Abstract
To assess baroreflex function under closed-loop conditions, a new approach was used to generate large and physiological perturbations in arterial pressure. Blood pressure (BP) and R-R interval were recorded continuously in 20 healthy young (33 ± 8 yr) and eight elderly subjects (66 ± 6 yr). Repeated squat-stand maneuvers at the frequencies of 0.05 and 0.1 Hz were performed to produce periodic oscillations in BP to provoke the baroreflex. To assess the effects of the muscle reflex and/or central command on the baroreflex, passive squat-stand maneuvers were conducted using a pulley system to assist changes in body position. Transfer function between changes in BP and R-R interval was estimated to assess the arterial-cardiac baroreflex. Relative to resting conditions, large and coherent oscillations in BP and R-R interval were produced during both active and passive squat-stand maneuvers. However, changes in BP were smaller during passive than during active maneuvers. Changes in R-R interval were reduced commensurately. Therefore, transfer function gain did not change between the two maneuvers. Compared with the young, transfer function gain was reduced and the phase became more negative in the elderly, demonstrating the well-known effects of aging on reducing baroreflex sensitivity. Collectively, these findings suggest that the changes in R-R interval elicited by BP perturbations during squat-stand maneuvers are mediated primarily by a baroreflex mechanism. Furthermore, baroreflex function can be assessed using the transfer function method during large perturbations in arterial pressure.
Keywords: blood pressure, heart rate, spectral analysis
baroreflex is essential for short-term arterial pressure stability (10). Impairment of baroreflex function may lead to orthostatic hypotension, resulting in brain hypoperfusion, falls, or syncope, a key problem in the elderly and patients with autonomic disease (9, 17, 39).
Assessment of baroreflex function is challenging for human studies. Baroreflex is a closed-loop feedback control system and is modulated by other reflex and nonreflex mechanisms (6). In this regard, a neck pressure chamber method has been developed to assess the carotid baroreflex function (5). Conversely, vasoactive drugs or performance of a Valsalva maneuver have been used to evaluate the integrated baroreflex function, which may involve multiple baroreceptors (25, 27). Recently, by taking the advantage of spontaneous oscillations in cardiovascular variables, baroreflex function has been assessed under resting conditions, using the sequence or transfer function methods (26, 29).
However, these methods, one way or another, have limitations. For example, the neck chamber method has been used extensively for research (6). However, the sophisticated techniques required for its use limit its value for daily practice in clinical medicine. Moreover, the linear regression used to link beat-to-beat changes in pulse interval (or heart rate) to changes in arterial pressure during vasoactive drug infusion or a Valsalva maneuver is static in nature (memory-less) and may not reveal dynamic properties of the baroreflex function. In addition, under closed-loop conditions, assessing baroreflex function from spontaneous changes in cardiovascular variables may be biased because of lack of sufficient external stimuli to provoke the baroreflex (16, 21). Finally, baroreflex function assessed under supine resting conditions may not be readily transferrable to the regulation of arterial pressure or heart rate during body postural change or other perturbations in daily life.
Squat followed by standing elicits transient changes in central blood volume and peripheral vascular resistance, leading to dramatic changes in arterial pressure and heart rate (23, 34, 39). Recently, it has been used as a model for studying the cardiovascular control during orthostatic stress (3, 39). In addition, heart rate response to changes in arterial pressure during a single squat-stand maneuver has been measured to assess the arterial-cardiac baroreflex function in patients with diabetes mellitus (22). However, assessment of baroreflex function during squat-stand maneuvers may involve not only arterial, but also cardiopulmonary, baroreflex due to changes in central blood volume/pressure (39).
In this study, we explored whether arterial-cardiac baroreflex function can be assessed during repeated squat-stand maneuvers at different frequencies. We hypothesized that coherent changes in heart rate (or R-R interval) would be produced in response to oscillations in arterial pressure, reflecting mainly a baroreflex mechanism. To reduce the potential effects of the muscle reflex and/or central command on the assessment of baroreflex function, passive squat-stand maneuvers were performed by using a pulley system to assist changes in body position. Finally, using this method, we demonstrated the well-known effects of aging on reducing baroreflex sensitivity in the elderly.
METHODS
Subjects.
Ten healthy young subjects with a mean age of 30 yr (range 23–39 yr, 6 women) and eight elderly subjects with a mean age of 66 yr (range 58–78 yr, 5 women) participated in one study of performing only active squat-stand maneuvers. Another group of 10 healthy subjects with a mean age of 35 yr (range 23–50 yr, 6 women) participated in a second study of performing both active and passive squat-stand maneuvers to assess the influence of activation of the muscle reflex and/or central command on the baroreflex function. Elderly subjects with normal cognitive function were recruited from the University of Texas Southwestern Alzheimer's Disease Center. No subject smoked or had known medical problems. Subjects were screened carefully with a medical history and a physical examination including 12-lead ECG. Two elderly subjects with leg or knee problems that prevented them from performing the squat-stand maneuvers were excluded. The protocol for this study was approved by and all subjects signed an informed consent form approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and the Presbyterian Hospital of Dallas.
Instrumentation and procedures.
An electrocardiogram (ECG) was recorded using a three-lead system (Hewlett-Packard). Beat-to-beat hear rate was obtained from each R-R interval identified from the QRS complexes. Arterial pressure was measured noninvasively at the middle finger of the right hand using Finapres (Ohmeda). The finger pressure cuff was positioned carefully at heart level with the hand being held in the left midaxillary line. The hand and arm were supported securely and comfortably with a custom-made vest-sling system using Velcro to ensure the stability of the pressure recordings during squat-stand maneuvers. Intermittent arterial pressure was measured in the left arm by electrosphygmomanometry (Suntech) to corroborate the measurement of finger arterial pressure. Respiratory CO2 was measured via a nasal cannula with capnography (Criticare Systems) to monitor the breathing frequency.
All experiments were performed in the morning in an environmentally controlled laboratory with an ambient temperature of 22°C. Subjects refrained from heavy exercise and caffeinated or alcoholic beverages at least 24 h before the tests.
For active squat-stand maneuvers, first, a single squat-stand maneuver was performed. After 10 min rest in the sitting position, subjects stood up for 2 min for stabilization followed by a squat for 1 min and then stand for 2 min (Fig. 1). During squatting, subjects could take either a tiptoe or a feet-flat position, depending on their choice for a comfortable performance. This maneuver was repeated three times separated by two 3-min recovery intervals. The purpose of performing this single squat-stand maneuver was to confirm the temporal characteristics of changes in arterial pressure and heart rate as observed in previous studies (22, 30). This maneuver was performed only in eight young and five elderly subjects because changes in arterial pressure and heart rate were highly reproducible. After a 10-min recovery in the sitting position, spontaneous changes in arterial pressure and R-R interval were recorded for 5 min for spectral analysis of cardiovascular variability to compare with those during repeated squat-stand maneuvers.
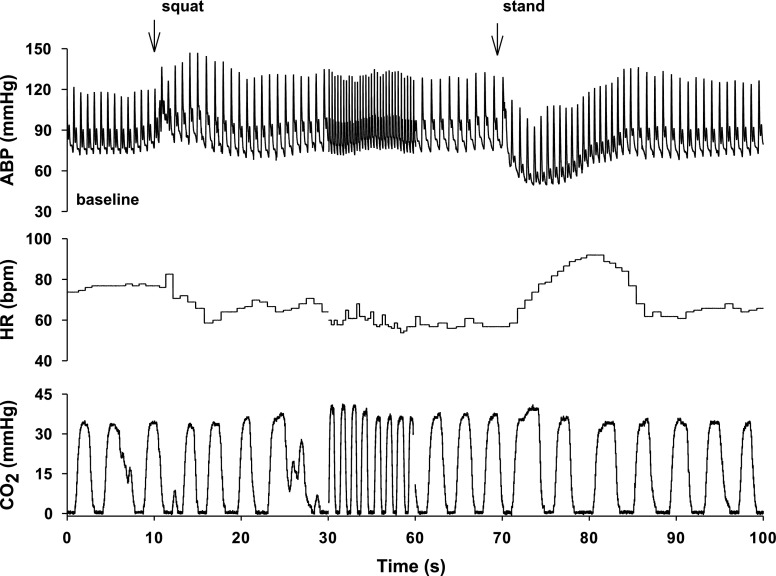
Fig. 1.
Representative changes in arterial blood pressure (ABP), heart rate (HR), and respiratory CO2 in a young subject during a single squat-stand maneuver. The time scale between 30 and 60 s is compressed to highlight the transient changes in ABP and HR during squatting and standing up, indicated by the arrows.
Subjects were instructed to perform repeated squat-stand maneuvers at the frequency of 0.1 Hz (5-s squat followed by 5-s stand) for 5 min. After a 10-min recovery, the maneuver was repeated at 0.05 Hz (10-s squat followed by 10-s stand) for 5 min (Fig. 2). During these tests, subjects were instructed to breathe normally to avoid performing a Valsalva maneuver while standing up. The breathing pattern was monitored continuously with respiratory CO2 (Fig. 2). When necessary, a stand was provided to the elderly to grab with the left hand to reduce the physical strain each time when they stood up from squatting (13). In addition, shorter durations of 3 min for 0.1 Hz and 4 min for 0.05 Hz were used to reduce their effort to perform these maneuvers.
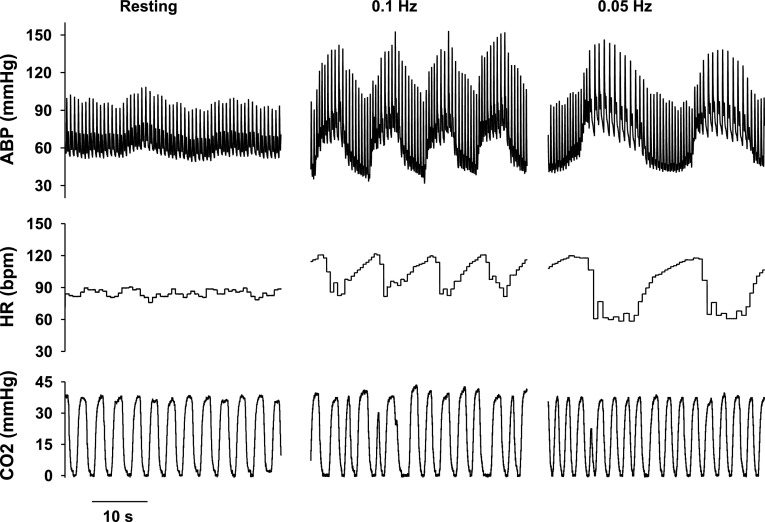
Fig. 2.
Changes in ABP, HR, and respiratory CO2 in a young subject under resting conditions and during active squat-stand maneuvers at 0.1 and 0.05 Hz. Note the large and coherent oscillations in ABP and HR during these maneuvers relative to resting conditions.
For passive squat-stand maneuvers, a pulley system attached to a rock-climbing harness worn by the subjects was used to support the body weight to reduce the leg muscle contractions during changes in body position. During the experiment, subjects were lowered down during squatting and pulled up during standing and were instructed to make no subjective effort to minimize the effects of muscle contraction and/or central command on arterial pressure and heart rate. The frequencies used for these maneuvers were the same as for active maneuvers. The order of active and passive maneuvers was randomized for each subject. A time interval of at least 20 min between passive and active maneuvers was used for recovery.
There are several reasons for us to select the 0.1 and 0.05 Hz frequencies for these maneuvers. First, these frequencies were below the respiratory frequencies of the subjects (Tables 1–3). Therefore, respiration should have minimal effects on changes in arterial pressure and heart rate (or R-R interval) at these frequencies. Second, oscillations in arterial pressure are likely to be enhanced at these frequencies because they are close to the baroreflex-mediated resonance frequencies observed in humans (11, 38). Finally, transfer function gain estimated during the 0.1-Hz maneuvers could be compared directly with that under resting conditions at the frequencies around 0.1 Hz (14).
Data analysis.
For the single squat-stand maneuver, increases in systolic blood pressure (SBP) and decreases in heart rate during squatting were obtained as the mean value during squat minus the baseline data (averaged 30-s data before squat). During standing, maximal reduction in SBP was obtained as the averaged 30-s data before standing minus the nadir of SBP after standing (Fig. 1). Maximal increases in heart rate were obtained similarly. The ratio of maximal changes in R-R interval to maximal changes in SBP was calculated to reflect baroreflex function. The use of R-R interval rather than heart rate to reflect baroreflex function would be consistent with most previous studies (14). In addition, animal studies indicate that changes in R-R interval rather than heart rate are related linearly to efferent vagal activity (15). It should be noted that assessment of baroreflex function using this index is explorative in this study. The purpose is to determine whether the effects of aging on the baroreflex function also can be revealed during a single squat-stand maneuver.
For repeated squat-stand maneuvers and those under resting conditions, the data segments of arterial pressure, heart rate, and respiratory frequency were averaged to assess the steady-state hemodynamics. Spectral power of SBP and R-R interval was estimated to quantify SBP and R-R variability in different frequency ranges. Transfer function between changes in SBP and R-R interval was estimated to quantify baroreflex function using the method described in our previous studies (14, 24). Briefly, under resting conditions, spectral power of SBP and R-R interval, mean values of transfer function gain, phase, and coherence were calculated in the very low (VLF; 0.0078–0.05 Hz), low (LF; 0.05–0.15 Hz), and high (HF; 0.15–0.35 Hz) frequency ranges (14, 24). Moreover, gain, phase, and coherence were calculated in the frequency range from 0.078 to 0.125 Hz to compare directly with those obtained during squat-stand maneuvers. For repeated squat-stand maneuvers, spectral power of SBP and R-R interval, mean values of transfer function gain, phase, and coherence were calculated in the frequency range from 0.078 to 0.125 Hz for the maneuvers at 0.1 Hz and from 0.031 to 0.078 Hz for the maneuvers at 0.05 Hz, because coherence was > 0.5 in these frequency ranges (Tables 2 and 3). Finally, peak value of coherence was identified during squat-stand maneuvers and the corresponding gain and phase were obtained.
Statistics.
An unpaired t-test was performed between the young and elderly to determine the effects of aging on spontaneous cardiovascular variability under resting conditions. Two-way ANOVA was used to determine the effects of aging and the frequencies of squat-stand maneuvers on the measurements. Two-way ANOVA also was used to determine the effects of the active and passive maneuvers as well as the frequencies of the maneuvers on the assessment of baroreflex function. Mann-Whitney rank sum tests were performed if the variables were not normally distributed or had unequal variances. The relationship between the transfer function gain in the frequency range from 0.078 to 0.125 Hz under resting conditions and the gain during active squat-stand maneuvers at 0.1 Hz was examined using Pearson's linear correlation, and the group means were compared with paired t-tests. This correlation was not conducted at 0.05 Hz because most subjects had low coherence at the very low frequencies for spontaneous oscillations (Table 1). Pearson's correlation also was used to examine the relationship between the estimates of transfer function gain between the active and passive squat-stand maneuvers. Statistical data analysis was performed using SigmaStat (version 3.1). Data are presented as means ± SD. The significance level was set to P < 0.05.
Table 1.
Spontaneous cardiovascular variability under resting conditions
| Variables | Young, n = 10 | Elderly, n = 8 |
|---|---|---|
| SBP, mmHg | 120±10 | 143±14* |
| DBP, mmHg | 68±10 | 70±6 |
| HR, beats/min | 71±16 | 71±14 |
| RF, Hz | 0.29±0.06 | 0.27±0.09 |
| ETCO2, mmHg | 37±4 | 38±3 |
| SBPVLF, mmHg2 | 13±9 | 16±16 |
| SBPLF, mmHg2 | 8±5 | 10±13 |
| SBPHF, mmHg2 | 2±1 | 3±3 |
| RRVLF, ms2 | 908±812 | 133±62* |
| RRLF, ms2 | 845±619 | 226±372* |
| RRHF, ms2 | 369±357 | 88±88* |
| Gain-VLF, ms/mmHg | 5.7±4.4 | 2.6±1.7 |
| Gain-LF, ms/mmHg | 9.3±4.0 | 3.7±2.4* |
| Gain-HF, ms/mmHg | 11.9±7.7 | 5.2±4.6* |
| Gain-SF, ms/mmHg | 9.8±3.8 | 3.6±2.2* |
| Phase-VLF, rads | −0.9±0.6 | −0.9±1.0 |
| Phase-LF, rads | −0.9±0.4 | −1.3±0.3 |
| Phase-HF, rads | −0.1±0.4 | −0.3±0.5 |
| Phase-SF, rads | −0.9±0.3 | −1.4±0.5* |
| Coherence-VLF | 0.38±0.11 | 0.45±0.13 |
| Coherence-LF | 0.65±0.09 | 0.62±0.15 |
| Coherence-HF | 0.61±0.10 | 0.48±0.15 |
| Coherence-SF | 0.70±0.18 | 0.66±0.16 |
Values are means ± SD. SBP and DBP, systolic and diastolic blood pressure. HR, heart rate. RF, respiratory frequency. ETCO2, end-tidal CO2. SBPVLF, SBPLF, SBPHF, RRVLF, RRLF and RRHF, spectral power of SBP and R-R interval at the very low (VLF), low (LF), and high frequencies (HF). Transfer function gain, phase, and coherence were estimated in the VLF, LF, and HF ranges and in the specific frequency range from 0.078 to 0.125 Hz (SF).
P < 0.05, comparisons between the young and elderly.
RESULTS
Representative changes in arterial pressure and heart rate during a single squat-stand maneuver are presented in Fig. 1. During squatting, arterial pressure increased acutely, then fell slightly, and stabilized at a level above baseline. Mean values of increases in SBP were 13 ± 5 mmHg for the young and 9 ± 16 mmHg for the elderly. In response to increases in arterial pressure, heart rate decreased by 8 ± 8 beats/min in the young and by 1 ± 4 beats/min in the elderly. During standing up from squat, SBP fell substantially by 42 ± 14 mmHg in the young and by 49 ± 10 mmHg in the elderly. In response to these large falls in pressure, heart rate increased by 37 ± 10 beats/min in the young but only by 8 ± 9 beats/min in the elderly. As expected, the ratio of maximal change in R-R interval divided by maximal change in SBP was reduced in the elderly (young, 7.8 ± 2.5 ms/mmHg; elderly, 1.3 ± 1.4 ms/mmHg, P = 0.0003), indicating reduced baroreflex sensitivity with aging.
Under resting conditions, relative to the young, R-R variability was reduced in the elderly, despite similar BP variability (Table 1). As expected, transfer function gain was reduced. During active squat-stand maneuvers, marked oscillations in arterial pressure and heart rate (or R-R interval) were produced at the frequencies of 0.05 and 0.1 Hz (Figs. 2 and 4, Tables 2 and 3). When compared with the young, induced R-R variability was reduced substantially in the elderly, consistent with reductions in transfer function gain (Table 2). In addition, the estimated phase was more negative in the elderly than in the young (Table 2). Finally, a weak but significant linear correlation was observed between the estimates of transfer function gain under resting conditions and those during squat-stand maneuvers in the frequency range from 0.078 to 0.125 Hz (r2 = 0.48, P = 0.001). However, gain in this frequency range under resting conditions was significantly higher than that during squat-stand maneuvers in both young and elderly subjects (P < 0.05, Tables 1 and 2).
Table 2.
Cardiovascular variability during active squat-stand maneuvers in young and elderly subjects
| Variables | Young, n = 10 |
Elderly, n = 8 | ||
|---|---|---|---|---|
| 0.1 Hz | 0.05 Hz | 0.1 Hz | 0.05 Hz | |
| SBP, mmHg | 134±15 | 129±15 | 170±28* | 153±27* |
| DBP, mmHg | 67±12 | 67±10 | 75±8 | 70±9 |
| HR, beats/min | 88±17 | 82±17 | 92±14 | 88±15 |
| RF, Hz | 0.37±0.05 | 0.37±0.06 | 0.38±0.12 | 0.37±0.13 |
| ETCO2, mmHg | 38±5 | 36±4 | 40±3 | 38±4 |
| SBP-SP, mmHg2 | 253±119 | 374±191 | 203±87 | 561±321† |
| RR-SP, ms2 | 8075±8930 | 24839±13829† | 497±677 | 3098±3726* |
| Gain, ms/mmHg | 5.4±2.9 | 7.6±2.8† | 1.2±1.0* | 2.2±2.1* |
| Gain-P, ms/mmHg | 5.3±2.9 | 8.6±3.4† | 1.1±0.8* | 2.1±2.2* |
| Phase, rads | −0.8±0.3 | −0.5±0.2 | −1.6±0.7* | −1.3±0.5*† |
| Phase-P, rads | −0.8±0.3 | −0.4±0.2† | −1.7±0.6* | −1.2±0.5*† |
| Coherence | 0.82±0.07 | 0.79±0.08 | 0.69±0.15 | 0.72±0.16 |
| Coherence-P | 0.97±0.03 | 0.98±0.01 | 0.89±0.14 | 0.92±0.08† |
Values are means ± SD. Transfer function gain, phase, and coherence were estimated in the frequency range from 0.078 to 0.125 Hz and from 0.031 to 0.078 Hz. Gain-P, Phase-P, and Coherence-P were estimated at the peak coherence.
P < 0.05, comparisons between young and elderly at the same frequency.
P < 0.05, comparisons between the frequencies for the same group.
Table 3.
Cardiovascular variability during active and passive squat-stand maneuvers in young subjects
| Variables | Active |
Passive | ||
|---|---|---|---|---|
| 0.1 Hz | 0.05 Hz | 0.1 Hz | 0.05 Hz | |
| SBP, mmHg | 133±13 | 128±11 | 138±17 | 128±11† |
| DBP, mmHg | 62±12 | 62±10 | 68±13 | 64±10 |
| HR, beats/min | 86±9 | 79±9† | 84±9 | 77±8† |
| RF, Hz | 0.35±0.7 | 0.32±0.06 | 0.40±0.09 | 0.37±0.09 |
| ETCO2, mmHg | 39±3 | 39±3 | 39±3 | 39±3 |
| SBP-SP, mmHg2 | 223±90 | 343±248† | 75±59* | 113±74* |
| RR-SP, ms2 | 4,224±2,872 | 16,933±6,236† | 1813±2130 | 6227±3608*† |
| Gain, ms/mmHg | 4.3±1.5 | 6.8±2.4† | 4.4±2.5 | 7.0±2.5† |
| Gain-P, ms/mmHg | 4.1±1.8 | 7.7±2.6† | 4.5±2.5 | 8.3±4.2† |
| Phase, rads | −0.9±0.6 | −0.6±0.3 | −0.9±0.5 | −0.9±0.5 |
| Phase-P, rads | −1.0±0.6 | −0.5±0.2† | −1.1±0.5 | −0.8±0.3*† |
| Coherence | 0.70±0.06 | 0.77±0.08 | 0.73±0.17 | 0.69±0.09 |
| Coherence-P | 0.99±0.01 | 0.99±0.01 | 0.96±0.09 | 0.96±0.05 |
Values are means ± SD (n = 10). Transfer function gain, phase, and coherence were estimated in the frequency range from 0.078 to 0.125 Hz and from 0.031 to 0.078 Hz. Gain-P, Phase-P and Coherence-P, estimated at the peak coherence.
P < 0.05, comparisons between active and passive maneuvers at the same frequency,
P < 0.05 comparisons between the frequencies under the same conditions.
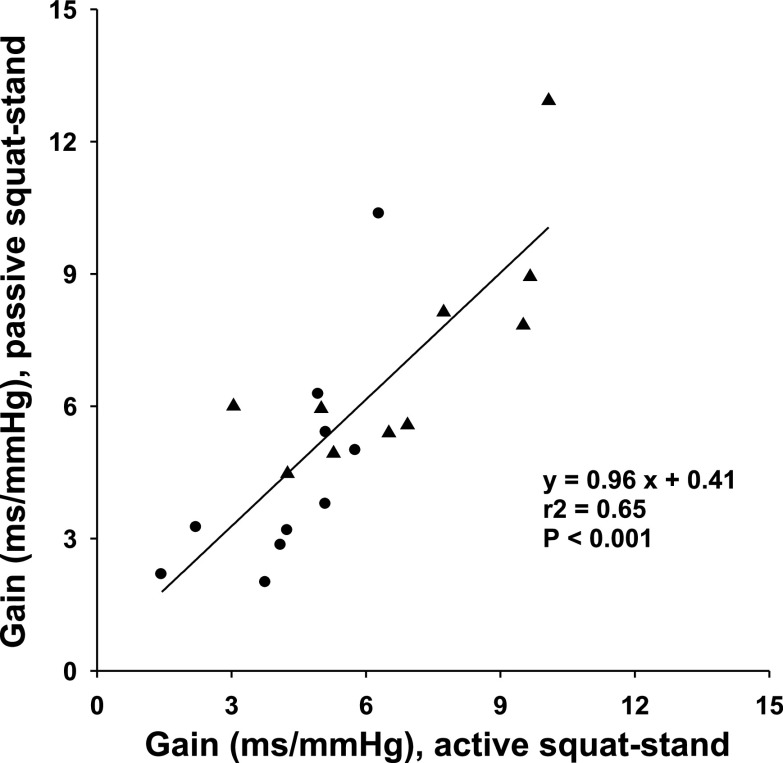
Passive squat-stand maneuvers at both 0.1 and 0.05 Hz also generated marked oscillations in arterial pressure and heart rate (or R-R interval) (Figs. 3 and 4). However, these oscillations were significantly smaller than those during active maneuvers (Fig. 4, Table 3). Notably, no difference in transfer function gain was observed between the two maneuvers (Table 3). Finally, a significant linear correlation of transfer function gain between the two maneuvers was observed (Fig. 5).
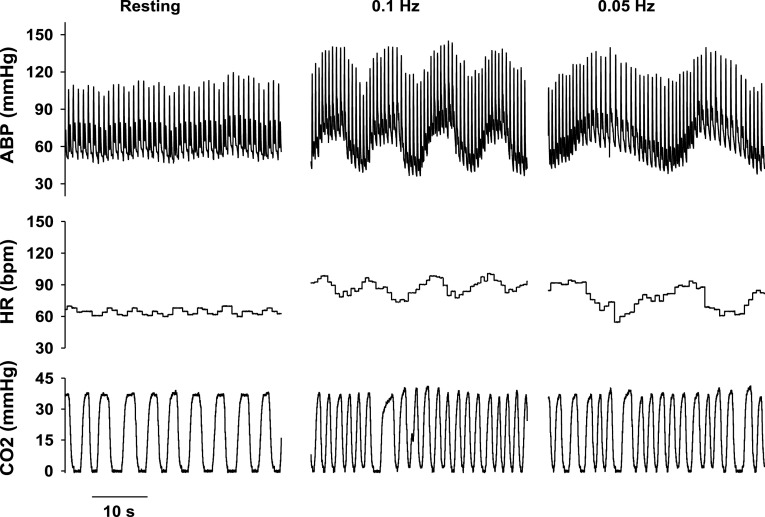
Fig. 3.
Changes in ABP, HR, and respiratory CO2 in a young subject under resting conditions and during passive squat-stand maneuvers at 0.1 and 0.05 Hz. Note the large and coherent oscillations in ABP and HR during these maneuvers relative to resting conditions.
Fig. 4.
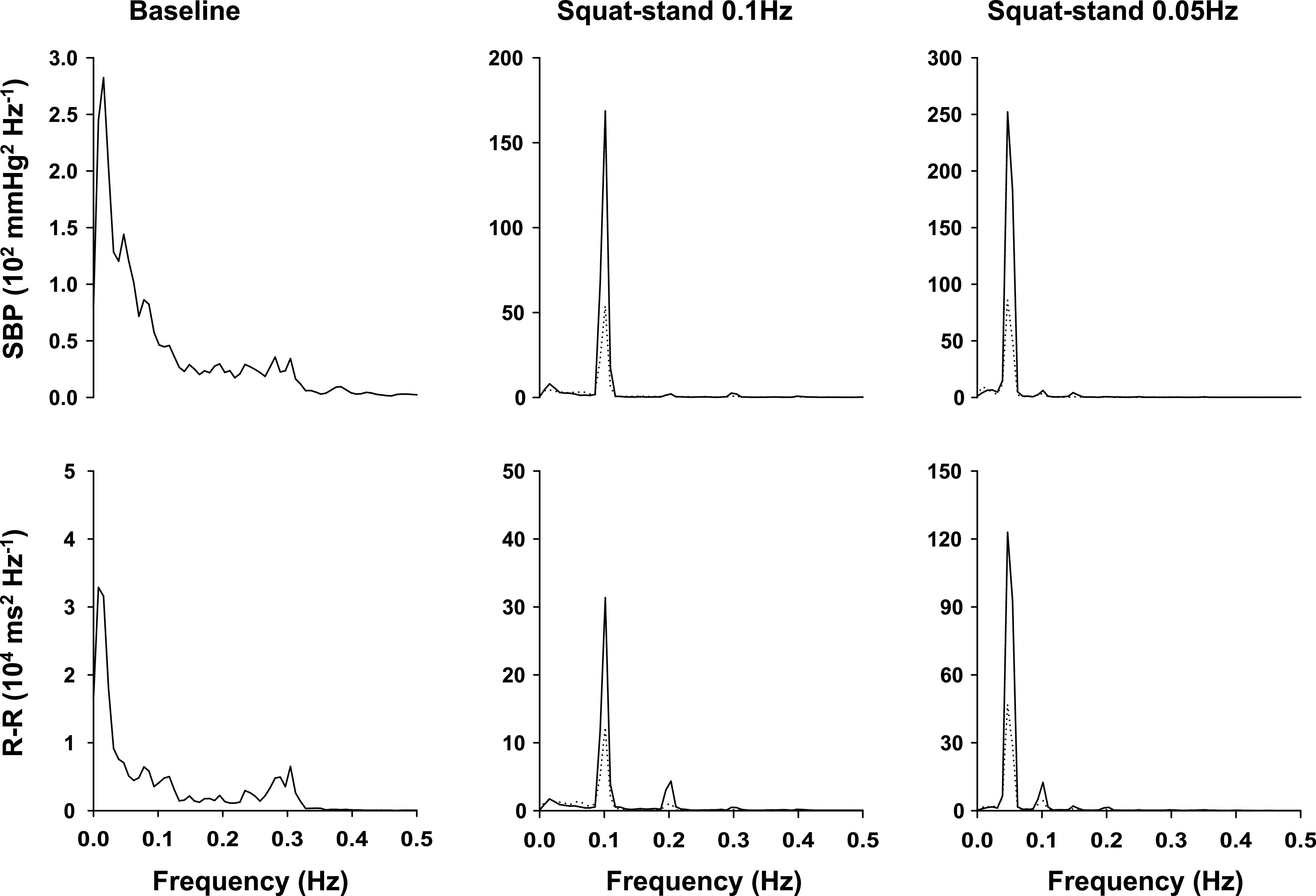
Group averaged autospectra of systolic blood pressure (SBP) and R-R interval variability obtained from young subjects under resting conditions and during squat-stand maneuvers at 0.1 and 0.05 Hz (n = 10). Solid lines are from active squat-stand maneuvers; dotted lines are passive squat-stand maneuvers. Note the scale differences in the y-axes under resting conditions and during squat-stand maneuvers. Large scales of y-axis are used to represent large oscillations in SBP and R-R during squat-stand maneuvers. The apparent coherent oscillations in SBP and R-R at 0.3 Hz under resting conditions suggest the synchronization of spontaneous changes in SBP and R-R with respiration.
Fig. 5.
Linear correlation of transfer function gain during active and passive squat-stand maneuvers. Note that gain at the 0.05 Hz appears to be higher than that at 0.1 Hz (Table 3). The y-intercept value of linear regression (0.41) is not significantly different from zero (P = 0.69). Data at 0.1 Hz (•); data at 0.05 Hz (▴).
In contrast to the trend toward a reduction in gain for lower frequencies under resting conditions, transfer function gain during squat-stand maneuvers was higher at 0.05 Hz than 0.1 Hz associated with a reduction in phase (Tables 2 and 3). However, despite a higher gain (higher baroreflex sensitivity), spectral power of BP at 0.05 Hz was larger than at 0.1 Hz (Tables 2 and 3).
Peak value of coherence was between 0.89 to 0.99 during squat-stand maneuvers under all conditions (Tables 2 and 3). Gain and phase at the peak value of coherence were similar to the mean values averaged over each of the corresponding frequency ranges (Tables 2 and 3).
DISCUSSION
The main findings of this study are threefold. First, repeated squat-stand maneuvers at the frequencies of 0.1 and 0.05 Hz produced large and coherent oscillations in arterial pressure and R-R interval. Second, oscillations in arterial pressure and R-R interval were smaller during passive than during active maneuvers. However, transfer function gain did not change between the two maneuvers. Finally, when compared with the young, transfer function gain was reduced and the phase became more negative in the elderly, demonstrating the well-known effects of aging on reducing baroreflex sensitivity. Collectively, these findings suggest that the changes in R-R interval elicited by BP perturbations during squat-stand maneuvers are mediated primarily by a baroreflex mechanism and that baroreflex function can be assessed using the transfer function method during large perturbations in arterial pressure.
Arterial pressure and heart rate during squat-stand maneuvers.
Squatting involves leg muscle contraction and compression of leg arteries and veins (23, 34). In addition, it brings the heart closer to the level of the feet, thus removing the force of gravity on the circulation below heart level, similar to the supine position (23). Thus, cardiac output increases due to increases in central blood volume and stroke volume (12, 19, 23). Interestingly, peripheral vascular resistance either was reduced slightly or did not change despite compression of the leg blood vessels (12, 19). Therefore, increases in arterial pressure during squat are determined mainly by increases in cardiac output.
Notably, increases in stroke volume and arterial pressure during squat in patients with heart transplantation (cardiac denervation) were similar to those in healthy individuals (12). Thus, increases in cardiac output most likely are mediated by the Frank-Starling mechanism. Moreover, these findings suggest that changes in heart rate have minimal, if any, effects on the increases in arterial pressure.
In this study, consistent with previous studies, heart rate was reduced during squatting in response to increases in arterial pressure (23, 39). The reduction in heart rate has been attributed to a arterial baroreflex mechanism since involvement of the cardiopulmonary reflex associated with changes in central blood volume/pressure is likely to play a minimal role in the regulation of heart rate (6, 34). In addition, autonomic blockade with atropine abolished changes in heart rate, suggesting an enhanced cardiac vagal activity in response to increases in arterial pressure during squatting (22).
Arterial pressure fell substantially upon standing up from a squat. This transient reduction in pressure has been attributed to a reduction in peripheral vascular resistance since cardiac output was increased, rather than decreased, during fall in pressure (20). The increase in heart rate in response to the fall in pressure suggests a baroreflex mechanism. However, the possibility of activation of the muscle mechanoreflex and/or central command associated with muscle contraction cannot be excluded (30, 39).
To assess the potential effects of the muscle reflex and/or central command on the baroreflex, passive squat-stand maneuvers were performed to reduce the rhythmic muscle contractions or the involvement of central command during change in body posture. During the passive maneuvers, oscillations of arterial pressure and R-R interval both were reduced when compared with active maneuvers. However, estimation of transfer function gain between these variables did not change. These findings suggest that activation of the muscle reflex and/or central command during squat-stand maneuvers do not alter arterial-cardiac baroreflex function.
The reduced pressure oscillations during the passive maneuvers could be attributed to a smaller change in cardiac output and/or peripheral vascular resistance or possibly less central command and less muscle reflex activity. In addition, vascular myogenic responses to BP oscillations and/or the activities of muscle pump also may play a role (39). Conversely, the commensurate reduction in R-R oscillations most likely reflect a baroreflex mechanism in response to smaller changes in arterial pressure.
Assessment of baroreflex function using the transfer function method.
Transfer function analysis of spontaneous changes in arterial pressure and R-R interval has been used for assessing baroreflex function (14, 29). However, the magnitude of spontaneous changes in BP and R-R interval may be small and variable among individual subjects and may not be mediated exclusively by the baroreflex (4, 6). In addition, changes in R-R interval may have feed-forward and/or feedback effects on arterial pressure, thus formulating a closed-loop relationship under resting conditions (36). Not surprisingly, using the open-loop transfer function method to assess baroreflex function was biased in animal studies (16). In this regard, both theoretical analysis and experimental data suggest that the accuracy of assessing baroreflex function could be improved by introducing external perturbations to arterial pressure (16, 21).
Based on this knowledge, repeated squat-stand maneuvers were employed to elicit large and clinically relevant perturbations in arterial pressure to provoke the baroreflex. As discussed above, changes in arterial pressure during squat-stand maneuvers are determined mainly by changes in central blood volume and peripheral vascular resistance (12, 39). Therefore, changes in heart rate (or R-R interval) should have minimal effects on changes in arterial pressure, providing justifications to use the open-loop transfer function method to assess baroreflex function (16, 21). Peak value of coherence was ≥ 0.89 during squat-stand maneuvers at both the frequencies of 0.05 and 0.1 Hz and for both young and elderly subjects, further supporting the validity of using the transfer function method.
In line with this discussion, a weak but significant linear correlation was observed between the estimates of transfer function gain under resting conditions and during squat-stand maneuvers. However, gain under resting conditions was significantly higher than that during squat-stand maneuvers. It is possible that estimation of transfer function gain under resting conditions may be biased if changes in R-R interval had significant feed-forward and/or feedback effects on spontaneous changes in arterial pressure (16, 21, 36).
Furthermore, we found that transfer function gain during squat-stand maneuvers at 0.05 Hz was higher than that at 0.1 Hz, associated with a reduction in phase. These findings are consistent with the low pass filter properties of baroreflex function (1, 32). However, despite a higher gain (higher baroreflex sensitivity), oscillations in BP were larger at 0.05 Hz than at 0.1 Hz, reflecting possibly an enhanced myogenic vascular response and/or larger changes in cardiac output at the 0.05 Hz (39). These observations also are consistent with the argument that changes in heart rate (or R-R interval) mediated by the baroreflex are not likely to be essential for buffering changes in BP during squat-stand maneuvers.
Effects of aging on baroreflex function.
In the elderly, not only was the magnitude of R-R interval response to changes in arterial pressure decreased, but also the estimate of phase between these variables became more negative relative to the young. The decreased R-R interval or heart rate response to changes in BP (reduction in transfer function gain) most likely reflect the well-known effects of aging on reducing baroreflex sensitivity (8). However, the underlying mechanisms for changes in phase are not clear.
The phase lag between changes in BP and R-R interval is likely to be determined not only by the baroreflex latency, but also the dynamic components (low pass filter) of the baroreflex function (7). Arterial-cardiac baroreflex latency is determined mainly by the vagal nerve activity (6). Moreover, heart rate response to sympathetic nerve stimulation is much slower than to vagal activity (1, 32). Aging is associated with a reduction in vagal activity and increases in sympathetic neural activity (33). Thus, the increased phase lag (more negative phase) in the elderly suggests that heart rate response to changes in BP was delayed, mediated by changes in autonomic neural activity (18, 33, 35).
However, despite a substantially decreased heart rate response, BP oscillations during squat-stand maneuvers were not enhanced significantly in the elderly (Table 2). Assuming similar changes in cardiac output during squat-stand maneuvers between the young and elderly subjects, these observations would suggest that a preserved arterial baroreflex and/or cardiopulmonary reflexes in control of the sympathetic neural activity, and hence vascular resistance, may play a more important role than the arterial-cardiac baroreflex for buffering changes in BP in the elderly (8). In addition, consistent with previous studies, these observations also suggest that changes in heart rate may not play an important role in orthostatic hypotension in the elderly (8, 13, 37).
Study limitations.
First, we cannot exclude completely the possibility that muscle reflex and/or central command may influence heart rate during squat-stand maneuvers either directly or indirectly through interactions with the baroreflex (28, 31). Particularly, activation of muscle reflex and/or central command may cause an overall vagal withdrawal and increase in sympathetic neural activity, leading to increases in steady-state heart rate and arterial pressure. However, reducing the potential effects of these confounding factors with passive squat-stand maneuvers did not alter the dynamic relationship between beat-to-beat changes in arterial pressure and R-R interval. These results are consistent with previous studies showing that R-R interval response to sinusoidal changes in neck pressure remained unchanged during dynamic knee-extension exercise (40). Furthermore, leg pulling and compression in the supine position (squat-lying) or squat in water produced little or no effect on arterial pressure and heart rate (2, 23). Thus, we suspect that activation of muscle reflex and/or central command plays a minor, if any, role in the regulation of heart rate in response to changes in arterial pressure during squat-stand maneuvers.
Second, similar to the Valsalva maneuver or infusion of vasoactive drugs, assessment of baroreflex function during squat-stand maneuvers may involve multiple receptors, including both arterial and cardiopulmonary baroreceptors (6). Contributions from each of the individual baroreceptors to changes in heart rate cannot be determined in this study. Thus, assessment of baroreflex function during squat-stand maneuvers is likely to reflect an integrated baroreflex function associated with changes in body posture in the daily life. In addition, we do not know the specific mechanisms for autonomic control of arterial pressure and R-R interval. Further studies using autonomic blockade may provide insight on these issues (22).
Finally, the frail elderly, obese individuals, or people with severe orthostatic hypotension may not be able to perform squat-stand maneuvers. Practically, a less stressful sit-stand maneuver may be suitable for generating oscillations in arterial pressure to assess the baroreflex function (39).
Perspectives and Significance
Large and coherent oscillations in BP and R-R interval were produced during repeated squat-stand maneuvers at different frequencies. Similar transfer function gain between changes in BP and R-R interval was observed during active and passive maneuvers. These findings suggest that changes in heart rate are mediated primarily by a baroreflex mechanism in response to changes in BP. Thus, transfer function analysis can be used to assess baroreflex function during large perturbations in arterial pressure. Application of this method to the elderly confirmed the well-known effects of aging on reducing baroreflex sensitivity. Further work is warranted to demonstrate the reproducibility and to compare this method with other established methods for assessing the baroreflex function. The new method presented in this study potentially may improve the accuracy as well as the reliability for assessing baroreflex function in human studies. Furthermore, in-depth understanding the mechanisms for changes in arterial pressure and heart rate during squat-stand maneuvers may shed light on the complexity of cardiovascular regulation, falls, or syncope during orthostasis.
GRANTS
This study was supported, in part, by the Kelly King Foundation and National Institute on Aging Grant P30-AG-12300 to the University of Texas Southwestern Alzheimer's Disease Center.
Acknowledgments
We thank all the subjects for their willingness to participate in this study. We thank Dr. Kazunobu Okazaki and Robin Shook for the technical support and Chester Wu and Arenda van Beek for excellent work in assisting data analysis.
REFERENCES
- 1.Berger RD, Saul JP, Cohen RJ. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am J Physiol Heart Circ Physiol 256: H142–H152, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Brotmacher L Haemodynamic effects of squatting during recovery from exertion. Br Heart J 19: 567–573, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Convertino VA, Ratliff DA, Crissey J, Doerr DF, Idris AH, Lurie KG. Effects of inspiratory impedance on hemodynamic responses to a squat-stand test in human volunteers: implications for treatment of orthostatic hypotension. Eur J Appl Physiol 94: 392–399, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cooley RL, Montano N, Cogliati C, van de Borne P, Richenbacher W, Oren R, Somers VK. Evidence for a central origin of the low-frequency oscillation in RR-interval variability. Circulation 98: 556–561, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med 85: 167–173, 1975. [PubMed] [Google Scholar]
- 6.Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford, UK: Clarendon, 1992.
- 7.Elghozi JL, Julien C. Sympathetic control of short-term heart rate variability and its pharmacological modulation. Fundam Clin Pharmacol 21: 337–347, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol 95: 2591–2597, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med 120: 841–847, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Guyton AC Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. Am J Physiol Regul Integr Comp Physiol 288: R1637–R1648, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hanson P, Slane PR, Rueckert PA, Clark SV. Squatting revisited: comparison of haemodynamic responses in normal individuals and heart transplantation recipients. Br Heart J 74: 154–158, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imholz BP, Dambrink JH, Karemaker JM, Wieling W. Orthostatic circulatory control in the elderly evaluated by non-invasive continuous blood pressure measurement. Clin Sci (Lond) 79: 73–79, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki K, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol 95: 1575–1583, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol 218: 1030–1037, 1970. [DOI] [PubMed] [Google Scholar]
- 16.Kawada T, Sugimachi M, Sato T, Miyano H, Shishido T, Miyashita H, Yoshimura R, Takaki H, Alexander J Jr, Sunagawa K. Closed-loop identification of carotid sinus baroreflex open-loop transfer characteristics in rabbits. Am J Physiol Heart Circ Physiol 273: H1024–H1031, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Kenny RA, Kalaria R, Ballard C. Neurocardiovascular instability in cognitive impairment and dementia. Ann NY Acad Sci 977: 183–195, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Keyl C, Schneider A, Dambacher M, Bernardi L. Time delay of vagally mediated cardiac baroreflex response varies with autonomic cardiovascular control. J Appl Physiol 91: 283–289, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Krediet CT, de Bruin IG, Ganzeboom KS, Linzer M, van Lieshout JJ, Wieling W. Leg crossing, muscle tensing, squatting, and the crash position are effective against vasovagal reactions solely through increases in cardiac output. J Appl Physiol 99: 1697–1703, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Krediet CT, Go-Schon IK, Kim YS, Linzer M, Van Lieshout JJ, Wieling W. Management of initial orthostatic hypotension: lower body muscle tensing attenuates the transient arterial blood pressure decrease upon standing from squatting. Clin Sci (Lond) 113: 401–407, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ljung L System Identification. Upper Saddle River, NJ: Prentice Hall, 1987.
- 22.Marfella R, Giugliano D, di Maro G, Acampora R, Giunta R, D'Onofrio F. The squatting test. A useful tool to assess both parasympathetic and sympathetic involvement of the cardiovascular autonomic neuropathy in diabetes. Diabetes 43: 607–612, 1994. [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell TV, Mc IM. The circulatory effects of squatting. Am Heart J 64: 347–356, 1962. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol 99: 1041–1049, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Palmero HA, Caeiro TF, Iosa DJ, Bas J. Baroreceptor reflex sensitivity index derived from Phase 4 of the Valsalva maneuver. Hypertension 3: II134–137, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12: 214–222, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Pickering TG, Gribbin B, Sleight P. Comparison of the reflex heart rate response to rising and falling arterial pressure in man. Cardiovasc Res 6: 277–283, 1972. [DOI] [PubMed] [Google Scholar]
- 28.Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol 91: 37–49, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Robbe HW, Mulder LJ, Ruddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 10: 538–543, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Rossberg F, Penaz J. Initial cardiovascular response on change of posture from squatting to standing. Eur J Appl Physiol Occup Physiol 57: 93–97, 1988. [DOI] [PubMed] [Google Scholar]
- 31.Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol Heart Circ Physiol 261: H1231–H1245, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharpey-Schafer EP Effects of squatting on the normal and failing circulation. Br Med J 1: 1072–1074, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi X, Wray DW, Formes KJ, Wang HW, Hayes PM, AHOY, Weiss MS, Reese IP. Orthostatic hypotension in aging humans. Am J Physiol Heart Circ Physiol 279: H1548–H1554, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation 93: 1527–1532, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Taylor JA, Hand GA, Johnson DG, Seals DR. Sympathoadrenal-circulatory regulation of arterial pressure during orthostatic stress in young and older men. Am J Physiol Regul Integr Comp Physiol 263: R1147–R1155, 1992. [DOI] [PubMed] [Google Scholar]
- 38.van de Vooren H, Gademan MG, Swenne CA, TenVoorde BJ, Schalij MJ, Van der Wall EE. Baroreflex sensitivity, blood pressure buffering, and resonance: what are the links? Computer simulation of healthy subjects and heart failure patients. J Appl Physiol 102: 1348–1356, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Wray DW, Fadel PJ, Keller DM, Ogoh S, Sander M, Raven PB, Smith ML. Dynamic carotid baroreflex control of the peripheral circulation during exercise in humans. J Physiol 559: 675–684, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]