Abstract
Translocator protein (TSPO), formerly known as the peripheral-type benzodiazepine receptor, is an 18-kDa drug- and cholesterol-binding protein localized to the outer mitochondrial membrane and implicated in a variety of cell and mitochondrial functions. To determine the role of TSPO in ischemia-reperfusion injury (IRI), we used both in vivo and in vitro porcine models: an in vivo renal ischemia model where different conservation modalities were tested and an in vitro model where TSPO-transfected porcine proximal tubule LLC-PK1 cells were exposed to hypoxia and oxidative stress. The expression of TSPO and its partners in steroidogenic cells, steroidogenic acute regulatory protein (StAR) and cytochrome P-450 side chain cleavage CYP11A1, as well as the impact of TSPO overexpression and exposure to TSPO ligands in vitro in hypoxia-ischemia conditions were investigated. Hypoxia induced caspase activation, reduction of ATP content, and LLC-PK1 cell death. Transfection and overexpression of TSPO rescued the cells from the detrimental effects of hypoxia and reoxygenation. Moreover, TSPO overexpression was accompanied by a reduction of H2O2-induced necrosis. TSPO drug ligands did not affect TSPO-mediated functions. In vivo, TSPO expression was modulated by IRI and during regeneration particularly in proximal tubule cells, which do not express this protein at the basal level. Under the same conditions, StAR and CYP11A1 protein and gene expression was reduced without apparent relation to TSPO changes. Pregnenolone was identified and measured in the pig kidney. Pregnenolone synthesis was not affected by the experimental conditions used. Taken together, these results indicate that changes in TSPO expression in kidney regenerating tissue could be important for renal protection and maintenance of kidney function.
Keywords: ischemia-reperfusion injury, translocator protein, renal regeneration
ischemia caused by arterial occlusion, shock, or transplantation often leads to kidney failure as a result of increased cell death in the affected kidney (2, 5, 23). Accumulating evidence points to a central role for mitochondria in this multifactorial process due to their pivotal role in energy production, generation of reactive oxygen species (ROS), and initiation of apoptosis (2, 24, 25, 34). Translocator protein 18 kDa (TSPO), formerly known as the peripheral-type benzodiazepine receptor (38), is a widely distributed transmembrane protein that is localized mainly in the outer mitochondrial membrane and binds with high-affinity drug ligands and cholesterol (36, 38, 39, 47, 48). TSPO and the TSPO homolog have been found throughout the animalia, plantae, and bacteria kingdoms (36, 48). An effort to generate a TSPO knockout mouse model resulted in early embryonic lethality, outlining the importance of TSPO (36, 37).
Expression of TSPO is ubiquitous, with a particularly high expression in steroid-producing tissues, as well as in the heart, liver, kidney, and blood cells (47). A broad spectrum of functions has been attributed to the TSPO including various host defenses, developmental processes, and mitochondrial functions. In steroid-producing tissues, TSPO has been implicated in the regulation of cholesterol transport into the mitochondria, where it is metabolized by CYP11A1 to pregnenolone, the precursor of all steroids (22, 37–39). Interactions with different proteins partners, such as the steroidogenesis acute regulatory protein (StAR), are important parts of this regulatory mechanism (10, 22, 39).
The minimal functional unit of TSPO is the 18-kDa monomer (38). The monomer has the ability to bind both drug ligands and cholesterol (28). However, TSPO has the ability to form functional stable homopolymers (12), to associate with other mitochondrial proteins, such as the voltage-dependent anion channel (VDAC) and the adenine nucleotide carrier (ANT) (32), and it has been shown to associate with the mitochondrial membrane transition pore (1). The role of TSPO, either in its polymeric or monomeric conformation in pathophysiological systems is under investigation in various laboratories (1, 12, 48).
It was previously shown that TSPO is relatively abundant in the kidney. In the rat, TSPO was localized by means of autoradiography using high-affinity radiolabeled drug ligands, within the thick ascending limb of the loop of Henle (TAL) and distal convoluted tubules (DCT) (19). Specific drug ligand binding was also present in the glomeruli and from the medullary TAL to the medullary collecting duct (8). Using drug ligands, several studies have been conducted to understand the role of TSPO under different experimental conditions of oxidative stress (9, 27, 29, 44–46). TSPO was also shown to be involved in the control of glomerular dynamics in an angiotensin II-induced hypertension model (7). Using the irreversible TSPO ligand SSR180575 (antiapoptotic), recent findings indicated a role for TSPO as a modulator of ischemia-reperfusion injury (IRI)-induced necrotic and apoptotic death (27). However, the diagnostic TSPO ligands PK 11195 and Ro5-4864 have relatively inconsistent and contradictory effects in regard to ischemia and when used at high concentrations these ligands may exert effects independently of TSPO (18, 26, 49). The modulation of TSPO per se has been poorly studied, particularly during ischemia or hypoxia. Recently, porcine TSPO was cloned, sequenced, and localized on chromosome 5 (51). We then showed that TSPO expression was modulated after severe warm ischemia (WI; 60 min of renal pedicle clamping) in the pig kidney during renal regeneration and temporarily expressed in the proximal tubule, where it is not usually detected under normal conditions (13, 52). TSPO expression was also studied after cold ischemia (4°C) in different preservation solutions in autotransplanted pig kidneys (21). Data from this work suggested that TSPO may serve as a potential marker of kidney preservation quality and an index of mitochondria viability, a major target of reperfusion injury (25). Interestingly, in normal and damaged renal tissue, TSPO was mainly expressed in its monomeric form (51, 52). Since TSPO can work as a monomer, a polymer, or a complex associated with other proteins (1, 12, 28, 32, 39), its functional conformation in renal IRI remains to be determined. We report herein the results from a series of experimental studies undertaken to clarify the role of TSPO in IRI. These studies were designed to 1) characterize the changes in TSPO and partner expression in the porcine kidney in response to different renal ischemia conditions; 2) assess the effect of TSPO overexpression in porcine proximal tubular LLC-PK1cells, a cell line which contains a very low level of TSPO, against hypoxia-induced changes in caspase activation, ATP levels, and cell viability; and 3) assess the effect of TSPO overexpression in LLC-PK1 cells against oxidative stress. The results obtained indicate that changes in TSPO expression are important for renal protection and maintenance of kidney function.
MATERIALS AND METHODS
Cell culture and transfection.
Porcine proximal tubular cells (LLC-PK1) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown in culture medium M199 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 3% fetal calf serum. Cells were maintained at 37°C in a 5% CO2 humidified atmosphere and were subcultured every 2 days.
The entire coding region of porcine TSPO cDNA (829 bp; access number: AY466517) was cloned into the mammalian expression vector pcDNA3.1/V5-His (Invitrogen Life Technology, Carlsbad CA). TSPO cDNA ligated in pcDNA3.1 or the empty pcDNA3.1 (control) vector was stably transfected into LLC-PK1 cells. Monolayers were grown to 80% confluence and transfected using Lipofectamine 2000 (Invitrogen Life Technology) according to the manufacturer's instructions. Briefly, plasmid DNA (4 μg), purified using Wizard Plus Minipreps kits (Promega, Madison, WI), was incubated at room temperature for 30 min with 10 μl of Lipofectamine in 500 μl of serum-free culture medium M199, then added to confluent cells cultured on six-well plates. After 6 h, the DNA-Lipofectamine mixture was removed. Cell cultures were then incubated in M199/3% fetal calf serum for 24 h and cultured in the same media containing 800 μg/ml geneticin (G418, Invitrogen-GIBCO) for selection.
Determination of transfection efficiency by confocal fluorescence microscopy.
Immunostaining and confocal fluorescence microscopy were performed to evaluate the expression of the fused protein, TSPO-V5/His, in transfected cells. Transfected cells were grown on glass slides for 24 h as detailed above, fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 in PBS, and coverslipped in mounting medium. TSPO-V5/His or native TSPO was stained indirectly using a primary monoclonal anti-V5 antibody (Invitrogen Life Technology) or a rabbit polyclonal anti-TSPO antibody (30) and a secondary FITC-conjugated goat anti-mouse IgG antibody and a secondary rhodamine-conjugated goat anti-rabbit IgG antibody (Jackson ImmunoResearch Laboratories, Westgrove, PA). Double staining was performed using FITC-conjugated phalloidin to stain actin. Samples were analyzed using a Bio-Rad MRC 1024 ES confocal microscope. To evaluate transfection efficiency, 20 high-power fields were randomly selected, and the ratio of transfected cells (stained) vs. nontransfected cells (unstained) was established.
Hypoxia model.
Media of confluent monolayers of either wild-type or transfected LLC-PK1 cells were replaced by fresh media previously incubated for 2 h at 37°C in an air/5% CO2 humidified atmosphere. Cultures were then exposed to hypoxia by placing them in a chamber continuously flushed with 95% N2/5% CO2 in which the oxygen concentration was <0.1%. In parallel, control cultures were maintained under normoxic conditions, air/5% CO2 at 37°C. In a second set of experiments, selective TSPO ligands PK 11195 (1, 10, and 100 nM), Ro5-4864 (1, 10, and 100 nM), and clonazepam (5 nM) were added to the culture medium during hypoxia. Following hypoxic conditions, cells were submitted to reoxygenation (air/5% CO2 humidified atmosphere) for 6 h.
Induction of proximal tubule cell necrosis with H2O2.
Confluent monolayers of LLC-PK1 proximal tubule cells grown in 6- or 24-well plates were treated with 0.5 and 2 mM H2O2 for 4 h at 37°C in a 95% air-5% CO2 incubator. Release of cytoplasmic lactate dehydrogenase (LDH) as an indicator of membrane damage was measured spectrophotometrically using a commercial cytotoxicity detection kit (Boehringer Mannheim, Mannheim, Germany). The percentage of total cellular LDH released into the supernatant was taken as a parameter of structural cell injury. Cell viability was assessed using the MTT assay, which is based on the conversion of MTT, a tetrazolium salt, into formazan, which depends on activity of NADH and NADH-dependent dehydrogenase. As a consequence, MTT is an indicator of cell metabolic activity. The data are presented as the percentage of undamaged control cells.
In a second set of experiments, the selective TSPO ligands PK 11195 (100 nM), Ro5-4864 (100 nM), and clonazepam (5 nM) were added to the culture medium before H2O2 treatment.
Ischemia-reperfusion model.
Large white male pigs, weighting 30–35 kg, were prepared as previously described (21, 51, 52). The surgical and experimental protocols were performed in accordance with the French Ministry of Agriculture for the use and care of laboratory animals. Different experimental conditions were used: 1) the WI model, where right kidneys were exposed 60 min of WI by pedicle clamping and then reperfused; 2) the autotransplanted model where right kidneys were harvested and cold flushed and preserved at 4°C for 24 h; and 3) where right kidneys were submitted to WI (1 h) and cold preservation (24 h). In each experimental group, the left kidney was removed to mimic the nephron mass in the transplanted situation. Experimental groups were compared with a sham-operated group (Sham; age- and weight mass-matched group) and an uninephrectomized group (Unif; age-, weight-, and nephron mass-matched group). Eight animals were studied in each group.
Analysis of caspase activity and ATP quantification assays.
Cell death was evaluated by LDH release as previously described (14). Activity of caspase 3 was determined by measuring the fluorescence signal produced by cleavage of the specific substrate, DEVD-AFC (R&D Systems, Minneapolis, MN) using a spectrofluorometer (Victor 3 TM Multilabel counter, PerkinElmer Life and Analytical Sciences, Shelton, CT) using an excitation and emission wavelength of 400 and 505 nm, respectively, as previously described (14). Caspase 8 and caspase 9 activity assays were performed with ApoAlert caspase fluorescent assay kits (Clontech, Palo Alto, CA). ATP levels were quantified using a luminometry ATP Assay kit (Thermo Labsystems, Franklin, MA) according to the manufacturer's instructions. After addition of perchloric acid (0.2 N), cells were harvested by scraping. After centrifugation (12,000 g for 5 min), the supernatant was neutralized with potassium carbonate (1 M). The ATP concentration in the supernatant was measured using the luciferase assay kit with a Victor 3 TM Multilabel counter (PerkinElmer).
Determination of AIF, Bax, and BcL-xL protein expression by Western blotting.
After treatment, cells were washed three times with cold PBS and lysed in Laemmli sample buffer containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Protein concentration in supernatants was determined using a Bicinchoninic Acid kit (Sigma-Aldrich). Samples were run on 4–20% SDS-PAGE gradient gels and electrophoretically transferred onto nitrocellulose membranes. Membranes were incubated overnight at 4°C with antibodies against AIF (1:1,000; BD Biosiences, San Jose, CA) and Bax and BcL-xL (1:200, Santa Cruz Biotechnology, Santa Cruz, CA). After several washes, membranes were incubated with the horseradish peroxidase-linked antibody for 1 h at room temperature. Bound antibodies were detected by chemiluminescence (ECL Hyperfilm and ECL Plus Reagent, Amersham Biosciences, Piscataway, NJ). Blots were stripped by submerging the membrane in a buffer containing 100 mM 2β-mercaptoethanol, 20% SDS, and 62.5 mM Tris·HCl, pH 6.8, at 55°C for 50 min followed by washes with TBS. Blots were then reprobed using an antibody directed against the total fraction of each protein. GAPDH or actin expression was used as a loading control. Image-densitometric analysis of the immmunoreactive protein bands was performed using Image J software.
Determination of cytochrome c release by Western blotting.
Cytochrome c release was determined as previously described (33). Briefly, from cell pellets obtained after centrifugation and resuspension, mitochondrial and cytosolic fractions were isolated. A total of 20 μg of proteins obtained from these different fractions was separated by 12% SDS-PGE and immunoblotted onto a nitrocellulose membrane and probed with a cytochrome c antibody (1:1,000). Bound antibodies were detected by chemiluminescence (ECL Hyperfilm and ECL Plus Reagent, Amersham Biosciences). The membranes were stripped and then probed with a cytochrome oxydase (COX) antibody for mitochondrial fractions and a β-actin antibody for cytosolic fractions as internal controls. Image-densitometric analysis of the immunoreactive protein bands was performed using Image J software.
Renal function determination.
Blood creatinine levels were measured 3 h and 1, 3, and 7 days after reperfusion using an automatic analyzer (modular automatic analyzer, Roche, Mannheim, Germany).
Histochemical and immunohistochemical studies.
Kidney fragments from the cortex and outer medulla were collected at 3 h and 3 and 7 days after reperfusion and processed as described (21). The magnitude of tubular epithelial cell loss, necrosis, intratubular debris, and tubular cast formation was scored on six levels based on the percentage of affected tubules in a high-power field under light microscopy as previously described (51, 52): 0, no abnormality; 0.5, <10%; 1, 10–25%; 2, 25–50%; 3, 50–75%; and 4, >75%.
Immunolocalization of TSPO was performed as previously described using rabbit polyclonal antibodies and anti-mouse TSPO (Department of Biochemistry and Molecular Biology, Georgetown University, Washington, DC) directed against amino acid sequences conserved across species (50). TSPO immunoreactivity was expressed in a semiquantitative load score as follows: 0, absence of significant staining; +, faint staining affecting <25% of tubules; ++, moderate staining affecting 25–50% of tubules; +++, diffuse staining affecting 50–75% of tubules; and ++++, strong staining affecting >75% of tubules. As a negative control, analysis was performed by omitting the primary antibody, and the positive control was performed using adrenal tissue.
Determination of CYP11A1, StAR, and TSPO protein expression by Western blotting.
Supplementary animals were added for determination of protein in renal tissue submitted to the different experimental conditions at 3 h (n = 3), 3 days (n = 3), and 7 days (n = 3) after reperfusion. Immediately after the kidney and testis were removed, tissue samples were dissected using a razor blade and stored at −80°C until assay. Tissue was minced and homogenized in a Ultra-turrax homogenizer at 4°C in the homogenization buffer (250 mM sucrose, 80 mM KCl, 20 mM tetrasodium pyrophosphate, 1 mM EGTA, 20 mM immidazole supplemented with protease inhibitor cocktail, pH 7.4). After centrifugation at 1,000 g for 15 min at 4°C, the supernatant was centrifuged at 150,000 g for 30 min at 4°C, separating membrane and cytosolic proteins. These fractions were used for StAR and CYP11A1 protein expression. Total proteins in the extract in each condition were measured by a BCA protein assay kit with BSA as a standard. Protein extracts was denatured in Laemmli buffer, separated by 10% SDS-PAGE, and electrotransferred onto Hybond C membranes (Amersham, Orsay, France). After an overnight blocking step at 4°C with 5% nonfat dry milk in Tris-buffered saline (TBS), proteins of the steroidogenic pathway were detected using rabbit polyclonal antibodies raised against CYP11A1 (1:1,000), StAR (1:1,000), and TSPO (1:1,000) in PBS-0.1% Tween 20 for 1.5 h at room temperature. Several antibodies against StAR were assessed (3, 20), produced in rabbits immunized with StAR proteins expressed with full-length StAR cDNA. The filter was then washed with TBS-0.1% Tween 20 and further incubated for 30 min at room temperature with a 1:10,000 dilution of goat horseradish peroxidase-conjugated anti-rabbit IgG antibody (Amersham Biosciences) in TBS-0.1% Tween 20. The membrane was washed as described above, and horseradish peroxidase activity was detected using a chemiluminescent technique to reveal the labeled bands (ECL kit, Amersham Biosciences). TSPO expression was determined in whole-cell extracts following a standardized procedure (14).
Determination of CYP11A1 and StAR mRNA expression by quantitative real-time PCR.
Tissues obtained similarly than for protein expression measurement were processed for RNA extraction using TRIzol (Invitrogen) according to the manufacturer's recommendations. Genomic DNA was removed using a DNA-free kit (PE Applied Biosystems, Foster City, CA). Each cDNA template for RT-PCR was prepared by first-strand reverse transcription using random primers (Applied Biosystems). TaqMan PCR assays were performed in duplicate/triplicate with 10 ng total cDNA templates in 96-well optical plates on an ABI Prism 7300 Sequence Detection System following the manufacturer's recommendations (Applied Biosystems). Porcine primers were designed on Primer Express (Applied Biosystems) with the following sequences: StAR forward AACCCCCAGTGTCAAGAAGATCAA and reverse AACTCATGGGTGATGACTGTGTCT; and CYP11A1 forward CATCAGGGAAGACCTGTTTCG and reverse GGCGCTCTCCAAATATGACATT. For each sample, an amplification plot was generated, displaying an increase in the reporter dye fluorescence (ΔRn) with each PCR cycle. A threshold cycle (Ct) value was calculated, which was the PCR cycle number at which fluorescence reached threshold, based on baseline variability data in the first 15 cycles. ΔCt for each gene target was obtained by subtracting the Ct value of L19 rRNA from that of the gene target. Finally, the level of mRNA expression relative to expression in isografts was obtained by the following equation: relative expression = 2−(ΔCt of target in treatment group −ΔCt of target in normal tissue).
Pregnenolone identification and measurement by gas chromatography/mass spectrometry.
Immediately after kidney removal, tissue samples were dissected using a razor blade and stored at −80°C until assay. This method was adapted from Liere et al. (31) and used [2H4] pregnenolone (Cluzeau, France) as an internal standard (2 ng). Briefly, the samples were weighed (≅800 mg) and homogenized in 5 ml methanol/acetic acid (95/5, vol/vol) in a Thomas potter. After evaporation of the organic phase, the residue was reconstituted in 2 ml of MeOH-H2O (40/60). Then, a clean-up step was performed by solid-phase extraction with C18 Sep Pak 500-mg cartridges (Waters, En Yvelines Cedex, France). The extract was dried and derivatized by heptafluorobutyric acid anhydride (Interchim, Montlucon Cedex, France). The reaction was then carried out at 67°C for 35 min. After evaporation, the residue was dissolved in 12 μl of heptane. Two microliters of the sample was applied on a Hewlett-Packard GC 6890 Series gas chromatograph coupled with a Hewlett-Packard 5973 mass-selective detector as previously described (15). For derivatized pregnenolone and deuterated standard, ions at m/z 298 and 302, respectively, were selected for quantification.
Statistical analysis.
All data are expressed as means ± SE. Differences between means were analyzed by ANOVA, followed by Dunnett's test or Student's t-test with Graph Pad Prism 3.02 software (Graph Pad Software, San Diego, CA). Probability values < 0.05 were accepted as significant.
RESULTS
Influence of renal ischemia conditions on renal function.
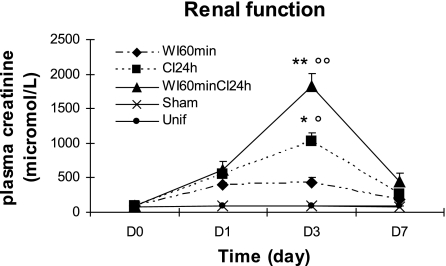
A significant increase in blood creatinine concentrations, a marker of declined renal function, was observed 1 (P < 0.01) and 3 days (P < 0.001) after reperfusion (Fig. 1). Creatinine concentrations remained significantly increased at day 7 (P < 0.01), although they began to return to baseline levels at this time. Creatinine concentrations were not changed in Sham and Unif groups compared with day 0 from the different experimental groups (Fig. 1).
Fig. 1.
Effect of ischemia-reperfusion on blood creatinine concentrations. Blood creatinine concentrations, an index of renal function, were measured 1, 3, and 7 days after different experimental conditions [warm ischemia for 60 min and reperfusion (WI60min)]; [cold ischemia for 24 h (CI24h)]; [warm ischemia for 60 min and cold ischemia for 24 h (WI60CI24h)]. Creatinine concentrations in sham-operated group (Sham) were also measured. Unif, uninephrectomized. Creatinine concentrations in experimental groups were significantly increased over control levels at day 3 and decreased to basal values by day 7 (°P < 0.05, °°P < 0.01 vs. Unif and Sham). Creatinine levels at day 3 were significantly greater in CI24h and WI60minCI24h than in WI60min (n = 3 for all time points; *P < 0.05, **P <0.01 vs. WI60min).
Influence of renal ischemia conditions on StAR and CYP11A1 protein and mRNA expression.
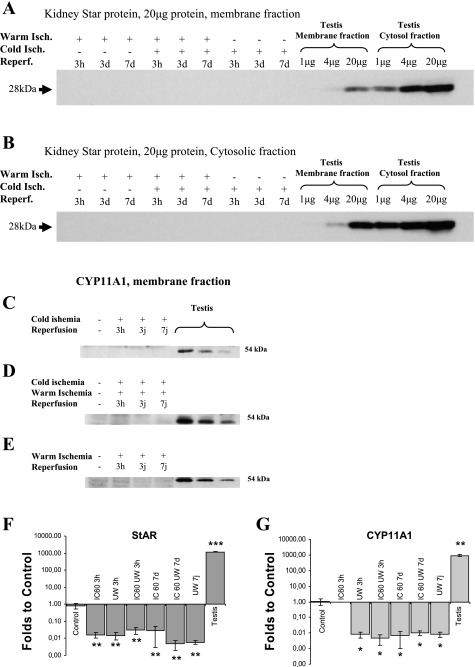
In steroid-synthesizing cells, StAR and CYP11A1 are proteins involved in cholesterol transfer into mitochondria through an interaction with TSPO and cholesterol metabolism into pregnenolone, respectively. As illustrated in Fig. 2 (A–E), neither StAR (A and B) nor CYP11A1 (C–E) proteins were detected in the cytosol or membrane fractions of pig kidney under any of the experimental conditions used. StAR and CYP11A1 proteins were found in control tissue like testis.
Fig. 2.
Representative Western immunoblotting analysis of kidney homogenate using anti-steroidogenic acute regulatory protein (StAR; A–C) and anti-CYP11A1 (D–F) and quantitative real-time PCR measurement of StAR and CYP11A1 (G and H). Kidneys were removed during reperfusion (3 h, 3 days, 7 days) after 24 h of cold preservation (A and D), 60 min of warm ischemia and cold preservation (B and E), or 60 min of warm ischemia without transplantation (C and F); n = 3/group. For StAR, each lane of renal tissue contained 20 μg of cytosolic proteins and, respectively, 2.8m 1.4, and 0.7 μg from testis tissue. For CYP11A1, each lane of renal tissue contained 35 μg of mitochondrial proteins and, respectively, 1.4, 0.7, and 0.35 μg from testis tissue. F: quantitation of StAR mRNA expression in normal kidney (control), kidneys which underwent warm ischemia at body temperature for 60 min (IC 60), kidneys conserved at 4°C for 24 h in University of Wisconsin solution (UW) after 60-min warm ischemia (IC 60 UW), and kidneys conserved at 4°C for 24 in UW (UW). Measurements were performed at both 3 h and 7 days postreperfusion. Testis is used as a positive control for the expression of StAR. G: expression of CYP11A1 in similar tissues and conditions. Values are means ± SE by t-test to control. *P < 0.05, **P < 0.01, and ***P < 0.001.
The presence of StAR and CYP11A1 mRNA and the impact of IRI on its levels were further investigated by quantitative PCR. StAR and CYP11A1 mRNAs were measured at two different time points (3 h and 7 days) following several experimental conditions, namely, 60-min warm ischemia, 24-h cold static conservation in University of Wisconsin solution preceded by 60-min warm ischemia in situ, and 24-h cold preservation in University of Wisconsin solution (Fig. 2, F and G). Overall, expression of StAR mRNA levels was reduced by the ischemic injury, to an average of 0.02 ± 0.01 of control (P < 0.01 for all conditions), whereas the expression in a steroidogenic organ such as the testis was 1,187.38 ± 60.00-fold over the kidney (P < 0.001).
Expression of CYP11A1 mRNA levels was found to follow that of StAR (Fig. 2G) expression. CYP11A1 mRNA levels were significantly reduced at all ischemic conditions used (0.01 ± 0.01 to control, P < 0.05 across conditions). CYP11A1 mRNA was found abundant in testis (936.29 ± 105.77-fold over control, P < 0.01).
Influence of renal ischemia conditions on kidney pregnenolone levels.
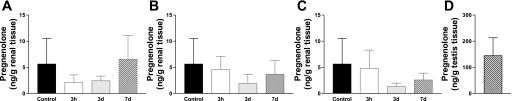
Pig kidney pregnenolone levels were measured and are presented in Fig. 3. Pregnenolone levels were 25-fold less than that found in steroidogenic testis (Fig. 3D). No significant modulation of kidney pregnenolone levels was observed under the different experimental conditions used.
Fig. 3.
Pregnenolone concentrations in renal (A–C) and in testis (D) tissue homogenate. Kidneys were removed during reperfusion (3 h, 3 days, 7 days) after 24 h of cold preservation (A), 60 min of warm ischemia and cold preservation for 24 h (B), or 60 min of warm ischemia without transplantation (C). Values are means ± SE (n = 6/group). Control is representative of the value determined in fresh kidney tissue.
Changes in TSPO expression after ischemia and reperfusion.
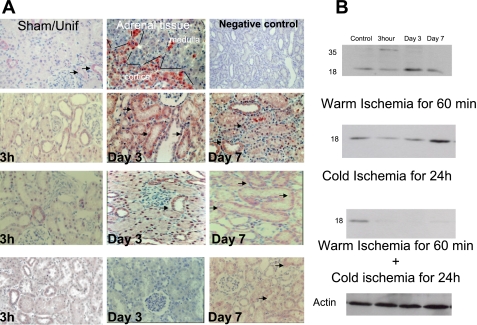
Table 1 represents a semiquantitative analysis of the morphological changes seen in renal injury. The scoring for TSPO immunolabeling is presented in Table 2. In normal kidney, TSPO was mainly expressed in the distal part of the nephrons and the collecting ducts (Fig. 2A, Sham and Unif groups). TSPO expression in injured proximal tubular cells appeared 24 h after reperfusion in the WI model. In the cold-ischemia model, TSPO was detected after 24 h and its levels increased progressively during the first week. Under conditions where the WI and cold preservation were associated, TSPO immunoreactivity appeared after 3 days of reperfusion. It is important to note that TSPO expression paralleled renal tissue regeneration (Fig. 4A). Immunoblot analysis performed with tissue extracts confirmed the immunohistochemical results (Fig. 4B). TSPO migrates as an 18-kDa protein. TSPO 36-kDa dimers were also observed.
Table 1.
Renal injury after reperfusion
|
Warm Ischemia for 60 Min | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control/Unif | 3 h | D3 | D7 | |||||
| ECL | 0 | 3.0±0.4 | 2.5±0.3 | 2.3±0.3 | ||||
| N | 0 | 3.1±0.6 | 2.5±0.3 | 2.2±0.2 | ||||
| ID | 0 | 3.0/0.3 | 2.3±0.3 | 2.0±0.3 | ||||
| CF | 0 | 3.1/0.3 | 2.5±0.3 | 2.1±0.3 | ||||
| Cold Preservation for 24 h | ||||||||
| ECL | 0 | 2.8±0.3 | 2.3±0.3 | 2.0±0.2 | ||||
| N | 0 | 2.9±0.3 | 2.5±0.3 | 1.9±0.3 | ||||
| ID | 0 | 3.0±0.3 | 2.4±0.3 | 2.0±0.2 | ||||
| CF | 0 | 3.0±0.2 | 2.5±0.3 | 1.8±0.3 | ||||
| Warm Ischemia for 60 min, Then Cold Preservation for 24 h | ||||||||
| ECL | 0 | 4.0±0.1 | 3.8±0.4 | 3.1±0.3 | ||||
| N | 0 | 3.9±0.3 | 3.5±0.3 | 3.0±0.3 | ||||
| ID | 0 | 4.0±0.2 | 3.6±0.3 | 3.0±0.3 | ||||
| CF | 0 | 3.5±0.2 | 3.3±0.3 | 2.8±0.2 | ||||
Values are means ± SE. Lesion grades are 0-5. Tubular epithelial cell loss, necrosis, intratubular debris and cast formation were examined in light microscopic studies. D, day; Unif, uninephrectomized; ECL, epithelial cell loss; N, necrosis; ID, intratubular debris; CF, cast formation.
Table 2.
Effect of experimental conditions on renal TSPO expression in the cortex and medulla
|
Warm Ischemia for 60 min | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sham/Unif | 3 h | D3 | D7 | |||||
| Cortex: | ||||||||
| PT (S1; S2) | 0 | 0 | +++ | + | ||||
| Distal tubule | +++ | + | ++ | +++ | ||||
| Medulla: | ||||||||
| Proximal tubule (S3) | 0 | 0 | +++ | + | ||||
| TAL | +++ | + | ++ | +++ | ||||
| TDL | + | + | ++ | + | ||||
| CD | +++ | + | ++ | +++ | ||||
| V | ++ | + | ++ | ++ | ||||
| Cold Ischemia for 24 h | ||||||||
| Cortex: | ||||||||
| PT (S1; S2) | 0 | 0 | ++ | ++ | ||||
| Distal tubule | +++ | + | ++ | +++ | ||||
| Medulla: | ||||||||
| Proximal Tubule (S3) | 0 | 0 | + | ++ | ||||
| TAL | +++ | + | ++ | +++ | ||||
| TDL | + | + | + | + | ||||
| CD | +++ | + | ++ | +++ | ||||
| V | ++ | + | ++ | ++ | ||||
| Warm Ischemia for 60 min and Cold Ischemia for 24 h | ||||||||
| Cortex: | ||||||||
| PT (S1; S2) | 0 | 0 | 0 | 0/+ | ||||
| Distal tubule | +++ | 0/+ | + | ++ | ||||
| Medulla: | ||||||||
| Proximal Tubule (S3) | 0 | 0 | 0/+ | + | ||||
| TAL | +++ | 0/+ | + | ++ | ||||
| TDL | + | 0 | + | + | ||||
| CD | +++ | 0/+ | + | ++ | ||||
| V | ++ | 0 | + | ++ | ||||
TSPO, translocator protein; TAL, thick ascending limb of Henle's loop; TDL, thin descending limb of Henle's loop; CD, collecting duct; PT, proximal tubule; DT, distal tubule; V, vessels.
Fig. 4.
Effect of ischemia-reperfusion on translocator protein 18 kDa (TSPO). TSPO staining is indicated by black arrows. Negative control was performed by omitting the primary antibody, and the positive control was performed using adrenal tissue. The representative staining concerns the cortical part of the adrenal where steroidogenesis is located. TSPO staining appears to be parallel with tissue regeneration in kidneys following ischemia-reperfusion lesions. Original magnification ×200.
Transfected TSPO protects LLC-PK1 cells from hypoxia-induced apoptosis.
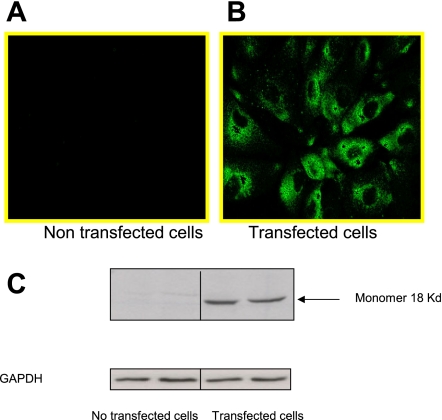
The intracellular distribution of TSPO-V5 fused protein, as determined by confocal microscopy, is shown in Fig. 5, A and B. Transfection efficiency was >70%, and TSPO staining was mainly localized in the cytoplasm. Western blotting of cell extracts obtained from native LLC-PK1 cells revealed that TSPO expression was very low in the native cells (Fig. 5C; nontransfected cells). After transfection, an 18-kDa protein was strongly recognized by the antibody directed against TSPO. This indicates that transfection resulted in the formation of the 18-kDa TSPO monomer in LLC-PK1 cells.
Fig. 5.
Localization of TSPO-V5-tagged protein in transfected LLC-PK1 cells. The intracellular distribution of TSPO-V5 protein was examined using immunostaining and confocal microscopy. A: basal expression in nontransfected cells. B: TSPO-V5 subcellular localization in transfected cells. C: representative immunoblot of 3 independent experiments. GAPDH was used as a loading control.
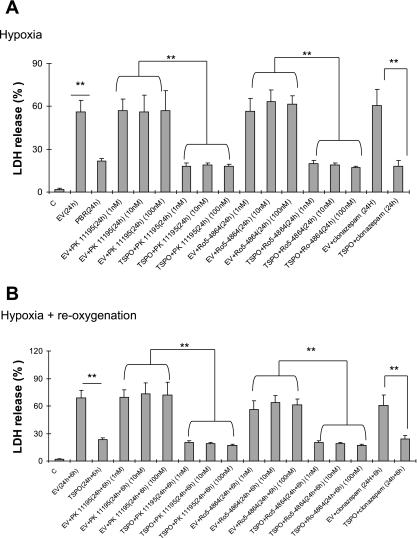
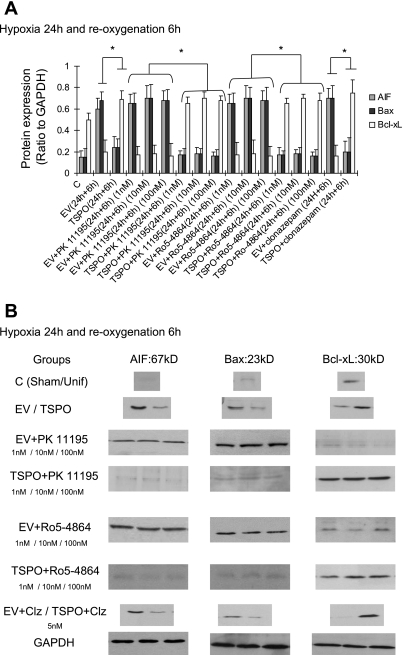
Interestingly, TSPO induced a protective effect on caspase 3 activation and ATP levels, as shown in Table 3. Hypoxia-induced caspase 3 activation was markedly reduced by ∼60% in TSPO-overexpressing cells compared with cells with the empty virus. Similarly, after 24 h of hypoxia, ATP levels decreased to 18% of normoxic levels in control cells while ATP levels were maintained at 41% of normoxic levels in TSPO-transfected cells. These results were correlated to a reduction of LDH release after hypoxia and reoxygenation (Fig. 6, A and B). Except at the highest 100 nM concentration, the TSPO ligands did not influence these parameters under hypoxic conditions in TSPO-transfected cells and native LLC-PK1 cells (Table 1). Clonazepam did not to induce any change in this model (Table 1). During reoxygenation, ATP increased significantly in TSPO-transfected cells compared with nontransfected cells, accompanied by a caspase 3 activity reduction in TSPO-transfected groups (Table 3). TSPO ligands reduced caspase 3 activity and ameliorated ATP synthesis level in a dose-dependent manner (Table 3). TSPO-transfected cells showed a decrease in AIF and Bax expression, while the antiapoptotic Bcl-xL was increased (Fig. 7, A and B).
Table 3.
Effect of 24 h of hypoxia on caspase 3 activity and ATP levels in pcDNA3.1 and pcDNA3.1 TSPO-transfected LLC-PK1 cells
|
Hypoxia |
||
|---|---|---|
| Caspase 3 Activity, % | ATP Level, % | |
| EV (24 h) | 100 | 18.21±1.1 |
| TSPO (24 h) | 37±6.2* | 41.21±17.9* |
| EV+PK11195 (24 h) | 97±5.6/96±6.3/95 | 19.30±1.4/18.25±1.6/8.80±2.1† |
| 1 nM/10 nM/100 nM | ||
| TSPO+PK11195 (24 h) | 35±4.6*/37±3.8*/38±4* | 43.11±14.2*/45±12*/46±11* |
| 1 nM/10 nM/100 nM | ||
| EV+Ro5-4864 (24 h) | 99±6.2/98±5.1/97±4.9 | 15.31±1.3/16.3±1.8/8.7±2.3† |
| 1 nM/10 nM/100 nM | ||
| TSPO EV+Ro5-4864 (24 h) | 41±6.4*/43±3.7*/46±4.1* | 41.21±17.9*/44.2±14*/45.3±13* |
| 1 nM/10 nM/100 nM | ||
| EV+clonazepam (24 h) | 96±5.8 | 16.66±1.8 |
| TSPO+clonazepam (24 h) | 38±5.3*/44±4.2*/45±3.9 | 42.3±13.1*/43.1±11*/43.8±16.3* |
| Reoygenation | ||
| EV (24 h) | 100 | 13±0,9 |
| TSPO (24 h) | 24±4,1* | 53±11.8* |
| EV+PK11195 (24 h) | 91±3.3/92±5.1/91±3.7 | 14.10±1.1/13.4±1.9/7.2±3.7 |
| 1 nM/10 nM/100 nM | ||
| TSPO+PK11195 (24 h) | 25±3.8*/17±1.2*/11±3.7* | 54.1±12.2*/65±9.4*/77±12.8* |
| 1 nM/10 nM/100 nM | ||
| EV+Ro5-4864 (24 h) | 90±3.4/92±4.3/93±3;3 | 12.5±2.3/11.53±2.5/6.3±2.0 |
| 1 nM/10 nM/100 nM | ||
| TSPO EV+Ro5-4864 (24 h) | 21±3.4*/12±1.7*/7±1.1 | 51.4±11.9*/69.2±10*/75.3±11* |
| 1 nM/10 nM/100 nM | ||
| EV+clonazepam (24 h) | 90±3.6 | 15.7±2.9 |
| TSPO+clonazepam (24 h) | 28±3.6*/24±3.8*/25±5.6 | 55±10.3*/56.5±10*/58.9±11.6* |
Values are means ± SE of 6 independent experiments. Caspase 3 activity results are expressed as percentage of activity at 24 h of hypoxia and ATP results as percentage between values measured at time 0 and after 24 h of hypoxia and 6 h of reoxygenation. PK 11195 and RO5-4864 were used at 3 different concentrations: 1, 10, 100 nM (left to right in the results). Clonazepam was used at 5 nM. EV, cells with empty virus; TSPO, cells which overexpressed TSPO.
P < 0.05 TSPO-transfected LLC-PK1 cells vs. LLC-PK1 with empty virus.
P <0.05 100 nM dose vs. 1 and 10 nM doses.
Fig. 6.
Effect of peripheral-type benzodiazepine receptor (PBR) transfection on cell death after hypoxia (A) and reoxygenation (B). LDH activity was measured spectrophotometrically, by following the rate of conversion of NADH to NAD+, at 340 nm. LDH release was calculated as [supernatant LDH/(supernatant LDH + cell lysate LDH) × 100]. Values are means ± SE of 6 independent experiments. **P < 0.05 TSPO-transfected LLC-PK1 cells vs. LLC-PK1 with empty virus (EV).
Fig. 7.
Renal protein densitometry (A) and representative immunoblots (B) of the regulation of apoptosis by AIF, Bax, and Bcl-xL in the different experimental groups. *P < 0.05 TSPO-transfected cells vs. nontransfected cells, transfected cells plus ligands vs. nontransfected cells plus ligands, and transfected cells plus clonazepam (Clz) vs. nontransfected cells plus ligands
Transfected TSPO protects LLC-PK1 cells from H2O2-induced necrosis.
The protective effects of TSPO against IRI were also accompanied by a reduction of LDH release (Fig. 8A). In addition, TSPO transfection induced a protective effect on caspase 8 and 9 activation and increased metabolic activity as determined by MTT activity (Fig. 8, B–D). TSPO ligands did not influence the effect on the different parameters (Fig. 8, A–D).
Fig. 8.
Effect of H2O2 in the different experimental groups. Cell injury was measured by LDH (A) and MTT assay (B). Caspase 8 (C) and -9 (D) activities were determined and are presented, respectively. *P < 0.05 transfected cells vs. nontransfected cells.
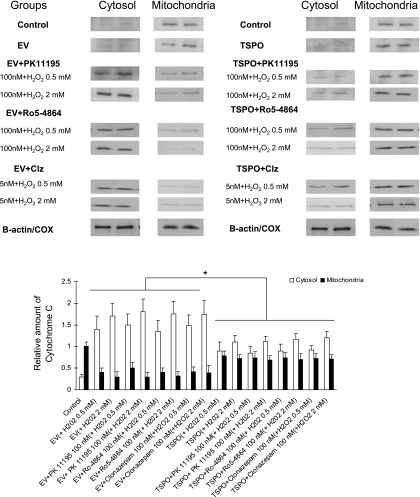
In nontransfected cells, an appreciable amount of cytochrome c was detected in the cytosolic fraction after 4-h treatment with H2O2 (Fig. 9, top and bottom). The effect of TSPO was associated with a reduction in cytochrome c release in the cytosolic fraction, suggesting a cytoprotective effect by preventing the apoptosis process (Fig. 9, top and bottom). In addition, no effect of TSPO ligands was observed.
Fig. 9.
Effect of TSPO transfection on cytochrome c release. Top: immunoblot analysis of cytosolic and mitochondrial fraction in the different experimental groups. β-Actin antibody was used as an internal control for cytosolic fractions, and a cytochrome oxydase (COX) antibody was used as an internal control of the mitochondrial fractions. Bottom: relative amount of normalized cytochrome c protein level. *P < 0.05 transfected cells vs. nontransfected cells.
DISCUSSION
In the kidney as well as other organs, IRI is associated with generation of ROS in parenchymal cells such as proximal tubules and endothelium (2, 6, 34, 42). Despite high blood flow and oxygen delivery, the countercurrent nature of the renal vasculature involves that the oxygen tensions in the kidney are lower than in other vascularized organs, particularly in the renal medulla (35, 43). The major finding of the present study is that in response to WI, TSPO protein levels increase in the kidney cortex and outer medulla, where the proximal tubular segments are predominantly located. Among the tubule segments, the most extensive cell injury develops in the proximal tubule. The particular susceptibility of these cells is attributable to both their low glycolytic capacity and their mandatory need for oxygen, making them dependent on mitochondrial metabolism for ATP synthesis (6, 34, 35, 43). TSPO expression was modulated by the experimental conditions. The combination of WI and cold preservation demonstrated the lowest TSPO expression level and was associated with delayed renal function and tissue injury. In addition, these changes in TSPO expression did not appear to be linked to either parallel changes in StAR and CYP11A1 mRNA or pregnenolone levels found in the kidney. It is unclear at present whether the identified pregnenolone is the result of local synthesis or tissue-specific accumulation. In summary, TSPO expression in the post-IRI kidney is a good marker of tissue generation and is associated with a better long-term outcome.
In the rainbow trout, StAR, TSPO, and CYP11A1 are present in the renal tissue (19). In the fetal rat kidney, it was demonstrated that local steroid hormone production was possible. However, the exact role of this local steroidogenesis remains to be clarified. It was previously shown that StAR is not expressed in adult and fetal renal porcine tissue (40). The data presented herein and these reported results suggest some interspecies specificity, which could be related to some particularities of renal and adrenal development. The absence of pregnenolone modulation by IRI does not formally exclude a steroidogenic role for the kidney; indeed, further studies are warranted to determine the potential role of peripheral steroidogenic pathways such as reductase, aldo-keto reductase, 3β-dehydroxysteroid dehydrogenase, or other CYP enzymes involved in the downstream metabolism of pregnenolone. In fish, the adrenocortical equivalent is embedded in the kidney and, consequently, the different partners are detectable in both tissues. Collectively, this in vivo model suggests that 1) TSPO is modulated by the different IRI conditions, 2) this modulation occurs independently of its steroidogenesis partners, and 3) the observed downregulation of steroidogenesis partners seems to be independent of pregnenolone levels found in the kidney.
To clarify the functional role of TSPO in IRI, we have utilized a cell model, the low-in-TSPO LLC-PK1 cells. As expected from previous studies, transfection of these cells with TSPO resulted in the expression of the 18-kDa TSPO protein (50, 51). TSPO in its native environment exists in various higher molecular mass complexes, ranging from 30 to 200 kDa. These complexes have been described as homopolymers of the 18-kDa TSPO monomer linked by dityrosine bonds or part of larger multiprotein complexes (12, 22, 36–39). In the present study, TSPO overexpression helped preserve mitochondrial integrity, as shown by the recovered ATP levels and reduced caspase 3 activity in response to 24 h of hypoxic stress and reoxygenation for 6 h. TSPO drug ligands (Ro5-4864 and PK 11195) have no effect (anti- or proapoptotic) during hypoxia and reoxygenation. Thus the frequently reported proapoptotic effects of Ro5-4864 and PK 11195 may be due to sites with low-affinity binding for these specific TSPO ligands and are not directly related to TSPO and its partners, namely, VDAC and ANT (50). Other studies have reported that the effect of TSPO ligands is dependent on the cell line studied and experimental conditions (4, 18, 26), and this may be due to the presence of distinct and at times various concentrations of endogenous ligands, such as the polypeptide diazepam binding inhibitor (11). Compared with this study, our contribution to an understanding of the role of TSPO in the pathophysiological process of IRI has focused on TSPO protein rather than the ligands. The mitochondria mass increases as cells pass through the cell cycle and the different steps of the repair process. This suggests a correlation between the increased presences of TSPO during the active phases of the cell cycle supported by effective mitochondrial biogenesis and the need for increased cholesterol levels for membrane formation.
A recent study employing TSPO-transfected Jurkat cells revealed that TSPO may regulate early death signals, leading to UV-induced apoptosis (43). Using a model of oxidative stress, TSPO transfection was also observed to be protective from free radical-induced damage and thereby modulate apoptosis in the hematopoietic system (9). These data suggest that TSPO may play a pivotal role in the cell death process triggered by different stresses. In the current study, during the hypoxic condition, TSPO transfection reduced ATP decline and caspase 3 activity, associated with a limitation of LDH release. In addition, TSPO-transfected cells expressed the antiapoptotic Bcl-xL close to the control level, while expression of the proapoptotic AIF and Bax was reduced. Recently, a study provided evidence for TSPO involvement in mitochondrial permeability transition pore opening in rat brain mitochondria, controlling the Ca2+-induced Ca2+efflux, and apoptosis-inducing factor release from mitochondria, important stages in the initiation of programmed cell death (1). In our hands, TSPO transfection was accompanied by a reduction in the deleterious effect of hypoxia and reoxygenation and exhibited an antiapoptotic influence. These data support a role of TSPO in IRI and in mitochondrial permeability transition pore opening, which is built during early hypoxia, suggesting that the protective role of TSPO transfection takes place during hypoxia (16). Such differences could be related to the cells used and dependent on the experimental conditions. In addition, in the study in rat brain mitochondria, isolated mitochondria could be preactivated and the effect of Ca2+ overload on mitochondrial permeability transition pore opening could be a specific effect in brain cells (1).
TSPO transfection also reduced H2O2-induced necrosis, suggesting a protective effect of this protein against stress. The proximal tubule epithelium is highly metabolic dependent and well endowed with mitochondria, a source of energy but also of oxidative stress. Consequently, the current data suggest that TSPO could be an essential contributor to limit cell damage. Because TSPO was shown to be involved in the mitochondrial protein import process, we surmise that TSPO abundance could be a factor in mitochondrial import of molecules such as superoxide dismutase, which plays a pivotal role in limiting IRI (24, 25, 50). These data confirm the protective effect of TSPO against stresses such as hypoxia and reoxygenation.
The TSPO localization data presented herein are in agreement with the basal TSPO localization in renal tubules, which has been previously observed within distal tubules. The renal distal tubular epithelial cells are well adapted to the hypoxic environment of the renal medulla and appear to be more able to withstand hypoxic stress than the epithelium of the proximal tubule (17, 35). Present data from different models outline the role of distal tubular epithelial cells as potential support for proximal tubular cell survival after renal injury (17). Renal distal tubule epithelial cells are a distinct segment of the renal nephron. The epithelial cells of the nephron are derived from the mesenchyme (metanephric blastema under the influence of the ureteric bud) during embryogenesis. We have previously observed the parallel expression of leukemia inhibitor factor and TSPO during embryogenesis and the regeneration process after IRI in pig tissue (51). Renal distal tubular epithelial cells have regenerative capacity after injury. Many proteins secreted by distal tubular epithelium may act positively on the ischemia-sensitive proximal tubular epithelium, which has receptors to many of these proteins but may not be able to synthesize them (17). In response to IRI, the renal distal tubular epithelial cells adaptively increase expression of survival factors, increase synthesis and secretion of reparative growth factors, and may help protect the sensitive proximal tubule from injury.
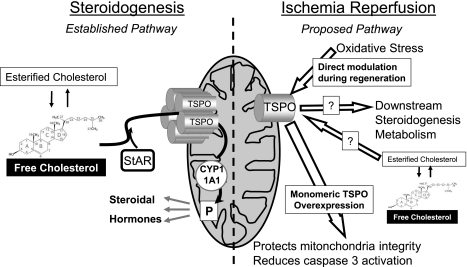
This report offers new insights into the participation of mitochondria in IRI and brings information on the role of TSPO in the mechanisms of renal IRI. TSPO is involved in the regeneration process after renal injury, independently of any of its drug ligands. The putative role of TSPO in IRI is summarized in Fig. 10 (right): oxidative stress during IRI favors the production of monomeric TSPO. In this conformation, TSPO offers protection against lesional mechanisms like apoptosis and maintains mitochondrial integrity. The transient phenotype change in proximal tubule cells could potentially permit a more functional adaptation of this segment of the nephron to IRI.
Fig. 10.
Schematic representation of the putative role of TSPO in ischemia-reperfusion injury (IRI). Left: classic steroidogenesis pathway, by which StAr transports free cholesterol to TSPO to transfer it inside the mitochondria to be processed by metabolic enzymes (among which is CYP11A1) into pregnenolone and further steroid hormones. Right: oxidative stress during IRI modulates the expression and conformation of TSPO, favoring the monomeric form. This specific conformation allows TSPO to have a protective effect against lesional mechanisms like apoptosis and maintain mitochondrial integrity. It may affect the downstream steroidogenic metabolism as well. The role of cholesterol in this conformation is yet to be determined.
These data also suggest that the role of intrinsic epithelial cells is predominant in the mechanisms of nephron repair. In addition, this transient expression is observed under different experimental conditions, suggesting a common process during renal repair. Further studies are required under preconditioning conditions where TSPO could also play a pivotal role. Because it is involved in major cell functions (36, 39, 41, 46–48), TSPO may represent a new pathophysiological key and a potential target for the prevention of the stress linked to IRI. In addition, the results presented herein suggest that TSPO could be of importance in the cellular protection from hypoxia and reoxygenation and oxidative stress, emphasizing its role in cell apoptosis during oxygen decrease.
GRANTS
This work was supported by grants from the Conseil Régional Poitou-Charente, University of Poitiers, Banque Tarneaud, University Hospital of Poitiers, Institut National de Recherche Agronomique (France), and the National Institutes of Health.
Acknowledgments
We thank Catherine Henry and William Hebrard (Département de Génétique Animale, INRA, Surgères), Jean Luc Boutin (technical support), Béatrice Fernandez, and Nathalie Quellard (Unité de Microscopie Electronique, Laboratoire d'Anatomo-pathologie) for expert technical assistance and Laurent Pradel (Banque Tarneaud). We thank also Sandrine Joffrion and Stéphanie André for technical support.
REFERENCES
- 1.Azarashvili T, Grachev D, Krestinina O, Evtodienko Y, Yurkov I, Papadopoulos V, Reiser G. The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium 42: 27–39, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Benitez-Bribiesca L, Gomez-Camarillo M, Castellanos-Juarez E, Mravko E, Sanchez-Suarez P. Morphologic, biochemical and molecular mitochondrial changes during reperfusion phase following brief renal ischemia. Ann NY Acad Sci 926: 165–179, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Blomberg LA, Zuelke KA. Expression analysis of the steroidogenic acute regulatory protein (STAR) gene in developing porcine concept uses. Mol Reprod Dev 72: 419–429, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bono F, Lamarche I, Prabonnaud V, Le Fur G, Herbert JM. Peripheral benzodiazepine receptor agonists exhibit potent antiapoptotic activities. Biochem Biophys Res Commun 265: 457–461, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med 332: 647–655, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Bribes E, Casellas P, Vidal H, Dussossoy D, Casellas D. Peripheral benzodiazepine receptor mapping in rat kidney. Effects of angiotensin II-induced hypertension. J Am Soc Nephrol 13: 1–9, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Butlen D Benzodiazepine receptors along the nephron: [3H]PK 11195 binding in rat tubules. FEBS Lett 169: 138–142, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Carayon P, Portier M, Dussossoy D, Bord A, Petitpretre G, Canat X, Le Fur G, Casellas P. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood 87: 3170–3178, 1996. [PubMed] [Google Scholar]
- 10.Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int 40: 475–486, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Costa E, Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci 49: 325–344, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Peranzi G, Yao ZX, Maccario J, Lacapere JJ, Papadopoulos V. In vivo and in vitro peripheral-type benzodiazepine receptor polymerization: functional significance in drug ligand and cholesterol binding. Biochemistry 42: 4506–4519, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Doucet C, Zhang K, Desurmont T, Hebrard W, Scepi M, Nadeau C, Cau J, Leyre P, Febrer G, Carretier M, Richer JP, Papadopoulos V, Hauet T, Burucoa C, Goujon JM. Influence of warm ischemia time on peripheral-type benzodiazepine receptor: a new aspect of the role of mitochondria. Nephron 107: e1–e11, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dutheil D, Rioja-Pastor I, Tallineau C, Goujon JM, Hauet T, Mauco G, Petit-Paris I. Protective effect of PEG 35,000 Da on renal cells: paradoxical activation of JNK signaling pathway during cold storage. Am J Transplant 6: 1529–1540, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Favreau F, Petit-Paris I, Hauet T, Dutheil D, Papet Y, Mauco G, Tallineau C. Cyclooxygenase 1-dependent production of F2-isoprostane and changes in redox status during warm renal ischemia-reperfusion. Free Radic Biol Med 36: 1034–1042, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gateau-Roesch O, Argaud L, Ovize M. Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res 70: 264–273, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol 39: 1551–1561, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Polo RA, Carvalho G, Braun T, Decaudin D, Fabre C, Larochette N, Perfettini JL, Djavaheri-Mergny M, Youlyouz-Marfak I, Codogno P, Raphael M, Feuillard J, Kroemer G. PK11195 potently sensitizes to apoptosis induction independently from the peripheral benzodiazepine receptor. Oncogene 24: 7503–7513, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Gravel A, Vijayan MM. Salicylate disrupts interrenal steroidogenesis and brain glucocorticoid receptor expression in rainbow trout. Toxicol Sci 93: 41–49, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hales KH, Diemer T, Ginde S, Shankar BK, Roberts M, Bosmann HB, Hales DB. Diametric effects of bacterial endotoxin lipopolysaccharide on adrenal and Leydig cell steroidogenic acute regulatory protein. Endocrinology 141: 4000–4012, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Hauet T, Han Z, Wang Y, Hameury F, Jayle C, Gibelin H, Goujon JM, Eugene M, Papadopoulos V. Modulation of peripheral-type benzodiazepine receptor levels in a reperfusion injury pig kidney-graft model. Transplantation 74: 1507–1515, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Hauet T, Liu J, Li H, Gazouli M, Culty M, Papadopoulos V. PBR, StAR, and PKA: partners in cholesterol transport in steroidogenic cells. Endocr Res 28: 395–401, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard WJ, Bland KI, Chaudry IH. The role of the mitochondrion in trauma and shock. Shock 22: 395–402, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Izumi M, McDonald MC, Sharpe MA, Chatterjee PK, Thiemermann C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock 18: 230–235, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Jassem W, Fuggle SV, Rela M, Koo DD, Heaton ND. The role of mitochondria in ischemia/reperfusion injury. Transplantation 73: 493–499, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kletsas D, Li W, Han Z, Papadopoulos V. Peripheral-type benzodiazepine receptor (PBR) and PBR drug ligands in fibroblast and fibrosarcoma cell proliferation: role of ERK, c-Jun and ligand-activated PBR-independent pathways. Biochem Pharmacol 67: 1927–1932, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Kunduzova OR, Escourrou G, De La Farge F, Salvayre R, Seguelas MH, Leducq N, Bono F, Herbert JM, Parini A. Involvement of peripheral benzodiazepine receptor in the oxidative stress, death-signaling pathways, and renal injury induced by ischemia-reperfusion. J Am Soc Nephrol 15: 2152–2160, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lacapere JJ, Delavoie F, Li H, Peranzi G, Maccario J, Papadopoulos V, Vidic B. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem Biophys Res Commun 284: 536–541, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Leducq N, Bono F, Sulpice T, Vin V, Janiak P, Fur GL, O'Connor SE, Herbert JM. Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia-reperfusion. J Pharmacol Exp Ther 306: 828–837, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Yao Z, Degenhardt B, Teper G, Papadopoulos V. Cholesterol binding at the cholesterol recognition/ interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc Natl Acad Sci USA 98: 1267–1272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liere P, Akwa Y, Weill-Engerer S, Eychenne B, Pianos A, Robel P, Sjovall J, Schumacher M, Baulieu EE. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J Chromatogr B Biod Med Sci Appl 739: 301–312, 2000. [DOI] [PubMed] [Google Scholar]
- 32.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA 89: 3170–3174, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagothu KK, Bhatt R, Kaushal GP, Portilla D. Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int 68: 2680–2693, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med 109: 665–678, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos V In search of the function of the peripheral-type benzodiazepine receptor. Endocr Res 30: 677–684, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids 62: 21–28, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27: 402–409, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol Cell Endocrinol 265–266: 59–64, 2007. [DOI] [PubMed]
- 40.Pilon N, Daneau I, Brisson C, Ethier JF, Lussier JG, Silversides DW. Porcine and bovine steroidogenic acute regulatory protein (StAR) gene expression during gestation. Endocrinology 138: 1085–1091, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Rey C, Mauduit C, Naureils O, Benahmed M, Louisot P, Gasnier F. Up-regulation of mitochondrial peripheral benzodiazepine receptor expression by tumor necrosis factor alpha in testicular Leydig cells. Possible involvement in cell survival. Biochem Pharmacol 60: 1639–1646, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol 23: 511–521, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Simmons MN, Schreiber MJ, Gill IS. Surgical renal ischemia: a contemporary overview. J Urol 180: 19–30, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Stoebner PE, Carayon P, Casellas P, Portier M, Lavabre-Bertrand T, Cuq P, Cano JP, Meynadier J, Meunier L. Transient protection by peripheral benzodiazepine receptors during the early events of ultraviolet light-induced apoptosis. Cell Death Differ 8: 747–753, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Stoebner PE, Carayon P, Penarier G, Frechin N, Barneon G, Casellas P, Cano JP, Meynadier J, Meunier L. The expression of peripheral benzodiazepine receptors in human skin: the relationship with epidermal cell differentiation. Br J Dermatol 140: 1010–1016, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med 186: 25–37, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol Ther 110: 503–524, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Veenman L, Papadopoulos V, Gavish M. Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des 13: 2385–2405, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Walter RB, Pirga JL, Cronk MR, Mayer S, Appelbaum FR, Banker DE. PK11195, a peripheral benzodiazepine receptor (pBR) ligand, broadly blocks drug efflux to chemosensitize leukemia and myeloma cells by a pBR-independent, direct transporter-modulating mechanism. Blood 106: 3584–3593, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright G, Reichenbecher V. The effects of superoxide and the peripheral benzodiazepine receptor ligands on the mitochondrial processing of manganese-dependent superoxide dismutase. Exp Cell Res 246: 443–450, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, Demeure O, Belliard A, Goujon JM, Favreau F, Desurmont T, Mauco G, Barriere M, Carretier M, Milan D, Papadopoulos V, Hauet T. Cloning, sequencing, and chromosomal localization of pig peripheral benzodiazepine receptor: three different forms produced by alternative splicing. Mamm Genome 17: 1050–1062, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Zhang K, Desurmont T, Goujon JM, Favreau F, Cau J, Deretz S, Mauco G, Carretier M, Papadopoulos V, Hauet T. Modulation of peripheral-type benzodiazepine receptor during ischemia reperfusion injury in a pig kidney model: a new partner of leukemia inhibitory factor in tubular regeneration. J Am Coll Surg 203: 353–364, 2006. [DOI] [PubMed] [Google Scholar]