOS proteins expression by 29% in cultured HGMEC, compared with the control (P < 0.01). There was a significant decrease by 28% in eNOS expression of HGMEC treated with VEGF plus SU5416 (10 μmol/l), compared with the control (P < 0.01).
Fig. 6.
Effects of VEGF or VEGF + SU5416 on the expression of endothelial nitric oxide synthase (eNOS) protein in cultured human glomerular microvascular endothelial cells (HGMEC). VEGF (10 ng/ml) significantly increased eNOS protein expression 29% in cultured HGMEC, compared with the control (P < 0.01). There was a significant decrease by 28% in eNOS expression of HGMEC treated with VEGF + SU5416 (10 μmol/l), compared with the control (n = 10; P < 0.01).
DISCUSSION
Clinical evidence links the inhibition of VEGF to hypertension. However, the role of VEGF in the pathogenesis of hypertension remains in question. We have recently reported that a long-term (8 wk) HS diet induces renal injury and hypertension, which are associated with decreased renal VEGF expression in SD rats (7). The major finding in this study is that chronic VEGF inhibition (SU5416) enhances dietary salt-induced hypertension, increases renal injury manifested by proteinuria, albuminuria, and renal histological abnormalities, and causes a significant decrease in slope of the pressure-natriuresis mechanism, which was characterized by a significantly decreased slope and a slightly increased intercept of the pressure-natriuresis mechanism in SD rats. The renal histological abnormalities include increased glomerular area, moderate glomeruloslcerosis, extracellular matrix expansion, tubulointerstitial injury, and tubulointerstial infiltration with inflammatory cells. Also, the cell culture studies have demonstrated that VEGF receptor inhibitor, SU5416, significantly reduces proliferation of renal proximal tubular epithelial cells and decreases eNOS expression in glomerular microvessel endothelial cells. These in vivo and in vitro findings support the hypothesis that attenuated response to VEGF in the kidneys contributes to HS-induced hypertension and renal injury.
Mounting evidence suggests that VEGF exerts a very important and unique role in maintaining renal structure and functions. VEGF binds two related receptors, Flt-1 and KDR/Flk-1 (4, 23), and both are expressed in glomerular and peritubular endothelial cells (23). Recently, Flt-1 and/or Flk-1 have been shown to be expressed in mesangial cells, podocyte cells, and renal tubular epithelial cells (20, 29, 31). Yuan et al. (34) have showed that the peritubular capillary loss resulting after mouse acute nephrotoxicity is correlated with the downregulation of VEGF and hypoxia-inducible factor-1α. VEGF was required for podocyte and mesangial cell survival (20, 31). More recently, Eremina et al. (2) demonstrated that a striking loss of mesangial cells occurred in postnatal mice that carry one hypomorphic VEGF-A allele and one podocyte-specific null VEGF-A allele. The role of VEGF in the kidneys is unlike that of any other organ. In addition to maintaining the functions and structure of endothelial cells, VEGF also exerts autocrine and paracrine actions for regulating the functions and structure of other renal cells such as podocytes, mesangial cells, and renal tubular epithelial cells. In the present study, 4 wk of HS diet did not increase blood pressure in SD rats, but chronic administration of SU5416 caused a significant increase in the MAP of SD rats fed with HS. SU5416 administration also caused renal injury supported by the evidence of a significant increase in proteinuria, albuminuria, renal histological abnormalities, and a rightward shift and rotation of the pressure-natriuresis curve due to VEGF receptor inhibition in SD rats. Thus, we believe that SU5416-induced hypertension in SD rats on an HS diet is due to the alteration of renal function and structure by VEGF receptor inhibition. However, further studies are needed to understand the molecular mechanisms underlying the role of VEGF in the renal mechanisms of hypertension.
The renal proximal tubules play a major role in maintaining renal function and hemodynamics because < 65% of the filtered load of sodium and water are reabsorbed by the proximal tubules, controlling salt delivery to the distal nephron, which is critical for tubuloglomerular feedback autoregulation and for fine control of salt excretion in the distal nephron. Tubular epithelial cells have the potential ability for repair after ischemic or toxic injury. When injured, cells undergo necrosis and/or apoptosis, the noninjured cells proliferate actively so that the integrity of tubules can be maintained, and renal function can be recovered (30, 33). Villegas et al. (31) reported that VEGF stimulated proliferation of rat proximal tubular cells in an autocrine manner. In the present study, we examined whether VEGF or SU5416 directly affects proliferation of cultured HRPTEC, compared with HUVEC and HASMC. We found that VEGF slightly but significantly increased proliferation of cultured HRPTEC, but there was a significant decrease in the proliferation of HRPTEC treated with VEGF plus SU5416, compared with the control. VEGF caused a twofold increase in the proliferation of HUVEC, compared with the control, but its action was completely abolished by SU5416. Neither VEGF nor SU5416 exerted any effect on the proliferation of cultured HASMC. These results suggest autocrine and paracrine functions of VEGF on renal tubular epithelial cells. The inhibition of proliferation of HRPTEC by SU5416 may contribute to renal injury. These findings are consistent with a recent report that the expression of VEGF in renal tubules is positively correlated with tubule regeneration in patients with acute tubular necrosis (33). However, further studies are necessary to determine whether a VEGF receptor inhibitor directly causes renal tubular epithelial cell injury by inhibiting proliferation and stimulating apoptosis by using an animal model.
We also speculate that other mechanisms related to VEGF receptor inhibition on renal tubular epithelial cells and vascular endothelial cells may contribute to hypertension caused by VEGF inhibition. Nitric oxide (NO), known as a renal vasodilator and a natriuretic agent, plays an important role in the kidneys to regulate renal hemodynamics and sodium retention (18–19). NO is synthesized endogenously from the amino acid l-arginine by the actions of the enzymes, NO synthases (NOS; 10, 16). It has been found that administration of VEGF increases renal endothelial NOS (eNOS) expression and stabilizes renal function in a mouse remnant kidney model (15). Recent studies show that an HS diet reduces renal eNOS activity and expression in Dahl salt-sensitive rats (36) and SD rats (27). We have recently reported that a long-term (8 wk) HS diet induces renal injury and hypertension, which are associated with decreased renal VEGF expression in SD rats (7). In the present study, we have demonstrated that chronic administration of VEGF receptor inhibitor, SU5416 enhances salt-induced hypertension and renal injury in SD rats, and that SU5416 significantly reduces proliferation of renal proximal tubular epithelial cells and decreases eNOS expression in glomerular microvessel endothelial cells. These new findings further suggest that the inhibition of VEGF and eNOS in the kidneys may contribute to the mechanisms of salt-induced hypertension. However, this should be further tested using an animal model.
The soluble VEGF receptor-1 (sFlt-1) is known to inhibit VEGF activity by binding VEGF. In a different setting, preeclampsia is associated with elevated sFlt-1, endothelial cell dysfunction, and proteinuria. More recently, some studies demonstrated that the administration of sFlt1 induced proteinuria in rats (9, 26). In this study, we have found that SU5416 treatment significantly increased renal excretion of sFlt-1 in SD rats on an HS diet, compared with the DMSO (vehicle)-treated animals fed with HS. An increase in renal excretion of sFlt-1 in SD rats induced by SU5416 may suggest that VEGF receptor blockade leads to a vicious cycle of VEGF inhibition. Hornig, et al. (13) showed that sFlt-1 was upregulated by hypoxia in cultured human placental villous cells. Thus, it is likely that the increased renal excretion of sFlt-1 in SD rats on an HS diet could be due to a renal injury caused by chronic administration of SU5416.
Perspectives and Significance
Clinical evidence links the inhibition of VEGF to hypertension. We have demonstrated that chronic VEGF inhibition (SU5416) enhances dietary salt-induced hypertension, increases proteinuria as well as abuminuria, causes the marked renal histological abnormalities, and changes the pressure-natriuresis curve characterized by a significantly decreased slope and a slightly increased intercept of the pressure-natriuresis mechanisms in male adult SD rats. This animal model may lead to further understanding of the mechanisms underlying VEGF inhibition-induced hypertension. All evidence supports the notion that SU5416-induced hypertension in SD rats on an HS diet is due to the alteration of renal functions and structure by VEGF receptor inhibition, possibly by direct damage on renal cells and decreasing NO production by eNOS. However, these in vitro findings on SU6416 blocking the proliferation of HRPTEC and eNOS expression in HGMEC are limited. Further studies should be focused on in vivo models to understand the relationship between hypertension, VEGF inhibition, renal injury, NO production and eNOS activity in the kidneys.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-51971, and National Institute on Alcohol Abuse and Alcoholism Grant AA-013821.
REFERENCES
- 1.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal disease. J Clin Invest 111: 707–716, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, Ema M, Rossant J, Jothy S, Miner JH, Quaggin SE. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol 17: 724–735, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Fan L, Wakayama T, Yokoyama S, Amano O, Iseki S. Downregulation of vascular endothelial growth factor and its receptors in the kidney in rats with puromycin aminonucleoside nephrosis. Nephron 90: 95–102, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56: 794–814, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, Louie SG, Hong W, Brega NM, Massimini G, Scigalla P, Berdel WE, Hossfeld DK. A phase I study of SU11248 in the treatment of patients with refractory of resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105: 986–993, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Gu JW, Bailey AP, Sartin A, Makey I, Brady AL. Ethanol stimulates tumor progression and expression of vascular endothelial growth factor in chick embryos. Cancer 103: 422–431, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Gu JW, Bailey AP, Tan Wei Shparago M, Young E. Long-term high-salt diet causes hypertension and decreases renal expression of vascular endothelial growth factor in Sprague-Dawley rats. J Am Soc Hypertens 2: 275–285, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD Jr. Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 291: R1817–R1824, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Hara A, Wada T, Furuichi K, Sakai N, Kawachi H, Shimizu F, Shibuya M, Matsushima K, Yokoyama H, Egashira K, Kaneko S. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int 69: 1986–1995, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Herrera M, Garvin JL. Recent advances in the regulation of nitric oxide in the kidney. Hypertension 45: 1062–1067, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol 16: 1391–1397, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Honkanen EO, Teppo AM, Gronhagen-Riska C. Decreased urinary excretion of vascular endothelial growth factor in idiopathic membranous glomerulo-nephritis. Kidney Int 57: 2343–2349, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest 80: 443–454, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Kang DH, Kim YG, Andoh TF, Gordon KL, Suga SI, Mazzali M, Jefferson JA, Hughes J, Bennett W, Schreiner GF, Johnson R. Post-cyclosporin-mediated hypertension and nephropathy: amelioration by vascular endothelial growth factor. Am J Physiol Renal Physiol 280: F727–F736, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson R. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kone BC Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. Semin Nephrol 24: 299–315, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of pre-eclampsia. N Engl J Med 350: 672–683, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Majid DSA, Nishiyama A, Jackson KE, Castillo A. Inhibition of nitric oxide synthase enhances superoxide activity in canine kidney. Am J Physiol Regul Integr Comp Physiol 287: R27–R32, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Rebecca RF, Rachel H, Karen A, et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am J Physiol Renal Physiol 284: F1263–F1273, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett 580: 2879–2887, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Sandler AB, Johnson DH, Hertst RS. Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res 10: 4258s–4262s, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Schrijvers BF, Flyvbjerg A, DeVriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in pre-eclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab 90: 4895–4903, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Siegel AK, Kossmehl P, Planert M, Schulz A, Wehland M, Stoll M, Bruijn JA, de Heer E, Kreutz R. Genetic linkage of albuminuria and renal injury in Dahl salt-sensitive rats on a high-salt diet: comparison with spontaneously hypertensive rats. Physiol Genomics 18: 218–225, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto H, Hamano Y, Charyta D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan JC, Smart EJ, Pollock DM, Pollock JS. Influence of salt on subcellular localization of nitric oxide synthase activity and expression in the renal inner medulla. Clin Exp Pharmacol Physiol 35: 120–125, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Thomas AL, Morgan B, Drevs J, Unger C, Wiedenmann B, Vanhoefer U, Laurent D, Dugan M, Steward WP. Vascular endothelial growth factor receptor tyrosine kinase inhibitors: PTK787/ZK 222584. Semin Oncol 30, Suppl 6: 32–38, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Thomas S, Vanuystel J, Gruden G, Rodriquez V, Burt D, Hartley B, Viberti G. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol 11: 1236–1243, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Toback FG Regeneration after acute tubular necrosis. Kidney Int 41: 226–246, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Villegas G, Lange-Sperandio B, Tufro A. Autocrine and paracrine functions of vascular endothelial growth factor (VEGF) in renal tubular epithelial cells. Kidney Int 67: 449–457, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Wolf G, Chen S, Ziyadeh F. From the periphery of the glomerular capillary wall toward the center of disease. Podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Li X, Wang H. Possible mechanisms explaining the tendency towards interstitial fibrosis in aristolochic acid-induced acute tubular necrosis. Nephrol Dial Transplant 22: 445–456, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia inducible factor-1α. Am J Pathol 163: 2289–2301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zangari M, Anaissie E, Stopeck A, Morimoto A, Tan N, Lancet J, Cooper M, Hannah A, Garcia-Manero G, Faderl S, Kantarjian H, Cherrington J, Albitar M, Giles FJ. Phase II study of SU5416, a small molecular vascular endothelial growth factor tyrosine kinase receptor inhibitor, in patients with refractory multiple myeloma. Clin Cancer Res 10: 88–95, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Zhou MS, Schuman IH, Jaimes EA, Raji L. Renoprotection by statins is linked to a decrease in renal oxidative stress, TGF-β, and fibronectin with concomitant increase in nitric oxide bioavailability. Am J Physiol Renal Physiol 295: F53–F59, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]