Abstract
The H+-K+-ATPase α2 (HKα2) gene of the renal collecting duct and distal colon plays a central role in potassium and acid-base homeostasis, yet its transcriptional control remains poorly characterized. We previously demonstrated that the proximal 177 bp of its 5′-flanking region confers basal transcriptional activity in murine inner medullary collecting duct (mIMCD3) cells and that NF-κB and CREB-1 bind this region to alter transcription. In the present study, we sought to determine whether the −144/−135 Sp element influences basal HKα2 gene transcription in these cells. Electrophoretic mobility shift and supershift assays using probes for −154/−127 revealed Sp1-containing DNA-protein complexes in nuclear extracts of mIMCD3 cells. Chromatin immunoprecipitation (ChIP) assays demonstrated that Sp1, but not Sp3, binds to this promoter region of the HKα2 gene in mIMCD3 cells in vivo. HKα2 minimal promoter-luciferase constructs with point mutations in the −144/−135 Sp element exhibited much lower activity than the wild-type promoter in transient transfection assays. Overexpression of Sp1, but not Sp3, trans-activated an HKα2 proximal promoter-luciferase construct in mIMCD3 cells as well as in SL2 insect cells, which lack Sp factors. Conversely, small interfering RNA knockdown of Sp1 inhibited endogenous HKα2 mRNA expression, and binding of Sp1 to chromatin associated with the proximal HKα2 promoter without altering the binding or regulatory influence of NF-κB p65 or CREB-1 on the proximal HKα2 promoter. We conclude that Sp1 plays an important and positive role in controlling basal HKα2 gene expression in mIMCD3 cells in vivo and in vitro.
Keywords: chromatin, transcription, collecting duct, transcription factor, gene expression
epithelial cells of the kidney and colon play critical roles in adjusting K+ and acid elimination to accommodate changes in intake and maintain cell function. Physiological and molecular biological studies in animals have pointed to the H+-K+-ATPase α2-subunit (HKα2) gene Atp12a1 , which is principally expressed in the distal colon and renal collecting duct, as a key participant in the control of body K+ homeostasis (8, 16, 17, 22). For example, mice with null mutations in this gene suffer hypokalemia during dietary K+ (10) and sodium restriction (18). HKα2 may also play a role in bicarbonate absorption by the kidney (11, 12) and distal colon (10), and increased ammonium secretion in the inner medullary collecting duct (IMCD) during chronic hypokalemia (20), and the chronic adaptation to changes in sodium (15) and aldosterone (5, 6, 13) balance. Most recently, studies in mice in which both the H+-K+-ATPase α1-subunit and HKα2 genes were genetically ablated indicated that both isoforms contribute to H+ secretion by collecting duct intercalated cells (9). Although it plays a central role in these important basal and adaptive responses, transcriptional control of the HKα2 gene remains insufficiently characterized.
We previously analyzed the structural organization and several regulatory points of the murine HKα2 gene. The HKα2 gene contains 23 exons, spans 23.5 kb of genomic DNA on chromosome 14C3, and shares an exon-intron organization comparable to that of human ATP12A (38). The proximal 177 bp of the 5′-flanking region of the murine HKα2 gene comprises the minimal promoter and confers basal collecting duct-selective expression in vivo (26). This region contains a TATA- like sequence (ATTTAA) at −46/−40 relative to the transcription start site and several consensus sequences for transcription factors that we have been systematically characterizing (Fig. 1A). We have shown that CRE-binding protein (CREB)-1, binding to one or more cAMP/Ca2+ response elements (CRE)-like elements in the −86/−60 region, elicits modest basal trans-activation and robust forskolin induction of the HKα2 gene (23). In contrast, NF-κB, binding to a κB DNA binding element at −104/−94, interacts with histone deacetylase-6 to trans-repress basal HKα2 transcription in mIMCD3 cells under basal conditions (25). Also present within this proximal promoter region at −144/−135 is the sequence 5′-ACCCCaCCCA-3′, which is a 9 of 10 nucleotide match (the single nucleotide mismatch is indicated by the lower case letter) to the reverse complement of the consensus binding element 5′-(G/T)GGGCGG(G/A)(G/A)(C/T)-3′ for specificity protein (Sp)-1. This site is highly conserved both in relative location and sequence in the promoters of the human (19), mouse (26), and rabbit (30) HKα2 genes (Fig. 1B).
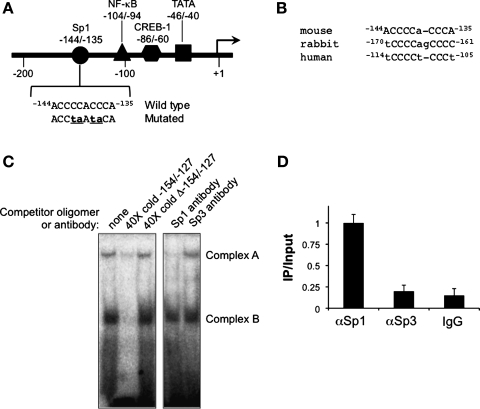
Fig. 1.
Analysis of Sp protein-DNA interactions associated with the proximal promoter region of the murine H+-K+-ATPase α2-subunit (HKα2) gene. A: map of the proximal 5′-flanking region of the murine HKα2 gene promoter. Consensus sites for the binding of selected transcription factors are indicated. Sequence of the −144/−135 region and the mutated sequence (mutations in lower case letters) used for EMSA and trans-activation assays are also indicated. B: cross-species alignment of the conserved Sp-binding element in the proximal HKα2 gene promoter. The lower case letters indicate sequence divergence from the reverse complement of the consensus binding element 5′-(G/T)GGGCGG(G/A)(G/A)(C/T)-3′. C: EMSA (autoradiograph to the left) in which a radiolabeled duplex probe −154/−127 of the mouse HKα2 gene was incubated with 10 μg of inner medullary collecting duct cell (IMCD3) nuclear extracts alone (lane 1) or in the presence of a 40-fold molar excess of unlabeled wild-type cold probe (lane 2) or mutant cold probe (lane 3). Antibody interference assays (autoradiograph to the right) were performed using 2 μg of rabbit polyclonal Sp1 or Sp3 antibody. Sp1 antibody decreased formation of complex A, whereas Sp3 antibody had no effect. Autoradiographs are representative of 4 experiments each for the EMSA and for the supershift/interference assays. D: chromatin immunoprecipitation (ChIP)/quantitative (q) PCR analysis of Sp1 and Sp3 occupancy of the proximal HKα2 promoter. ChIP assays were performed with anti-Sp1, anti-Sp3 antibody, or nonimmune IgG followed by qPCR with primer sets designed to amplify −194 to +56 of the HKα2 gene. The value for the final amplicon/input DNA with anti-Sp1 was set as 1, and the other values were normalized to it. Values are means ± SE of 4 separate experiments, each performed in triplicate.
Sp1 is a member of the Sp/Krüppel-like factor (KLF) transcription factors (Sp/KLF factors hereafter) that regulate the expression of a large number of genes that have GC-rich promoters, including those encoding structural proteins, metabolic enzymes, cell cycle regulators, transcription factors, growth factors, and signaling receptors (reviewed in Ref. 21). Sp1 binds its target DNA sequence via three tandem zinc finger motifs in its COOH terminus. In some promoter contexts, Sp1 cooperatively activates genes through interactions with other trans-acting factors (reviewed in Ref. 2). Functional studies have shown that Sp1 and its close relative Sp4 generally act as transcriptional activators, while Sp3 acts either to repress or activate transcription. In this study, we identified Sp1 as a nuclear protein that binds to the −144/−135 cis-element of the HKα2 proximal promoter to contribute significantly to the basal HKα2 promoter activity and transcription levels in mIMCD3 cells.
MATERIALS AND METHODS
Cell culture and reagents.
mIMCD3 cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a 5% CO2 environment. Drosophila Schneider's Line-2 (SL2) cells (American Type Culture Collection, Manassas, VA) were grown at 23°C in Schneider's Drosophila medium with 10% heat-inactivated fetal bovine serum. The QuikChange Multi site-directed mutagenesis system was from Stratagene (La Jolla, CA). The Dual-Luciferase Reporter Assay System and the luciferase vectors pGL3-Basic and pRL-TK were from Promega (Madison, WI). The DyNAmo SYBR green qPCR KIT was from Finnzymes Oy (Espoo, Finland). LipofectAMINE 2000 Reagent was from Invitrogen (Carlsbad, CA). The siPORT Amine Transfection Reagent [a reagent optimized for small interfering RNA (siRNA) transfections], the Silencer siRNA transfection kit, scrambled siRNA, and siRNA designed to target murine Sp1 (catalog no. 4390771) were from Ambion (Austin, TX). Rabbit polyclonal antibodies directed against Sp1 (catalog no. sc-59 X) and Sp3 (catalog no. sc-644 X) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Plasmids and constructs.
pGL3–0.18mHKα2, which contains +253 to −177 of the murine HKα2 proximal promoter fused to the luciferase gene, has been described (25). pGL3–0.18mHKα2Δ−144/−135, harboring a mutated Sp1 element−144ACCtaAtaCA−135 (mutated nucleotides in lower case letters; Fig. 1A), was generated by PCR from pGL3–0.18mHKα2 as a template using a Quick Change Multi site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's protocols. Sp expression vectors pCMV-Sp1, pCMB-Sp3, pPac0, pPacSp1, and pPacSp3 were a generous gift from Dr. Robert Tjian (University of California Berkeley). pPac0 is a control vector lacking the Sp coding region, whereas pPacSp1 and pPacSp3 contain coding sequences for Sp1 and Sp3, respectively, driven by the actin promoter (3). The NF-κB p65 expression vector pRSV-p65 was a gift from Dr. Bharrat Agarwal (University of Texas M. D. Anderson Cancer Center). The constitutively active CREB mutant CREB-VP16 (24) was a gift from Dr. D. D. Ginty (Department of Neuroscience, Howard Hughes Medical Institute, The Johns Hopkins University School of Medicine, Baltimore, MD).
EMSA and supershift/interference assays.
Native (−154CCAATCCTACCCCACCCAGGCTTGCGTG−127; Sp element core sequence underlined) and mutant (CCAATCCTACCtaAtaCAGGCTTGCGTG; mutated nucleotides in lower case letters) double-stranded oligomers of the mouse HKα2 gene were end-labeled with [γ-32]ATP using T4 polynucleotide kinase. Nuclear extracts were prepared from mIMCD3 cells, and EMSA was conducted as detailed in our earlier work (25). For supershift/antibody interference assays, 2 μg of rabbit polyclonal antibodies against Sp1 or Sp3 or nonimmune IgG were preincubated with nuclear extracts for 20 min at 4°C; then, the radiolabeled probes were added in the binding reactions and incubated at room temperature for 15 min before electrophoresis.
Transient transfection of plasmid DNA and reporter gene assays.
mIMCD3 cells grown in 24-well plates were transiently transfected with plasmid DNA using LipofectAMINE 2000 Reagent as detailed in our laboratory's previous work (25). In firefly luciferase reporter gene assays, the cells were cotransfected with the Renilla luciferase expression plasmid pRL-TK (20 ng/well) to normalize for transfection efficiency. The ratio of firefly to Renilla luciferase activities was reported and taken as an index of HKα2 promoter activity. Each determination was performed in triplicate, and the mean value was recorded as a single independent observation. Three to four independent observations were conducted for each experimental protocol. For trans-repression/trans-activation experiments in mIMCD3 cells, the cells were transiently transfected with 0.8 μg of pGL3–0.18mHKα2 and 0.1 μg of expression plasmids for Sp1 or Sp3 (3) or insertless expression vector. For Drosophila SL2 cells, DNAs were cotransfected using 2 μg of the target promoter DNA in the presence or absence of the Sp expression vectors. The transfection conditions used in the two cell types were those that reproducibly provided the highest expression levels of Sp1 and Sp3 proteins detected on immunoblots. Forty-eight hours later, firefly and Renilla luciferase activities were measured.
Sp1 silencing by RNA interference.
RNA interference in mIMCD3 cells was performed with murine Sp1 siRNA (50 nM) or scrambled siRNA, the siPORT Amine Transfection Reagent, and the Silencer siRNA transfection kit (Ambion) as previously described (7). Transfection optimization was achieved by immunoblot comparison (anti-GAPDH antibody, catalog no. AM4300, Ambion) to the degree of protein knockdown achieved with the positive control GAPDH vs. the scrambled negative control siRNAs included in the Silencer siRNA transfection kit. The degree and specificity of Sp1 knockdown were then assessed in each experiment by immunoblot analysis with β-actin as a loading and target-specificity control.
Quantitative RT-PCR, chromatin immunoprecipitation, sequential chromatin immunoprecipitation, and quantitative PCR.
Quantitative (q) real-time RT-PCR assays were performed using a DyNAmo SYBR green qPCR kit, primers to amplify nucleotides +28 to +476 of murine HKα2 (forward, 5′-GGTGCCTTGTCTCTGTAAC-3′, and reverse, 5′-GACCCTGGATGATGTTTG-3′) and +25 to +564 of the housekeeping control murine β-actin (forward, 5′-GTGGGCCGCTCTAGGCACCAA-3′, and reverse, 5′-CTCTTTGATGTCACGCACGATTTC-3′), and cDNAs synthesized from mIMCD3 cell total RNA with the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen), as described in our previous work (23). Standard curves were plotted with the threshold cycles vs. log template quantities. For quantification, HKα2 expression was normalized to the expressed level of β-actin. Chromatin immunoprecipitation (ChIP) and sequential ChIP assays were performed essentially as previously described (27). Purified chromatin was immunoprecipitated using 10 μg of anti-Sp1, 10 μg of anti-Sp3, or 5 μl of rabbit nonimmune serum; eluted DNA fragments were purified to serve as templates for PCR with primers constructed to amplify the region −194 to +56 of the HKα2 gene (forward 5′-ATCAAGAGGAGTCCCCACCT-3′ and reverse 5′-CGATGTTGGCGTTACAGAGA-3′). qPCRs were run in triplicate, and the values were transferred into copy numbers of DNA using a standard curve of genomic DNA with known copy numbers. The resulting transcription values were then normalized for primer pair amplification efficiency using the qPCR values obtained with input DNA. For sequential ChIP, immunoprecipitates obtained with anti-Sp1 were subjected to a second immunoprecipitation with antibodies against Sp1, NF-κB p65, or CREB-1. The samples were processed and subjected to qPCR with the −194/+56 primer set for HKα2 (27). Data are means ± SE from four independent experiments, with each experiment carried out in triplicate.
Data analysis.
Quantitative data are expressed as means ± SE and were analyzed for statistical significance by one-way ANOVA. P values <0.05 were taken as significant.
RESULTS
Sp1, but not Sp3, binds the −144/−135 element in the HKα2 promoter in vitro and in vivo.
To determine whether endogenous Sp1 and/or Sp3 transcription factors bind to the −144/−135 cis-element in the proximal promoter region of the mouse HKα2 gene, EMSAs with mIMCD3 cell nuclear extracts were performed. Two DNA-protein complexes were formed using mIMCD3 nuclear extracts and the −154/−127 oligomer, which contains the −144/−135 Sp site (Fig. 1C). The abundance of complex A was greatly diminished by addition of a 40-fold molar excess of nonradiolabeled wild-type oligomer, but not by the addition of cold oligomer in which the −144/−135 site had been mutated (Fig. 1C). These results indicate that complex A represents a specific Sp protein DNA-binding sequence. Complex B was only diminished by a wild-type cold probe, indicating it is likely composed of a protein-binding element other than an Sp1 binding sequence. In supershift/interference assays, an antibody against Sp1 almost completely eliminated complex A, whereas anti-Sp3 antibody was without effect (Fig. 1C). This result is consistent with an effect of an anti-Sp1 antibody to disrupt the DNA-protein complex A. Moreover, since we did not detect Sp4 protein on immunoblots of nuclear lysates of mIMCD3 cells (data not shown), we did not pursue supershift assays for this protein. Collectively, these EMSA and antibody interference data indicate that endogenous Sp1, but not Sp3 or Sp4, is involved in the formation of the specific DNA-protein complex A.
ChIP assays coupled with qPCR were then performed to examine in vivo whether Sp1 and/or Sp3 protein binds to the region −194/+56 of the HKα2 gene, which contains the −144/−135 Sp site, in mIMCD3 cells. Chromatin containing this HKα2 promoter region was consistently immunoprecipitated by anti-Sp1 antibody (Fig. 1D). This value of final amplicon/input DNA was set equal to 1 for comparative purposes. In contrast, only negligible amounts of chromatin containing the HKα2 proximal promoter region were immunoprecipitated by the anti-Sp3 antibody or control IgG (Fig. 1D). These results indicate that Sp1 but not Sp3 binds to the HKα2 proximal promoter region in chromatin of mIMCD3 cells.
The −144/−135 element is important for HKα2 promoter activity.
To evaluate the functional role of Sp1 binding to the −144/−135 element, the same site-specific mutations used in the EMSA that prevented the Sp1 interaction with the probe (Fig. 1, A and C) were introduced into pGL3–0.18mHKα2 to generate pGL3–0.18mHKα2ΔSp1−144/−135. The wild-type and mutant constructs or the empty parent vector pGL3 were transiently transfected along with the Renilla luciferase expression plasmid pRL-TK into mIMCD3 cells. After 24 h, the firefly and Renilla luciferase activities were measured, and their ratios were determined and used as an index of HKα2 promoter activity. The value for the vector control was set at 1, and the other values were normalized to this. The results, shown in Fig. 2A, revealed robust promoter activity of pGL3–0.18mHKα2, suggesting the activating effects of endogenous Sp1. However, the promoter activity for the pGL3–0.18mHKα2ΔSp1−144/−135 construct was ∼35% lower, indicating the functional importance of the mutated element in basal promoter function. The fact that pGL3–0.18mHKα2ΔSp1−144/−135 still generated greater promoter activity than the vector-transfected controls indicates that elements outside the −144/−135 element also contribute to 0.18mHKα2 promoter activity, presumably the positive regulatory element for CREB-1 binding at −86/−60 (23).
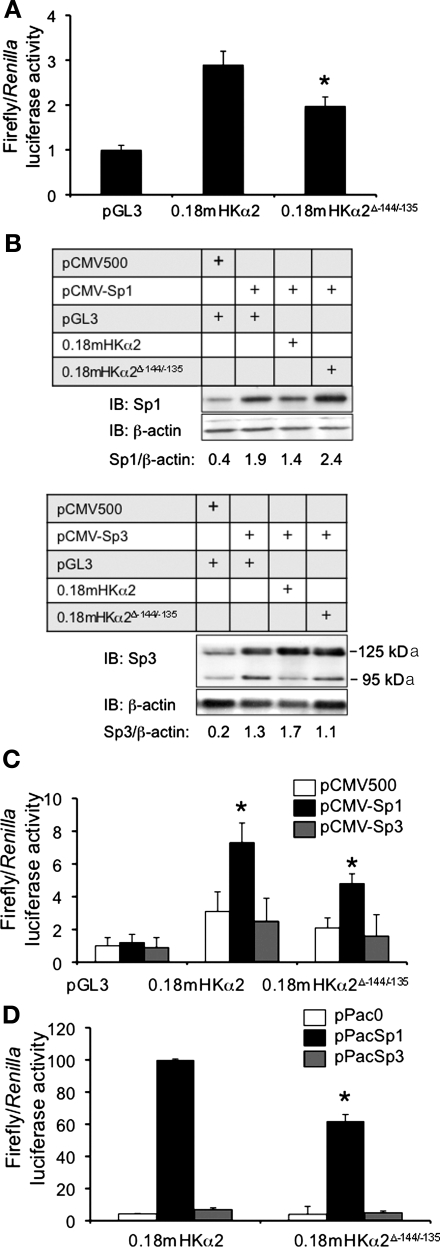
Fig. 2.
Functional analysis of the −144/−135 Sp binding site in the proximal 5′-flanking region of the murine H+-K+-ATPase α2 gene. A: functional analysis of the −144/−135 Sp binding site in the proximal 5′-flanking region of the murine HKα2 gene. Core nucleotides of the Sp protein binding site in the pGL3–0.18mHKα2 were mutated by point mutagenesis to create pGL3–0.18mHKα2Δ−144/−135 as described in materials and methods. Each construct was cotransfected with pRL-TK into mIMCD3 cells, and firefly and Renilla luciferase activities were measured in triplicate for each sample. Error bars indicate ± SE. *P ≤ 0.05 vs. pGL3–0.18mHKα2; n = 4. B: representative (n = 4) immunoblot of Sp1 and Sp3 expression levels obtained in trans-activation assays in which mIMCD3 cells were cotransfected with empty vector, an Sp1 expression vector, or an Sp3 expression vector together with pGL3–0.18mHKα2 or pGL3–0.18mHKα2Δ−144/−135 and pRL-TK. The densitometric ratio of Sp1 or Sp3 expression to that of the housekeeping control β-actin on the blots is presented. For Sp3 expression, the sum of the values for the density of the 125- and 95-kDa bands were used. C: Sp1, but not Sp3, trans-activates the proximal promoter of the mHKα2 gene in mIMCD3 cells via the −144/−135 element. The bar graph plots the luciferase activities in the various transfection conditions. Error bars indicate ± SE. *P ≤ 0.05 vs. pGL3–0.18mHKα2; n = 4. D: Sp1, but not Sp3, trans-activates HKα2 promoter activity in Drosophila SL2 cells. pPac0, a control vector lacking the Sp coding region, or Sp expression vectors pPacSp1 or pPacSp3 were cotransfected with pGL3–0.18mHKα2 or pGL3–0.18mHKα2Δ−144/−135, respectively. Values are means ± SE of 4 separate experiments. *P < 0.05 vs. pGL3–0.18mHKα2.
To determine directly whether Sp1 trans-activates the HKα2 promoter, cotransfection assays with empty vector or expression vectors for Sp1 or Sp3 together with pGL3 (as a control), pGL3–0.18mHKα2, or pGL3–0.18mHKα2ΔSp1−144/−135 in mIMCD3 cells were performed. The total amounts of DNA transfected were held constant. The value generated by the cotransfection of the control plasmids pGL3 and pcDNA3 was set at 1, and the values obtained in the other transfections were normalized to this value in both the immunoblots (Fig. 2B) and the luciferase activity assays (Fig. 2C). As shown in the representative immunoblots in Fig. 2B, modest levels of endogenous 95-kDa Sp1 and the 125- and 95-kDa forms of Sp3 (presumably arising from alternative translation initiation sites) were observed in cells transfected with the empty parent plasmid pCMV500 together with pGL3. This result is consistent with studies in nontransfected mIMCD3 cells (29). In contrast, much higher levels of Sp1 and Sp3 protein expression were observed when pCMV-Sp1 and pCMV-Sp3, respectively, were transfected with the pGL3–0.18mHKα2 or pGL3–0.18mHKα2ΔSp1−144/−135 promoter-reporter plasmids (Fig. 2B). As shown in the bar graph of reporter gene activity in Fig. 2C, Sp1 overexpression trans-activated the activity of pGL3–0.18mHKα2 (to a level ∼3-fold of the control), but not that of pGL3–0.18mHKα2ΔSp1−144/−135. In contrast, Sp3 overexpression did not significantly alter the activity of any of the promoter-reporter constructs (Fig. 2C). These functional results are consistent with the fact that Sp3 was not evident in the DNA-protein complexes formed in mIMCD3 cells in the supershift/interference assays (Fig. 1C) or in the ChIP assays (Fig. 1D).
Given the basal expression of endogenous Sp1 in mIMCD3 cells, however, these Sp1 overexpression experiments may not represent the full impact of Sp1 on basal HKα2 transcription. We addressed this question in two ways. First, we repeated the trans-activation assays in Drosophila SL2 cells (Fig. 2D). SL2 cells are devoid of endogenous Sp factors and have been used as a model system to analyze the effects of exogenously introduced factors on the activities of cotransfected promoters from various genes. As expected, pGL3–0.18mHKα2 promoter activity was negligible in SL2 cells cotransfected with the control expression vector pPac0 (Fig. 2D). However, overexpression of Sp1 protein, but not Sp3, dramatically increased pGL3–0.18mHKα2 promoter activity. Again, these results are internally consistent with the other assays.
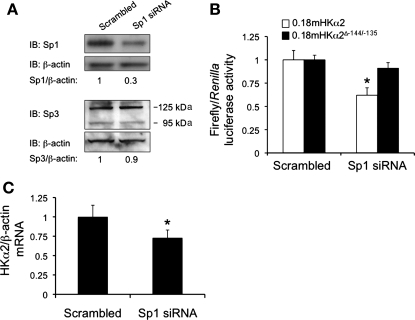
In the second approach, we used RNA interference to knock down endogenous Sp1 protein expression and then tested the basal activity of pGL3–0.18mHKα2 and pGL3–0.18mHKα2ΔSp1−144/−135. To confirm successful knockdown, protein blots were prepared from nuclear extracts of mIMCD3 cells that had been transfected with a scrambled control siRNA or with an siRNA specifically targeting Sp1. The blots were probed with antibodies against Sp1 or β-actin, or, in similarly prepared blots, Sp3 or β-actin, as a control for specificity (Fig. 3A). As shown in Fig. 3A, mIMCD3 cells transfected with the siRNA targeting Sp1 exhibited ∼70% lower levels of Sp1 nuclear protein compared with mIMCD3 cells transfected with an equimolar amount of negative control siRNA. In contrast, Sp3 protein expression was virtually the same in the two conditions. Consistent with our hypothesis that Sp1 trans-activates the 0.18mHKα2 promoter via the −144/−135 element, pGL3–0.18mHKα2 activity was ∼40% lower in the cells transfected with Sp1 siRNA compared with the control transfected cells (Fig. 3B). Moreover, the pGL3–0.18mHKα2ΔSp1−144/−135-transfected cells did not exhibit any significant difference in promoter activity with Sp1 knockdown compared with cells in which Sp1 expression was unperturbed (scrambled control) (Fig. 3B). Consistent with these results, cells transfected with Sp1 siRNA also exhibited substantially less HKα2 mRNA (as measured by qRT-PCR and normalized to the levels of β-actin mRNA (obtained by qRT-PCR in the same samples) compared with cells transfected with negative control siRNA (Fig. 3C). The levels of β-actin mRNA, used as a specificity control, were invariant across the samples.
Fig. 3.
Knockdown of Sp1 expression by small interfering (si) RNA results in downregulation of HKα2 promoter activity and endogenous HKα2 mRNA expression in mIMCD3 cells. mIMCD3 cells were treated with scrambled control siRNA or Sp1-specific siRNA and then subjected to immunoblot analysis to verify Sp1 protein knockdown, but not Sp3 knockdown (n = 3; A), pGL3–0.18mHKα2 and pGL3–0.18mHKα2Δ−144/−135 promoter activities (n = 4; B; *P < 0.05 vs. scrambled siRNA), and qRT-PCR analysis of endogenous HKα2 mRNA expression (n = 4; C; *P < 0.05 vs. Sp1 siRNA).
Sp1 binding is concurrent with and does not influence NF-κB or CREB-1 binding and regulation of the HKα2 proximal promoter.
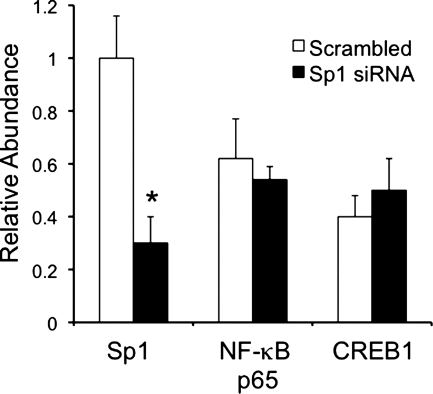
Our collective data suggest that NF-κB, CREB-1, and Sp1 each play a role in the basal activity of the HKα2 proximal promoter. To determine whether these three transcription factors co-occupy the promoter in the native chromatin environment of mIMCD3 cells, and whether Sp1 binding is required for or antagonizes binding of NF-κB and CREB-1, we performed sequential ChIP experiments. In these experiments, we also employed RNA interference to deplete Sp1 protein levels in the cells (as in Fig. 3A) to determine any synergism or antagonism in binding of Sp1 with the other two transcription factors without any possible structural alteration to the promoter organized as chromatin. Cells were treated with scrambled siRNA or Sp1 siRNA and then subjected to the first ChIP with Sp1 antibody as in Fig. 1D. The sample immunoprecipitated with the Sp1 antibody was then subjected to sequential ChIP with IgG or antibodies directed against NF-κB p65 or CREB-1. Reverse cross-linked DNA from both ChIPs was analyzed using qPCR and HKα2 primers targeting the −194/+56 region. Results from the first ChIP and from the second ChIP are represented as percentage of the input DNA (Fig. 4). Cells in which Sp1 had been depleted by RNA interference showed levels of NF-κB p65 and CREB-1 in the sequential ChIP comparable to that of cells treated with the scrambled siRNA (Fig. 4). This qPCR result indicates that NF-κB p65, CREB-1, and Sp1 concurrently occupy the HKα2 proximal promoter under basal conditions in vivo. These results further suggest that Sp1 does not facilitate or hinder the binding of NF-κB p65 and CREB-1 to the HKα2 proximal promoter under basal conditions in vivo.
Fig. 4.
ChIP and sequential ChIP assays demonstrating that siRNA knockdown of Sp1 does not affect the abundance of NF-κB or CREB-1 at the proximal HKα2 promoter in vivo. mIMCD3 cells were cotransfected with scrambled siRNA or Sp1-specific siRNA followed by sequential immunoprecipitation with anti-Sp1 and anti-NF-κB p65 or anti-CREB-1 antibodies. qPCR was then performed with a primer set designed to amplify −194/+56 of the HKα2 gene. Relative sequential ChIP efficiency was defined as the amount of reimmunoprecipitated material compared with that of the initial input sample and was assigned as 1 for the scrambled siRNA-transfected cells (n = 4). *P < 0.05 vs. scrambled control.
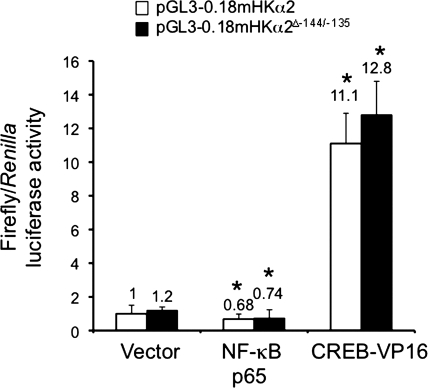
To delineate further the potential interaction of these three cis-elements, we compared the effects of overexpression of NF-κB p65 or the constitutively active CREB-1 mutant CREB-VP16 on the luciferase activities generated by transfected pGL3–0.18mHKα2 or pGL3–0.18mHKα2ΔSp1−144/−135. In previous work, we demonstrated that overexpression of NF-κB p65 in mIMCD3 cells inhibited by ∼25% the activity of the proximal HKα2 promoter (25), whereas overexpression of CREB-VP16 increased by ∼16-fold the activity of the proximal HKα2 promoter (23). We chose to use CREB-VP16 to achieve the maximal CREB-mediated effect on proximal HKα2 promoter activity. We hypothesized that an effect of Sp1 at the −144/−135 binding element to exert functional synergism or antagonism with these other transcription factors would be lost in pGL3–0.18mHKα2ΔSp1−144/−135. However, we observed that the mutant promoter yielded the same responses to overexpression of NF-κB p65 or CREB-VP16 as the wild-type HKα2 promoter (Fig. 5). Taken together with the sequential ChIP assay results (Fig. 4), these data strongly suggest that Sp1 acts independently from NF-κB and CREB-1 to regulate the activity of the proximal HKα2 promoter.
Fig. 5.
Mutations in the −144/−135 Sp1 element do not limit the CREB or NF-κB-mediated regulation of HKα2 proximal promoter activity. pGL3–0.18mHKα2 or pGL3–0.18mHKα2Δ−144/−135 and the Renilla luciferase expression plasmid pRL-TK were cotransfected in the presence of the expression vector for NF-κB p65 or the constitutively active CREB1 mutant CREB-VP16. Forty-eight hours after transfection, cell lysates were prepared and firefly and Renilla luciferase activities in lysates of the cells were assayed. Values are means ± SE of 4 separate experiments. *P < 0.05 vs. pGL3–0.18mHKα2.
DISCUSSION
Analysis of the regulation of active K+ reabsorption and H+ extrusion in the renal collecting duct and distal colon requires knowledge of transcriptional control mechanisms governing HKα2 gene expression. Promoter regions of eukaryotic genes are generally composed of multiple binding sites for transcriptional activators and repressors that act in combination to regulate the expression of a linked gene. Our analysis of the proximal promoter of the HKα2 gene has previously revealed that its basal activity requires at least two functional elements: a κB element at −104/−94 that binds NF-κB and interacts with HDAC6 (25) and the CRE-like element at −86/−60 that binds CREB-1 (23). In this study, we build on our earlier work defining such control elements by demonstrating in mIMCD3 cells that Sp1 is required as a trans-activator of the HKα2 gene by binding to an Sp1 element at −144/−135 of the HKα2 proximal promoter under basal conditions and that its binding does not appear to have interdependence with that of NF-κB and CREB-1 to this region in chromatin.
Sp1 was the first C2H2-type zinc finger-containing transcriptional factor to be purified and cloned from mammalian cells (4). Currently, the Sp/Kruppel-like factor (Sp/KLF) superfamily consists of two subfamilies: the Sp subfamily has 8 members and favors binding to GC-rich regions, whereas the KLF subfamily has 15 members and generally prefers GT-boxes (1). The Sp5-Sp8 proteins consist of truncated versions of Sp1. The Sp1, Sp3, and Sp4 proteins share highly conserved primary and domain structures. In addition to three zinc fingers, each of these proteins possesses two glutamine-rich modules that comprise their transcriptional activation domains (AD). A serine/threonine (S/T)-rich domain that may be targeted for posttranslational modifications neighbors the AD. These posttranslational modifications appear to regulate not only the expression level, intracellular compartmentalization, DNA-binding activity, and trans-activation potential of Sp1 but also its interaction with other transcription factors (14). In contrast, Sp2 contains one glutamine-rich AD and an S/T module proximal to the zinc fingers. The COOH-terminal domain of Sp1 interacts with several transcriptional activators and repressors (14).
The effect of Sp1 on the HKα2 promoter in mIMCD3 cells was likely direct and mediated principally by DNA binding, since mutation of these −144/−135 element sequences disrupted the Sp1 DNA-binding activity in vitro (Fig. 1C) and in vivo (Fig. 1D), partially abrogated Sp1-mediated trans-activation of the HKα2 promoter in overexpression experiments (Fig. 2, C and D), and rendered the HKα2 promoter completely unresponsive to the inhibitory effects of Sp1 siRNA (Fig. 3B). The binding of Sp1 to this region was specific since the formation of the major complex A was inhibited by the addition of an excess of unlabeled sequence, but not by an excess of unlabeled sequence in which the Sp1 element was mutated. The fact that overexpressed Sp1 still trans-activated the mutated HKα2 promoter, albeit to a much lower degree than the wild-type promoter, suggests several possibilities: 1) there are other, functionally more minor, Sp1 elements within the region of the HKα2 promoter tested; 2) Sp1 interacts with DNA-binding proteins other than NF-κB p65 or CREB-1 (Figs. 4 and 5) at sites outside the −144/−135 area to trans-activate HKα2 promoter; or 3) either or both of these two possibilities (1 and 3) occur as an artifact of overexpression itself. The fact the activity of the mutant promoter was not significantly less than that of the wild-type promoter when Sp1 was knocked down by RNA interference suggests that the latter hypothesis may be correct. Further experiments will be needed to test this.
Our collective data support the hypothesis that all three transcription factors interact concurrently with their respective cis-elements in the HKα2 proximal promoter under basal conditions and that binding of NF-κB or CREB-1 does not appear to be nucleated, increased, or limited by the absolute level of Sp1 binding to the −144/−135 site: 1) depletion of Sp1 by RNA interference yielded no significant difference in occupancy of the neighboring NF-κB and CREB-1 sites in chromatin associated with the HKα2 promoter (Fig. 4); 2) the HKα2 proximal promoter harboring a mutated −144/−135 Sp1 element exhibited responses to overexpressed NF-κB p65 and CREB-VP16 comparable to that of the wild-type HKα2 promoter (Fig. 5); and 3) as we previously reported (23), an HKα2 promoter harboring a mutated −104/−94 κB element exhibited trans-activation by CREB-VP16 comparable to that of the wild-type HKα2 promoter. However, since Sp1, although greatly downregulated, was still expressed in the RNA interference studies, it remains possible that a low threshold amount of Sp1 is required for cooperative binding of NF-κB and CREB-1 to their sites.
Since Sp3 was not detected in supershift/interference assays (Fig. 1C) or ChIP assays (Fig. 1D) of mIMCD3 cells using the involved HKα2 promoter region, and since overexpression of Sp3 did not trans-activate the pGL3–0.18mHKα2 reporter gene in mIMCD3 cells (Fig. 2C) or in Drosophila SL2 cells (Fig. 2D), we conclude that Sp3 does not play a significant role in regulating the basal activity of the HKα2 proximal promoter. Furthermore, we did not detect expression of Sp4 on immunoblots of nuclear lysates of mIMCD3 cells, eliminating this homolog as a transcriptional regulator. However, we cannot exclude the possibility, which we believe is remote, that related Sp/KLF family members participate in HKα2-regulatory control in this circumstance.
Our collective analysis of the HKα2 proximal promoter indicate opposing, but apparently independent actions of Sp1 and CREB-1, which function as basal trans-activators, and NF-κB, which recruits HDAC-6 to limit HKα2 transcriptional activity in mIMCD3 cells. These results should be helpful in understanding the molecular mechanisms governing HKα2 gene expression. Whether and how the balance of these opposing functions is altered during states of altered HKα2 transcription, such as occurs during HKα2 up-regulation in the collecting duct during hypokalemia, remain to be established. Furthermore, whether this complement of transcription factors is required for the collecting duct-specific expression driven by the proximal HKα2 promoter that we observed in transgenic mice in vivo (28) requires further analysis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-47981.
Acknowledgments
The authors thank Dr. Lei Zou, Dr. Diana Jalal, and Teresa Kuncewicz for contributing to manuscript.
Footnotes
Atp12a has been designated by HUGO as the official name for the murine gene. However, given the multiple references to past publications and DNA constructs using the alias “HKα2,” we refer to this gene as HKα2 in this manuscript for clarity.
REFERENCES
- 1.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol 195: 27–38, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Brayer KJ, Segal DJ. Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys 50: 111–131, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55: 887–898, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Dynan WS, Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell 35: 79–87, 1983. [DOI] [PubMed] [Google Scholar]
- 5.Foster ES, Jones WJ, Hayslett JP, Binder HJ. Role of aldosterone and dietary potassium in potassium adaptation in the distal colon of the rat. Gastroenterology 88: 41–46, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Jaisser F, Escoubet B, Coutry N, Eugene E, Bonvalet JP, Farman N. Differential regulation of putative K+-ATPase by low-K+ diet and corticosteroids in rat distal colon and kidney. Am J Physiol Cell Physiol 270: C679–C687, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Jalal DI, Kone BC. Src activation of NF-kappaB augments IL-1beta-induced nitric oxide production in mesangial cells. J Am Soc Nephrol 17: 99–106, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Kone BC Renal H,K-ATPase: structure, function and regulation. Miner Electrolyte Metab 22: 349–365, 1996. [PubMed] [Google Scholar]
- 9.Lynch IJ, Rudin A, Xia SL, Stow LR, Shull GE, Weiner ID, Cain BD, Wingo CS. Impaired acid secretion in cortical collecting duct intercalated cells from H-K-ATPase-deficient mice: role of HK-α isoforms. Am J Physiol Renal Physiol 294: F621–F627, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Meneton P, Schultheis PJ, Greeb J, Nieman ML, Liu LH, Clarke LL, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Increased sensitivity to K+ deprivation in colonic H,K-ATPase-deficient mice. J Clin Invest 101: 536–542, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura S, Amlal H, Galla JH, Soleimani M. Colonic H+-K+-ATPase is induced and mediates increased HCO3− reabsorption in inner medullary collecting duct in potassium depletion. Kidney Int 54: 1233–1239, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S, Wang Z, Galla JH, Soleimani M. K+ depletion increases HCO3− reabsorption in OMCD by activation of colonic H+-K+-ATPase. Am J Physiol Renal Physiol 274: F687–F692, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Perrone RD, McBride DE. Aldosterone and Pco2 enhance rubidium absorption in rat distal colon. Am J Physiol Gastrointest Liver Physiol 254: G898–G906, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Safe S, Kim K. Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog Nucleic Acid Res Mol Biol 77: 1–36, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Sangan P, Rajendran VM, Mann AS, Kashgarian M, Binder HJ. Regulation of colonic H-K-ATPase in large intestine and kidney by dietary Na depletion and dietary K depletion. Am J Physiol Cell Physiol 272: C685–C696, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Shull GE, Miller ML, Schultheis PJ. Lessons from genetically engineered animal models VIII. Absorption and secretion of ions in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 278: G185–G190, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am J Physiol Renal Physiol 276: F799–F811, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Spicer Z, Clarke LL, Gawenis LR, Shull GE. Colonic H+-K+-ATPase in K+ conservation and electrogenic Na+ absorption during Na+ restriction. Am J Physiol Gastrointest Liver Physiol 281: G1369–G1377, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Sverdlov VE, Kostina MB, Modyanov NN. Genomic organization of the human ATP1AL1 gene encoding a ouabain-sensitive H,K-ATPase. Genomics 32: 317–327, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Wall SM, Truong AV, DuBose TD Jr. H+-K +-ATPase mediates net acid secretion in rat terminal inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1037–F1044, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Wierstra I Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 372: 1–13, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Wingo CS, Smolka AJ. Function and structure of H-K-ATPase in the kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F1–F16, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Zhang W, Kone BC. CREB trans-activates the murine H+-K+-ATPase α2-subunit gene. Am J Physiol Cell Physiol 287: C903–C911, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Yan G, Chen X, Bancroft C. A constitutively active form of CREB can activate expression of the rat prolactin promoter in non-pituitary cells. Mol Cell Endocrinol 101: R25–30, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Kone BC. NF-κB inhibits transcription of the H+-K+-ATPase α2-subunit gene: role of histone deacetylases. Am J Physiol Renal Physiol 283: F904–F911, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Kuncewicz T, Higham SC, Kone BC. Structure, promoter analysis, and chromosomal localization of the murine H+/K+-ATPase α2 subunit gene. J Am Soc Nephrol 12: 2554–2564, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117: 773–783, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Xia X, Zou L, Xu X, LeSage GD, Kone BC. in vivo expression profile of a H+-K+-ATPase α2-subunit promoter-reporter transgene. Am J Physiol Renal Physiol 286: F1171–F1177, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Li Y, Dai C, Yang J, Mundel P, Liu Y. Sp1 and Sp3 transcription factors synergistically regulate HGF receptor gene expression in kidney. Am J Physiol Renal Physiol 284: F82–F94, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Zies DL, Gumz ML, Wingo CS, Cain BD. Characterization of the rabbit HKalpha2 gene promoter. Biochim Biophys Acta 1759: 443–450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]