Abstract
ADP ribosyl (ADPR) cyclases comprise a family of ectoenzymes recently shown to influence cytosolic Ca2+ concentration in a variety of cell types. At least two ADPR cyclase family members have been identified in mammals: CD38 and CD157. We recently found reduced renal vascular reactivity to angiotensin II (ANG II), endothelin-1 (ET-1), and norepinephrine (NE) in the presence of the broad ADPR cyclase inhibitor nicotinamide. We hypothesized that CD38 mediates effects attributed to ADPR cyclase. We found expression of ADPR cyclases CD38 and CD157 mRNA in spleen, thymus, skin, and preglomerular arterioles of wild-type (WT) animals. Mice lacking CD38 showed decreased CD157 expression in most tissues tested. No difference in systolic or mean arterial pressure was observed between strains in either conscious or anesthetized states, whereas heart rate was reduced 10–20% in CD38−/− animals (P < 0.05). During anesthesia, CD38−/− mice had reduced basal renal blood flow (RBF) and urine excretion (P < 0.05). RBF responses to intravenous injection of ANG II, ET-1, and NE were attenuated ∼50% in CD38−/− vs. WT mice (P < 0.01 for all). The systemic pressor response to ANG II was decreased in the absence of CD38 (P < 0.01), whereas that to NE was normal (P > 0.05); ET-1 was administered at a nonpressor dose. Nicotinamide effectively inhibited ANG II-induced renal vasoconstriction in WT mice (P < 0.001), but had no effect on renal responses to ANG II in CD38−/− mice (P > 0.5). Overall, our observations indicate the presence of two ADPR cyclase family members in renal preglomerular resistance arterioles and the importance of CD38 participation in acute vascular responses to all three vasoconstrictors in the renal microcirculation.
Keywords: renal circulation, glomerular arterioles, Ca2+ signaling, vascular smooth muscle, ryanodine receptor
adp ribosyl (ADPR) cyclases are a family of homodimeric transmembrane glycoproteins capable of transforming NAD+ and NADP+ to cyclic ADP ribose (cADPR) and nicotinic acid ADP (NAADP), respectively (22). cADPR and NAADP regulate cytosolic Ca2+ concentration ([Ca2+]i) via direct or indirect activation of ryanodine receptors (RyR). cADPR produced by plasma membrane ADPR cyclase(s) is thought to act on RyR directly, either by binding or by removing FK506 (tacrolimus) binding proteins (FKBP12 and/or FKBP12.6) (29). NAADP produced in intracellular acidic vesicles likely activates RyR more indirectly by stimulating release of vesicular Ca2+, possibly from late endsomes or lysosomes near the sarcoplasmic or endoplasmic reticulum and subsequent activation of Ca2+-induced Ca2+ release (CICR) (21, 40).
Initially characterized from the ovotestis of the sea hare, Aplysia californica (22), the existence of two ADPR cyclases was soon discovered in mammals. Interestingly, these two enzymes had already been extensively studied as surface antigens on immune cells and termed CD38 and CD157 (the latter also known as bone marrow stromal antigen-1 or BST-1) (24). Although the two proteins are expressed in many of the same tissues, enzymatic activity of CD38 is thought to predominate over CD157 based on greater expression levels and enzymatic activity (30).
The activities of CD38 and CD157 have been studied in a variety of tissues including most immune cells, neurons, and pancreas (30); however, less is known about the expression and function of these two enzymes in vascular smooth muscle cells (VSMC). Aortas from CD38−/− mice exhibit decreased contractile responses to norepinephrine (NE) and phenylephrine (PE), indicating a role for CD38 in mediating α-adrenergic receptor responses in VSMC (26). Interestingly, these investigators noted normal vasoconstrictor responses to other G protein-coupled receptor (GPCR) agonists [e.g., 5-hydroxytryptamine, endothelin-1 (ET-1)] as well as caffeine, KCl, and thapsigargin. Other studies on aorta suggest the presence of a novel ADPR cyclase family member specific to VSMC in addition to CD38. Unlike CD38, ADPR cyclase activity in rat aortic VSMC is inhibited by gangliosides, Zn2+, and Cu2+, unaltered by a CD38 antibody, and stimulated by retinoids and tri-iodothyronine (8). The identity of this putative family member and its possible role in resistance arterioles await investigation.
Whether ADPR cyclase activity in VSMC is attributed to CD38, CD157, or another cyclase family member, the importance of ADPR cyclase and its second messengers in mediating increases in [Ca2+]i is generally accepted for VSMC from arteries of various sizes. ADPR cyclase activity has been observed in vessels of all sizes from aorta (8, 26) to glomerulus (4) and is thought to contribute to the regulation of basal [Ca2+]i as well as Ca2+ responses to GPCR stimulation (14, 30). cADPR injection into permeabilized porcine coronary VSMC increases [Ca2+]i, an action dependent on RyR, but not the Ca2+ mobilizing second messenger inositol trisphosphate (IP3) (1). In bovine coronary artery, inhibition of ADPR cyclase produces vasodilation dependent on cADPR and RyR (30). In pulmonary artery VSMC, cADPR is produced under hypoxic conditions and likely mediates hypoxia-induced vasoconstriction (9). In addition, NAADP elicits [Ca2+]i responses in coronary (29), pulmonary (21), and renal arterial VSMC (40). Although small Ca2+ release events can be initiated by NAADP during RyR inhibition, it is currently thought that RyR participate in NAADP responses via CICR in VSMC (21).
Small-diameter resistance arterioles in the kidney are responsible for the regulation of renal blood flow (RBF) and glomerular filtration rate (GFR) and participate in the regulation of salt and water balance and arterial pressure (AP) (27). Several studies investigated the contribution of ADPR cyclase to Ca2+ signaling and constriction of these vessels. Small rat renal arteries produce cADPR that increases [Ca2+]i (23). CICR in these vessels relies largely on cADPR subject to pharmacological inhibition (1), a process likely due to sensitization of RyR by endogenous cADPR. Furthermore, blockade of ADPR cyclase, cADPR, or RyR attenuates [Ca2+]i responses to angiotensin II (ANG II) and ET-1 in physiologically important isolated rat afferent arterioles (13, 14). ADPR cyclase also regulates resting vascular tone in a rat kidney, as demonstrated by dilatory actions of the ADPR cyclase inhibitor nicotinamide. Additional evidence for ADPR cyclase participating in CICR responses in the renal microcirculation is based on ADPR cyclase inhibition attenuating renal vasoconstrictor responses in vivo and increased [Ca2+]i to stimulation of Ca2+ entry through L-type voltage-gated channels (39). Acute renal vasoconstriction elicited by the GPCR agonists ANG II, ET-1, and NE is also attenuated during inhibition of ADPR cyclase, indicating a role for the enzyme in cell signaling and contraction downstream of three classes of GPCR linked to the G protein Gqα (38, 39). Interestingly, the vasodilator action of agmatine on preglomerular vessels is also mediated by cADPR and RyR, apparently by stimulation of endothelial production of nitric oxide (34).
No previous study analyzed the contribution of individual ADPR cyclase family members to Ca2+ signaling and/or vascular reactivity in renal resistance vessels. To this end, we determined the expression of CD38 and CD157 mRNA in afferent arterioles and the functional consequence of a genetic deficiency of CD38 in systemic pressor and renal vascular responsiveness. We postulated that CD38 contributes importantly to systemic and renal vascular reactivity to administered ANG II, ET-1, and NE in wild-type mice and that hemodynamic responses to these agents are attenuated in CD38−/− mice.
METHODS
Animals were used in accordance with protocols approved by our Institutional Animal Care and Use Committee. Animals were fed standard laboratory chow and given tap water ad libitum and kept on a 12:12-h light-dark cycle.
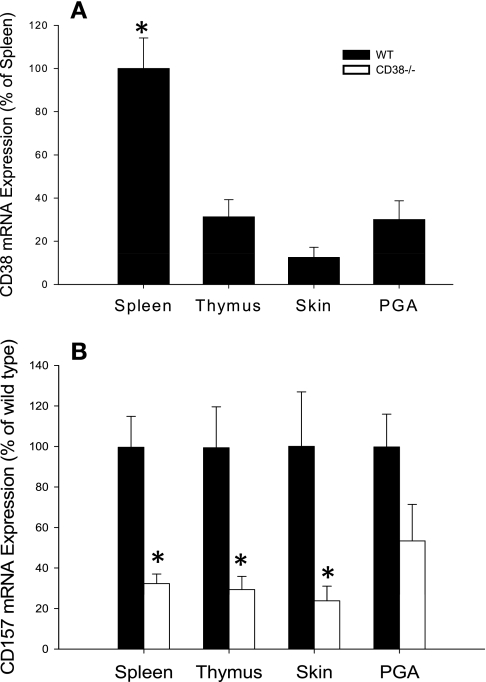
A total of 88 male mice 7–9 wk of age were studied. Wild-type mice of C57black6 background, initially purchased as breeder pairs from Jackson Laboratories, were bred in our in-house breeding facility. CD38−/− mouse breeder pairs, backcrossed onto a C57black6 background, were a kind gift from F. Lund (Trudeau Institute, Ithaca, NY) (5). The CD38−/− animals we studied were bred locally. Real-time quantitative RT-PCR (Fig. 1) confirmed the presence of CD38 mRNA in spleen, thymus, skin, and preglomerular resistance arterioles in wild-type control animals and its absence in CD38−/− mice.
Fig. 1.
Expression of mRNA for ADP ribosyl (ADPR) cyclases in spleen, thymus, skin, and preglomerular arterioles (PGA). A: expression of CD38 mRNA in wild-type (WT) mice. Values are normalized to the grand mean for spleen. Undetectable levels were found in all tissues of CD38-deficient mice. *P < 0.05 vs. PGA, n ≥ 6. B: CD157 mRNA expression in tissues from WT and CD38−/− mice. Values are normalized to WT. *P < 0.05 vs. the same tissue from WT animals, n ≥ 6.
Systolic blood pressure measurement.
Systolic AP and heart rate were measured in conscious animals by tail-cuff methodology using the Hatteras MC-4000 system (Cary, NC). Mice were trained for 3 days followed by 2 days of measurements. Mice were placed in a dark chamber on a heated platform (92°F) for 15–20 min before measurement. Five training cycles were performed at the start of each experiment followed by 15 measurement cycles. The values of those 15 cycles were averaged to generate one value for that day. The values for the last 2 days were averaged to obtain means for systolic AP and heart rate for a single mouse.
Tissue isolation.
Preglomerular resistance arterioles were isolated for RT-PCR analysis using a modification of the iron oxide method described previously for rats (3, 12, 31, 32). Mice were anesthetized using pentobarbital sodium (80–90 mg/kg ip). Hair was removed from a sample of skin using forceps and skin was removed and placed in PBS. A midline incision was made and the aorta was clamped above the level of the renal arteries and cannulated at the bifurcation of the left and right common iliac arteries with a blunted 25-gauge butterfly needle. The left renal vein was punctured and the kidneys were perfused with PBS containing 5 mM glucose and 0.1 mM CaCl2 until all blood had been removed from the kidneys as evidenced by clear solution exiting the left renal vein (∼5 ml). Kidneys were then perfused with 5 ml of 1% iron oxide followed by 1–2 ml of PBS solution and immediately excised and placed in PBS, followed by removal of spleen and thymus. Skin, thymus, and spleen samples sat on ice during the preparation of arterioles. Kidneys were decapsulated and the cortical slices were minced for 3 min using a razor blade and homogenized using a tissuemizer for 10 s. Vessels were removed by magnet and passed through a bent 22-gauge needle followed by three passages through straight 25-gauge needles and sieving on a 55-μm sieve to remove tubular fragments and glomeruli. Vessels were placed in PBS containing 312.5 digestion units of collagenase (type 1A, Sigma, 2.5 mg/25 ml) for 30 min followed by magnet removal of vessels and passage through two 25-gauge needles and a 27-gauge needle. Preglomerular resistance arterioles were obtained by centrifugation at 5,000 rpm for 30 min at 4°C. After preparation, all tissues were weighed, transferred to RNAlater solution to preserve mRNA, and sent off for analysis.
Real-time quantitative RT-PCR.
Generation of primers and probes, isolation of mRNA, and real-time qRT-PCR were performed by the Gene Expression Core Facility at the University of North Carolina at Chapel Hill (2, 28). Forward primers were 5′-GTG GAG ACC CTA GTA CTT CT-3′, 5′-GGA AAA GTG CAT CCA TGC AG-3′, and 5′-CTG CCT GAC GGC CAG GTC-3′ for CD38, CD157, and β-actin, respectively. Reverse primers were 5′-GAA CAC AGT AAT AGG GTT GTT G-3′, 5′-TCC TTT TGG CTC CGA GCC AT-3′, and 5′-CAA GAA GGA AGG CTG GAA AAG A-3′. Probes were conjugated upstream to reporter dye FAM (6-carboxyfluorescein) and downstream to quencher dye TAMRA (6-carboxytetramethyl1-rhodamine) and were 5′-CT ATG TCT CTT GCC CAC ATT GGA GTG-3′, 5′-CA TGA CGT TGA TCA CCC CTG AAC TG-3′, and 5′-CAC TAT TGG CAA CGA GCG GTT CCG-3′ for CD38, CD157, and β-actin. Before normalization, wild-type animals had Ct values for CD38 in skin of 24-31, thymus 18-22, spleen 17-30, and PGA 21-30 and for CD157 in skin of 26-33, thymus 21-26, spleen 21-29, and PGA 23-35. In CD38−/− animals, Ct values for CD157 in skin were 24-35, spleen 19-29, thymus 21-30, and PGA 24-27. Ct values were >38 for all tissues for CD38−/− mice.
In vivo measurement of RBF.
Mice were anesthetized using pentobarbital sodium (80–90 mg/kg ip) and placed on a warm table (37°C). A pulled PE-50 catheter was inserted into a femoral artery and attached to a pressure transducer to measure mean AP (MAP). Hematocrit of femoral arterial blood was measured periodically. Three pulled PE-10 catheters were inserted into a femoral vein for infusion of 2.45% bovine serum albumin (continuous at 10 μl/min throughout an experiment) and intravenous administration of pharmacological agents. A catheter was inserted close to the neck of the bladder to collect urine. A PE-50 catheter served as a tracheal tube to facilitate breathing. The animal was then turned onto its side. A subcostal incision was made to expose the left kidney. An ultrasonic flow probe (Transonic System TS420, Ithaca, NY; 0.5-V probe) was positioned around the left renal artery to measure RBF. Animals were allowed to stabilize 1 h before observations were made.
Pharmacological agents.
ANG II (Sigma, St. Louis, MO), ET-1 (American Peptide, Sunnyvale, CA), and NE (Levophed, Abbott Labs, Chicago, IL) were given as bolus injections (10–50 μl) into a femoral vein. Nicotinamide (Sigma) was infused intravenously for 3 min before and 2 min after injection of ANG II. All agents were dissolved in isotonic saline. As all animals quickly responded to vasoactive agents or inhibitors and returned to control values, multiple measurements were made in each animal.
Data analysis and statistics.
Student's t-test assessed group differences for baseline values presented in Table 1 using SigmaStat software.
Table 1.
Renal and cardiovascular variables measured in wild-type and CD38−/− mice during basal conditions
| Wild-Type | CD38−/− | |
|---|---|---|
| Conscious animals | ||
| Systolic arterial pressure, mmHg | 137±4 | 133±5 |
| Heart rate, beats/min | 611±15 | 542±25* |
| Anesthetized animals | ||
| Mean arterial pressure, mmHg | 71±4 | 65±3 |
| Heart rate, beats/min | 464±18 | 378±18† |
| Renal blood flow, ml·min−1·g kidney wt−1 | 8.6±1.0 | 5.4±0.6* |
| Renal vascular resistance, mmHg·ml−1·min | 51±10 | 64±7 |
| Left kidney weight, g | 0.2±0.01 | 0.2±0.01 |
| Urine output, μl/min | 5.2±0.7 | 2.2±0.4† |
| Heart weight, g | 0.15±0.007 | 0.16±0.004 |
| Body weight, g | 26±1.1 | 26±0.6 |
| Hematocrit, % | 36±2.6 | 36±2.3 |
Data are means ± SE; n = 15 of each strain of anesthetized mice and n = 8 of each strain of conscious mice.
P < 0.05.
P < 0.005.
For real-time quantitative RT-PCR studies, Ct values >38 were considered to be a lack of expression. Ct values for detectable mRNA levels were normalized to β-actin and the grand mean for the control group (either wild-type spleen for CD38 studies or matched wild-type tissue for CD157 studies) was set to 100%. Data for other groups were normalized to this value. Individual groups were compared using a Student's t-test (SigmaStat).
In vivo RBF and MAP data were sampled using Labtech Notebook. Transient responses of RBF and MAP are presented as the total integrated area of the response curve using a summation between 31 and 150 s after injection, expressed as a fraction of a hypothetical total response calculated from the maximum change from baseline for the entire postinjection time of 119 s. Significant effects of a given agonist were determined by two-way ANOVA with repeated measures for overall dose and CD38 effects, with post hoc Bonferroni testing of comparisons between individual groups (GraphPad). Studies of nicotinamide effects were analyzed within a given group by one-way ANOVA with repeated measures and post hoc individual comparisons via the Holm-Sidak method (SigmaStat).
RESULTS
Results are presented for a total of 15 wild-type and 15 CD38−/− mice between 7 and 13 wk of age. Body weight, kidney weight, hematocrit, and heart weight did not differ between groups (P > 0.05; Table 1). Notably, basal RBF and urine excretion were lower in CD38−/− animals during anesthesia (P < 0.05). Renal vascular resistance did not differ between strains.
CD38 and CD157 are expressed in preglomerular resistance arterioles.
We tested expression of mRNA for two ADPR cyclase family members in several tissues using real-time quantitative RT-PCR. CD38 and CD157 mRNA are both expressed in spleen and thymus (24, 25), so these tissues served as positive controls. In wild-type animals, CD38 expression was detected in all tissues. As expected, spleen expressed the largest amount of CD38 mRNA (Fig. 1A). Preglomerular resistance arterioles expressed less CD38 than spleen, a level similar to that of thymus and skin (Fig. 1A). No CD38 mRNA was detected in any CD38−/− tissue tested (data not shown). To determine whether CD157 could possibly be compensating for loss of CD38 in CD38−/− animals, we compared CD157 mRNA levels in several tissues isolated from wild-type and CD38−/− mice. Interestingly, CD157 was decreased in skin, spleen, and thymus isolated from CD38−/− mice compared with wild-type controls (P < 0.05). Although preglomerular resistance arterioles from CD38−/− mice did not express significantly less CD157 mRNA than those isolated from wild-type animals, levels of CD157 in CD38−/− renal arterioles did not differ statistically from that in other tissues tested (P > 0.1). These data indicate localization of CD38 and CD157 mRNA in preglomerular resistance arterioles of wild-type animals and an overall decrease, rather than an increase, of CD157 mRNA in tissues from CD38−/− mice, suggesting a lack of compensatory upregulation in the absence of CD38.
CD38−/− mice have normal arterial blood pressure and decreased heart rate.
Due to the importance of ADPR cyclases in [Ca2+]i regulation in cardiac myocytes (17, 42) and VSMC (8, 14, 30), we measured systolic AP and heart rate in conscious mice via the tail-cuff method. Systolic AP did not differ between groups (P > 0.5), but heart rate was lower in CD38−/− mice (Table 1).
Loss of CD38 leads to attenuated renal vascular reactivity.
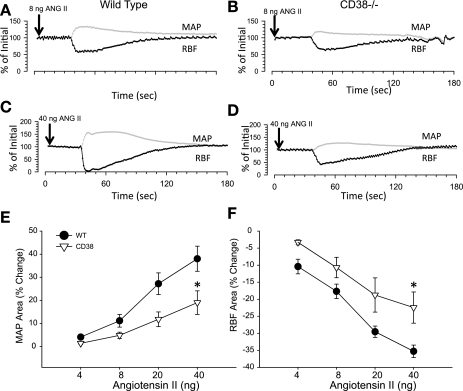
To determine the role of CD38 in systemic and renal vascular reactivity to vasoconstrictor agents, we assessed acute RBF and MAP responses to intravenous injection of ANG II, NE, and ET-1. MAP and RBF responses to ANG II were clearly evident in a dose-dependent manner in control animals and were markedly reduced in CD38−/− mice (ANOVA P < 0.01 for both; Fig. 2). There was a strain difference in renal and systemic vascular responses assessed as RBF and MAP effects of 40 ng ANG II. Wild-type animals exhibited integrated MAP and RBF response curves that were increased 38.0 ± 5.4 and decreased 35.3 ± 1.8% from baseline, respectively. CD38−/− animals, on the other hand, displayed weaker responses: 22.6 ± 5.7 and −24.2 ± 5.5%, respectively (P < 0.05). Analysis of maximum peak changes in RBF and MAP produced similar differences between groups as the reported integrated area analyses of the response curves.
Fig. 2.
Acute effect of ANG II injected intravenously on renal blood flow (RBF) and mean arterial pressure (MAP) in WT and CD38−/− mice. A-D: representative tracings of RBF (black) and MAP (gray) in WT (A and C) and CD38−/− mice (B and D) in response to 8 (A and B) and 40 (C and D) ng ANG II injection. E: average MAP response to ANG II in WT (•) and CD38−/− (▿). F: average RBF response to ANG II in WT (filled symbols) and CD38−/− mice (filled symbols). *P < 0.05 compared with WT, n ≥ 8.
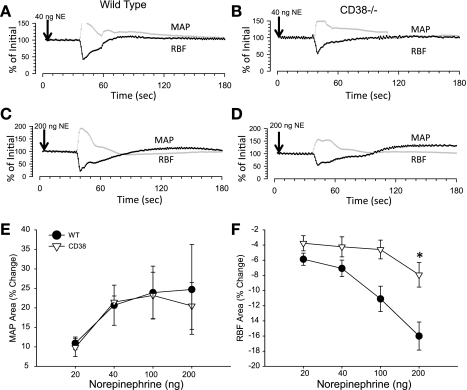
Attenuated dose-dependent RBF responses to NE were also observed in CD38−/− mice (ANOVA P < 0.005; Fig. 3). On the average, intravenous injection of 200 ng NE reduced RBF almost twofold less in CD38-deficient than in wild-type animals (−16.0 ± 1.8 vs. −9.2 ± 1.8%, P < 0.05). In marked contrast, MAP responses to multiple doses of NE did not differ between strains (P > 0.05). These results suggest more of a specific role for CD38 in renal vasoconstriction vs. global systemic vascular responses to NE.
Fig. 3.
Norepinephrine (NE)-induced changes in RBF and MAP in WT and CD38−/− mice. A and C: individual RBF (black) and MAP (gray) responses to NE injected intravenously into a WT mouse at 40 (A) and 200 (C) ng NE. B and D: sample RBF and MAP response in a CD38−/− mouse at 40 (C) and 200 (D) ng NE. E and F: average MAP (E) and RBF (F) responses to varying doses of NE injected into WT (•) and CD38−/− (▿) mice. *P < 0.05 compared with WT, n ≥ 8.
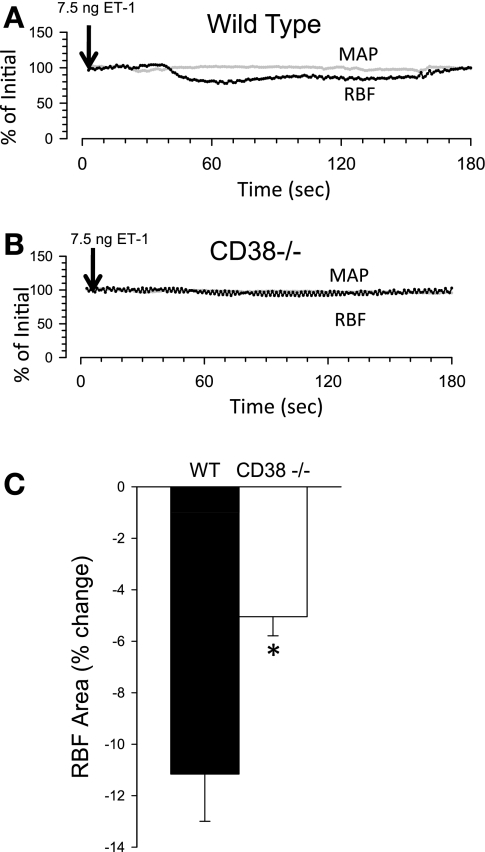
We also investigated the contribution of CD38 to renal vascular ET-1 responses (Fig. 4). Due to the long-lasting action of ET-1, one dose of ET-1 was given systemically to an animal. By design, the relatively small amount of ET-1 given intravenously did not produce a consistent change in MAP in wild-type mice; this was also the case in CD38 mutants (data not shown). In contrast, this amount of ET-1 elicited discernable renal vasoconstriction in both groups. ET-1 reduced RBF roughly twofold more in wild-type than in CD38−/− mice (−11.2 ± 1.8 vs. −5.1 ± 0.8%, P < 0.01). Collectively, these data indicate a major contribution of CD38 to renal vascular responses to ANG II, NE, and ET-1 in wild-type control mice and attenuated responsiveness of the renal microcirculation in CD38-deficient mice.
Fig. 4.
RBF responses to endothelin-1 (ET-1) injected intravenously in WT and CD38−/− mice. A and B: typical tracings of RBF (black circles) and MAP (gray circles) responses to ET-1 injection (7.5 ng) in WT (A) and CD38−/− mice (B). C: average RBF response to ET-1. *P < 0.05 compared with WT, n ≥ 8.
Nicotinamide has no effect on ANG II-induced RBF responses in CD38−/− mice.
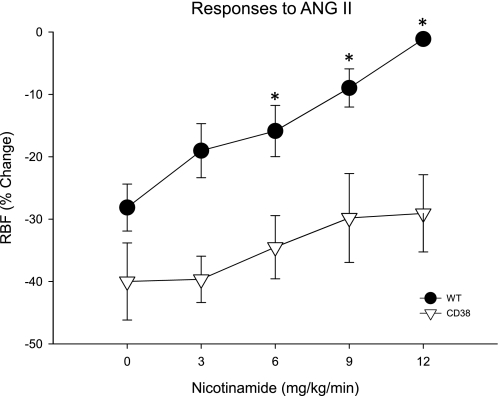
We previously showed that acute nicotinamide inhibition of renal ADPR cyclase activity attenuates ANG II-induced renal vasoconstriction in Sprague-Dawley rats (39). To test whether this effect was primarily due to inhibition of CD38, we compared the ability of nicotinamide to affect ANG II-induced decreases in RBF between wild-type and CD38−/− animals. To compensate for impaired renal vascular reactivity to ANG II seen in CD38−/− mice, we adjusted doses of ANG II to produce a similar degree of renal vasoconstriction in the two groups. For this purpose, we compared bolus intravenous injection of 4 ng ANG II in wild-type animals to 12 ng ANG II in CD38−/− animals. Before administration of nicotinamide, the reduction in RBF was similar in both strains (P > 0.1; Fig. 5). Intravenous infusion of multiple doses of nicotinamide before injection of ANG II effectively attenuated ANG II-induced renal vasoconstriction in wild-type animals in a dose-dependent manner (P < 0.001 via ANOVA). ANG II reduced RBF by −28.1 ± 3.8% during control conditions. In the experimental period, nicotinamide (6, 9, or 12 mg·kg−1·min−1) reduced integrated RBF responses to ANG II to −15.9 ± 4.1, −9.0 ± 3.1, and −1.1 ± 0.6%, respectively (P < 0.05 for each). In marked contrast, similar amounts of nicotinamide failed to inhibit ANG II-induced renal vasoconstriction in CD38−/− mice (P > 0.5). Importantly, no dose of nicotinamide tested produced significant changes in baseline RBF or MAP in either strain. Collectively, these data suggest that the primary action of nicotinamide on ANG II-induced RBF responses is due to inhibition of CD38 in control animals.
Fig. 5.
Effect of acute intravenous administration of nicotinamide on ANG II-induced renal vasoconstriction in WT and CD38−/− mice. Average changes in RBF produced by ANG II in WT (•) and CD38−/− mice (▿) before and during administration of nicotinamide. ANOVA results: *P < 0.05 compared with control ANG II (no nicotinamide), n ≥ 5.
DISCUSSION
We tested the hypothesis that the ADPR cyclase CD38 contributes to renal vasoconstriction produced by three classes of GPCR agonists: the neurohormonal agents ANG II and NE, and the endothelial-derived paracrine factor ET-1. We document, for the first time, expression of CD38 mRNA in preglomerular resistance arterioles and demonstrate a significant role for the enzyme in RBF responses to each of these vasoconstrictors in wild-type mice. The absence of CD38 mRNA is demonstrated in all tissues examined including the renal vasculature of gene-targeted mice. Our novel data show decreased overall MAP responses to ANG II, but not NE, in CD38-null mice. We present new evidence characterizing cardiovascular and renal status under resting conditions in CD38−/− mice, highlighted by values for MAP and RBF in the normal range in the absence of CD38. Systolic AP was also with normal limits in conscious animals. On the other hand, heart rate was lower in CD38-deficient mice during conscious and anesthetized states. Basal RBF and urinary excretion were reduced in the absence of CD38 function following anesthesia and surgery in CD38−/− animals. The present study extends our previous work showing attenuated Ca2+ signaling and renal vascular reactivity during pharmacological inhibition of ADPR cyclase in isolated renal afferent arterioles and kidneys of anesthetized Sprague-Dawley rats (13, 14, 38, 39). We now conclude specific involvement of the ADPR cyclase CD38 in mediating renal vascular responses. Collectively, our results indicate an important functional role for the ADPR cyclase CD38 in the renal microcirculation and systemic pressor responses.
Our real-time quantitative RT-PCR establishes for the first time CD38 and CD157 mRNA expression localized to preglomerular arterioles. Previous studies showed CD38 expression in glomeruli (15). Interestingly, it has been suggested that ADPR cyclase in VSMC is not CD38, but rather, a novel ADPR cyclase characterized by responses to all-trans-retinoic acid, Zn2+, and Cu2+ that are distinct from the conventional cyclase enzyme of rat aortic VSMC (7, 8). In demonstrating the presence of CD38 mRNA we do not exclude the possibility of other family members. A previous study showed expression of CD157 in whole kidney homogenates (19); its function and localization are not known.
We document the absence of CD38 mRNA in the renal vasculature of CD38-null mice. In this regard, our results differ from the earlier suggestion that ADPR cyclase enzyme activity and cADPR levels are not altered in whole kidneys of CD38−/− animals, whereas they are reduced in heart and brain (44). In contrast to the study of whole kidney homogenates, our study focused specifically on renal microvessels. Importantly, our functional data reinforce the idea that a renal phenotype of these animals is due to altered ADPR cyclase enzyme activity in the renal microcirculation.
To our knowledge, we are original in examining CD157 mRNA expression levels in any tissue of CD38−/− animals. If CD157 were to compensate for the loss of CD38, one would expect increased expression. However, CD157 mRNA levels were found to be reduced in multiple CD38−/− tissues, indicating a lack of positive compensation by CD157. It is possible that knockout of CD38 resulted in decreased CD157. The genes for both enzymes are located on chromosome 5 in mice, only 25 kb apart (13). It seems possible that the neomycin selection cassette interfered with CD157 expression. Future studies are required to elucidate the role, if any, of CD157 enzymatic activity in cardiovascular and renal function in wild-type as well as in CD38-deficient mice.
Based on our understanding of the importance of ADPR cyclase and cyclic ADPR in acute Ca2+ signaling and contraction of intrarenal arteries as well as coronary and pulmonary arteries to vasoconstrictor substances (30), we predicted that CD38−/− mice would be hypotensive with increased baseline RBF due to the lack of the CD38 vasoconstrictor signaling pathway. The long-term absence of CD38 did not agree with our predictions. The CD38-deficient mice were normotensive relative to wild-type controls when we compared systolic AP measured by the tail-cuff method in conscious animals or MAP determined by an in-dwelling arterial catheter during anesthesia. Resting RBF was lower, not higher, in CD38-mutant animals. The mechanisms involved are not known at present. Urine output and heart rate were also decreased in CD38−/− mice. It is important to mention that urine output was measured under anesthesia in acute experiments and further studies need to be performed to evaluate long-term alterations in fluid excretion in these animals. One cause of the strain difference in heart rate may be due to the effects of CD38 on neurotransmitter release from sympathetic nerves. CD38 is localized to postganglionic nerve terminals of mesenteric artery and vein (35) and cADPR modulates Ca2+ signaling RyR and transmitter release in sympathetic neurons (16, 18). Differences in urine output may be due to reduced RBF and perhaps GFR. An increase in activity of the renin-angiotensin system is consistent with the reported ability of cADPR to suppress renin release from As4.1 cells (43). Little is known about ADPR cyclase and CICR activity and function in native juxtaglmerular granular cells and renal tubular epithelial cells.
We conducted RBF studies on CD38−/− animals and showed attenuated acute renal vascular responses to ANG II, NE, and ET-1 compared with wild-type mice. These findings strongly support our previous results obtained using pharmacological inhibitors of ADPR cyclase in rat kidneys and the second messengers cADPR and NAADP in isolated rat afferent arterioles (13, 14) and earlier consequences of inhibiting RyR in rat arterioles in vitro and in rat kidneys in vivo (38, 39). Our evidence extends our current pool of knowledge by implicating CD38 specifically in the regulation of GPCR-induced renal vasoconstriction.
Our observations were made on CD38-deficient mice created by Cockayne et al. (5). We observed similar heart weights in 2- to 3-mo-old CD38−/− mice that had normal systolic AP and reduced heart rate compared with wild-type (Table 1). On the other hand, CD38 knockout mice generated by Kato et al. (36) present with cardiac hypertrophy at 3–9 mo of age. Noteworthy differences between our studies were that isolated aortas from CD38−/− mice created by Kato et al. exhibit decreased responsiveness to α-adrenergic receptor signaling elicited by NE and PE, but not to ET-1, 5-hydroxytyptamine, caffeine, or high KCl (26). In contrast, we found reduced renal vascular reactivity to three distinct classes of GPCR, including α-adrenoceptors and those responsive to ANG II and ET-1 in CD38−/− mice of Cockayne et al.
The variable vascular reactivity to ET-1 may indicate a difference between vascular beds. Large-diameter vessels tend to rely more on endothelin ETA receptors to produce contraction (41), whereas renal microvessels express functional ETA and ETB receptors and with evidence of synergic interactions between the two ET receptors (20). We previously found a major contribution of ADPR cyclase to both ETA and ETB receptor signaling participating in constriction of the renal vasculature (38).
Another intriguing comparison between groups is that we show that MAP responses to NE were not attenuated in CD38−/− mice, although RBF responses were attenuated. NE may affect heart rate more than ANG II. While low-dose ANG II increases MAP without affecting heart rate, NE increase heart rate, even at low doses. However, peak NE-induced increases in heart rate were comparable between groups (data not shown). Thus, the similar MAP pressor response to NE in wild-type and CD38-null mice suggests similar increases in total peripheral vascular resistance. Why the systemic vasculature of CD38−/− mice is relatively more responsive to the vasoconstrictor actions of NE than the renal microcirculation of these animals is not known. A possible explanation is the distribution of adrenergic receptor subtypes. α1-Adrenergic receptors predominate in renal cortical blood vessels while α2 receptors are the major type in the medulla (10). Preglomerular resistance arterioles specifically express predominately α1A receptors (33). Large vessels such as aorta express α1A, B, and D with α1A predominating in adventitia and α1D being more prevalent in media (11). It is possible that CD38 contributes more to signaling downstream of adrenoceptors in renal arterioles than systemic resistance vessels.
To ask whether an ADPR cyclase family member other than CD38 plays an important role in renal vascular reactivity, we used nicotinamide to antagonize ADPR cyclase in control vs. CD38−/− mice. Nicotinamide is a byproduct of the ADPR cyclase reaction (22) and, when added in sufficient quantities, shifts the reaction such that production of NAD+/NADP+ prevails over that of cADPR/NAADP. Although inhibitory efficacy on various ADPR cyclase family members has not been performed, nicotinamide should inhibit all members as all ADPR cyclases catalyze the same reaction and nicotinamide should therefore have a similar effect. We found dose-dependent nicotinamide inhibition of ANG II-induced renal vasoconstriction in wild-type animals, with almost complete blockade at high concentrations. In marked contrast, nicotinamide over the same dose range was ineffective in CD38−/− mice. Such results using a global inhibitor of ADPR cyclase activity strongly suggest that CD38 is the predominant ADPR cyclase mediating renal vascular actions of ANG II and support the notion that CD157 has a minimal influence of the renal hemodynamic responses observed CD38−/− mice. Nevertheless, we cannot exclude the possibility that the observed reduction in CD157 mRNA was a contributing factor. Importantly, our findings indicate that effects of CD38 knockout on RBF responses to ANG II are due to ADPR cyclase activity and not some unintended nonspecific effect. Thus, we show, for the renal vasculature, at least, the contribution of ADPR cyclase to ANG II signaling is due largely to CD38.
Interestingly, mice lacking CD38 had a RBF response to ANG II that was ∼50% of that seen in wild-type mice, a greater degree of renal vasoconstriction than predicted based on the ability of nicotinamide to inhibit up to 70% of the RBF response in wild-type mice and rats (38). As a result, it is likely that some other Ca2+ signaling pathway compensates in the absence of CD38. An attractive candidate is upregulation of RyR function and CICR. Since ADPR cyclases work largely by sensitization of RyR to Ca2+, it is possible that increased RyR expression and Ca2+ signaling would lead to a less pronounced phenotype in CD38−/− mice. In addition, multiple ADPR cyclase family members are emerging in different cells (6, 7), some of which may be modulated in an attempt to normalize renal vascular responses in CD38−/− animals. Another possibility is upregulation of IP3 receptor channels or plasma membrane Ca2+ entry channels. Clearly, a more comprehensive understanding of signaling pathways responsible for systemic pressor and renal vasoconstriction in CD38−/− mice is worthy of future investigation.
In conclusion, we present novel evidence demonstrating an important role for the ADPR cyclase CD38 in mediating acute renal vascular responses to three vasoconstrictors, ANG II, ET-1, and NE, and the systemic pressor response to ANG II but not NE. We document expression of CD38 and CD157 mRNA in preglomerular resistance arterioles of control mice. Mice lacking CD38 have attenuated renal vasoconstrictor responses to ANG II, NE, and ET-1 as evidenced by decreased transient RBF responses in the face of increases in MAP. These RBF data coupled with our finding that the ADPR cyclase inhibitor nicotinamide has no effect on renal vascular reactivity in CD38−/− animals implicate CD38 as the predominant ADPR cyclase mediating renal vasoconstriction. Due to the importance of the renal vasculature in pathogenesis of hypertension and renal disease, our results have implications in the pathophysiology of disease states. At present, little is known about the functional significance of CD38 in the long-term regulation of renal function and AP.
GRANTS
This work was supported by National Institutes of Health (NIH) Research Grant HL-02334. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH.
Acknowledgments
We acknowledge Dr. F. Lund for the kind gift of CD38−/− breeder mice.
REFERENCES
- 1.Bartz JA, McInnes LA. CD38 regulates oxytocin secretion and complex social behavior. Bioessays 29: 837–841, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Brosnihan KB, Hodgin JB, Smithies O, Maeda N, Gallagher P. Tissue-specific regulation of ACE/ACE2 and AT1/AT2 receptor gene expression by oestrogen in apolipoprotein E/oestrogen receptor-alpha knock-out mice. Exp Physiol 93: 658–664, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatziantoniou C, Dussaule JC, Arendshorst WJ, Ardaillou R. Angiotensin II receptors and renin release in rat glomerular afferent arterioles. Kidney Int 46: 1570–1573, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Chini EN, Klener P Jr, Beers KW, Chini CC, Grande JP, Dousa TP. Cyclic ADP-ribose metabolism in rat kidney: high capacity for synthesis in glomeruli. Kidney Int 51: 1500–1506, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Cockayne DA, Muchamuel T, Grimaldi JC, Muller-Steffner H, Randall TD, Lund FE, Murray R, Schuber F, Howard MC. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood 92: 1324–1333, 1998. [PubMed] [Google Scholar]
- 6.Davis LC, Morgan A, Ruas M, Wong J, Graeff R, Poustka A, Lee H, Wessel G, Parrington J, Galione A. Ca2+ signaling occurs via second messenger release from intraorganelle synthesis sites. Curr Biol 18: 1612–1618, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Toledo F, Cheng J, Dousa TP. Retinoic acid and triiodothyronine stimulate ADP-ribosyl cyclase activity in rat vascular smooth muscle cells. Biochem Biophys Res Commun 238: 847–850, 1997. [DOI] [PubMed] [Google Scholar]
- 8.De Toledo F, Cheng J, Liang M, Chini EN, Dousa TP. ADP-ribosyl cyclase in rat vascular smooth muscle cells: properties and regulation. Circ Res 86: 1153–1159, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res 89: 77–83, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Eppel GA, Lee LL, Evans RG. α-Adrenoceptor subtypes mediating regional kidney blood flow responses to renal nerve stimulation. Auton Neurosci 112: 15–24, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Faber JE, Yang N, Xin X. Expression of alpha-adrenoceptor subtypes by smooth muscle cells and adventitial fibroblasts in rat aorta and in cell culture. J Pharmacol Exp Ther 298: 441–452, 2001. [PubMed] [Google Scholar]
- 12.Facemire C, Mohler P, Arendshorst W. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol 286: F546–F551, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Renal Physiol 288: F785–F791, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Fellner SK, Arendshorst WJ. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 292: F175–F184, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez J, Deaglio S, Donati D, Beusan I, Corno F, Aranega A, Forni M, Falini B, Malavasi F. Analysis of the distribution of human CD38 and of its ligand CD31 in normal tissues. J Biol Regul Homeost Agents 12: 81–91, 1998. [PubMed] [Google Scholar]
- 16.Higashida H, Egorova A, Higashida C, Zhong Z, Yokoyama S, Noda M, Zhang J. Sympathetic potentiation of cyclic ADP-ribose formation in rat cardiac myocytes. J Biol Chem 274: 33348–33354, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Higashida H, Zhang J, Hashii M, Shintaku M, Higashida C, Takeda Y. Angiotensin II stimulates cyclic ADP-ribose formation in neonatal rat cardiac myocytes. Biochem J 352: 197–202, 2000. [PMC free article] [PubMed] [Google Scholar]
- 18.Hua S, Tokimasa T, Takasawa S, Furuya Y, Nohmi M, Okamoto H, Kuba K. Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron 12: 1073–1079, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Itoh M, Ishihara K, Tomizawa H, Tanaka H, Kobune Y, Ishikawa J, Kaisho T, Hirano T. Molecular cloning of murine BST-1 having homology with CD38 and aplysia ADP-ribosyl cyclase. Biochem Biophys Res Commun 203: 1309–1317, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Just A, Olson A, Arendshorst WJ. Dual constrictor and dilator actions of ETB receptors in the rat renal microcirculation: interactions with ETA receptors. Am J Physiol Renal Physiol 286: F660–F668, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans A. Lysosome-sarcoplasmic reticulum junctions: a trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J Biol Chem 279: 54319–54326, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Lee HC Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol 41: 317–345, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Teggatz EG, Li PL, Allaire R, Zou AP. Formation and actions of cyclic ADP-ribose in renal microvessels. Microvasc Res 60: 149–159, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88: 841–886, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Mehta K, Shahid U, Malavasi F. Human CD38, a cell-surface protein with multiple functions. FASEB J 10: 1408–1417, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Mitsui-Saito M, Kato I, Takasawa S, Okamoto H, Yanagisawa T. CD38 gene disruption inhibits the contraction induced by alpha-adrenoceptor stimulation in mouse aorta. J Vet Med Sci 65: 1325–1330, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Nishikimi T, Hagaman JR, Takahashi N, Kim HS, Matsuoka H, Smithies O, Maeda N. Increased susceptibility to heart failure in response to volume overload in mice lacking natriuretic peptide receptor-A gene. Cardiovasc Res 66: 94–103, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J Biol Chem 272: 3133–3136, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Ortolan E, Vacca P, Capobianco A, Armando E, Crivellin F, Horenstein A, Malavasi F. CD157, the Janus of CD38 but with a unique personality. Cell Biochem Funct 20: 309–322, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Peng Z, Dang A, Arendshorst W. Increased expression and activity of phospholipase C in renal arterioles of young spontaneously hypertensive rats. Am J Hypertens 20: 38–43, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Ruan X, Chatziantoniou C, Arendshorst WJ. Impaired prostaglandin E2/prostaglandin I2 receptor Gs protein interactions in isolated renal resistance arterioles of spontaneously hypertensive rats. Hypertension 34: 1134–1140, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Salomonsson M, Oker M, Kim S, Zhang H, Faber JE, Arendshorst WJ. α1-Adrenoceptor subtypes on rat afferent arterioles assessed by radioligand binding and RT-PCR. Am J Physiol Renal Physiol 281: F172–F178, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Satriano J, Cunard R, Peterson OW, Dousa T, Gabbai FB, Blantz RC. Effects on kidney filtration rate by agmatine requires activation of ryanodine channels for nitric oxide generation. Am J Physiol Renal Physiol 294: F795–F800, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Smyth LM, Breen LT, Yamboliev IA, Mutafova-Yambolieva VN. Novel localization of CD38 in perivascular sympathetic nerve terminals. Neuroscience 139: 1467–1477, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi J, Kagaya Y, Kato I, Ohta J, Isoyama S, Miura M, Sugai Y, Hirose M, Wakayama Y, Ninomiya M, Watanabe J, Takasawa S, Okamoto H, Shirato K. Deficit of CD38/cyclic ADP-ribose is differentially compensated in hearts by gender. Biochem Biophys Res Commun 312: 434–440, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Teggatz E, Zhang G, Zhang A, Yi F, Li N, Zou A, Li P. Role of cyclic ADP-ribose in Ca2+-induced Ca2+ release and vasoconstriction in small renal arteries. Microvasc Res 70: 65–75, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Thai TL, Fellner SK, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptor activity contribute to basal renal vasomotor tone and agonist-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol 293: F1107–F1114, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Thai TL, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptors mediate endothelin ETA and ETB receptor-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol 295: F360–F368, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thai TL, Churchill GC, Arendshorst WJ. NAADP receptors mediate calcium signaling stimulated by endothelin-1 and norepinephrine in renal efferent arterioles. Am J Physiol Renal Physiol In Press. [DOI] [PMC free article] [PubMed]
- 41.Williams DL, Jones KL, Pettibone DJ, Lis EV, Clineschmidt BV. Sarafotoxin S6c: an agonist which distinguishes between endothelin receptor subtypes. Biochem Biophys Res Commun 175: 556–561, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Xie GH, Rah SY, Kim SJ, Nam TS, Ha KC, Chae SW, Im MJ, Kim UH. ADP-ribosyl cyclase couples to cyclic AMP signaling in the cardiomyocytes. Biochem Biophys Res Commun 330: 1290–1298, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Yi F, Zhang AY, Li N, Zhang F, Xia M, Li PL. Role of cyclic ADP-ribose-Ca2+ signaling in mediating renin production and release in As4.1 cells. Cell Physiol Biochem 19: 293–302, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the CD38−/− mouse. Biochem Biophys Res Commun 346: 188–192, 2006. [DOI] [PubMed] [Google Scholar]