Abstract
Prenatal administration of dexamethasone and a low-protein diet has been shown to result in hypertension in the offspring when they are adults. The cause for the hypertension is unknown. The purpose of this study was to examine whether there was prenatal programming of thick ascending limb transport. Rats were administered either dexamethasone for 4 days (0.2 mg/kg body wt) by intraperitoneal injection daily between the 15th and 18th day of gestation, or they were fed a low-protein diet (6% protein) or an isocaloric normal protein diet (20% protein) from day 12 gestation until birth. The offspring were studied as adults. Prenatal dexamethasone and dietary protein deprivation resulted in an increase in blood pressure. Offspring of mothers fed a low-protein diet had an increase in medullary but not cortical bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2) protein abundance (P < 0.01). There was not a statistically significant increase in medullary NKCC2 by prenatal dexamethasone (P = 0.07). Both prenatal administration of dexamethasone and a low-protein diet resulted in an increase in medullary thick ascending limb chloride transport compared with control (298 ± 33 pmoles·mm−1·min−1, 280 ± 26 pmoles·mm−1·min−1, and 191 ± 21 pmoles·mm−1·min−1, respectively P < 0.05). There was a higher lumen-positive transepithelial potential difference in the prenatal dexamethasone and low-protein group compared with control as well. Administration of furosemide for 24 h resulted in a decrease in blood pressure in the low-protein group but not the control group. This study demonstrates that insults administered to the fetus can program altered sodium transport. Increased tubular sodium transport is a likely cause for the hypertension by prenatal programming.
Keywords: prenatal programming, hypertension, microperfusion, furosemide
prenatal insults to the developing fetus have been shown to cause hypertension and cardiovascular disease in later life (2–5, 43, 43). Animal models have been used to elucidate the pathogenesis of the hypertension, as well as other sequela of prenatal insults. The two best studied prenatal insults that cause postnatal hypertension in rodents are maternal dietary protein deprivation and prenatal administration of glucocorticoids (14, 15, 28, 30–34, 38, 40–42). In both prenatal dietary protein deprivation and prenatal administration of dexamethasone, there are distinct times during gestation when these insults are associated with hypertension. We have shown that prenatal dexamethasone-programmed hypertension in rats when administered between 15 and 18 days of gestation, but not before or after this time (41). Similarly, dietary protein deprivation results in hypertension when administered in the second half, but not the first half, of pregnancy when the offspring are studied as adults (50).
The mechanism whereby prenatal insults cause hypertension has been elusive. Animal and human studies have shown that intrauterine growth retardation results in a reduction in the number of nephrons (25–27, 36, 41, 42, 50), which has been postulated to cause hypertension in later life (11–13, 35). There is also evidence that the renin-angiotensin system and renal nerves may play a role in mediating the hypertension with prenatal programming (31, 39). Prenatal programming of hypertension could also be mediated by altered renal sodium transport (7, 15, 37).
Recently, maternal dietary protein deprivation has been shown to increase renal bumetanide-sensitive NKCC2 cotransporter and the thiazide-sensitive NaCl cotransporter mRNA and protein abundance in rats (16, 37). Whether this translates into an increase in sodium transport in these nephron segments is unknown. The rat thick ascending limb, but not the distal convoluted tubule, can be dissected and perfused in vitro. The purpose of the present study was to examine directly whether prenatal programming alters sodium transport in the thick ascending limb.
METHODS
Animals.
Pregnant Sprague-Dawley rats were fed either a 6% protein diet (low protein) or an isocaloric diet containing 20% protein (normal protein) from day 12 gestation until birth as previously described by Manning and colleagues (37, 38) to examine the effect of prenatal dietary protein deprivation on thick ascending limb chloride transport. After birth, the mother was placed on a normal protein diet, as were the offspring when weaned. The rats were studied at ∼6 wk of age. In experiments designed to examine the effect of prenatal dexamethasone on thick ascending limb chloride transport, rats were fed the normal protein diet and were administered dexamethasone (0.2 mg/kg body wt) by intraperitoneal injection daily between 15th and 18th day of gestation (15). We have previously shown that rats develop hypertension when dexamethasone is administered during this period of gestation (15, 41, 42). Rats from multiple litters were studied in each group. Only males were studied to lessen the variability. Females were not studied since one laboratory found that at 6 wk of age, only males were hypertensive (47), and another laboratory found that modest maternal protein restriction did not result in hypertension in female offspring (49). We wanted to ensure that we were studying hypertensive animals, and 6 wk is as old as it is possible to dissect tubules in a rat due to interstitial fibrosis. These studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Measurement of blood pressure and electrolytes.
Blood pressure was measured by tail cuff in rats using an International Instrumentation and Technical Consulting model 179 blood pressure analyzer (Woodland Hills, CA) at 6 wk of age. Rats were trained for 4 days by placing rats in a Lucite tube and inflating the tail blood pressure cuff several times a day prior to the actual measurement of blood pressure on the 5th day. Blood pressure was measured at least four times in each rat, and the means of these values were used as the blood pressure for that rat. The prenatal low-protein and prenatal dexamethasone groups were studied at different times. An equivalent number of controls were measured at the same time as the blood pressure was measured in prenatal low-protein and prenatal dexamethasone groups (15). These rats were then used for other experiments in this study. We studied the effect of furosemide on blood pressure in rats on the low-protein and control diets as well. In these experiments, rats were trained, and blood pressure was measured as above. Rats were then placed on furosemide in their drinking water at a dose of 12 mg/100 ml, as previously described, and their blood pressure was measured 24 h later (48). Serum electrolytes were measured using Critical Care Xpress (Nova Biomedical, Waltham, MA).
In vitro microperfusion flux studies.
Approximately 5–10 min prior to administration of anesthesia, rats were given intraperitoneal furosemide (2 mg/100 g body wt). Rats were given anesthesia using intraperitoneal Inactin (10 mg/100 g body wt). The abdomen was opened to expose the left kidney, which was bathed in ice-cold PBS for ∼1 min. The kidney was then quickly removed, and the kidney was cut into several coronal slices for microdissection. These maneuvers have been shown previously to preserve the viability of the thick ascending limb for in vitro microperfusion (18–20, 29).
Isolated segments of rat medullary thick ascending limbs (mTAL) were dissected free hand in Hanks' solution containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2 10 tris (hydroxymethyl) aminomethane hydrochloride, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. Dissected tubules were transferred to a 1-ml temperature-controlled bathing chamber.
Tubules were perfused with an ultrafiltrate-like solution that contained (in mM) 115 NaCl, 25 NaHCO3, 4.0 Na2HPO4, 10 Na acetate, 1.8 mM CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, 5 alanine, 2 lactate, and 2 glutamine at 3–4 nl/min. The tubules were bathed in a similar solution that contained 6 g/dl of BSA. The osmolality of all solutions was adjusted to 300 mosmol/kg H2O. The bathing solution was heated to 38°C and exchanged at 0.5 ml/min to maintain a constant pH and osmolality.
Since the rate of volume absorption is zero in the mTAL, the rate of chloride transport was calculated as JCl = (Vo) (Clo − Cll)Po/CloL, where Vo is the collection rate; Clo and Cll were the chloride concentrations in the perfusion solution and the collected fluid, respectively, in counts per minute per nanoliter; Po is the chloride concentration in mM; and L is the tubular length in mm. The tubule lengths were measured with an eyepiece micrometer. The perfusion solution contained 36Cl at a concentration of 10 μCi/ml. The collection rate was measured with a 30-nl constant-volume pipette. Tubules were incubated for ∼10 min before initiation of the measurements for chloride absorption. There were three measurements per tubule, and the mean of the three collections was used to represent the chloride absorption for that tubule. In some tubules, the bathing solution was cooled to <20°C to inhibit active transport to demonstrate that chloride transport could be totally inhibited.
The transepithelial potential difference was measured using the perfusion pipette as the bridge into the lumen of the tubule and referenced to the bathing solution using a World Precision Instruments Electrometer (Sarasota, FL). In this segment, the luminal positive potential is due to luminal potassium secretion and reflective of net chloride transport (21, 23).
Protein isolation and analysis.
Rats were anesthetized with intraperitoneal Inactin (100 mg/kg), and the kidneys were quickly removed and placed in ice-cold PBS. The rat was then killed. Under a magnifying glass, the cortex and medulla were dissected. For immunoblot studies, the dissected tissue was put in sucrose buffer containing 250 mM sucrose and 10 mM triethanolamine, 1 μl/1 ml of protease inhibitor cocktail (Sigma, St. Louis, MO), and 100 μg/ml phenylmethylsulfonyl fluoride (Calbiochem, La Jolla, CA) (pH = 7.4). The tissue was homogenized with 15 strokes of polytetrafluoroethylene-glass homogenizer at 4°C. The homogenate was centrifuged at 1000 g for 15 min, and the supernatant protein concentration was assayed using the Bradford method (10).
SDS-PAGE and immunoblotting.
Protein samples (50 μg/lane) were denatured and separated on a 7.5% polyacrylamide gel using SDS-PAGE as previously described (6, 44). Proteins were then transferred to polyvinylidene diflouride membranes at 100 mV at 4°C for 1 h. The blots were subsequently blocked using Blotto (1% nonfat milk and 0.1% Tween-20 in PBS, pH 7.4) for 1 h before incubation with primary antibody to NKCC2 (a generous gift from H. M. Kwon) that was added to the Blotto at 1:1,500 dilution. The blots were then washed in Blotto followed by the addition of the secondary antibody, a horseradish peroxidase-conjugated donkey anti-rabbit at 1:10,000 dilution. The bound antibody was detected using enhanced chemiluminescence (Perkin-Elmer, Boston, MA). β-actin was used to verify equal loading of proteins at 1:10,000 dilution (Sigma Biochemicals and Reagents, St. Louis, MO). Relative NKCC2 and β-actin protein abundance were quantitated using densitometry.
Statistics.
ANOVA was used to determine statistical significance for studies with more than two groups. A Student's t-test was used to compare studies with two groups. Data are expressed as means ± SE.
RESULTS
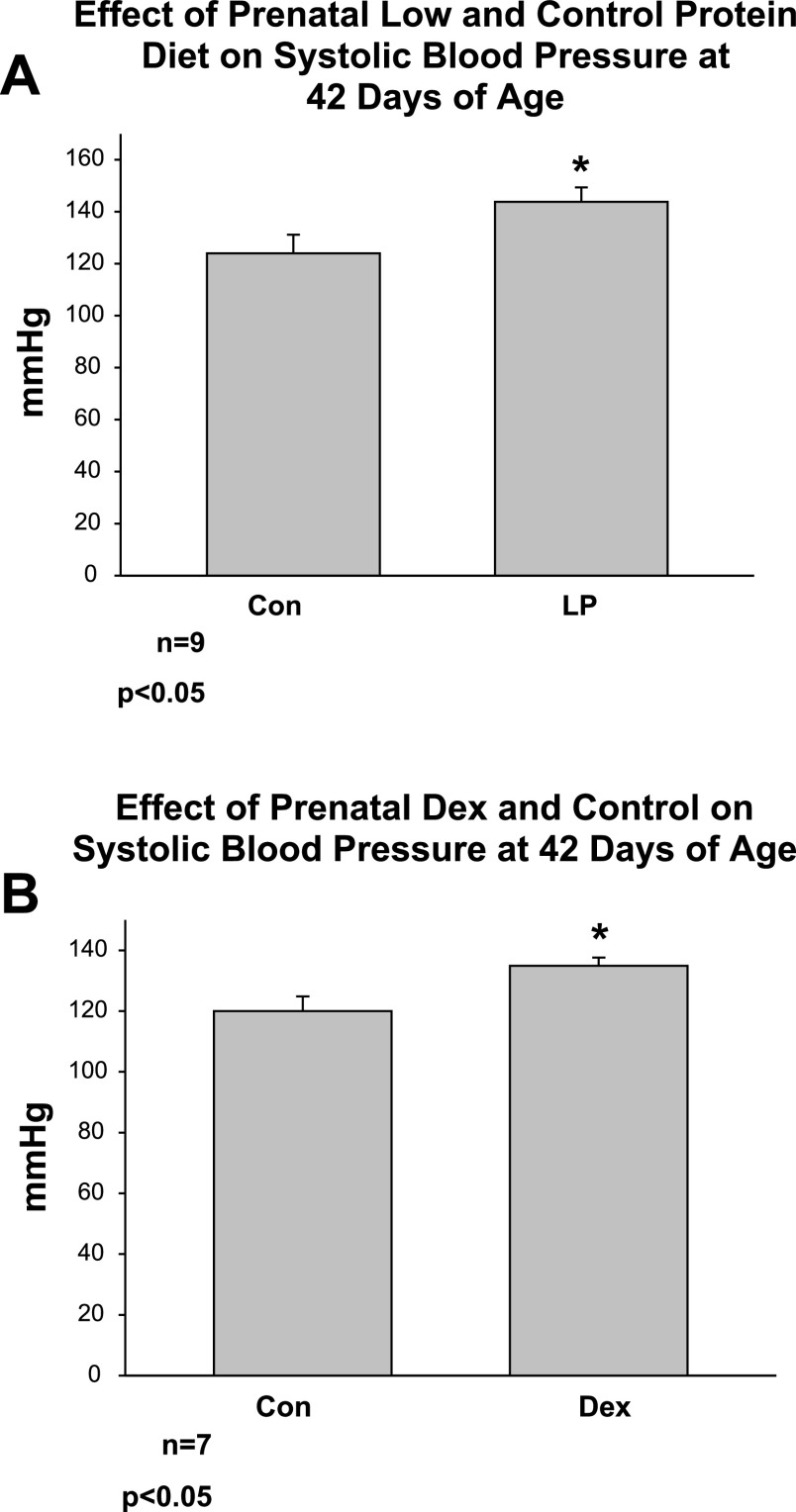
In the first group of experiments, we examined the effect of prenatal low-protein diet and prenatal dexamethasone on systolic blood pressure compared with controls at 6 wk of age. This age was chosen, as this was the average age of the rats studied in the experiments below. Both prenatal low-protein diet and prenatal dexamethasone resulted in an increase in blood pressure in 42-day-old rats compared with controls studied at the same time as shown in Fig. 1.
Fig. 1.
A: effect of prenatal low and control protein diet on systolic blood pressure at 42 days of age. Blood pressure was measured in rats whose mothers were fed a low-protein (LP) and control protein diet. The offspring of the LP diet group had a higher blood pressure than the control group. B: effect of prenatal dexamethasone on systolic blood pressure at 42 days of age. Rats whose mother received prenatal dexamethasone had a higher blood pressure than that of control rats. *P < 0.05.
Serum electrolytes were measured in 6-wk-old rats whose mothers received normal protein or a low-protein diet. The results are shown in Table 1. The prenatal low-protein group had a higher serum bicarbonate, sodium, and blood urea nitrogen (BUN) than the control group. The higher BUN is consistent with the reduction in nephron number in the low-protein group (47, 50). We have previously shown an increase in proximal tubule acidification with prenatal programming by dexamethasone that may, in part, explain the higher serum bicarbonate level in the prenatal dietary protein deprivation group (15). The higher serum sodium in the low-protein group may be due to prenatal programming of altered thirst or a renal concentration defect, a subject that will require future investigation.
Table 1.
Measurements of serum electrolytes in 6-wk-old rats whose mothers received normal protein or a low-protein diet
| Control diet, n = 5 | Low-protein diet, n = 5 | P value | |
|---|---|---|---|
| Na, mmol/l | 141.7±0.5 | 144.0±0.4 | 0.01 |
| K, mmol/l | 5.17±0.07 | 5.07±0.06 | 0.34 |
| Cl, mmol/l | 103.1±0.9 | 100.8±0.5 | 0.06 |
| HCO3, mmol/l | 26.4±1.2 | 29.7±0.6 | 0.04 |
| BUN, mg/dl | 15±0.4 | 20±0.5 | <0.001 |
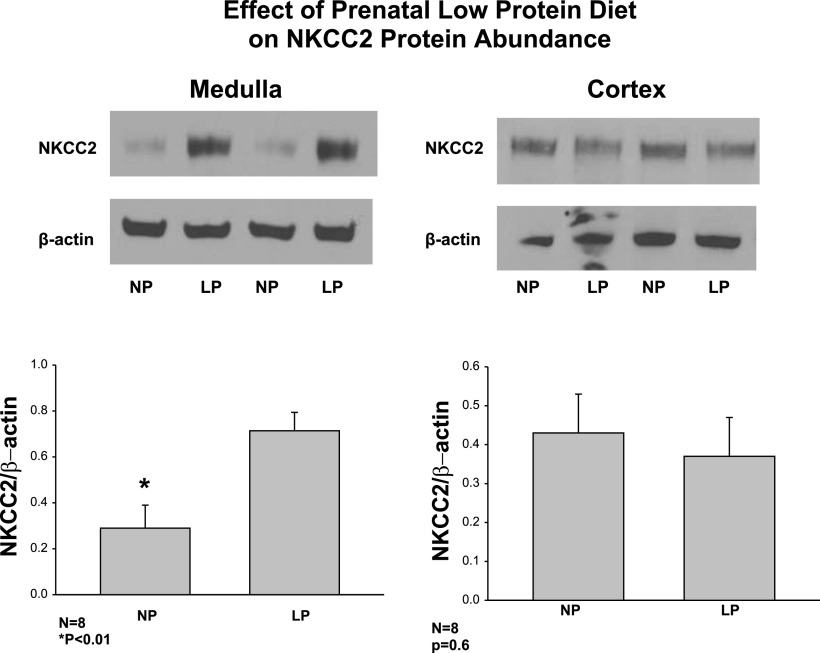
In the next series of experiments, we examined the effect of maternal administration of a low-protein diet on postnatal NKCC2 protein abundance in both medulla and cortex in rats 6 wk of age. As shown in Fig. 2, there was over a twofold increase in NKCC2 protein abundance in the medulla of the low-protein group compared with the control group. Interestingly, there was no difference in NKCC2 protein abundance in the cortex in the low-protein and control groups.
Fig. 2.
Effect of maternal LP diet on postnatal bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2) protein abundance in cortex and medulla. NKCC2 protein abundance was assessed using SDS-PAGE and immunoblot from medulla and cortex of rats whose mothers were administered a LP diet compared with a control normal protein diet. There was an increase in NKCC2 protein abundance in the medulla but not in the cortex. *P < 0.01.
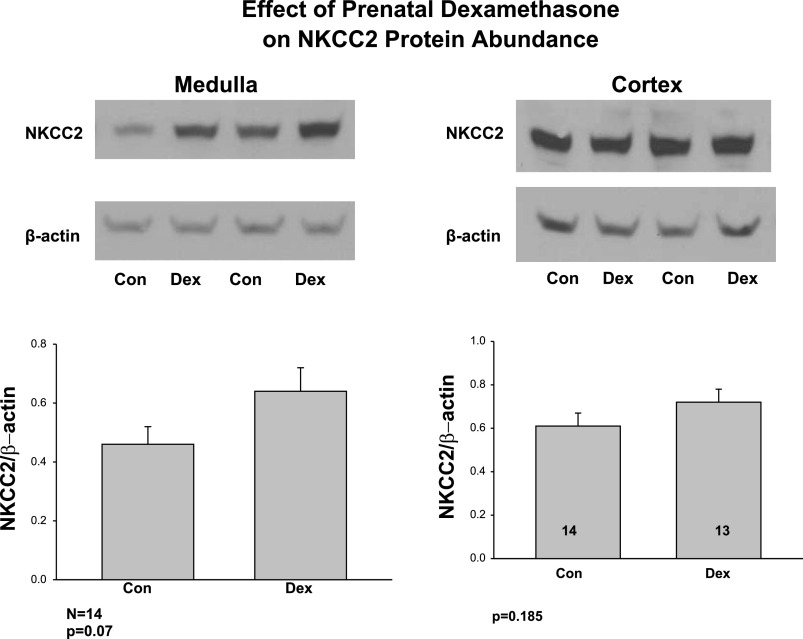
We next examined the effect of prenatal dexamethasone on NKCC2 protein abundance in the medulla and cortex (Fig. 3). Medullary and cortical NKCC2 protein abundance was not significantly higher in the prenatal dexamethasone group than the control group.
Fig. 3.
Effect of prenatal dexamethasone on postnatal NKCC2 protein abundance in cortex and medulla. NKCC2 protein abundance was assessed using SDS-PAGE and immunoblot from medulla and cortex of control rats and rats whose mothers were administered prenatal dexamethasone. Prenatal dexamethasone did not cause a statistically significant increase medullary NKCC2 protein abundance in the offspring.
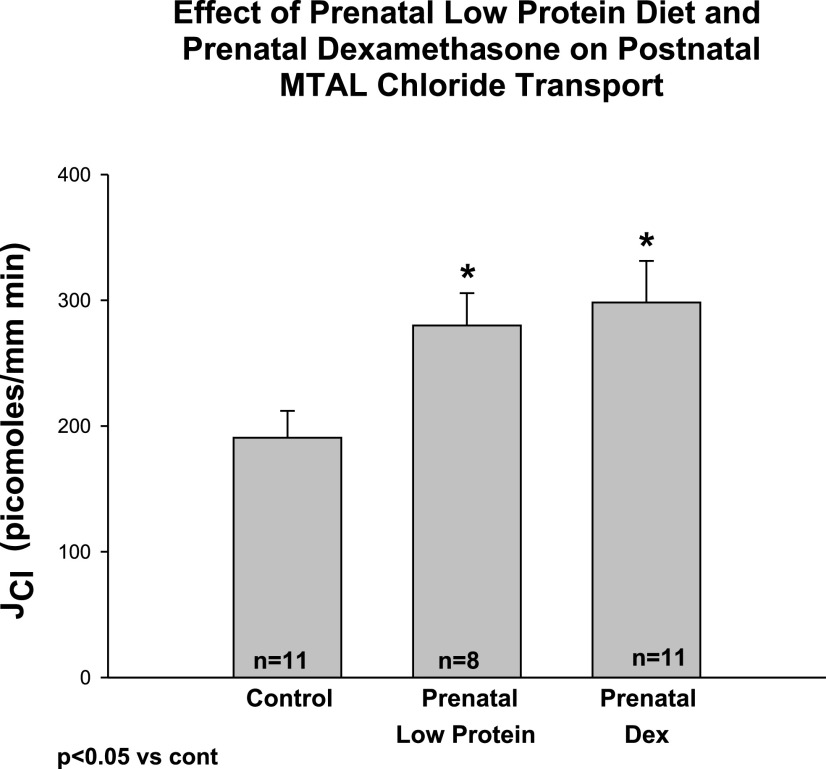
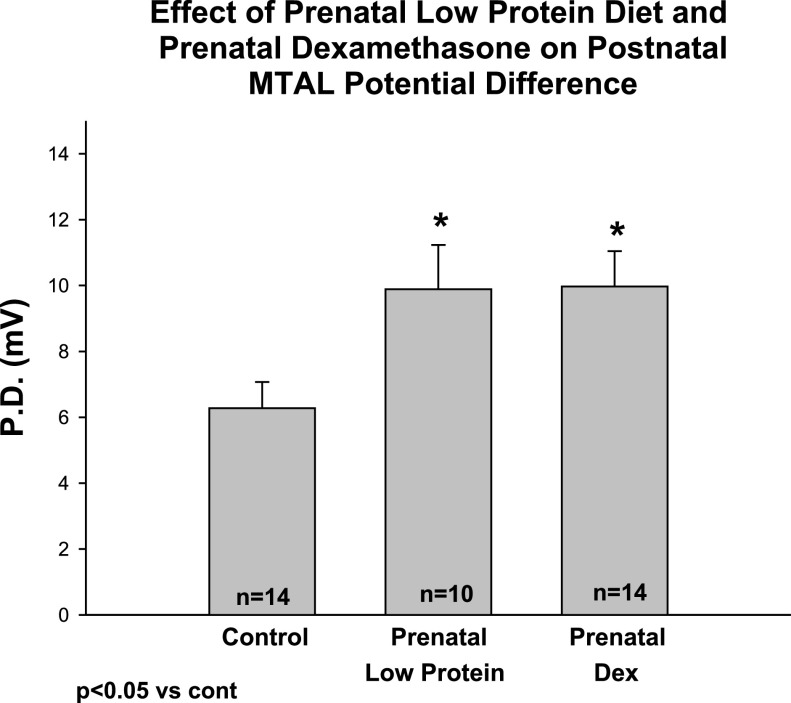
In the next series of experiments, we examined the effect of maternal dietary protein deprivation and prenatal dexamethasone on chloride transport and the transepithelial potential difference in the mTAL. Control tubules were dissected and perfused in rats who were 45 ± 3 days of age in the control group, 44 ± 5 days of age in the prenatal low-protein group, and 40 ± 1 days of age in the prenatal dexamethasone group. The tubular lengths in the control, prenatal dexamethasone, and maternal low-protein group were 0.53 ± 0.06 mm, 0.46 ± 0.04 mm, and 0.59 ± 0.06 mm, respectively (P = ns). The effect of prenatal dietary protein deprivation and prenatal dexamethasone administration on postnatal chloride transport is shown in Fig. 4. As can be seen, prenatal dietary protein deprivation and prenatal dexamethasone resulted in an increased chloride transport compared with control. To demonstrate that we were, indeed, measuring active chloride transport, we cooled the tubules to ·mm−1·min−1 (n = 15) or <90% of the transport measured before cooling. We validated our transport findings by measuring the transepithelial potential difference. Both the prenatal low-protein and prenatal dexamethasone groups had a higher potential difference than the control group as shown in Fig. 5.
Fig. 4.
Effect of prenatal low-protein diet and prenatal dexamethasone on medullary thick ascending limb (mTAL) chloride transport. mTAL were dissected and perfused in vitro from adult rats whose mothers were administered a normal protein diet, a low-protein diet (6%), or prenatal dexamethasone (0.2 mg/kg body wt) by intraperitoneal injection daily between 15th and 18th day of gestation. The 6-wk-old offspring of mothers that received prenatal dexamethasone or a low-protein diet had a significant increase in the rate of mTAL chloride transport. *P < 0.05 vs. Control.
Fig. 5.
Effect of prenatal low-protein diet and prenatal dexamethasone (PD) on mTAL transepithelial potential difference. Transepithelial potential difference was assessed in tubules using the perfusion pipette as the bridge in the tubular lumen and comparing the potential to the bathing solution. The potential difference was higher in the groups from rats in the prenatal dexamethasone and prenatal low-protein group compared with the control group. *P < 0.05 vs. Control.
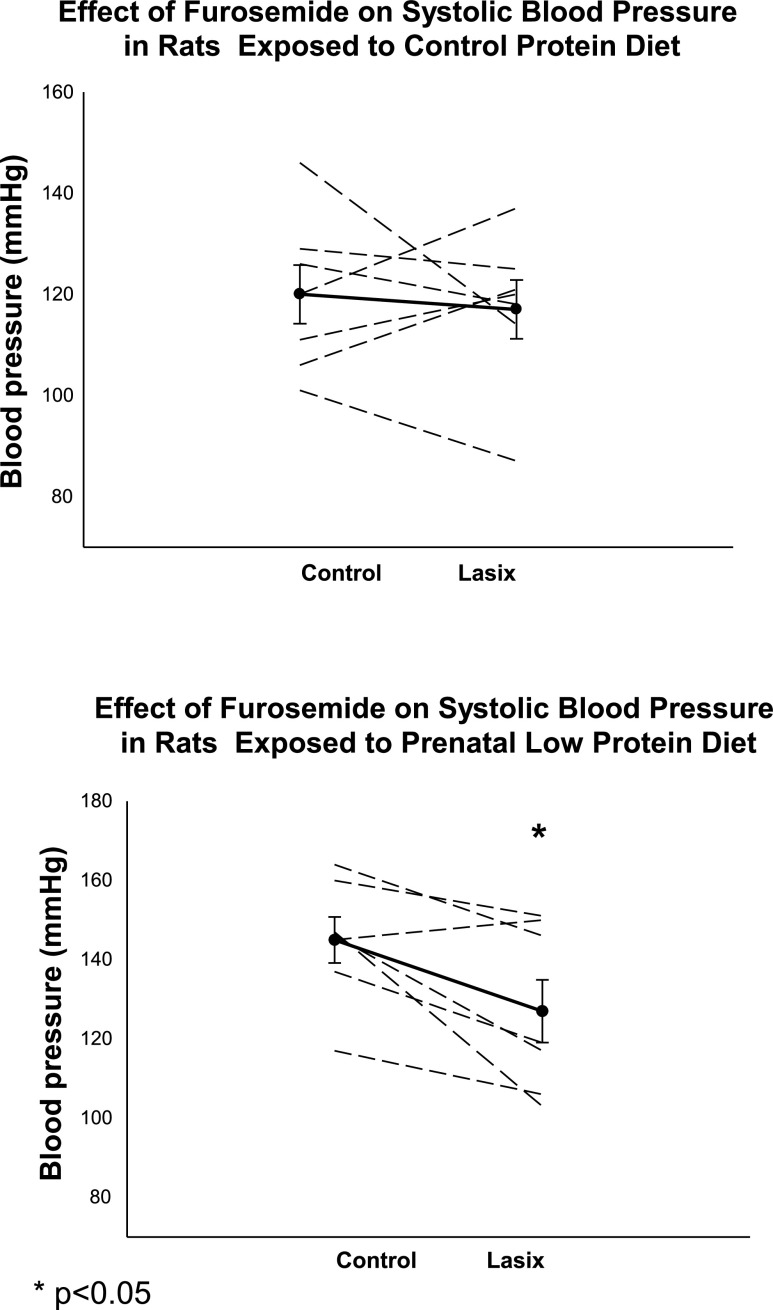
In the next experiment, we examined the effect of furosemide (12 mg/100 ml drinking water) on blood pressure in control rats and adult rats whose mothers ate a low-protein diet during the second half of pregnancy. As shown in Fig. 6, there was a consistent decrease in blood pressure in the low-protein group, while there was no significant change in blood pressure in the control group.
Fig. 6.
Effect of furosemide (Lasix) on blood pressure in adult rats whose mothers ate a control protein diet (top) or a low-protein diet (bottom). Systolic blood pressure was measured by tail cuff in control rats and rats whose mothers received a low-protein diet during the last half of pregnancy. Offspring was then given water that contained 12 mg furosemide/100 ml and blood pressure measured again 24 h later (48). *P < 0.05 vs. Control.
Finally, we examined the effect of maternal dietary protein deprivation in 3-wk-old rats at an age prior to the development of hypertension (47). The blood pressures were 99 ± 5 mmHg in the control group and 98 ± 5 mmHg in the group whose mothers received prenatal low-protein during the second half of pregnancy. Unlike the hypertensive older rats above, there was no difference in NKCC2 protein abundance/β actin (1.10 ± 0.15 control vs. 0.94 ± 0.19 low-protein group) or the medullary thick ascending limb transepithelial potential difference (3.4 ± 0.8 mV in control vs. 2.8 ± 0.7 mV in the low-protein group).
DISCUSSION
Prenatal insults whether from dietary protein deprivation or prenatal dexamethasone result in hypertension in the offspring when they are studied as adults (14, 28, 30–34, 38, 40–42). Prenatal insults have been shown to result in prenatal programming of hypertension and cardiovascular disease in humans (2–5, 43, 43). The cause for the hypertension caused by prenatal insults is unknown, and elucidating the pathogenesis of the hypertension may lead to more directed therapy.
In previous studies examining maternal dietary protein deprivation and maternal administration of dexamethasone, there was a decrease in nephron number (14, 27, 41, 42, 50), which Brenner and colleagues have hypothesized to be the main factor for the generation of hypertension due to prenatal insults (11–13, 35). However, there are several lines of evidence that the paucity of nephrons is not the sole or predominant cause for the hypertension in prenatal programming. In most studies, prenatal dexamethasone and prenatal dietary protein deprivation result in less than a 50% reduction in nephron number (27, 41, 42, 50). A 20–30% reduction in nephron number in rats treated with prenatal dexamethasone did not affect inulin clearance, demonstrating that renal insufficiency is not associated with an increase in blood pressure (41, 42). In addition, prenatal administration of dexamethasone at specific points of gestation results in hypertension without a reduction in nephron number (42). In cross-breeding experiments between Wistar-Kyoto and spontaneously hypertensive rats, which have a reduced nephron number and are hypertensive, the F2 generation was examined to determine whether there was a correlation of nephron number and blood pressure. Although there was a significant variation in glomerular number from ∼20,000 to ∼37,000, there was no correlation between glomerular number and blood pressure (8). Finally, postnatally enhanced nutrition by decreasing the litter size has been shown to increase glomerular number, but nonetheless is associated with hypertension (9).
A potentially important clue for the pathogenesis of the hypertension in prenatal programming came from the work of Manning et al. (37), in which they found that prenatal protein deprivation resulted in the upregulation in both NKCC2 cotransporter and the thiazide-sensitive cotransporter protein expression when the rats were studied at 4 wk of age (37). However, this group also showed that at 4 wk of age, the rats that were the offspring of mothers fed a low-protein diet were not hypertensive and did not have an elevated blood pressure until 6 wk of age (47). Our study provides evidence that an upregulation of the NKCC2 is not only important in the generation of hypertension but also in the maintenance of the hypertension, as these rats have an increase in NKCC2 at the point they are hypertensive. An upregulation in either transporter with a concomitant increase in transport would focus therapy on salt intake and diuretics specifically targeted to the affected nephron segment.
As shown by by immunoblot analysis, there was an increase in NKCC2 cotransporter abundance in the medulla but not in the cortex of rats that were the offspring of mothers fed a low-protein diet. The reason for the nephron heterogeneity between the upregulation of the NKCC2 cotransporter by dietary protein deprivation is unknown. There is nephron heterogeneity between the cortical thick acending limb (cTAL) and the mTAL. Only the mTAL responds to vasopressin with an increase in sodium transport (24). In addition, the mTAL is more highly innervated than the cTAL (1). We have recently shown that denervation results in normalization of blood pressure in rats administered prenatal dexamethasone (16). It is possible that renal innervation is the cause for the increase in mTAL sodium transport, but that has yet to be determined.
In the present study, we found that the increase in NKCC2 protein abundance in 6-wk-old rats that were the offspring of mothers treated with prenatal dexamethasone was not statistically significant. We have recently found that prenatal dexamethasone resulted in a significant increase in NKCC2 in rats studied at 8 wk of age (16). This suggests that the effect of prenatal insults on transporter abundance may be age dependent. The increase in transport likely signifies that it is a more sensitive measure of transporter activity than the total protein abundance. Alternatively, prenatal dexamethasone may be associated with a greater percentage of NKCC2 on the apical membrane than in the cytosol.
The fact that previous studies had demonstrated that there was an increase in NKCC2 cotransporter protein abundance by no means translates into an increase in NaCl transport by the thick ascending limb in the offspring of rats whose mothers were treated with a low-protein diet during pregnancy. In this study and in the previous studies (16, 37), NKCC2 was assayed from a protein homogenate, which does not guarantee that there is increased protein abundance on the apical membrane. In addition, for there to be increased sodium transport, there must also be a concomitant increase in apical ROMK (rat outer-medullary potassium channel) so that the potassium absorbed by the NKCC2 can recycle into the tubular lumen and the basolateral chloride channel (CLCNKB) to allow basolateral chloride exit. Mutations in ROMK or CLCNKB, as well as loss of the accessory chloride channel protein barttin, leads to loss of function of the thick ascending limb and Bartter's syndrome (17, 45, 46). There must also be a concomitant supply of fuel and increase in basolateral Na/K-ATPase activity for there to be an increase in chloride transport. Thus, an increase in chloride transport in the thick ascending limb is dependent on more than an increase in NKCC2 protein abundance. This study directly demonstrates for the first time that there is an increase in NaCl transport by the mTAL. We found a parallel increase in the lumen-positive transepithelial potential difference that is generated by luminal potassium diffusion through the apical potassium channel (ROMK), which is due to and parallels NKCC2 activity (21, 22). Importantly, the increase in sodium chloride transport was seen at a time when there was an increase in blood pressure. Thus, the elevated blood pressure seen in prenatal programming is likely mediated, at least in part, by increased sodium absorption. Consistent with this is the fact that furosemide decreased blood pressure in the prenatal low-protein group but not in the control group.
This is not the first study that has demonstrated prenatal programming can affect sodium transport. We have recently shown that rats exposed to prenatal dexamethasone using the same protocol as that used in earlier studies resulted in an increase in proximal tubule volume absorption, Na+/H+ exchanger activity, and brush-border membrane NHE3 protein abundance (15). This study, however, is the first to show that there is another nephron segment where sodium transport is increased in offspring that are exposed to prenatal dexamethasone. Furthermore, it demonstrates that there is nephron heterogeneity on the effect of prenatal programming and that not all nephron segments likely have an increase in transport.
In summary, we found that both prenatal dexamethasone and a low dietary protein intake cause an increase in medullary thick ascending limb transport. The mechanism for this effect is unknown at present and whether other distal nephron segments are affected will need to be determined.
Perspectives and Significance
It is now clear that prenatal insults can lead to hypertension in the offspring in animal models and in humans. The mechanism(s) for the generation and maintenance of hypertension by prenatal programming is an area of intense investigation and of clinical significance. This study shows that there is an increase in transport in the mTAL, a segment responsible for a substantial fraction of renal sodium absorption. The final determinant of urinary sodium excretion lies in more distal nephron segments and whether transport in the distal nephron segments are affected by prenatal programming is unknown and yet to be investigated. The increase in serum sodium observed in this study suggests that there may be a concentrating defect or an abnormality in the thirst mechanism, which is an issue for future investigation. Furthermore, how prenatal insults program the initiation and maintenance of hypertension remains unclear at present. Nonetheless, this study shows that this altered sodium transport may be an effective therapeutic target for the treatment of patients whose hypertension is the result of a prenatal insult.
GRANTS
This work was supported by National Institutes of Health Grant DK-41612 and DK-078596 to M. Baum, T32 DK-07257 (P. Igarashi and M. Baum), and the O'Brien Center P30DK-079328 (P. Igarashi, principal investigator).
REFERENCES
- 1.Barajas L, Powers KV. Innervation of the thick ascending limb of Henle. Am J Physiol Renal Fluid Electrolyte Physiol 255: F340–F348, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ Fetal nutrition and cardiovascular disease in later life. Br Med Bull 53: 96–108, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J 301: 259–262, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 31: 1235–1239, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Baum M, Dwarakanath V, Alpern RJ, Moe OW. Effects of thyroid hormone on the neonatal renal cortical Na+/H+ antiporter. Kidney Int 53: 1254–1258, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11β-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology 142: 2841–2853, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Black MJ, Briscoe TA, Constantinou M, Kett MM, Bertram JF. Is there an association between level of adult blood pressure and nephron number or renal filtration surface area? Kidney Int 65: 582–588, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Boubred F, Buffat C, Feuerstein JM, Daniel L, Tsimaratos M, Oliver C, Lelievre-Pegorier M, Simeoni U. Effects of early postnatal hypernutrition on nephron number and long-term renal function and structure in rats. Am J Physiol Renal Physiol 293: F1944–F1949, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens 2: 691–695, 1993. [PubMed] [Google Scholar]
- 12.Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994. [PubMed] [Google Scholar]
- 13.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Celsi G, Kistner A, Aizman R, Eklof AC, Ceccatelli S, de Santiago A, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 44: 317–322, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol 295: F29–F34, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ. Barttin is a Cl- channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414: 558–561, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Good DW Effects of potassium on ammonia transport by medullary thick ascending limb of the rat. J Clin Invest 80: 1358–1365, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good DW Adaptation of HCO-3 and NH+4 transport in rat MTAL: effects of chronic metabolic acidosis and Na+ intake. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1345–F1353, 1990. [DOI] [PubMed] [Google Scholar]
- 20.Good DW, Knepper MA, Burg MB. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F35–F44, 1984. [DOI] [PubMed] [Google Scholar]
- 21.Greger R, Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 392: 92–94, 1981. [DOI] [PubMed] [Google Scholar]
- 22.Greger R, Weidtke C, Schlatter E, Wittner M, Gebler B. Potassium activity in cells of isolated perfused cortical thick ascending limbs of rabbit kidney. Pflügers Arch 401: 52–57, 1984. [DOI] [PubMed] [Google Scholar]
- 23.Hebert SC, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle: II. Determinants of the ADH-mediated increases in transepithelial voltage and in net Cl-absorption. J Membr Biol 80: 221–233, 1984. [DOI] [PubMed] [Google Scholar]
- 24.Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs.1. Functional nephron heterogeneity and Adh-stimulated NaCl cotransport. Am J Physiol Renal Fluid Electrolyte Physiol 241: F412–F431, 1981. [DOI] [PubMed] [Google Scholar]
- 25.Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99: 296–301, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991. [PubMed] [Google Scholar]
- 27.Hoppe CC, Evans RG, Bertram JF, Moritz KM. Effects of dietary protein restriction on nephron number in the mouse. Am J Physiol Regul Integr Comp Physiol 292: R1768–R1774, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) 103: 633–639, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Knepper MA Urea transport in isolated thick ascending limbs and collecting ducts from rats. Am J Physiol Renal Fluid Electrolyte Physiol 245: F634–F639, 1983. [DOI] [PubMed] [Google Scholar]
- 30.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 86: 217–222, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A 110: 223–228, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Langley-Evans SC, Jackson AA. Rats with hypertension induced by in utero exposure to maternal low-protein diets fail to increase blood pressure in response to a high salt intake. Ann Nutr Metab 40: 1–9, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 91: 607–615, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: The fetal flaw in essential hypertension? Kidney Int Suppl 55: S30–S34, 1996. [PubMed] [Google Scholar]
- 36.Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 58: 770–773, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol 283: F202–F206, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol 16: 417–422, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Comp Physiol 288: R80–R84, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero. Br J Nutr 83: 79–85, 2000. [PubMed] [Google Scholar]
- 41.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 20: 345–352, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Shah M, Gupta N, Dwarakanath V, Moe OW, Baum M. Ontogeny of Na+/H+ antiporter activity in rat proximal convoluted tubules. Pediatr Res 48: 206–210, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17: 171–178, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SK, Solez K, Heptinstall RH. The failure of furosemide-induced salt and water loss to convert benign to malignant hypertension in the rat. Am J Pathol 101: 303–318, 1980. [PMC free article] [PubMed] [Google Scholar]
- 49.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R1131–R1136, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int 65: 1339–1348, 2004. [DOI] [PubMed] [Google Scholar]