Abstract
The vast majority (∼99%) of all known cases of autosomal dominant polycystic kidney disease (ADPKD) are caused by naturally occurring mutations in two separate, but genetically interacting, loci, pkd1 and pkd2. pkd1 encodes a large multispanning membrane protein (PKD1) of unknown function, while pkd2 encodes a protein (TRPP2, polycystin-2, or PKD2) of the transient receptor potential (TRP) superfamily of ion channels. Biochemical, functional, and genetic studies support a model in which PKD1 physically interacts with TRPP2 to form an ion channel complex that conveys extracellular stimuli to ionic currents. However, the molecular identity of these extracellular stimuli remains elusive. Functional studies in cell culture show that TRPP2 can be activated in response to mechanical cues (fluid shear stress) and/or receptor tyrosine kinase (RTK) and G protein-coupled receptor (GPCR) activation at the cell surface. Recent genetic studies in Chlamydomonas reinhardtii show that CrPKD2 functions in a pathway linking cell-cell adhesion and Ca2+ signaling. The mode of activation depends on protein-protein interactions with other channel subunits and auxiliary proteins. Therefore, understanding the mechanisms underlying the molecular makeup of TRPP2-containing complexes is critical in delineating the mechanisms of TRPP2 activation and, most importantly, the mechanisms by which naturally occurring mutations in pkd1 or pkd2 lead not only to ADPKD, but also to other defects reported in model organisms lacking functional TRPP2. This review focuses on the molecular assembly, function, and regulation of TRPP2 as a cell surface cation channel and discusses its potential role in Ca2+ signaling and ADPKD pathophysiology.
Keywords: TRP channels, autosomal dominant polycystic kidney disease, Ca2+ signaling
trpp2 (polycystin-2, PC2, or PKD2) is the protein encoded for by the pkd2 gene originally identified as one of the genes responsible for autosomal dominant polycystic kidney disease (ADPKD) (50). ADPKD is one of the most common genetic diseases, affecting 1 in 400–1,000 individuals (24, 28) with the development of large fluid-filled kidney, liver, and, pancreatic cysts. In addition to defects associated with polycystic kidney disease (91, 92), homozygous deletion of pkd2 in mice results in randomization of embryonic turning manifested as abnormal left-right axis patterning and abnormal heart looping morphogenesis (67). These data highlight the indispensable role of TRPP2 in vertebrate development and health.
Structurally, TRPP2 belongs to the transient receptor potential (TRP) superfamily of channel proteins (87). Mammalian TRP channels have been grouped into seven categories (TRPC, TRPM, TRPP, TRPV, TRPML, TRPN, and TRPA1) based on primary sequence homology (54). All TRP channels span the plasma membrane six times with their NH2 and COOH termini located in the cytoplasm. The region responsible for forming the ionic pore is believed to be between transmembrane segments five and six. With the exception of TRPV5 and TRPV6, which are highly Ca2+ selective, and TRPM4, which is practically impermeable to Ca2+, all other members of the TRP superfamily form nonselective cation channels, which are permeable to divalent cations to various degrees (61). One of the most exciting properties of TRP channels is their ability to heteromultimerize, resulting in the formation of channels with new biophysical properties, modes of activation, or simply more efficient trafficking of the ion conducting subunit to the plasma membrane (87).
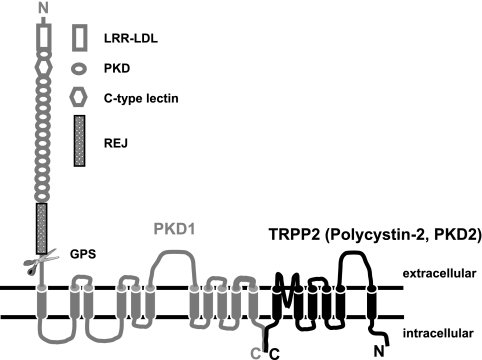
TRPP2 has been shown to physically interact with PKD1 (75, 85), resulting in the formation of a functional ion channel complex (29). The interaction between the two proteins is mediated through their COOH termini and involves amino acid residues 822–895 of human TRPP2 (84). PKD1, the gene product of pkd1, is a large protein of ∼4,300 amino acids with an NH2-terminal extracellular domain of ∼2,500 residues (32). The extracellular portion of PKD1 contains several domains present in known cell adhesion molecules (Fig. 1). Of particular interest is a domain similar to the receptor of egg jelly (REJ) in sea urchin (55). This domain is believed to mediate egg-sperm interactions in this organism. Just before the first transmembrane segment, there is a G protein-coupled receptor (GPCR) proteolytic site (GPS) (69), which is responsible for the proteolytic cleavage of full-length PKD1 into two pieces (49, 90). The NH2-terminal fragment encompasses almost the entire extracellular domain (90), while the COOH-terminal fragment contains the remaining molecule, which is predicted to span the plasma membrane 11 times with the COOH terminus residing in the cytoplasm. The two fragments have been suggested to be held together via noncovalent protein-protein interactions (90). In this context, it has been proposed that PKD1 acts as a receptor activated by a yet to be identified ligand, in a signal transduction scheme that is vital for normal tubulogenesis.
Fig. 1.
Diagram illustrating the membrane topology of PKD1 and TRPP2. LRR-LDL, leucine-rich repeats and low-density lipoprotein homology domains; PKD, polycystic kidney disease domain; C-type lectin: C-type lectin homology domain; REJ, receptor of egg jelly; GPS, G protein-coupled receptor (GPCR) proteolytic site.
While there are multiple lines of independent evidence that TRPP2 and PKD1 form a heteromultimeric ion channel complex with an indispensable role in kidney development, there are still outstanding questions on the activation mechanisms of TRPP2/PKD1 channel, their exact cellular functions, and their specific roles in ADPKD pathophysiology. Data accumulated over the last 5 years support three major modes of TRPP2 activation: activation by fluid shear stress (mechanosensitive TRPP2), activation by cell surface receptor stimulation (receptor-operated TRPP2), and activation by cell adhesion (cell adhesion-mediated activation of TRPP2). This review summarizes data of current modes of TRPP2 activation at the plasma membrane and discusses the relevance of each one of these modes in the pathophysiology of ADPKD. For further reading on the function of TRPP2, the reader is directed to many excellent reviews (1, 11, 15, 16, 35, 38, 86).
Mechanosensitive TRPP2
The identification of the primary cilium as perhaps the most relevant organelle in ADPKD pathophysiology (65, 94), along with the expression of TRPP2 and PKD1 there (66, 95), prompted investigations on whether these proteins have a ciliary function. Cilium bending has been shown to be associated with increased Ca2+ entry into the cell (70, 71, 73). Initially, Nauli et al. (56) showed that fluid shear stress applied to ciliated cells resulted in Ca2+ entry into the cell body, which was dependent on TRPP2 or PKD1. Kidney epithelial cells derived from mice lacking pkd1 did not respond to fluid flow when they were allowed to form cilia. Similarly, a TRPP2-specific antibody raised against the first extracellular loop of TRPP2 blocked fluid flow-induced Ca2+ entry in ciliated cells. Nauli et al. (56) proposed that TRPP2 and PKD1 play a mechanosensitive role in kidney cells and that loss of fluid flow-induced PKD1/TRPP2-mediated Ca2+ entry through the cilium may be the primary defect in ADPKD.
Consistent with the notion that TRPP2 could function as a mechanosensitive channel, the Cantiello and Chen groups have shown that the actin cytoskeleton can directly modulate TRPP2 activity in several in vitro systems. TRPP2 activity was identified and characterized in membrane vesicles derived from apical membranes of human syncytiotrophoblasts (hSTs) (26). Lipid bilayer reconstituted TRPP2 displayed eightfold-increased activity in response to cytochalasin D, an F actin depolymerizing agent, added to the cis (intracellular) compartment of the recording chamber (26). Moreover, gelsolin, an actin cytoskeleton severing protein, also increased TRPP2 activity in the same experimental setting (52). Because gelsolin was effective only in the presence of intracellular Ca2+, the authors proposed an interesting mechanism by which Ca2+ entry through TRPP2 could activate endogenous gelsolin to induce actin cytoskeleton remodeling that could also affect TRPP2 by a positive feedback mechanism (52). The implication of these data is that TRPP2 activity can modulate the actin cytoskeleton and this modulation may account for downstream effects of TRPP2 signaling. This intriguing hypothesis awaits further support from whole cell-based systems. In agreement with a role of actin cytoskeleton in modulating TRPP2 activity, Li et al. (44) identified α-actinin-2 as an interacting partner with an NH2-terminal domain (M1-K215) of human TRPP2, using a yeast two-hybrid screen. Directed protein-protein interaction studies identified a second interaction domain within the COOH-terminal cytosolic region of TRPP2 encompassing residues 821-878 (44). The interaction of TRPP2 and actinin was confirmed by multiple in vitro and in vivo binding and yeast two-hybrid assays. Most importantly, Li et al. (44) showed that α-actinin modulated TRPP2 single-channel activity. Using lipid bilayer reconstitution of TRPP2 from hST membranes, these investigators showed that purified α-actinin added to the cis compartment increased TRPP2 single-channel activity by almost 15-fold. Using the same approach, Montalbetti et al. (51) showed that changes in osmotic pressure or hydrostatic pressure affected TRPP2 activity, and this modulation was again dependent on an intact actin cytoskeleton. In sum, these studies provided evidence for the direct coupling of TRPP2 to the actin cytoskeleton and set forth the hypothesis that TRPP2 activity could be modulated by mechanical changes transferred to the channel through the actin network. However, this type of regulation is expected to occur mainly at the plasma membrane and not at the microtubule-based cilium, which lacks actin. In regard to regulation of TRPP2 by microtubules, it was recently shown that taxol, a microtubule-stabilizing agent, increased while colchicine, a microtubule-destabilizing agent, decreased TRPP2 activity (53). Purified tubulin and GTP increased reconstituted TRPP2 activity from apical hST membrane, but they were without effect on purified TRPP2, suggesting that the regulation by tubulin was indirect. KIF3B, a kinesin motor, was found to be present in the apical membrane of hSTs and also to physically interact with TRPP2 (53, 93). TRPP2 interacted with KIF3B through its COOH-terminal cytosolic domain encompassing amino acid residues 682-968 of human TRPP2 (93). Therefore, a model was proposed in which microtubular structures could regulate TRPP2 activity via KIF3B/KIF3A (53). While this type of regulation was described in a preparation derived from the apical surface of hSTs, it could be also extrapolated to the primary cilium, which is rich in tubulin, KIF3A, and TRPP2. Overall, a great deal of effort has been employed to test whether TRPP2 is a mechanosensitive channel. Supporting evidence comes from studies using measurements of intracellular Ca2+ concentration in response to fluid flow (47, 56, 57, 89) and single-channel currents in response to osmotic and hydrostatic pressure (51). Physical interaction of TRPP2 and actin cytoskeleton and microtubules is also supportive (53). However, whether TRPP2 is a bona fide mechanosensitive channel, according to certain criteria recently set for mechanosensitive channels (13), still remains an outstanding question. Furthermore, it is also unknown whether the mechanosensitive mode of TRPP2 is relevant to ADPKD.
In regard to the role of mechanosensitive TRPP2 in ADPKD, Kottgen et al. (37) recently showed that TRPP2 formed a heteromultimeric channel with TRPV4, which was required for fluid flow-induced Ca2+ entry in Madin-Darby canine kidney (MDCK) cells. Interestingly, TRPP2 alone or in association with endogenous PKD1 could not support fluid-flow induced Ca2+ entry, as knockdown of TRPV4 eliminated Ca2+ entry in response to fluid flow. Heterologous expression of TRPP2 and TRPV4 in Xenopus laevis oocytes resulted in the formation of a mechano- and thermosensitive channel complex. However, kidney cysts were not formed in mice or zebrafish lacking TRPV4. Kottgen et al. (37) concluded that while TRPP2 can form a mechanosensitive channel in association with TRPV4, loss of this activity cannot account for cyst formation.
Receptor-Operated TRPP2
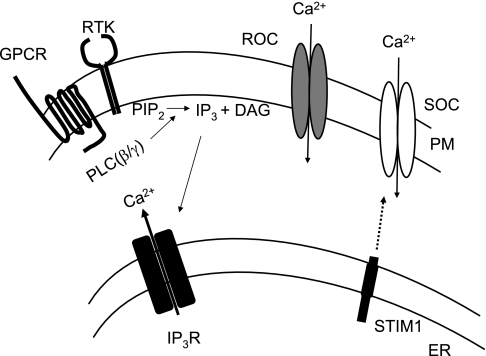
GPCRs and receptor tyrosine kinases (RTKs) comprise a very large group of cell surface receptors that elicit their physiological responses through the production of inositol 1,4,5-trisphosphate (IP3) (6) (Fig. 2). Upon receptor activation, newly synthesized IP3 acts on IP3 receptors (IP3Rs) to trigger a rapid increase in intracellular Ca2+ concentration by releasing free Ca2+ from intracellular stores (9). Intracellular Ca2+ concentration returns to normal levels by extrusion of cytoplasmic Ca2+ to the extracellular space by plasma membrane Ca2+-ATPase and Na+/Ca2+ exchangers, readmission of Ca2+ into the endoplasmic reticulum (ER) by the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pump, and Ca2+ entry via the store- and receptor-operated Ca2+ channels (20, 74).
Fig. 2.
Receptor- and store-operated Ca2+ signaling in nonexcitable cells. Activation of GPCRs or receptor tyrosine kinases (RTKs) results in the activation of phospholipase C-β (PLC-β) or -γ (PLC-γ) isoforms, respectively. Activated PLCs catalyze the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds and activates IP3 receptors (IP3Rs) in the endoplasmic reticulum (ER) membrane, resulting in the release of Ca2+ from the intracellular stores. Depletion of Ca2+ from the ER triggers the translocation of ER-resident STIM1 to the vicinity of the plasma membrane (PM) to activate store-operated Ca2+ channels (SOC). Receptor-operated channels (ROC) are activated by second messengers other than store depletion (i.e., reduction of PIP2, production of DAG, etc).
While there is general agreement that store-operated channels are defined by their ability to open directly in response to the depletion of the internal stores, the term receptor-operated channels is loosely defined (also discussed in Ref. 63). To avoid confusion, receptor-operated channels will be considered here as the channels activated in response to GPCR/RTK-induced activation of second messengers, but not store depletion. Therefore, cell surface receptor stimulation will result in the activation of both store- and receptor-operated channels, whereas pharmacological inhibition of the SERCA pump by thapsigargin or passive depletion of internal stores by cell dialysis with Ca2+ chelators will result in the activation of only the store-operated channels.
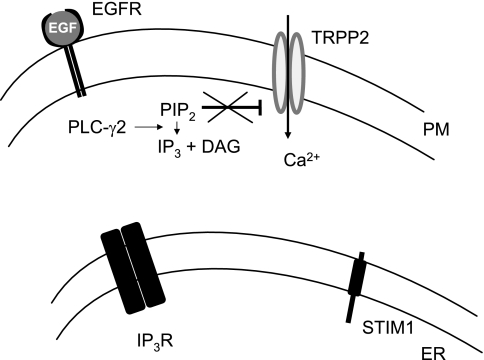
Consistent with the definition of receptor-operated Ca2+ channels, TRPP2 can be activated in response to epidermal growth factor (EGF) in the kidney epithelial cell line LLC-PK1 (45). In these cells, addition of EGF in the extracellular solution caused a rise in cytosolic Ca2+ concentration that was entirely dependent on extracellular Ca2+ and did not involve depletion of intracellular stores and activation of store-operated Ca2+ channels (Fig. 3). The physiological relevance of EGF-induced activation of TRPP2 in LLC-PK1 cells was supported by whole animal studies in which homozygous deletion of the egfr gene resulted in cystic dilatation of collecting ducts (83), an area that was also predominantly affected by pkd2 mutations (92). Mechanistically, TRPP2 overexpression increased EGF-induced conductance in LLC-PK1 kidney epithelial cells, while knockdown of endogenous TRPP2 by RNA interference (RNAi) or expression of the pathogenic missense variant TRPP2-D511V blunted the EGF-induced response. Pharmacological experiments indicated that the EGF-induced activation of TRPP2 occurred independently of store depletion but required the activity of phospholipase C (PLC) and phosphatidylinositol 3-kinase (PI3K). Pipette infusion of purified phosphatidylinositol 4,5-bisphosphate (PIP2) suppressed the TRPP2-mediated effect on EGF-induced conductance, while pipette infusion of phosphatidylinositol 3,4,5-trisphosphate (PIP3) had no detectable effect on this conductance. Overexpression of type Iα phosphatidylinositol 4-phosphate 5-kinase [PIP(5)Kα], which catalyzes the formation of PIP2, suppressed EGF-induced TRPP2 currents. Biochemically, TRPP2 interacted with PLC-γ2 and colocalized the primary cilium with EGF receptor (EGFR) and PIP2. Overall, TRPP2 functioned as a bona fide receptor-operated ion channel downstream of EGFR activation in the plasma membrane of LLC-PK1 cells. These studies identified a cellular, physiological signal transduction pathway of which TRPP2 was an important component and had implications of RTK-mediated Ca2+ signaling in ADPKD.
Fig. 3.
Function of TRPP2 as a ROC at the PM of LLC-PK1 kidney epithelial cells. Activation of epidermal growth factor (EGF) receptor (EGFR) by EGF results in the activation of PLC-γ2 and conversion of PIP2 to IP3 and DAG. In this cell line, EGF-induced IP3 is not sufficient to activate intracellular Ca2+ release from the ER and subsequent activation of SOCs. However, EGF-induced activation of PIP2 breakdown results in the release of PIP2-mediated inhibition of TRPP2. In this case, TRPP2 behaves as a bona fide ROC.
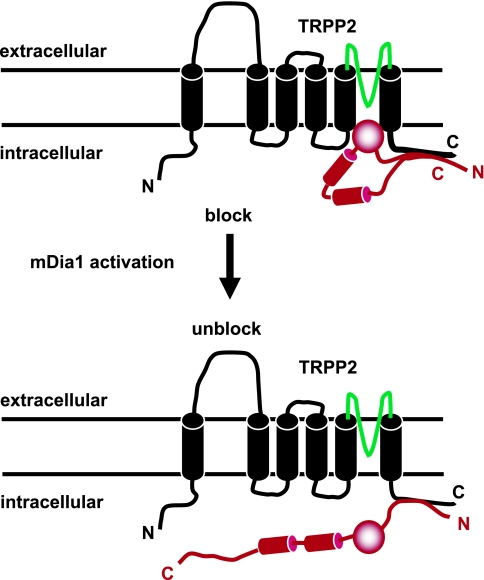
Following up on the activation of TRPP2 by EGF, Bai et al. (4) recently showed that native TRPP2 was gated by an intracellular protein, the mammalian homolog of the Diaphanous related formin 1 (mDia1). mDia1 exists in an autoinhibited or “closed” state in which the COOH terminus loops around to bind the NH2 terminus (30, 88). In response to RhoA-C activation, activated GTP-bound forms of RhoA-C proteins bind to the NH2 terminus of mDia1, resulting in mDia1 unwinding by disruption of the intramolecular interaction between its NH2 and COOH termini. The unwinding of mDia1 results in the exposure of internal formin homology domains 1 and 2 (FH1 and 2), which form docking sites for multiple effector molecules containing SH3 and WW domains. MDia1 was previously shown to physically associate with the very COOH-terminal tail of TRPP2 (amino acid residues 872<-968 of human TRPP2) and to mediate anchoring of TRPP2 to the mitotic spindles during metaphase (78). In a recent study (4), mDia1 was shown to function as an intracellular voltage-dependent gate for TRPP2 at the plasma membrane in cells in interphase. Specifically, at resting physiological potentials, mDia1 was shown to bind to and block TRPP2. Membrane depolarization or EGF stimulation resulted in the sequential activation of RhoA and mDia1. It was then hypothesized that activated mDia1 dissociated or “swung away” from TRPP2, releasing its block on the channel. This model of TRPP2 activation implies a “ball and chain” mechanism for TRPP2 activation (Fig. 4), which has never been proposed for any of the known 28 mammalian TRP channels. However, it still remains to be investigated how the PLC-γ2/PIP2 pathway functionally interacts with the RhoA/mDia1 pathway to regulate TRPP2 activation by EGF and possibly other ligands acting through RTKs.
Fig. 4.
Activation of TRPP2 by mammalian homolog of Diaphanous related formin 1 (mDia1). At resting membrane potentials, autoinhibited mDia1 (red) binds and blocks TRPP2 activity. In response to EGF or membrane depolarization, mDia1 switches from the autoinhibited state to the activated state, releasing the block on TRPP2. Predicted pore-forming region in TRPP2 is shown in green. Formin homology domains 1 and 2 (FH1 and FH2) are shown as red cylinders.
A new study reported that TRPP2 could function downstream of PLC activation, in a GPCR-activated mode. Bai et al. (3) showed that TRPP2 complexed with TRPC1, but not PKD1, was able to be activated in response to bradykinin (BK) stimulation, acting through PLC-coupled BK receptor subtypes. This study followed up an earlier report that transfected TRPP2 could physically interact with TRPC1 (84). The interaction involved both their COOH termini and transmembrane segments. Detailed structure-function analysis identified residue D886 in the cytosolic tail of TRPP2 as responsible for the interaction with TRPC1, but not PKD1 (84). Subsequently, a completely independent study identified an affected family with a missense mutation at the very same residue (76). Tsiokas et al. (84) proposed that the interaction through the soluble cytosolic domains may have a regulatory role, while the interaction through the transmembrane segments may have a more structural role in forming the ionic pore of the heteromultimeric channel. Consistently, the TRPP2/TRPPC1 channel complex displayed unique biophysical properties in terms of single-channel conductance, ion permeability, and pharmacology (3). TRPP2/TRPC1 showed intermediate single-channel conductance from TRPP2/PKD1 and TRPC1 homomultimers, higher permeability to Ca2+, and amiloride sensitivity similar to TRPP2/PKD1 but not to TRPC1 alone (3). As the properties of TRPP2/TRPC1 were distinct from those of PKD1/TRPP2, these new data indicated that the TRPP2 mode of activation was conferred through interaction with specific subunits. The identification of TRPP2/TRPC1 was the first example of a functional channel complex containing members of nonhomologous groups, such TRPP and TRPC, as all known interactions between TRP channels have been shown to occur exclusively between members of the same group. It also extended the functional diversity of TRP channels to an even larger number of heteromultimeric channels than thought before. Importantly, the identification of heteromultimeric TRPP2/TRPC1 has implications in purported roles of TRPP2 in fluid flow-mediated mechanosensation. It was recently shown that endothelial cells sensed fluid shear stress through the activation of bradykinin 2 (BK-2) receptor, a GPCR (12). Fluid shear stress activated BK-2 receptor through a conformational change in the receptor, in a ligand-independent manner (12). Mechanical bending of the primary cilium has been shown to induce Ca2+ influx through the activation of PLC (72, 73). Therefore, it is quite possible that fluid shear stress or cilium bending could activate a channel complex containing TRPP2 and TRPC1 through GPCR activation. This type of TRPP2-mediated mechanotransduction would be indirect and would involve ligand-independent activation of endogenous GPCRs present in the cell rather than activation of true mechanosensitive channels. Therefore, it is conceivable that a pool of TRPP2 associated with TRPC1 could be activated in response to cilium bending through PLC-mediated signaling. In support of the idea that ligand-independent activation of Gq-coupled receptors can activate TRP channels, TRPC6 was recently shown to be activated by the ligand-independent activation of angiotensin II receptors in vascular smooth muscle cells in response to membrane stretch (48). Interestingly, TRPP2/TRPC4 heteromultimers were shown to form an angiotensin II-activated plasma membrane channel in glomerular mesangial cells (19).
According to Bai et al. (3), GPCR-mediated activation of TRPP2 required TRPC1 and was independent of PKD1. In fact, PKD1 competed with TRPC1 for binding to TRPP2 and activation by BK. An initial analysis of mice lacking TRPC1 did not reveal obvious symptoms of polycystic kidney disease (18). Thus, while TRPP2 could be responsible for Ca2+ entry in response to cilium bending, defective fluid shear stress- and/or cilium bending-induced mechanotransduction is unlikely to be solely responsible for ADPKD, at least based on current data. This idea is supported by Kottgen et al. (37), as discussed above, and also by recent data on the temporal inactivation of pkd1, Ift88, or Kif3a in the mouse (14, 68). Ift88 or Kif3a genes encode the proteins IFT88/polaris or Kif3A, which are essential components of the intraflagellar transport (IFT) machinery and required for cilium formation (77). Conditional inactivation of Ift88 or Kif3A, at different times during postnatal life (newborn vs. 16-wk-old mice) resulted in complete loss of primary cilia independently of the time of gene inactivation, yet in marked differences in cyst formation (14). Inactivation of these genes in newborn mice resulted in massive cysts, while inactivation in 16-wk-old mice resulted in a much weaker cystic phenotype. These data led Davenport et al. (14) to conclude that defective mechanosensation cannot simply account for cyst formation. In aggregate, recent results from multiple independent investigations question the loss of cilium-based and, possibly, TRPP2-mediated mechanotransduction/mechanosensation as the initiating event in cystogenesis (3, 14, 37, 68). However, more studies are needed to definitively address the role of mechanosensation in ADPKD.
The identification of EGFR, TRPP2, and PIP2 in the cilium is worth discussing, as it raises the possibility that receptor-operated TRPP2 could function at the ciliary membrane. The presence of EGFR in the primary cilium and basal body of ovary epithelial cells has been shown previously (43). It is therefore conceivable that TRPP2 may function as an EGF-activated Ca2+ channel at the primary cilium (Fig. 5). EGF/EGFR signaling in the kidney has been a mystery since the discovery of EGF. While human EGF was identified in the urine (41, 42) and is known to be secreted from the apical surface of kidney tubular cells (41, 80), EGFR has never been detected in the apical surface of kidney tubular cells. EGFR is almost exclusively present at the basolateral surface of epithelial cells of the thick ascending loop of Henle and distal convoluted tubules (25, 79). Therefore, the question of whether EGF present in the urine was capable of signaling has been a longstanding one. The data of Ma et al. (45) raise the possibility that EGF could signal through cilium-based EGFR and, thus, may provide an answer to this old question. A possible signaling scheme is shown in Fig. 5: prepro-EGF is anchored to the apical surface of the plasma membrane of the cells lining the thick ascending limb of Henle and distal convoluted tubules (59), and it is believed to be cleaved by urine proteases and released to the tubular lumen (21, 33, 62). It should be noted that membrane-bound prepro-EGF is also biologically active (8). TRPP2 is highly expressed in the thick ascending loop of Henle and distal convoluted tubules (22). Therefore, cilium-resident TRPP2 could be activated by secreted EGF in the urine and/or plasma membrane-bound prepro-EGF. If indeed prepro-EGF is in close proximity to EGFR and TRPP2 channel, it could, in principle, preferentially activate TRPP2 at the base of the cilium. This could generate a gradient of partially activated (sensitized) TRPP2 along the cilium. This type of channel sensitization may have implications in cilium-based mechanotransduction, as it may reduce its threshold of activation by mechanical stimulation and/or other models of TRPP2 activation that work independently of PIP2 breakdown (for example, cell adhesion-mediated activation of TRPP2, see below).
Fig. 5.
Hypothetical regulation TRPP2 by EGF in the cilium in vivo. TRPP2 colocalizes with PIP2 and EGFR along the cilium, where it is kept insensitive to flow stimulation due to PIP2-mediated inhibition. Liberated EGF from prepro-EGF at the apical surface of the PM through the action of locally acting proteases releases TRPP2 from PIP2-mediated inhibition, generating a gradient in sensitized TRPP2 with a higher level of sensitization at the base (shown as yellow) and a lower level of sensitization at the tip of the cilium (shown as white). Highly sensitized TRPP2 at the base of the cilium mediates efficient mechanotransduction secondary to fluid flow changes.
Although not the focus of this review, the identification of TRPP2 as an intracellular Ca2+ release channel (39) may have also implications in receptor-operated Ca2+ signaling in some of the TRPP2 phenotypes reported in model organisms. Knockdown of TRPP2 in zebrafish has been shown to result in three distinct phenotypes: pronephric cysts, defects in body axis curvature, and loss of left-right asymmetry (or laterality defects) (23, 40, 60). Mutation of S812 to A or D in human TRPP2 was shown to result in higher expression in the plasma membrane or trapping in the ER, respectively (36), while Cai et al. (10) showed that S812A reduced the sensitivity of TRPP2 to intracellular Ca2+ without affecting trafficking of the mutant channel from the ER to the plasma membrane. Despite the discrepancy in the trafficking properties of TRPP2-S812A between the two studies, both studies are consistent with the fact that mutation of S812 to A resulted in impaired function of TRPP2 at the ER, either by a reduced amount in this organelle (36) or by reduced activity of TRPP2 at the ER (10). Introduction of TRPP2-S812A or TRPP2-S812D into zebrafish embryos lacking endogenous TRPP2 resulted in somewhat surprising results. TRPP2-S812A rescued the kidney cystic phenotype more efficiently than the body axis and laterality phenotypes rescued by the TRPP2-S812D construct (23), suggesting that the reduced Ca2+ sensitivity of this construct was irrelevant in respect to the rescue of cystogenesis. It is more likely that the increased plasma membrane expression of this construct was responsible for rescuing the cystic phenotype. Conversely, TRPP2-S812D rescued the body axis and laterality phenotypes more efficiently than the cystic phenotype compared with TRPP2-S812A (23). A previous study using a TRPP2 mutant lacking an NH2-terminal GSK3 phosphorylation side that was required for targeting to the plasma membrane showed that while this mutant was efficiently targeted to the cilium, it did not rescue the cystic phenotype caused by native TRPP2 knockdown (82). These data suggest that proper function of TRPP2 at the plasma membrane is primarily responsible for normal tubulogenesis and prevention of cyst formation.
In regard to the role of TRPP2 in body axis formation and left-right asymmetry, it is tempting to propose a model (Fig. 6) in which cilia bending could trigger GPCR activation, which would then lead to PLC activation and transient release of Ca2+ from the internal stores. The function of TRPP2 as an intracellular Ca2+ release channel could amplify this release, and this function could be physiologically relevant in terms of mechanotransduction directly impacting on the left-right asymmetry phenotype. This model is consistent with the independence of ER-resident TRPP2 by PKD1 and lack of a laterality phenotype in pkd1-knockout mice (34).
Fig. 6.
Hypothetical role of TRPP2 in mechanotransduction. Cilium bending in response to fluid shear stress may activate GPCR(s) in a ligand-independent fashion, probably through mechanical stretching of the PM and/or associated cytoskeletal structures (arrows). Activation of GPCR would result in intracellular Ca2+ release from the ER through the IP3 pathway and activation of SOCs. ROCs, including plasma membrane TRPP2, could also be activated, resulting in a massive and long-lasting increase in intracellular Ca2+ concentration. Dashed arrow denotes changes in membrane tension in response to cilium bending.
Cell Adhesion-Mediated Activation of TRPP2
Because of the presence of multiple domains in vertebrate PKD1 that are predicted to have a role in cell adhesion (32), it has been thought that cell adhesion events could trigger activation of the PKD1/TRPP2 heteromultimer. This idea is also supported by the identification of a heteromultimeric PKD2/PKD1 complex exclusively at the plasma membrane of the acrosomal vesicle in sea urchin sperm (58). While direct evidence for such an activation mechanism is lacking, recent studies on the PKD2 Chlamydomonas reinhardtii homolog (CrPKD2) may lend support to this concept. CrPKD2 was first identified as a component of the C. reinhardtii flagellum with a proteomic approach (64). CrPKD2 was subsequently found to exist in two fragments produced by proteolytic cleavage, occurring at a site close to the COOH terminus of the first extracellular loop (31). It was shown that CrPKD2 cleavage occurred at the cell body and the two fragments traveled to the axonemal membrane mostly by the IFT mechanism. CrPKD2 was essential for gamete mating, a process known to depend on extracellular Ca2+ as an initial step (27). RNAi experiments in this organism demonstrated that loss of CrPKD2 resulted in a block in an earlier step in mating, which could be rescued by restoring downstream components of the mating cascade (31). The proposed model is consistent with several aspects of polycystic kidney disease. Because gamete mating is initiated by cell adhesion through agglutinin molecules present in the flagella of gametes of opposite mating strains (5) and CrPKD2 causes a block in an early stage of mating (31), it is conceivable that CrPKD2 could be activated, directly or indirectly, by cell adhesion. This idea is consistent with the activation of mammalian TRPP2 by cell adhesion mediated, perhaps, by one or more of the predicted cell adhesion domains in PKD1. However, it should be noted that a PKD1 homolog in C. reinhardtii has yet to be identified, and there are significant differences in the primary amino acid sequence between CrPKD2 and vertebrate TRPP2. Nevertheless, it is possible that in contrast to Caenorhabditis elegans, mice, and humans, CrPKD2 may function independently of PKD1 in Chlamydomonas. It is conceivable that in Chlamydomonas, homophilic interactions between agglutinin molecules, CrPKD2 itself, and/or other yet to be identified molecules sitting on the flagella of gametes of opposing strains could activate CrPKD2. In higher organisms, such as mammals, the initial cell adhesion step may be mediated through PKD1. While this is a highly hypothetical model, it explains well how CrPKD2-mediated Ca2+ signaling could result in an increase in intracellular cAMP concentration, an observation that has been reported in cystic cells in humans (46). In addition, the proposed model does not require Ca2+ diffusion from the tip of the flagella to the cell body to initiate cell signaling, which would be inconsistent with the physical properties of the ion (for discussion see Ref. 86). Because of these reasons, these data form an attractive model for the cellular function of CrPKD2 that could also be extrapolated to its mammalian relatives. However, the exact role of Ca2+ influx in the mating process of C. reinhardtii is not immediately clear, as most of the evidence is indirect and is based on the use of general cation channel blockers such as lidocaine, La3+, and Cd2+ (27, 81). In this regard, while mammalian TRPP2 has been shown to be blocked by trivalents such as La3+ (17, 26, 45) or Gd3+ (2, 19, 26), it would be interesting to test whether all these inhibitors used to block mating could inhibit CrPKD2 channel activity in vitro. The most consistent data in terms of Ca2+ fluxes during the mating process are data showing Ca2+ efflux rather than influx (7, 27). This Ca2+ efflux is not associated with release from the ER but rather release from cell walls and is downstream of cAMP accumulation, and its physiological relevance to mating is unknown (27). Moreover, mating occurred normally in Ca2+-free extracellular solution, and in one report there was at most ∼25% reduction in mating in an extracellular solution containing 5 mM EGTA (7). One of the prevailing models is that Ca2+ influx is one of the earliest steps following cell adhesion. Incoming Ca2+ binds to Ca2+-binding proteins in the flagella and is released to the mating medium only after a rise in intraflagellar cAMP concentration (27). CrPKD2 may function as the Ca2+ channel activated by cell adhesion, although direct demonstration for such a role is currently missing. Nevertheless, the identification of a Ca2+-dependent cellular process requiring CrPKD2 in a whole organism is of great value as it could provide mechanistic insights into its function in mammalian systems.
Conclusions
TRPP2 has been proposed to function downstream of mechanical stimulation, cell surface receptor activation, and cell adhesion. These functions can be carried out at the plasma membrane and/or the primary cilium and require physical interactions with other channel subunits such as PKD1, TRPC1, TRPC4, or TRPV4 and auxiliary proteins (i.e., mDia1, actinins, KIF3A/B). Most of these protein-protein interactions are likely to be relevant to ADPKD, as they are eliminated in pathogenic mutants of TRPP2. The mechanosensitivity of TRPP2 has been supported by its involvement in intracellular Ca2+ changes associated with fluid flow changes, association, and regulation by actin cytoskeleton and microtubules. The receptor-operated mode of TRPP2 activation has been demonstrated in EGF and GPCR signal transduction cascades in native and overexpressed systems. Activation by cell adhesion is speculated based on its interaction with PKD1 and recent data on the role of CrPKD2 in the process of Chlamydomonas mating. However, it still remains to be established how each mode contributes to the biological functions of TRPP2. It is very possible that different modes of TRPP2 activation may serve different biological functions. Closer examination of the phenotypes associated with inactivation of the genes of known interacting partners, mechanisms of functional interaction with PKD1 and other proteins, and the role of cilium in the each mode of TRPP2 activation will be critical in establishing a causative role of each mode in ADPKD and other developmental defects resulting from the loss of functional TRPP2.
Acknowledgments
Support for research on TRPP2 was provided by the Oklahoma Center for the Advancement of Science and Technology, the PKD Foundation, the John S. Gammill Endowed Chair in Polycystic Kidney Disease, and the National Institutes of Health (DK-59599-07 and P20-RR01-7703-06).
REFERENCES
- 1.Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun 322: 1364–1373, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Anyatonwu GI, Ehrlich BE. Organic cation permeation through the channel formed by polycystin-2. J Biol Chem 280: 29488–29493, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T, Tsiokas L, Delmas P. Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472–479, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai CX, Kim S, Li WP, Streets AJ, Ong AC, Tsiokas L. Activation of TRPP2 through mDia1-dependent voltage gating. EMBO J 27: 1345–1356, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman K, Goodenough UW, Goodenough DA, Jawitz J, Martin H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J Cell Biol 67: 606–622, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Bloodgood RA, Levin EN. Transient increase in calcium efflux accompanies fertilization in Chlamydomonas. J Cell Biol 97: 397–404, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breyer JA, Cohen S. The epidermal growth factor precursor isolated from murine kidney membranes. Chemical characterization and biological properties. J Biol Chem 265: 16564–16570, 1990. [PubMed] [Google Scholar]
- 9.Burgess GM, Godfrey PP, McKinney JS, Berridge MJ, Irvine RF, Putney JW Jr. The second messenger linking receptor activation to internal Ca release in liver. Nature 309: 63–66, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE, Somlo S. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279: 19987–19995, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Cantiello HF Regulation of calcium signaling by polycystin-2. Am J Physiol Renal Physiol 286: F1012–F1029, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 17: 1586–1594, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmas P Polycystins: from mechanosensation to gene regulation. Cell 118: 145–148, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Delmas P Polycystins: polymodal receptor/ion-channel cellular sensors. Pflügers Arch 451: 264–276, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J 18: 740–742, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich A, Kalwa H, Storch U, Mederos YSM, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Du J, Ding M, Sours-Brothers S, Graham S, Ma R. Mediation of angiotensin II-induced Ca2+ signaling by polycystin 2 in glomerular mesangial cells. Am J Physiol Renal Physiol 294: F909–F918, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci 15: 77–83, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Fisher DA, Salido EC, Barajas L. Epidermal growth factor and the kidney. Annu Rev Physiol 51: 67–80, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol 11: 814–827, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Fu X, Wang Y, Schetle N, Gao H, Putz M, von Gersdorff G, Walz G, Kramer-Zucker AG. The subcellular localization of TRPP2 modulates its function. J Am Soc Nephrol 19: 1342–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabow PA Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Gesualdo L, Di Paolo S, Calabro A, Milani S, Maiorano E, Ranieri E, Pannarale G, Schena FP. Expression of epidermal growth factor and its receptor in normal and diseased human kidney: an immunohistochemical and in situ hybridization study. Kidney Int 49: 656–665, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodenough UW, Shames B, Small L, Saito T, Crain RC, Sanders MA, Salisbury JL. The role of calcium in the Chlamydomonas reinhardtii mating reaction. J Cell Biol 121: 365–374, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grantham JJ The etiology, pathogenesis, and treatment of autosomal dominant polycystic kidney disease: recent advances. Am J Kidney Dis 28: 788–803, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Higgs HN Formin proteins: a domain-based approach. Trends Biochem Sci 30: 342–353, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol 179: 501–514, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet 10: 151–160, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Journe F, Wattiez R, Piron A, Carion M, Laurent G, Heuson-Stiennon JA, Falmagne P. Renal epidermal growth factor precursor: proteolytic processing in an in vitro cell-free system. Biochim Biophys Acta 1357: 18–30, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Karcher C, Fischer A, Schweickert A, Bitzer E, Horie S, Witzgall R, Blum M. Lack of a laterality phenotype in Pkd1 knock-out embryos correlates with absence of polycystin-1 in nodal cilia. Differentiation 73: 425–432, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Kottgen M TRPP2 and autosomal dominant polycystic kidney disease. Biochim Biophys Acta 1772: 836–850, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J 24: 705–716, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182: 437–447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kottgen M, Walz G. Subcellular localization and trafficking of polycystins. Pflügers Arch 451: 286–293, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development 132: 1907–1921, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Lakshmanan J, Salido EC, Lam R, Barajas L, Fisher DA. Identification of pro-epidermal growth factor and high molecular weight epidermal growth factors in adult mouse urine. Biochem Biophys Res Commun 173: 902–911, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Lakshmanan J, Salido EC, Lam R, Fisher DA. Epidermal growth factor prohormone is secreted in human urine. Am J Physiol Endocrinol Metab 263: E142–E150, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Lei ZM, Rao CV. Expression of epidermal growth factor (EGF) receptor and its ligands, EGF and transforming growth factor-alpha, in human fallopian tubes. Endocrinology 131: 947–957, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Montalbetti N, Shen PY, Dai XQ, Cheeseman CI, Karpinski E, Wu G, Cantiello HF, Chen XZ. Alpha-actinin associates with polycystin-2 and regulates its channel activity. Hum Mol Genet 14: 1587–1603, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mangoo-Karim R, Uchic M, Lechene C, Grantham JJ. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci USA 86: 6007–6011, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114: 61–73, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mengerink KJ, Moy GW, Vacquier VD. suREJ3, a polycystin-1 protein, is cleaved at the GPS domain and localizes to the acrosomal region of sea urchin sperm. J Biol Chem 277: 943–948, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Montalbetti N, Li Q, Gonzalez-Perrett S, Semprine J, Chen XZ, Cantiello HF. Effect of hydro-osmotic pressure on polycystin-2 channel function in the human syncytiotrophoblast. Pflügers Arch 451: 294–303, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Montalbetti N, Li Q, Timpanaro GA, Gonzalez-Perrett S, Dai XQ, Chen XZ, Cantiello HF. Cytoskeletal regulation of calcium-permeable cation channels in the human syncytiotrophoblast: role of gelsolin. J Physiol 566: 309–325, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montalbetti N, Li Q, Wu Y, Chen XZ, Cantiello HF. Polycystin-2 cation channel function in the human syncytiotrophoblast is regulated by microtubular structures. J Physiol 579: 717–728, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montell C The TRP superfamily of cation channels. Sci STKE 2005: re3, 2005. [DOI] [PubMed]
- 55.Moy GW, Mendoza LM, Schulz JR, Swanson WJ, Glabe CG, Vacquier VD. The sea urchin sperm receptor for egg jelly is a modular protein with extensive homology to the human polycystic kidney disease protein, PKD1. J Cell Biol 133: 809–817, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17: 1015–1025, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Neill AT, Moy GW, Vacquier VD. Polycystin-2 associates with the polycystin-1 homolog, suREJ3, and localizes to the acrosomal region of sea urchin spermatozoa. Mol Reprod Dev 67: 472–477, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Nouwen EJ, De Broe ME. EGF and TGF-alpha in the human kidney: identification of octopal cells in the collecting duct. Kidney Int 45: 1510–1521, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J Am Soc Nephrol 17: 2706–2718, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of trp channels. Annu Rev Physiol 68: 685–717, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Parries G, Chen K, Misono KS, Cohen S. The human urinary epidermal growth factor (EGF) precursor. Isolation of a biologically active 160-kilodalton heparin-binding pro-EGF with a truncated carboxyl terminus. J Biol Chem 270: 27954–27960, 1995. [DOI] [PubMed] [Google Scholar]
- 63.Patterson RL, van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, Kurosaki T, Snyder SH, Gill DL. Phospholipase C-gamma is required for agonist-induced Ca2+ entry. Cell 111: 529–541, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol 170: 103–113, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol 12: 551–555, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–380, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel Polycystin-2 is required for left-right axis determination in mice. Curr Biol 12: 938–943, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ponting CP, Hofmann K, Bork P. A latrophilin/CL-1-like GPS domain in polycystin-1. Curr Biol 9: R585–R588, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Praetorius HA, Frokiaer J, Nielsen S, Spring KR. Bending the primary cilium opens Ca2+-sensitive intermediate-conductance K+ channels in MDCK cells. J Membr Biol 191: 193–200, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517–520, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Putney JW, McKay RR. Capacitative calcium entry channels. Bioessays 21: 38–46, 1999. [DOI] [PubMed] [Google Scholar]
- 75.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183, 1997. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds DM, Hayashi T, Cai Y, Veldhuisen B, Watnick TJ, Lens XM, Mochizuki T, Qian F, Maeda Y, Li L, Fossdal R, Coto E, Wu G, Breuning MH, Germino GG, Peters DJ, Somlo S. Aberrant splicing in the PKD2 gene as a cause of polycystic kidney disease. J Am Soc Nephrol 10: 2342–2351, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol 3: 813–825, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 in PKD2 localization to mitotic spindles. J Biol Chem 279: 29728–29739, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Salido EC, Barajas L, Lechago J, Laborde NP, Fisher DA. Immunocytochemical localization of epidermal growth factor in mouse kidney. J Histochem Cytochem 34: 1155–1160, 1986. [DOI] [PubMed] [Google Scholar]
- 80.Salido EC, Fisher DA, Barajas L. Immunoelectron microscopy of epidermal growth factor in mouse kidney. J Ultrastruct Mol Struct Res 96: 105–113, 1986. [DOI] [PubMed] [Google Scholar]
- 81.Snell WJ, Buchanan M, Clausell A. Lidocaine reversibly inhibits fertilization in Chlamydomonas: a possible role for calcium in sexual signalling. J Cell Biol 94: 607–612, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC. Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet 15: 1465–1473, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234, 1995. [DOI] [PubMed] [Google Scholar]
- 84.Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA 96: 3934–3939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal 19: 444–453, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 76: 387–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol 13: 435–446, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei W, Hackmann K, Xu H, Germino G, Qian F. Characterization of cis-autoproteolysis of polycystin-1, the product of human polycystic kidney disease 1 gene. J Biol Chem 282: 21729–21737, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Wu G, D'Agati V, Cai Y, Markowitz G, Park JH, Reynolds DM, Maeda Y, Le TC, Hou HJ, Kucherlapati R, Edelmann W, Somlo S. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell 93: 177–188, 1998. [DOI] [PubMed] [Google Scholar]
- 92.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 24: 75–78, 2000. [DOI] [PubMed] [Google Scholar]
- 93.Wu Y, Dai XQ, Li Q, Chen CX, Mai W, Hussain Z, Long W, Montalbetti N, Li G, Glynne R, Wang S, Cantiello HF, Wu G, Chen XZ. Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum Mol Genet 15: 3280–3292, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Yoder BK Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol 18: 1381–1388, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516, 2002. [DOI] [PubMed] [Google Scholar]