Abstract
We previously found that the Ca2+-sensing receptor (CaR) interacts with and inactivates the inwardly rectifying K+ channel Kir4.2 that is expressed in the kidney cortex and that has a COOH-terminal PDZ domain. To identify potential scaffolding proteins that could organize a macromolecular signaling complex involving the CaR and Kir4.2, we used yeast two-hybrid cloning with the COOH-terminal 125 amino acids (AA) of Kir4.2 as bait to screen a human kidney cDNA library. We identified two independent partial cDNAs corresponding to the COOH-terminal 900 AA of MUPP1, a protein containing 13 PDZ binding domains that is expressed in the kidney in tight junctions and lateral borders of epithelial cells. When expressed in human embryonic kidney (HEK)-293 cells, Kir4.2 coimmunoprecipitates reciprocally with MUPP1 but not with a Kir4.2 construct lacking the four COOH-terminal amino acids, Kir5.1, or the CaR. MUPP1 and Kir4.2 coimmunoprecipitate reciprocally from rat kidney cortex extracts. Coexpression of MUPP1 with Kir4.2 in HEK-293 cells leads to reduced cell surface expression of Kir4.2 as assessed by cell surface biotinylation. Coexpression of MUPP1 and Kir4.2 in Xenopus oocytes results in reduced whole cell currents compared with expression of Kir4.2 alone, whereas expression of Kir4.2ΔPDZ results in minimal currents and is not affected by coexpression with MUPP1. Immunofluorescence studies of oocytes demonstrate that MUPP1 reduces Kir4.2 membrane localization. These results indicate that Kir4.2 interacts selectively with MUPP1 to affect its cell surface expression. Thus MUPP1 and Kir4.2 may participate in a protein complex in the nephron that could regulate transport of K+ as well as other ions.
Keywords: PDZ binding proteins, potassium transport, calcium sensing, Xenopus oocyte expression
in epithelial cells, PDZ domain-containing proteins contribute to segregation of proteins, including membrane-bound transporters and ion channels, to the apical and basolateral domains of cells as well as subdomains of the plasma membrane and cell organelles (6). This segregation of transporters, channels, and pumps to apical or basolateral membranes is essential for vectoral transport of ions and solutes. Moreover, the localization of specific sets of proteins permits precise regulation of transport and signaling proteins in specialized domains such as synapses, cell junctions, and areas rich in receptors.
PDZ domain-containing proteins dock proteins that contain PDZ recognition sequences that have a characteristic four-amino acid (AA) sequence usually found at the COOH terminus of the protein. Intracellular localization of proteins by PDZ domains can occur via a number of mechanisms including mediating transport, retention in intracellular compartments, and retention on the plasma membrane (6). In renal epithelial cells, various PDZ proteins localize selectively to the apical membrane (NHERF-1, NHERF-2, and PDZK1), the basolateral membrane (MINT 1, MINT 2–3, CASK, MAGI-1a, and MALS/Veli-1–3), the Golgi network (GOPC), and tight junctions and basolateral membrane (MAGI-1 and MUPP1) (6, 13, 35, 49). Interaction of transport and signaling proteins with PDZ proteins has both physical and functional consequences. For example, in the apical membrane, NHERF-1 and -2 interact with both ROMK (Kir1.1) and CFTR, resulting in increased cell surface expression of ROMK, increased coimmunoprecipitation of ROMK with CFTR, and altered channel characteristics of ROMK (sensitivity to inhibition by glibenclamide) (56). Interaction of NHERF-1 or -2 with the Na+/H+ exchanger NHE3, NaPi-2a, and other transporters in a multiprotein complex brings them into association with PKA, facilitating their regulation by cAMP (6). PDZ proteins associate with each other via PDZ domains as well as other scaffolding proteins to create networks that organize high-order protein complexes to localize them in the cell and to ensure integrated regulation of their activities (6).
The mechanisms by which PDZ proteins localize in cells are not fully established but probably include interaction with membrane-bound proteins and chaperone proteins. Two PDZ proteins, MUPP1 and MAGI-1, normally associated with tight junctions, appear to require additional proteins for tight junctions and plasma membrane localization (10, 21, 54). These mechanisms are important for regulating renal function, but in many cases the components and mechanisms are not fully defined. Although critical for these functions, PDZ interactions alone may not be sufficient to explain all localization phenomena (33, 46).
Recently, we (17) found that the Ca2+-sensing receptor (CaR) interacts with and inactivates two K+ channels, Kir4.1 and Kir4.2. Kir4.1, probably in a complex with Kir5.1 and interacting with the PDZ protein MAGI-1a, is a component of the basolateral K+ conductance in the distal nephron (25, 49). Kir4.2, an inwardly rectifying K+ channel that is closely related to Kir4.1, is also expressed in the distal convoluted tubule of the distal nephron and probably other nephron segments, although its membrane distribution and the full extent of its expression along the nephron are not established (31). Kir4.2 is expressed in a number of other tissues and cell types including the central nervous system (neurons) and Calu 3 cells, an airway epithelial cell type (55). Kir4.2 has biophysical characteristics similar to those of Kir4.1, interacts with Kir5.1, and may have functions similar to those of Kir4.1 (31, 37, 50).
To identify proteins that interact with Kir4.2 and that could contribute to its regulation, we used the COOH-terminal 125 AA of Kir4.2 that contain a PDZ domain to screen a human kidney cDNA library using the yeast two-hybrid approach. We found that Kir4.2 interacts with a ubiquitously expressed PDZ domain-containing protein, MUPP1, in a PDZ-dependent manner but that overexpression of MUPP1 reduces cell surface expression of Kir4.2 and currents attributable to it in Xenopus oocytes. These results demonstrate that Kir4.2 interacts with MUPP1 such that MUPP1 is a potential scaffolding protein for Kir4.2.
MATERIALS AND METHODS
Materials.
All chemicals were purchased from Sigma or Fisher Scientific unless specified otherwise. The SuperSignal West Pico chemiluminescent substrate and BCA protein assay reagent were obtained from Pierce. The polyclonal anti-Myc (A-14) and monoclonal anti-Myc (9E10) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal anti-Kir4.2 antibody was provided by Dr. C. E. Hill (Queen's University, Kingston, ON, Canada) (14). The monoclonal anti-CaR antibody was described previously (3).
Yeast two-hybrid cloning.
A cDNA representing the COOH-terminal 125 AA of the rat Kir4.2 (AA 250–375) in the bait plasmid pAS2.1 was used to screen a human adult kidney cDNA library in pACT2 (Invitrogen). The bait plasmid was transformed into Saccharomyces cerevisiae strain AH109 by electroporation, mated with the pretransformed library in S. cerevisiae strain Y187, and then plated on medium deficient in leucine, tryptophan, and histidine (triple dropout medium) (3, 17). The plates were replicated on medium lacking histidine, tryptophan, leucine, and adenine (quadruple dropout medium). The colonies that grew under these conditions were tested for β-galactosidase activity. Yeast expressing the library plasmid pACT2 without an insert and the pACT2 vector with β-galactosidase were used as controls. The positive colonies were analyzed further by purifying the plasmids and sequencing the inserts from either end. The cDNA sequences were analyzed by searching the GenBank data base for homology to known DNA sequences. These positive clones were mated with the Kir4.2 construct plated on medium lacking histidine, leucine, tryptophan, and adenine to confirm the interaction.
Construction of plasmids.
To investigate the relationship between Kir4.2 and Kir5.1, without the limitation of specific antibodies, we made Myc- and hemagglutinin (HA)-tagged constructs of each channel, engineering epitope tags into the NH2 terminus of the proteins. The cDNA coding for rat Kir4.2 (Kir4.2) was a generous gift from Dr. C. E. Hill (14). The Kir4.2pGem construct was digested with EcoRI and NotI and then ligated into EcoRI and NotI sites of pCruz-HA or pCruz-Myc vector. The cDNA coding for rat Kir5.1 (Kir5.1) was generously provided by Stephen Tucker (University Laboratory of Physiology, Oxford, UK) (38). The Kir5.1pBF construct was digested with ApaI, blunt ended, and then digested with KpnI. The pCruz-Myc or -HA constructs were digested with NotI, blunt ended, and then digested with KpnI. The fragments were ligated into pCruz-Myc or pCruz-HA vector. All cDNA constructs were verified by direct sequencing. FLAG-tagged MUPP1 was generously provided by Dr. David Clapham (Harvard University, Cambridge, MA) (23).
Preparation of anti-MUPP1 antibodies.
Rabbit polyclonal antibodies were made using a peptide from the 210-AA region of MUPP1 between PDZ domains 5 and 6 (AA 780–793: PLSPEEGYVSAKED) (51). The sequence of this peptide is conserved in the human, mouse, rat, dog, and cow proteins, and it does not show significant homology to other proteins in the National Center for Biotechnology Information protein database. Two rabbits were injected. Specificity of the antisera was demonstrated as follows: 1) the sera from both rabbits recognized the same 220-kDa protein; 2) both sera recognized the FLAG-tagged MUPP1 expression construct from Dr. David Clapham (23); and 3) preincubation of the antisera with the antigenic peptide blocked recognition of native and expressed MUPP1.
Transfection, immunoblotting, immunoprecipitation, and cell surface biotinylation.
Human embryonic kidney (HEK)-293 cells were transiently transfected with the epitope-tagged channel constructs indicated (HA-tagged Kir4.2pCruz, Myc-tagged Kir4.2pCruz, HA-tagged Kir5.1pCruz, Myc-tagged Kir5.1pCruz, CaRpcDNA3, or FLAG-tagged MUPP1) using the FuGene 6 reagent and were incubated at 37°C for 24–48 h. The medium was removed and washed once with 1× PBS. The cells were lysed with 1× RIPA buffer, and the lysates were centrifuged at 15,000 rpm for 1 h at 4°C. The protein concentrations in the cell lysates were determined using the BCA protein assay reagent with BSA as a standard and were then adjusted to the same concentration with buffer. The samples were subjected to 11% SDS-PAGE and processed for immunoblotting, and the levels of protein expression were determined. The cell lysates were used for coimmunoprecipitation as described previously (16). Briefly, a monoclonal anti-FLAG, a polyclonal anti-Myc (A-14), our rabbit anti-MUPP1, or an anti-Kir4.2 antibody (Dr. C. E. Hill) was separately loaded onto the Dynabead-protein A complex and slowly rotated for 2 h (17). The antibody-loaded Dynabead-protein A complex was rinsed twice, and the beads were mixed with the various cell lysates, and rotated in the cold room overnight. The supernatants were discarded, and the Dynabead-protein A complex was washed. Loading buffer was added and vortexed vigorously. The tubes were placed in Dynal-MPC to collect the sample buffer. The samples were heated at 55°C for 20 min and then subjected to SDS-PAGE for immunoblotting using the antibodies indicated. For coimmunoprecipitation studies using rat kidney cortex membrane extracts, rat kidneys were isolated and decapsulated. The cortex was separated from the medulla, cut to small pieces, and homogenized in a buffer containing 20 mM HEPES, pH 8.0, 2 mM MgCl2, 1 mM EDTA, and protease inhibitors and centrifuged at 2,000 rpm for 10 min. The supernatant was centrifuged at 13,500 rpm for 60 min to obtain crude membrane and cytosol fractions. The samples were used for immunoblotting to confirm the presence of the endogenous proteins. The crude membranes were extracted in 1× RIPA buffer for 60 min and centrifuged at 13,500 rpm for 60 min, the resulting supernatants were used for immunoprecipitation with the anti-MUPP1 or anti-Kir4.2 antibodies, and the immunoprecipitated samples were processed for immunoblotting using antibodies against MUPP1 and Kir4.2 (17). Peptide blocking of antiserum was performed by incubating MUPP1 or Kir4.2 antiserum at a 1:500 dilution with 50 μg/ml of the antigenic peptide for 3 h in blotto at 4°C before incubation with the blots. Cell surface expression of Kir4.2 was measured using cell surface biotinylation. Cells are washed with PBS and incubated with sulfo-NHS-SS-biotin (biotin; 0.5 mg/ml) for 5 min on ice. The cells are washed three times with PBS, quenched with 100 mM glycine in PBS for 5 min, dissolved in immunoprecipitation buffer (125 mM NaCl, 62.5 mM NaH2PO4, pH 7.2, 0.625% lubrol, and protease inhibitors), and incubated overnight with avidin-agarose. The agarose-bound complexes are precipitated and size-fractionated by SDS-PAGE, and proteins were identified by immunoblotting (12).
Immunocytochemistry.
Xenopus oocytes were injected with H2O or cRNAs coding for MUPP1 and Kir4.2 as indicated. On the second day after injection, the oocytes were fixed in 4% formaldehyde for 1 h followed by 100 mM glycine in PBS for 30 min. They were stored in 30% sucrose and frozen in liquid nitrogen overnight, after which 10-μm cryostat sections were cut. The sections were blocked in 10% donkey serum, 1% BSA, and 0.1% Tween 20 in PBS for 1 h. Oocytes stained with only the secondary antibody were served as controls. Rabbit polyclonal anti-Kir4.2 antibody was used at a dilution of 1:100 overnight, followed by incubation with a secondary anti-rabbit Cy3 antibody at a dilution of 1:1,000. Sections were observed using fluorescent microscopy (Zeiss Axiovert 200), and images were collected using the AxioVision program (Zeiss).
Preparation of Xenopus oocyte membranes.
Eight to ten oocytes were suspended in Tris buffer (5 mM Tris, pH 8, 1 mM EDTA, and 1 mM EGTA) and disrupted by passage through a 25-gauge needle. The suspension was centrifuged at 1,200 g. The lipids were removed from the top of the tube, and the fraction containing the membranes and cytosol was removed and centrifuged at 15,000 g to separate membrane and cytosolic fractions.
Electrophysiology.
Oocytes were harvested and prepared as previously described (41). Kir4.2 and Kir4.2ΔPDZ were subcloned into the oocyte expression vectors, pGEMSH. Linearized plasmids were used for in vitro cRNA synthesis. Forty-six nanoliters of H2O or cRNA mixtures were injected into stage VI oocytes (Kir4.2 at 5 ng and MUPP1 at either 2.5 or 10 ng). Oocytes were studied 1–5 days after cRNA injection. We voltage-clamped oocytes to 0 mV in a K+ Ringer (98 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, and 5 mM HEPES, pH 7.5). Since intracellular [K+] is 80–90 mM (Romero MF and Boron WF, unpublished observations), this high-K+ solution results in a slightly inward chemical gradient for K+ and a reversal potential near 0 mV. From 0 mV, oocytes were stepped by 20 mV from −120 to +80 mV using 75-ms voltage pulses. Current-voltage curves were generated by averaging the final 10 ms of each resulting current response as previously described (11, 41).
RESULTS
Interaction of MUPP1 with Kir4.2 in yeast.
Based on our finding that the CaR interacts with the inwardly rectifying K+ channel Kir4.2 and that the COOH terminus of Kir4.2 contains a class I PDZ binding domain, we screened an adult human kidney cDNA library using the yeast two-hybrid system to identify additional proteins that interact with the channel and that could form additional parts of a signaling complex controlled by the CaR. With the COOH-terminal 125 AA of Kir4.2 as bait, we identified two cDNAs that code for MUPP1 (multi-PDZ protein 1), a ubiquitously expressed protein that contains 13 tandem PDZ binding domains (Fig. 1) (51). We did not identify other PDZ domain-containing proteins in this screen of >4 × 106 cDNA clones. MUPP1 is 2,054 AA in length, with a molecular mass of ∼220 kDa (44, 51). Our clones were partial, beginning in the predicted seventh PDZ domain at AA 1154 and 1160 (nt 3506 and 3494) and extending through the COOH terminus (AA 2054, nt 6173) so that they are ∼900 AA in length (Fig. 1A) (51). Sequencing revealed that these clones are identical to the sequences in GenBank. The COOH-terminal 900 AA of MUPP1 include part of PDZ domain 7 and PDZ domains 8–13.
Fig. 1.
MUPP1 (multi-PDZ protein 1) and renal protein expression. Diagram shows the 2 cDNAs isolated in relation to the full-length MUPP1 sequence. The top scale shows the amino acid (AA) number (1 to 2054) for the full-length protein. The diagram of the protein shows the NH2 (N) and COOH (C) termini and the position of each of the PDZ domains (1–13). The bottom scale represents the length and position of the cDNAs isolated in relation to the full-length protein (AA 1154 or 1164 to 2054). The predicted molecular mass of MUPP1 is 220 kDa.
These results indicate that Kir4.2 interacts with PDZ domains 8–13, although it also could interact with PDZ domains 1–7, since these were not specifically tested. To identify other proteins with which MUPP1 could interact and characterize the selectivity of its interaction with Kir4.2, we tested the COOH-terminal 125 AA of Kir5.1, another K+ channel expressed in the distal nephron with a COOH-terminal PDZ sequence (ESQM), and the COOH-terminal 210 AA of the CaR that contain two potential internal PDZ sequences (RSNV AA 891–894 and QSTV AA 1060–1063) in the yeast two-hybrid assay (7). Clonal yeast strains (AH109) containing the cDNAs corresponding to the COOH termini of these proteins in the bait plasmid pAS2.1 were mated with the yeast strain Y187 containing the MUPP1 clone isolated from the cDNA library. The yeast from these matings were plated on quadruple dropout-selective medium, and interactions were identified by the yeast that survived. The COOH terminus of Kir4.2, but not that of Kir5.1 or the CaR, interacted with MUPP1, demonstrating selectivity of the interaction of Kir4.2 for MUPP1 (not shown).
Selective interaction of MUPP1 with Kir4.2 in mammalian cells.
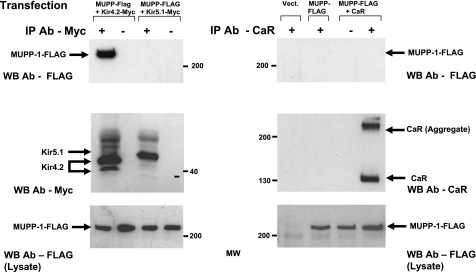
We obtained a full-length human MUPP1 cDNA with a FLAG epitope tag from Dr. David Clapham (23). We tested MUPP1 with Kir4.2 and Kir5.1, because both channels have COOH-terminal PDZ domains (Kir4.2 QSNV and Kir5.1 ESQM for both human and mouse sequences), and with the CaR, because it has potential internal PDZ sequences, in immunoprecipitation assays. We cotransfected Kir4.2-Myc, Kir5.1-Myc, or the CaR individually with MUPP1-FLAG into HEK-293 cells and processed them for immunoprecipitation. The samples containing Kir4.2 or Kir5.1 were immunoprecipitated with a polyclonal anti-Myc antibody, and the immunoprecipitates were immunoblotted with an anti-FLAG antibody to detect MUPP1-FLAG. The samples containing the CaR were immunoprecipitated with the anti-CaR antibody and then immunoblotted with the anti-FLAG antibody to recognize MUPP1. Figure 2 shows that Kir4.2 coimmunoprecipitated with MUPP1 but that Kir5.1 and the CaR did not. Figure 2 also shows that the immunoprecipitation antibodies in fact isolated their target proteins. These results demonstrate that MUPP1 interacts with Kir4.2 but not with Kir5.1 or the CaR in mammalian cells. We also tested NBCe1 (a sodium-dependent bicarbonate transporter) and WTIP (Wilms tumor-interacting protein), two other proteins with COOH-terminal PDZ domains, but they did not coimmunoprecipitate with MUPP1 (not shown), demonstrating that the interaction of Kir4.2 with MUPP1 is selective in mammalian cells.
Fig. 2.
Selective coimmunoprecipitation of Kir4.2 with MUPP1-FLAG coexpressed human embryonic kidney (HEK)-293 cells. HEK-293 cells were transiently cotransfected with MUPP1-FLAG and Kir4.2-Myc, Kir5.1-Myc, or the Ca2+-sensing receptor (CaR) as indicated. Immunoprecipitations (IP) were carried out with the anti-Myc or CaR antibodies (Ab) as indicated and then blotted with the anti-FLAG Ab to determine whether MUPP1-FLAG immunoprecipitated with any of the target proteins (top panels). Middle panels show IP of Kir4.2 and Kir5.1 with the anti-Myc Ab (left) or IP of CaR with the anti-CaR Ab (right). Bottom panels demonstrate that similar amounts of MUPP1-FLAG were expressed in all samples. Kir4.2 coimmunoprecipitated with MUPP1, but Kir5.1 and the CaR did not.
Figure 3 shows that the interaction of Kir4.2 with MUPP1 in reciprocal coimmunoprecipitation assays depends on the COOH-terminal four AA of the channel, its PDZ binding domain (QSNV). cDNAs coding for Myc-tagged wild-type Kir4.2 (Kir4.2-myc) and Myc-tagged Kir4.2 with deletion of the four COOH-terminal AA (Kir4.2ΔPDZ-Myc) were coexpressed in HEK-293 cells with MUPP1. The Kir4.2Δ-Myc construct did not coimmunoprecipitate with MUPP1 efficiently whether the anti-MUPP1 antibody or the anti-Myc antibody (directed at Kir4.2) was used. A small amount of Kir4.2ΔPDZ-Myc seen in the coimmunoprecipitation with the MUPP1 antibody may be due to interaction of MUPP1 with additional regions of the channel and the efficiency of immunoprecipitation of the expressed MUPP1-FLAG and the endogenous MUPP1 (52). Similar amounts of MUPP1 and Kir4.2-myc or Kir4.2ΔPDZ-Myc were immunoprecipitated in all lanes and extracts.
Fig. 3.
Dependence of the Kir4.2-MUPP1 interaction on the Kir4.2 COOH-terminal PDZ domain. HEK-293 cells were transiently cotransfected with vector (Vect) or MUPP1-FLAG with Kir4.2-Myc or Kir4.2ΔPDZ-Myc (Kir4.2-Δ-Myc) and immunoprecipitated with the anti-MUPP1 Ab (left panels) or the anti-Myc Ab (right panels) as indicated. The immunoprecipitates were immunoblotted (WB) with the anti-MUPP and anti-Myc Abs (top panels) to document successful IP. At bottom left, the immunoprecipitate was blotted with the anti-Myc Ab, and at bottom right, it was blotted with the anti-MUPP1 Ab. Reciprocal coimmunoprecipitation requires an intact COOH-terminal PDZ domain on Kir4.2.
To study MUPP1 in native tissues, we produced a rabbit polyclonal antibody to MUPP1 directed at a region between PDZ domains 5 and 6 (AA 780–793). This region is conserved in mouse, human, rat, dog, and cow proteins. Figure 4 shows that the antibody recognized a 220-kDa protein in extracts from kidney cortex and medulla and in HEK-293 cells (A) and in Xenopus oocytes (B). In the extracts from the mammalian cells, a predominant band was seen at ∼220 kDa, and a lower band at ∼130 kDa was also seen only in the samples from rat kidney. The majority of the signal in the lower band was blocked by incubation with peptide. The identity of the 130-kDa band is not certain, but it could represent either a splice variant or a proteolytic product. A band of this size in kidney has been reported by others (44). Similar findings were obtained with antiserum from a second rabbit (not shown).
Fig. 4.
Coimmunoprecipitation of MUPP1 and Kir4.2 from rat kidney cortex extracts and specificity of the antisera. A and B: specificity of MUPP1 Ab determined by peptide blocking. A: 10 μg of tissue or cell extract (lane 1, rat kidney cortex; lane 2, rat kidney medulla; lane 3, HEK-293 cells). B: 5 μg of Xenopus oocyte cytosol (lane 4) and membrane (lane 5). The filters were blotted the MUPP1 Ab (1:500) as indicated (−Pep) or with the MUPP1 Ab that had been preincubated with the antigenic peptide (+Pep; 50 μg/ml peptide). Molecular marker positions and the position of MUPP1 are shown at left for each set of panels. C: reciprocal coimmunoprecipitation of Kir4.2 and MUPP1 from kidney cortex extracts. In the left lanes (no IP), the IP was carried out with agarose beads but without an IP antibody; in the middle lanes (IP Kir4.2), duplicate samples were immunoprecipitated with the Kir4.2 Ab; and in the right lanes (IP MUPP2), duplicate samples were immunoprecipitated with the MUPP1 Ab. The top panel is from an 11% gel and was blotted with the anti-MUPP1 Ab; the bottom panel is from a 6% gel (necessary to separate Kir4.2 from the IgG heavy chain) and was blotted with the anti-Kir4.2 Ab. The Kir4.2 bands are more fuzzy than normal because of the 6% gel. The positions of MUPP1 and Kir4.2 were determined by size and the position of these proteins expressed in HEK-293 cells and run in separate lanes. D: specificity of the Kir4.2 Ab in rat kidney extracts determined using peptide blocking as described for the MUPP1 Ab.
MUPP1 and Kir4.2 coimmunoprecipitated reciprocally from rat kidney cortex extracts as shown in Fig. 4C. In the first lanes for both proteins, the immunoprecipitation was carried out with agarose beads but without attached immunoprecipitation antibody. The next two lanes show duplicate samples where the Kir4.2 antibody was used to immunoprecipitate proteins from the rat kidney extract, and in the last two duplicate lanes, the MUPP1 antibody was used. The top panel was immunoblotted with the anti-MUPP1 antibody, and the bottom panel was blotted with the Kir4.2 antibody. Both antibodies immunoprecipitated their targets, and the targets isolated their partners. As would be expected, the bands representing the immunoprecipitation target proteins, MUPP1 (top right) and Kir4.2 (bottom left) are denser than those representing the partner proteins [MUPP1 (top left) and Kir4.2 (bottom right)], because some proportion of the immunoprecipitation target proteins did not interact with the partners we were studying. Figure 4D shows that the anti-Kir4.2 antibody recognized a specific band in rat kidney extracts. Coexpression of MUPP1 and Kir4.2-Myc resulted in reduced cell surface expression of Kir4.2-Myc, as shown in Fig. 5. With increasing expression of MUPP1, the amount of Kir4.2 reaching the cell surface was progressively reduced. These results indicate that Kir4.2 interacts with MUPP1 in a PDZ-dependent manner, that the interaction of Kir4.2 with MUPP1 is selective, and that increased expression of MUPP1 leads to reduced levels of Kir4.2 at the cell surface.
Fig. 5.
Cell surface expression of Kir4.2 is reduced by coexpression with MUPP1. The first (top) panel shows summary data from 3 experiments as a percentage of Kir4.2 cell surface expression (surf. exp.) without MUPP1. The second panel shows representative results of cell surface expression of Kir4.2-Myc [Kir4.2 (cell surface)]. The third panel shows increasing expression of MUPP1-FLAG with increased transfected MUPP1-FLAG cDNA in the cell extracts (MUPP1-FLAG). The fourth (bottom) panel shows Kir4.2 expression in the cell extracts [Kir4.2 (extract)]. The level of Kir4.2 expression was similar in all extracts. HEK-293 cells were transiently cotransfected with 0.5 μg of Kir4.2-Myc cDNA and vector or MUPP1-FLAG cDNA in the amounts indicated (0, 0.5, 1.0, or 2.0 μg). After 48 h, the cells were incubated with the biotin reagent, extracts were prepared and normalized for protein content, and the cell surface proteins were isolated with avidin-agarose. The Myc Ab was used to identify Kir4.2 in the blots, and the FLAG Ab was used to identify MUPP-1. *P < 0.05, different from Kir4.2 alone (ANOVA). **P < 0.05, different from Kir4.2 with 0.5 μg MUPP1 cDNA (ANOVA).
Functional consequences of coexpression of MUPP1 and Kir4.2 channels in Xenopus oocytes.
To determine whether the interaction of Kir4.2 with MUPP1 affects inwardly rectifying K+ currents, we coexpressed the two proteins in Xenopus oocytes and measured whole cell currents. As shown in Fig. 6, A and B, increasing amounts of injected MUPP1 cRNA decreased whole cell currents. Injection of 5 ng of cRNA coding for Kir4.2 resulted in maximum K+ currents, ∼2.5 μA at −80 mV. Coinjection of 2.5 ng of MUPP1 cRNA resulted in an ∼60% reduction in current. In oocytes injected with 10 ng of cRNA, currents were reduced to a degree similar to those with 2.5 ng of MUPP1 cRNA. In individual experiments, the reduction appeared greater with 10 ng of MUPP1, but the difference was not statistically significant. Expression of Kir4.2ΔPDZ (failed to interact with MUPP1, see Fig. 3) resulted in lower currents than an equivalent amount of Kir4.2, indicating that the PDZ domain is important for channel function. However, in contrast to Kir4.2, expression of MUPP1 had no significant effect on currents attributable to Kir4.2ΔPDZ (Fig. 6, A and B). Figure 6C shows the level of Kir4.2 expression in oocyte membranes and the effects of MUPP1 coexpression under the conditions used for the electrophysiological studies. Increasing MUPP1 expression corresponded to reduced oocyte membrane expression of Kir4.2, consistent with the reduction in whole cell currents, as corroborated in Fig. 7. Kir4.2ΔQSNV is present in the oocyte membranes, and the level of MUPP1 expression did not affect its level of expression. The presence of Kir4.2ΔQSNV protein in the membrane fraction does not mean that it is functional or at the cell surface, because it may be present in a submembrane vesicle compartment (Kleyman TR and Frizzell RA, personal communication). These results clearly demonstrate that the effects of MUPP1 on localization and function of Kir4.2 require its PDZ domain. These results are corroborated by immunofluorescent staining of sections of Xenopus oocytes for Kir4.2 (Fig. 7) that show progressive loss of membrane staining for Kir4.2 with coexpression of increasing amounts of MUPP1. Oocyte sections were stained for either Kir4.2 or MUPP1. In the absence of MUPP1 injection, Kir4.2 is shown as bright staining at the membrane. With increasing MUPP1 injection, Kir4.2 staining at the membrane becomes less prominent. With injection of 10 ng of MUPP1 cRNA, membrane staining of Kir4.2 is difficult to see, consistent with the absence of Kir4.2 currents seen under the same conditions. The MUPP1 antibody recognized the endogenous protein (MUPP1; Fig. 4), but the staining also increased with increasing amounts of MUPP1 cRNA injected. These results suggest that under these experimental conditions, MUPP1 prevents Kir4.2 from reaching the cell surface in a form that it is functional.
Fig. 6.
Coexpression of Kir4.2 and MUPP1 leads to reduced whole cell currents in Xenopus oocytes. Xenopus oocytes were injected with H2O (control), Kir4.2 (5 ng), Kir4.2 (5 ng) and MUPP1 (2.5 ng), Kir4.2 (5 ng) and MUPP1 (10 ng), Kir4.2ΔQSNV (5 ng), or Kir4.2ΔQSNV (5 ng) and MUPP1 (10 ng). Electrophysiological studies were performed 2 days after injection. Two-electrode voltage clamp was used to measure whole oocyte currents. Oocyte membranes were voltage clamped to 0 mV and pulsed for 75 ms between −120 and +80 mV. A: current-voltage (I-Vm) plot of oocytes resulting from the basal currents of Kir4.2 with MUPP1 as shown. B: summary data showing Kir4.2 currents at −80 mV for each condition [Kir4.2, n = 8; Kir4.2 and MUPP1 (2.5 ng), n = 6; Kir4.2 5 and MUPP1 (10 ng), n = 5; Kir4.2ΔQSNV (5 ng), n = 5; or Kir4.2ΔQSNV and MUPP1 (10 ng), n = 3, where n indicates the number of oocytes studied and represents separate injections and studies on separate days]. The currents in the oocytes expressing Kir4.2 alone were statistically different from the other conditions, but the currents in the oocytes expressing Kir4.2 and MUPP1, Kir4.2ΔQSNV, and MUPP1 were statistically indistinguishable. C: expression of Kir4.2 or Kir4.2ΔPDZ in oocytes. Western blots were performed with 3 sets of pooled oocytes (n = 8–12) from separate injections. A representative Western blot for Kir4.2 is shown at top, and the bar graph (bottom) shows summary data for Kir4.2 expression (n = 3).
Fig. 7.
Coexpression of Kir4.2 and MUPP1 leads to reduced Kir4.2 expression at the cell membrane in Xenopus oocytes. Xenopus oocytes were injected with H2O (control) or Kir4.2 (5 ng), Kir4.2 (5 ng) and MUPP1 (2.5 ng), or Kir4.2 (5 ng) and MUPP1 (10 ng). Sections were processed for immunofluorescence microscopy 2 days after injection with the MUPP1 (top row) and Kir4.2 Ab (bottom row) indicated, both at dilutions of 1:100. Representative sections are shown at ×100 magnification. Scale bar, 100 μm.
DISCUSSION
Many membrane-bound transporters and ion channels contain COOH-terminal PDZ sequences that permit their localization and organization in specific plasma membrane domains by PDZ domain-containing proteins. In many cases, the interaction of a transporter or channel with a PDZ protein results in increased cell surface expression and activity (6, 15, 34, 42, 43, 45, 48, 56). In the kidney, the PDZ proteins responsible for basolateral distribution of K+ channels are not fully characterized. MALS/Veli/Lin-7b leads to basolateral localization of Kir2.3, and MAGI-1a interacts with the Kir4.1/Kir5.1 heterodimer in the distal nephron (25, 34, 39). In addition to Kir2.3, Kir4.1, and Kir5.1, both Kir4.2 and Kir7.1 are also localized in the distal nephron (8, 18, 31, 34, 36, 47). The precise distribution of Kir4.2 in the distal nephron is not defined due to lack of appropriate antibodies, although it is clearly present in the distal convoluted tubule based on RT-PCR of microssected tubules (31). The additional proteins with which it interacts and that localize it in the cell are not known.
On the basis of yeast two-hybrid cloning and co-immunoprecipitation studies, we found that Kir4.2 interacts selectively with MUPP1, a ubiquitously expressed PDZ protein. The interaction probably involves one or more of PDZ domains 8–13 but also could involve domains 1–7. In our screen of the human kidney cDNA library, we did not identify other PDZ domain-containing proteins, a result that does not prove that Kir4.2 cannot interact with other PDZ domain-containing proteins (it does interact with CIPP, a central nervous system-specific PDZ protein) but suggests that in the kidney, Kir4.2 may preferentially interact with MUPP1 (24). The ability to coimmunoprecipitate Kir4.1 and MUPP1 from kidney cortex extracts suggests that this interaction may take place in vivo, although the interaction in this setting could occur as a result of the homogenization and extraction procedure. The interaction is selective, because Kir5.1, NBCe1, and WTIP (a podocyte slit diaphragm protein), all proteins that contain COOH-terminal PDZ sequences, do not coimmunoprecipitate with MUPP1. Kir4.1 and Kir4.2 both form heterodimers with Kir5.1 (26, 38). Kir4.1, expressed on the basolateral membrane of the distal nephron, interacts with MAGI-1a. Through this interaction, Kir4.1 may interact with a unique set of additional proteins, although sequences within Kir4.1 and Kir5.1 are sufficient for heterodimerization (22, 25, 49). Through its interaction with MUPP1, Kir4.2 may interact with a different set of proteins from Kir4.1, may be subject to different modes of regulation, or may be found in different subdomains of epithelial cells. The interaction of Kir4.2 with MUPP1 affects membrane currents attributable to the channel through cell surface expression under the conditions of our studies. These results suggest that through interactions with MUPP1, Kir4.2 may contribute to the basolateral K+ conductance.
MUPP1 is a large (2054 AA, 220 kDa) protein that contains an NH2-terminal L27 motif and 13 PDZ domains that bind a number of proteins including other scaffolding proteins (PSD-95), viral oncoproteins, receptors, other signaling molecules, and tight junction proteins, particularly claudins (1, 4, 5, 9, 13, 21, 23, 32, 40). Presumably, MUPP1 organizes these proteins into specific complexes affecting cell surface expression and function. In the kidney, particularly the medulla, expression of MUPP1 RNA and protein are increased by hypertonicity. Reduction in MUPP expression is associated with reduced transepithelial resistance, indicating a role for it in tight junction function (27). MUPP1 is found in tight junctions, postsynaptic densities, and Schwann cell incisures (related to tight junctions) and associates with the plasma membrane (5, 13, 23, 29). In polarized Madin-Darby canine kidney and inner medullary collecting duct cells (IMCD), it is present not only in tight junctions but also the lateral borders of cells (13, 27, 28). MUPP1 has a similar domain structure and overlapping and distinct functions with another tight junction protein, Patj (1).
This pattern of MUPP1 expression is similar to that in initial descriptions of MAGI-1a, which has a tight junction and basolateral distribution and interacts with the Kir4.1-Kir5.1 heterodimer in the distal nephron (19, 49). MAGI-1 has a submembrane localization and requires additional proteins for tight junction localization, at least one of which is endothelial cell-selective adhesion molecule (ESAM) (19, 20, 54). MUPP1 appears to behave in a similar manner. We found that it is membrane associated but that the majority is cytosolic in HEK-293 cells (Huang C and Miller RT, unpublished results). In addition, in one situation, MUPP1 requires the coxsackievirus and adenovirus receptor (CAR) for membrane and tight junction localization, whereas in another, it requires claudin-4 (28). When CAR expression in Caco-2 cells was knocked down with small interfering RNA, MUPP1 was not membrane associated and was found in cytoplasmic aggregates (10, 53). Under some circumstances, Patj, a protein with similar structure and function, also requires additional proteins for tight junction association (1).
All the cells we studied, including Xenopus oocytes, have significant basal levels of MUPP1 that appear to permit cell surface expression of Kir4.2. Our finding that overexpression of MUPP1 in HEK-293 cells and Xenopus oocytes reduces Kir4.2 cell surface expression and Kir4.2 currents suggests that the expressed MUPP1 is binding the expressed Kir4.2 and preventing it from reaching the cell surface. This situation is different from that of other PDZ proteins including hLin7b, CIPP, PSD-93δ, and PSD-95 and their partner membrane proteins (15, 24, 30, 34). TIP-1, essentially a single PDZ domain, appears to function as a true scaffold antagonist (2).
One possibility to explain this unusual effect of MUPP1 is that additional proteins or cell structures required for cell surface localization are absent or present in insufficient quantities in the two cell types we studied, HEK-293 cells and Xenopus oocytes, so that coexpression of MUPP1 with Kir4.2 does not result in increased cell surface expression of the channel. HEK-293 cells and Xenopus oocytes could lack structures such as tight junctions that result in localization of MUPP1 in a manner that permits it to increase cell surface expression of proteins with which it interacts, such as Kir4.2. An additional possibility is that binding sites for MUPP1 at the plasma membrane are saturated with endogenous protein and that additional expressed MUPP1 with Kir4.2 cannot reach sites that result in functional cell surface expression of Kir4.2. In EpH4 cells, a mammary epithelial cell line, endogenous Patj and MUPP1 appear to require their NH2-terminal L27 domains for localization in tight junctions, because overexpression of these domains displaces the proteins. Overexpression of either MUPP1 or Patj protein can displace the other from tight junctions (1). These results suggest that a limited number of “binding sites” may be available for these two proteins in polarized epithelial cells and also, possibly, in nonpolarized cells. This observation by Adachi et al.(1) is compatible with our findings that overexpression of MUPP1 “removes” Kir4.2, possibly bound to endogenous or previously expressed MUPP1, from a cell location where the channel is active or blocks the channel's access to a site where it can be active so that in the absence of free MUPP1 binding sites, the channel does not reach the cell surface and is inactive.
In the setting of hypertonicity, when the level of expression of MUPP1 increases substantially (up to 10-fold in IMCD3 cells, perhaps analogous to our overexpression of the protein), it may displace preexisting MUPP1 or other proteins such as Patj from their sites in tight junctions or lateral cell borders (1, 27). In the case of Kir4.2, the result could be to reduce the K+ conductance of the lateral cell border, an effect that would be advantageous to a cell trying to preserve its intracellular solutes and volume.
Our data demonstrate that Kir4.2, a K+ channel that is expressed in the distal convoluted tubule and possibly other regions of the renal cortex, interacts selectively with MUPP1, a PDZ protein with 13 PDZ domains that is present in tight junctions and lateral cell borders in renal epithelial cells. MUPP1 is of great interest because it is the type of protein that could organize Kir4.2 with other transport and signaling proteins that contain COOH-terminal PDZ domains such as CaMKII, PLC, receptors, and proteins that regulate small GTP-binding proteins into a complex to permit coordinate and precise control of their activities. The identities of the additional proteins that bind MUPP1 in the kidney, particularly the distal convoluted tubule, where Kir4.2 is expressed, are not known. In addition, all of the factors that contribute to the cellular localization of MUPP1 remain to be identified. Despite the fact that the picture of how the CaR, Kir4.2, and MUPP1 interact is incomplete at this time, understanding how these proteins interact and identifying additional members of the complex will contribute to an understanding of the regulation of distal nephron ion transport.
GRANTS
This work was supported by Veterans Affairs Merit Review Awards (to R. T. Miller and C. Huang), National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK059985 (to R. T. Miller), R01 DK56218, and R01071239 (both to M. F. Romero), and funds from the Leonard Rosenberg Research Foundation.
Acknowledgments
Present address of A. Sindić: Dept. of Physiology, School of Medicine, Croatian Institute for Brain Research, University of Zagreb, Zagreb, Croatia.
REFERENCES
- 1.Adachi M, Hamazaki Y, Kobayashi Y, Itoh M, Tsukita S, Furuse M, Tsukita S. Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol Biol Cell 29: 2372–2389, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alewine C, Olsen O, Wade JB, Welling PA. TIP-1 has PDZ scaffold antagonist activity. Mol Biol Cell 17: 4200–4211, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the Ca-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem 276: 34871–34879, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold MUPP1 alters receptor stability and function. J Biol Chem 282: 4162–4171, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem 276: 12974–12982, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brone B, Eggermont J. PDZ proteins retain and regulate membrane transporters in polarized epithelial membranes. Am J Physiol Cell Physiol 288: C20–C29, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Cassamassima M, D'Amato MC, Pessia M, Tucker SJ. Identification of a heterotrimeric interaction that influences the rectification, gating, and pH sensitivity of Kir4.1/Kir5.1 potassium channels. J Biol Chem 278: 43533–43540, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Chung CH, Frese KK, Weiss RS, Prasad BVV, Javier RT. A new crucial protein interaction element that targets the adenovirus E4-ORF1 oncoprotein to membrane vesicles. J Virol 81: 4787–4797, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyne CB, Voelker T, Pichla SL, Bergelson JM. The coxsackievirus and adenovirus receptor interacts with the multi-PDZ domain protein1 (MUPP-1) within the tight junction. J Biol Chem 279: 48079–48084, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Flagg TP, Yoo D, Sciortino CM, Tate M, Romero MF, Welling PA. Molecular mechanisms of a COOH-terminal gating determinant in the ROMK channel revealed by a Bartter's disease mutation. J Physiol 544: 351–362, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol Renal Fluid Electrolyte Physiol 268: F285–F295, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. MultiPDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 277: 455–461, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hill CE, Briggs M, Lu JJ, Magtanong L. Cloning, expression, and localization of a rat hepatocyte inwardly rectifying potassium channel. Am J Physiol Gastrointest Liver Physiol 282: G233–G240, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. J Biol Chem 272: 12885–12888, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Huang C, Handlogten ME, Miller RT. Parallel activation of phosphoinositol 4-kinase and phospholipase C by the extracellular calcium-sensing receptor. J Biol Chem 277: 20293–20300, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Sindić A, Hill CE, Hujer KM, Chan KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF, Miller RT. Interaction of the Ca-sensing receptor with the inwardly-rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol 292: F1073–F1081, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by βγ. Nature 391: 803–806, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Ide N, Hata Y, Nishioka H, Hirao K, Yao I, Deguchi M, Mizoguchi A, Nishimori H, Tokino T, Nakamura Y, Takai Y. Localization of membrane-associated guanlylate kinase (MAGI)-1/BAI-associated protein (BAP) at tight junctions of epithelial cells. Oncogene 18: 7810–7815, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Ivanova S, Repnik U, Banks L, Turk V, Turk B. Cellular localization of MAGI-1 caspase cleavage products and their role in apoptosis. Biol Chem 388: 1195–1198, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kimber WA, Trinkle-Mulcahy L, Cheung PFC, Deak M, Marsden LJ, Keiloch A, Watt S, Javier RT, Gray A, Downes PC, Lucocq JM. Evidence that the tandem-pleckstrin-homology-domain-containing protein TAPP-1 interacts with Ptd(3,4)P2 and the multi-PDZ-domain-containing protein MUPP1 in vivo. Biochem J 361: 525–536, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konstas AA, Korbmacher C, Tucker SJ. Identification of domains that control the heteromeric assembly of Kir5.1/Kir4.0 potassium channels. Am J Physiol Cell Physiol 284: C910–C917, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron 43: 563–574, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kurschner C, Mermelstein PG, Holden WT, Surmeier DJ. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Mol Cell Neurosci 11: 161–172, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Lam HD, Lemay AM, Briggs MM, Yung M, Hill CE. Modulation of Kir4.2 rectification properties and pHi-sensitive run-down by association with Kir5.1. Biochim Biophys Acta 1758: 1837–1845, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lanaspa MA, Almeida NE, Andres-Hernando A, Rivard CJ, Capasso JM, Berl T. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidneys. Proc Natl Acad Sci USA 104: 13672–13677, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanaspa MA, Andres-Hernando A, Rivard CJ, Dai Y, Berl T. Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proc Natl Acad Sci USA 105: 157979–15802, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latorre IJ, Roh MH, Freese KK, Weiss RS, Margolis B, Javier R. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J Cell Sci 118: 4283–4293, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leyland ML, Dart C. An alternatively spliced isoform of PSD-93/chapsyn 110 binds to the inwardly rectifying potassium channel, Kir2.1. J Biol Chem 42: 43427–43436, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lourdel S, Paulais M, Cluzeaud F, Bens M, Masayuki T, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol 538: 391–404, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancini A, Koch A, Stefan M, Niemann H, Tamura T. The direct association of multiple PDZ domain containing proteins (MUPP-1) with the human c-Kit C-terminus is regulated by tyrosine kinase activity. FEBS Lett 482: 54–58, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Milewski MI, Lopez A, Jurkwoska M, LaRusch J, Cutting GR. PDZ-binding motifs are unable to ensure correct polarized protein distribution in the absence of additional localization signals. FEBS Lett 579: 483–487, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Olsen O, Liu H, Wade JB, Merot J, Welling PA. Basolateral membrane expression of the Kir2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex. Am J Physiol Cell Physiol 282: C183–C195, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Olsen O, Wade JB, Morin N, Bredt DS, Welling PA. Differential localization of mammalian Lin-7 (MALS/Veli) PDZ proteins and the kidney. Am J Physiol Renal Physiol 288: F345–F352, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Ookata K, Tojo A, Suzuki Y, Nakamura N, Kimura K, Wilcox CS, Hirose S. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Nephrol 11: 1987–1994, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Pearson WL, Dourado M, Schreiber M, Salkoff L, Nichols CG. Expression of a functional Kir4 family inward rectifier K channel from a gene cloned from mouse liver. J Physiol 514: 639–653, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pessia M, Imbrici P, D'Adamo Mc Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerization with Kir5.1. J Physiol 532: 359–367, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heterotrimeric inwardly rectifying K channels. EMBO J 15: 2980–2987, 1996. [PMC free article] [PubMed] [Google Scholar]
- 40.Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol 159: 361–371, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na-HCO3 cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Sheng M, Wyszynski M. Ion channel targeting in neurons. Bioessays 19: 847–853, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Shenolikar S, Voltz JW, Cunningham R, Weinman EJ. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology 19: 362–369, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Sitek B, Poschmann G, Schmidtke K, Ullmer C, Maskri L, Andriske CC, Zhu XR, Luebbert H. Expression of MUPP1 in mouse brain. Brain Res 970: 178–187, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Staub O, Rotin D. Regulation of ion transport by protein-protein interaction. Curr Opin Nephrol Hypertens 6: 447–454, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Tanemoto M, Abe T, Ito S. PDZ-binding and dihydrophobic motifs regulate distribution of Kir4.1 channels in renal cells. J Am Soc Nephrol 16: 2608–2614, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Tanemoto M, Abe T, Onogawa T, Ito S. PDZ binding motif-dependent localization of K channel on basolateral-side in distal tubules. Am J Physiol Renal Physiol 287: F1148–F1153, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Tanemoto M, Fujita A, Higashi K, Kurachi Y. PSD-95 mediates formation of a functional homomeric Kir5.1 channel in the brain. Neuron 34: 387–397, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Tanemoto M, Toyohara T, Abe T, Ito S. MAGI-1a functions as a scaffolding protein for the distal renal tubular basolateral K channels. J Biol Chem 283: 12241–12247, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Thiery E, Gosset P, Damotte D, Delezoide AL, de Saint-Sauveur N, Vayssetes C, Creau N. Developmentally regulated expression of the murine orthologue of the potassium channel Kir 4.2 (KCNJ15). Mech Dev 95: 313–316, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Ullmer C, Schmuck K, Figge A, Lübbert H. Cloning and characterization of MUPP1, a novel PDZ domain protein. FEBS Lett 424: 63–68, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Van de Pavert SA, Kantardzhieva A, Malysheva A, Meuleman J, Versteeg I, Levelt C, Klooster J, Geiger S, Seeliger MW, Rashbass P, Le Bivic A, Wijnholds J. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci 117: 4169–4177, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Vogelman R, Amieva MR, Falkow S, Nelson WJ. Breaking into the epithelial-junctional complex - news from pathogen hackers. Curr Opin Cell Biol 16: 86–93, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wegmann F, Ebnet K, Du pasquier L, Vestweber D, Butz S. Endothelial adhesion molecule EASM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res 300: 121–133, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Wu JV, Krowlewski AS, Rustagi A, Joo NS, Wine JJ. An inwardly rectifying potassium channel in apical membrane of Calu-3 cells. J Biol Chem 279: 46558–46565, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Yoo D, Flagg TP, Olsen O, Raghuram V, Foskett JK, Welling PA. Assembly and trafficking of a multiprotein ROMK (Kir1.1) channel complex by PDZ interaction. J Biol Chem 279: 6863–6873, 2005. [DOI] [PubMed] [Google Scholar]