Abstract
The RNA-binding protein human antigen R (HuR) participates in the posttranscriptional regulation of mRNAs bearing 3′ AU-rich and U-rich elements, which HuR can stabilize under conditions of cellular stress. Using the LLC-PK1 proximal tubule cell line model, we recently suggested a role for HuR in protecting kidney epithelia from injury during ischemic stress (Jeyaraj S, Dakhlallah D, Hill SR, Lee BS. J Biol Chem 280: 37957–37964, 2005; Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Am J Physiol Renal Physiol 291: F1255–F1263, 2006). Here, we have extended this work to show that small interfering RNA-mediated suppression of HuR in LLC-PK1 cells increased apoptosis during energy depletion, while overexpression of HuR diminished apoptosis. Suppression of HuR also resulted in diminished levels of key cell survival proteins such as Bcl-2 and Hsp70. Furthermore, rat kidneys were subjected in vivo to transient ischemia followed by varying periods of reperfusion. Ischemia and reperfusion (I/R) affected intensity and distribution of HuR in a nephron segment-specific manner. Cells of the proximal tubule, which are most sensitive to I/R injury, demonstrated a transient shift of HuR to the cytoplasm immediately following ischemia. Over a 14-day period following the onset of reperfusion, nuclear and total HuR protein gradually increased in cortical and medullary proximal tubules, but not in non-proximal tubule cells. HuR mRNA was expressed in two forms with alternate transcriptional start sites that increased over a 14-day I/R period, and in vitro studies suggest selective translatability of these two mRNAs. Baseline and I/R-stimulated levels of HuR mRNA did not parallel those of HuR protein, suggesting translational control of HuR expression, particularly in medullary proximal tubules. These findings suggest that alterations in distribution and expression of the antiaptotic protein HuR specifically in cells of the proximal tubule effect a protective mechanism during and following I/R injury in kidney.
Keywords: acute kidney injury, proximal tubule, RNA-binding proteins
renal ischemia-reperfusion (I/R) injury is a major cause of acute kidney failure, a clinical condition that despite decades of basic research and technical advances remains associated with high morbidity and mortality (12, 40). Thus understanding the molecular mechanisms of renal cell injury is of particular importance to the prevention or treatment of acute renal failure. Various cellular events contribute to the I/R injury, including reduced cellular ATP levels, damage to the cytoskeleton, generation of reactive oxygen species, accumulation of inflammatory cells, and other shifts in cellular activity (12, 36, 48). A number of recent studies have examined whether activation or inhibition of specific intracellular signaling pathways provides protection from I/R injury. It has been reported that preconditional activation of hypoxia-inducible factors (7), gene silencing of complement 3 and caspase 3 (51), and adenovirus-mediated Bcl-2 and Bcl- XL gene transfer (10, 11) ameliorate ischemic acute renal failure, whereas pretreatment with cyclooxygenase (COX)-2 inhibitor augments the degree of renal dysfunction and injury caused by I/R (38).
Our previous results using the proximal tubule cell line LLC-PK1 subjected to ATP depletion and recovery suggested a role for human antigen R (HuR) in protecting kidney epithelia from injury during ischemic stress (19, 20). HuR is a ubiquitously expressed member of the ELAV family of proteins, participating in posttranscriptional regulation of mRNAs bearing uridine-rich or adenine- and uridine-rich elements (AREs) in their 3′-untranslated sequences (27). Through identification of its targets, HuR has emerged as an important regulator in cell 3′-untranslated division, carcinogenesis, immune responsiveness, and the response to cellular stress (15, 26). The protective role of HuR during cellular stress is effected, at least partially, through its positive influence on the expression of antiapoptotic genes, as well as its negative effect on proapoptotic genes. HuR was shown to stabilize SIRT, a major antiapoptotic protein, the human ortholog of Sir2 (the stress-response and chromatin-silencing factor in S. cerevisae) (2). Prothymosin α (ProTα), an inhibitor of apoptosome formation, is regulated by HuR via elevation of its translation (23). More recently, two other HuR target mRNAs that encode antiapoptotic proteins, Bcl-2 and Mcl-1, have been identified (1). Available evidence indicates that HuR contributes to maintaining their elevated levels, although the precise mode of its action remains to be elucidated. HuR also modulates the expression of numerous proteins that indirectly prevent apoptosis by involvement in regulation of cell signaling (24, 31), cell division (47), and other functions. Under normal cellular growth conditions, HuR is localized predominantly in the nucleus, while stabilization and regulation of ARE-containing mRNA is associated with translocation of HuR to cytoplasm. In the nucleus, HuR has been postulated to participate in the processing of pre-mRNA (likely splicing and export), although these functions remain poorly understood. Recent data about the function of HuR as an alternative Fas pre-mRNA splicing regulator provide new insight about HuR as an antiapoptotic and survival factor (18).
In our previously published work on the LLC-PK1 proximal tubule cell model, energy depletion at the cellular level caused detectable net movement of HuR out of the nucleus, followed by net movement of HuR back into the nucleus on reversion to normal growth medium. Changes observed in HuR protein and mRNA levels during ATP depletion and recovery also suggested a role for HuR in preconditioning LLC-PK1 cells to multiple ischemic insults (20). To further explore HuR's function in protecting renal epithelia from stress, we examined the role of HuR expression in the protection of LLC-PK1 cells from apoptosis, as well as its expression and distribution in rat kidneys subjected to I/R injury.
MATERIALS AND METHODS
Antibodies.
A mouse monoclonal antibody against HuR was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), as was a rabbit polyclonal against Bcl-2. Mouse monoclonal antibodies against aquaporin-1 (AQ1) and β-actin were purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibodies against aquaporin 2 (AQ2), thiazide-sensitive NaCl cotransporter (TSC), and the chloride channel CLC-K were purchased from Chemicon International (Temecula, CA). A rabbit polyclonal antibody against Tamm-Horsfall glycoprotein (THP) was purchased from Biomedical Technologies (Stoughton, MA). A mouse monoclonal against Hsp70 was purchased from BD Biosciences (San Jose, CA).
Culture of LLC-PK1 cells and modulation of HuR levels.
LLC-PK1 cells (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium containing penicillin/streptomycin and supplemented with 10% fetal bovine serum at 37°C in 5% CO2. For ATP depletion experiments, when cells became confluent the culture medium was replenished and cells were incubated overnight. The next morning, cells were rinsed twice with phosphate-buffered saline and the culture medium was replaced with prewarmed Dulbecco's modified Eagle's medium base supplemented with l-glucose, sodium bicarbonate, and 0.1 μM antimycin A for 0–6 h. For small interfering RNA (siRNA)-mediated knockdown of HuR, LLC-PK1 cells were plated at 40–50% confluence on six-well plates and transfected transiently with a previously described HuR siRNA (19) of the following sequence: 5′-GGAGGAGUUACGAAGUCUGtt-3′ (sense); 5′-CAGACUUCGUAACUCCUCCtg-3′ (antisense). The HuR siRNA or a nontargeting control (Ambion, Austin, TX) was transfected at 50 nM using Lipofectamine with Plus reagent (Invitrogen, Carlsbad, CA). For overexpression of a FLAG-tagged HuR cDNA, addition of FLAG to the COOH-terminal end of the full-length murine HuR coding region was performed using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's recommendations. This cDNA, subcloned in expression vector pcDNA3.1 (Invitrogen), or pcDNA3.1 alone as a control, was stably transfected into LLC-PK1 cells. Transfections were performed using Lipofectamine and Plus reagent (Invitrogen). Forty-eight hours posttransfection, cells were treated with 800 μg/ml geneticin (Invitrogen); following expansion of cells, individual colonies were selected and Flag-tagged HuR expression was proved by Western blotting and immunocytochemical analysis, using an anti-Flag M2 antibody (Sigma-Aldrich, St. Louis, MO).
Apoptosis assays.
For caspase assays of siRNA-treated cells, 5 × 103 LLC-PK1 cells were seeded in 96-well plates, grown to confluence, and transfected with HuR or control siRNAs, as above. Cells were subjected to ATP depletion 48 h posttransfection and assayed for relative caspase 3/7 activity using the Caspase Glo 3/7 Assay kit (Promega, Madison, WI). HuR-overexpressing cells or controls were seeded at 12 × 103/well, grown to confluence, subjected to ATP depletion, and assayed for caspase 3/7 activity using the same kit. Each treatment was performed in triplicate, and three such independent experiments were performed. For enumeration of apoptotic nuclei, siRNA-treated, HuR-overexpressing, or control cells were cultured on glass coverslips in 24-well plates. Following ATP depletion, cells were permeabilized, fixed with 4% paraformaldehyde, and stained with Hoechst stain (1 μg/μl). Samples were examined under a Nikon Eclipse 80i epifluorescent microscope. Condensed or fragmented nuclei were scored as apoptotic, and three similar fields of cells were enumerated for each time point.
I/R injury of rats.
Care of the rats before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols had received prior approval by the Institutional Animal Care and Use Committee at Indiana University. Male Sprague-Dawley rats (∼250 g) were housed in pairs in standard shoe-box cages with a 12:12-h light-dark cycle. Animals were acclimated to standard laboratory chow (Teklad 2018S, Harlan). Food and water were available ad libitum. To induce acute renal failure, rats were anesthetized with ketamine (100 mg/kg ip) and pentobarbital sodium (25 mg/kg ip) and placed on a heated surgical table. Following a midline incision, the blood supply to the kidneys was interrupted by applying microvascular clamps on the renal pedicles of both kidneys for a period of 30 min (42). One group of animals was killed immediately following the ischemic period, while in other groups, clamps were removed to allow reperfusion and recovery for 1, 6, or 24 h or 14 days. To demonstrate preconditioning against multiple insults, one group of rats was subjected to I/R treatment for 14 days before a second treatment for 24 h.
Measurement of renal function.
For measurement of serum creatinine, tail blood samples (0.5 ml) were collected into heparinized tubes and plasma was obtained by centrifugation. Serum creatinine values were determined using a Beckman Creatinine II analyzer (Beckman Coulter, Fullerton, CA). At the indicated times, rats were anesthetized with ketamine-HCl (60 mg/kg), xylazine (6 mg/kg), and acepromazine maleate (0.9 mg/kg). The kidneys were quickly removed and cut longitudinally; representative kidney pieces were fixed by immersion in 10% formalin and processed in paraffin for histological analysis. Other pieces were separated into renal cortex and medulla and were snap-frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analysis.
Immunohistochemistry.
Paraffin-embedded sections (5-μm thick) were heated at 65°C for 1 h. After deparaffinization in xylene and rehydration through an ethanol series, then H2O, slides were placed in 10 mM citric buffer (pH 6.0, 0.05% Tween 20) and microwaved three times for 3 min each at 3-min intervals to unmask antigen epitopes. After microwaving, slides were allowed to cool for 20 min at room temperature. For detection of THP, antigen retrieval was performed by treatment with proteinase K (Dako, Carpinteria, CA) for 5 min at room temperature. Endogenous peroxidase activity was quenched by treatment with 3% hydrogen peroxide in absolute methanol for 30 min. Slides then were washed in PBS containing 0.05% Triton X-100 and incubated in normal blocking serum (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) for 30 min. After washing in PBS, slides were incubated with the primary antibodies. Tissue sections again were washed in PBS with 0.05% Triton X-100, and immunostaining was performed using a species-specific biotinylated secondary antibody, avidin/biotinylated horseradish peroxidase complex (Vectastain Elite ABC kit), and substrate-chromogen mixture (Vectastain Elite ABC kit). Immunoreactivity was visualized using Vector VIP (purple color) and Vector DAB (brown color) peroxidase substrates as chromogens. After a washing with distilled water, the sections were dehydrated in a graded series of ethanol followed by xylene and mounted with VectaMount permanent mounting media (Vector Laboratories).
For single labeling with antibodies against HuR (1:500 dilution), AQ1 (1:500 dilution), AQ2 (1:250 dilution), or TSC (1:250 dilution), sections were incubated overnight at 4°C. Antibodies against CLC-K1 (1:250 dilution) and THP (1:500 dilution) were applied for 1 h at room temperature. All antibodies for immunohistochemistry were diluted in PBS. DAB substrate was used as the chromogen for the single labeling.
Double staining was performed with antibodies against nephron segment-specific markers (AQ1, AQ2, TSC, THP, CLC-K1) and antibodies against HuR. First, sections were incubated with nephron segment-specific markers, as described for the single staining, and developed with Vector VIP peroxidase substrate to give a purple color. Sections were then incubated in 2% hydrogen peroxide in absolute methanol to remove any remaining peroxidase from the first staining. After avidin/biotin blocking for 30 min, each followed by a wash in PBS, sections were incubated overnight at 4°C with antibodies against HuR diluted as described above. The second staining was developed with Vector DAB peroxidase substrate to give a brown color. As a negative control, rat kidney sections were probed with antibody against an irrelevant epitope (V5 epitope, Invitrogen), and no staining was seen (data not shown). The intensity of nuclear staining was quantified using ImageJ software (39).
Western blot analysis.
Protein extracts from frozen kidneys were homogenized in 15 vol of 50 mM Tris·HCl, pH 8.0, containing 10% DMSO, 1 mM PMSF, 0.5 mM DTT, and 1 μM leupeptin. After centrifugation at 10,000 rpm for 5 min, the supernatant protein content was determined using a BCA protein assay kit (Pierce, Rockford, IL). LLC-PK1 cell lysates were prepared by incubating cells in MPER with protease and phosphatase inhibitor cocktails (Pierce). For Western blot analysis, proteins were separated by 12.5% SDS-PAGE (Bio-Rad, Hercules, CA) and transferred onto Hybond-P membrane (Amersham Biosciences, Piscataway, NJ). The membranes were blocked with 3% milk for 1 h and incubated with primary antibodies diluted in Blotto. Anti-mouse horseradish peroxidase-conjugated antibody and SuperSignal West Pico Chemiluminescent substrate (Pierce) were used for detection. For rat kidney Western blots, after stripping with 0.2 M NaOH for 5 min, membranes were stained in Ponceau S (Sigma-Aldrich) to verify equal loading. Quantitation of Western blots was performed using the Chemi-Doc image-analysis system with Quantity One software (Bio-Rad). Statistical analysis was performed using Student's t-test.
In situ hybridization.
A 300-base pair cDNA fragment of rat HuR cDNA from position 504 to 804 was generated by RT-PCR from rat whole kidney and cloned into the pCRII-TOPO vector (Invitrogen). The resulting construct, pCRII-TOPO-rHuR, was linearized by XhoI digestion and served as a DNA template to transcribe a digoxigenin (DIG)-labeled antisense probe with SP6 RNA polymerase, using DIG RNA Labeling MIX (Roche Applied Science, Indianapolis, IN) and a MAXIscript SP6 Kit (Ambion). The region of sequence that was used to produce the HuR riboprobe did not show significant homology to any known rat sequences.
In situ hybridization was performed on 5-μm sections using an IsHyb In Situ Hybridization (ISH) Kit (BioChain Institute, Hayward, CA) according to instructions from the manufacturer with modifications. Dewaxing, rehydration, and antigen retrieval of sections were done as described for immunohistochemistry. Tissues were then incubated in diethyl pyrocarbonate (DEPC)-treated PBS and fixed in 4% paraformaldehyde in PBS for 20 min. After being rinsed twice with PBS, the slides were acetylated in 0.1 M triethanolamine-HCl buffer (pH 8.0) with 0.25% acetic anhydride for 10 min. Slides were washed in DEPC-PBS, rinsed with DEPC-H2O, and prehybridized with ready-to-use prehybridization solution (BioChain Institute) for 3 h at 50°C. The DIG-labeled probe (0.15 μg/μl) was diluted in ready-to-use hybridization buffer (BioChain Institute) to 20 ng/μl and applied to the prehybridized tissues. Sections then were incubated at 80°C for 2 min and hybridized at 48°C for 18 h. Posthybridization washing and immunological detection, using anti-DIG-alkaline phosphatase with NBT/BCIP as substrates were performed exactly as recommended by the manufacturer. AP-conjugated anti-DIG antibodies (1:100 PBS diluted, BioChain Institute), were incubated with slides for 2 h. After staining was developed, the reaction was stopped by incubation of slides in 10 mM Tris buffer, pH 8.0, 1 mM EDTA. Finally, slides were rinsed in distilled H2O and mounted in AquaPerm mounting medium (ThermoScientific, Waltham, MA). To test for any nonspecific hybridization, adjacent sections were incubated in a mixture of labeled antisense probe with excess (×100) unlabeled antisense probe. As a further control, the antisense probe was assessed in sections treated with RNAse A (Sigma-Aldrich). In both cases, no visible specific signal was observed (data not shown).
RNase protection assay.
An RNase protection assay (RPA) was performed by using the RPA III kit (Ambion) according to the manufacturer's specifications. For RPA of rat HuR, a 32P-labeled probe was generated that consisted of 512 bases of 5′ untranslated region (UTR) and upstream sequences and 60 bases of the coding region. Total RNA from frozen kidney was isolated and reverse transcribed using a Super Script first-strand synthesis system (Invitrogen). The resulting cDNA was subjected to PCR with the following primers: 5′-ACTTCGGAAGTGAGCGGCAG-3′ (sense) and 5′-CGTTCTCCCAATGTCATCCCTG-3′ (antisense). The resulting PCR product was cloned into the pCR4-TOPO vector (Invitrogen), and the construct was used as a DNA template for in vitro transcription after linearization with NcoI. In vitro transcription of the labeled riboprobe was performed with 40 μCi [32P]UTP (800 Ci/mmol), 15 μM UTP, and T3 RNA polymerase using a Maxiscript kit (Ambion). Transcription was terminated by adding DNase I to the reaction, and labeled RNA was purified by loading the sample onto a 5% acrylamide-urea gel. The labeled full-length RNA probe was excised and eluted from the gel as specified by the manufacturer. The HuR-specific probe (4 × 104 cpm) was mixed with 20 μg of total rat RNA in the presence of the hybridization buffer, heat denatured, and hybridized for 16 h at 56°C. As a control, the HuR-specific probe was incubated with 20 μg of yeast tRNA. Following digestion with RNAses A and T1, protected double-stranded RNA was precipitated, harvested by centrifugation, and run in a 5% polyacrylamide-urea gel. Protected RNA fragments were visualized by autoradiography and quantified using the Chemi-Doc image-analysis system with Quantity One software (Bio-Rad).
In vitro transcription/translation.
In vitro transcription of uncapped and capped RNA was performed using the large-scale T7 MEGAscript and mMESSAGE mMACHINE transcription kits (Ambion), respectively, according to the supplier's instructions. DNA templates were generated by PCR amplification of total rat RNA extracted from rat kidney tissues as described above. Accuracy of PCR amplification was verified by sequencing. One microgram of each RNA transcript, purified by a MEGAclear purification kit (Ambion), was translated using a Retic Lysate IVT translation kit (Ambion) in the presence of [35S]-methionine according to the manufacturer's recommendations. Translation products were separated by 12.5% SDS-PAGE (precast gels, Bio-Rad) and visualized by autoradiography.
RESULTS
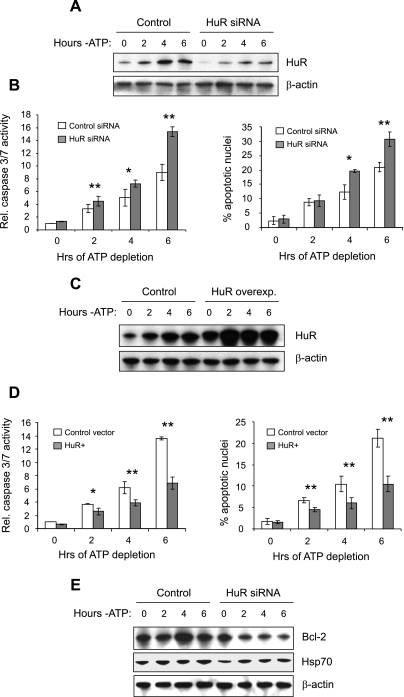
HuR is generally considered to prevent apoptosis in cells undergoing stress, although under some circumstances, a proapoptotic activity has been noted (29). We previously demonstrated that HuR expression is upregulated by independent translational and transcriptional mechanisms during ATP depletion and recovery in a proximal tubule cell line, suggesting a protective effect in renal epithelia (20). To determine whether expression of HuR is protective of renal cells undergoing stress, we knocked down or overexpressed HuR in LLC-PK1 cells undergoing ATP depletion. ATP-depleted LLC-PK1 cells mimic alterations found in ischemic kidney proximal tubule cells, including disruption of the actin cytoskeleton (9) and activation of heat shock proteins (30) and protein kinase pathways (37). We previously demonstrated the ability to use siRNA technology to suppress HuR in LLC-PK1 cells under normal growth conditions (19), and Fig. 1A demonstrates that knockdown was similarly efficient in cells undergoing ATP depletion. Measurement of caspase 3/7 activation or quantitation of condensed/fragmented nuclei demonstrated that ATP depletion of control cells resulted in increasing levels of apoptosis over a 6-h period (Fig. 1B). Suppression of HuR expression resulted in even greater apoptosis during this period. Similar results were obtained using the human proximal tubule cell line HK-2 (data not shown). Conversely, overexpression of HuR (Fig. 1C) showed that increased HuR levels were strongly protective against apoptosis during ATP depletion (Fig. 1D). Although only one HuR-overexpressing clonal line is shown here, a second clonal line produced very similar results (data not shown). We then wished to determine whether HuR might play a role in modulating expression levels of proteins known to play protective roles in in vivo and in vitro models of renal ischemia. The expression of Bcl-2, which is upregulated in regenerating proximal tubule cells and is protective against renal ischemic injury (6, 43), was previously shown to be modulated by HuR (1). As demonstrated in Fig. 1E, siRNA-mediated suppression of HuR resulted in diminished Bcl-2 expression during ATP depletion in LLC-PK1 cells. In addition, suppression of HuR mildly diminished expression of Hsp70, a heat shock protein known to be upregulated primarily in proximal tubule cells by renal ischemia and involved in a cellular-protective response (5, 41, 50). This is consistent with other studies demonstrating the Hsp70 mRNA to be a target of HuR binding (4, 27). Thus HuR appears to play a protective role in energy-depleted renal epithelia in vitro, leading us to propose that it will have similar functions in vivo.
Fig. 1.
Antiapoptotic activity of human antigen R (HuR) in a proximal tubule cell line. Knockdown of HuR (average knockdown of ∼75%) in LLC-PK1 cells with a specific small interfering (si)RNA (A) resulted in increased caspase 3/7 activity and condensation/fragmentation of nuclei (B). Conversely, overexpression of FLAG-tagged HuR by 2- to 3-fold (C) produced diminished caspase 3/7 activity and fewer apoptotic nuclei (D). A FLAG-tagged version of HuR was used to confirm that the exogenously expressed protein underwent appropriate nucleocytoplasmic trafficking and localization. In E, LLC-PK1 cells with suppressed HuR levels showed diminished expression of Bcl-2 and Hsp70. In B and D, values are means ± SD; n = 3. β-Actin was included as a loading control in A, C, and E. *P < 0.05. **P < 0.01.
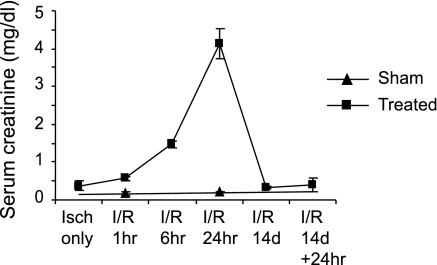
We previously demonstrated that HuR expression increases by independent transcriptional and translation mechanisms during ATP depletion and recovery in LLC-PK1 cells (20). To determine how HuR levels are modulated in native kidneys subjected to I/R injury, rats were sham-operated or subjected to 30 min of renal ischemia followed by reperfusion for 0, 1, 6, or 24 h or 14 days. At least three animals were prepared for each time point. The mean serum creatinine value at 24 h post-reperfusion was 4.14 mg/dl, indicating evidence of injury (Fig. 2). In animals that were allowed to recover for 14 days, serum creatinine values returned to sham-operated control levels (0.3 mg/dl), indicating recovery. These animals also showed evidence of preconditioning, since a second 24-h I/R treatment did not significantly increase serum creatinine levels (Fig. 2) or cause extensive damage to proximal tubules as seen in histological sections (data not shown).
Fig. 2.
Serum creatinine levels of animals used in this study. Serum creatinine levels of ischemia-reperfusion (I/R)-injured rats were measured as described in materials and methods. At least 3 animals were used for each group. Values are means ± SD.
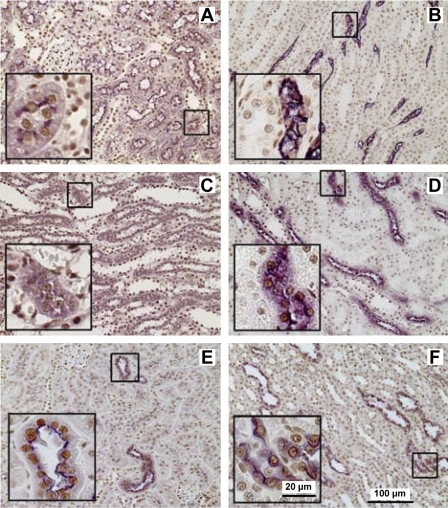
We evaluated expression and distribution of HuR protein in kidneys from sham-operated rats by double immunostaining of paraffin-embedded sections for HuR- and nephron segment-specific markers (Fig. 3). AQ1 was used as a marker of proximal tubules in the cortex (Fig. 3A) and the thin descending limb of Henle in the medulla (Fig. 3B) (3). The thin ascending limb of Henle (Fig. 3C) was distinguished as ClC-K1-positive cells in the medulla (44). Although the anti-ClC-K antibody we used recognized both ClC-K1 and ClC-K2, in the medulla only the thin ascending limb of Henle is known to express ClC-K1 (49). The thick ascending limb of Henle (Fig. 3D) was identified by staining with anti-THP antibodies (17). TSC-positive cells (Fig. 3E) were characteristic of distal convoluted tubules in the cortex (34). AQ2 (Fig. 3F) was used as a marker of the collecting duct (13). As expected, in sham-operated kidneys, HuR staining was predominantly nuclear along the length of the nephron.
Fig. 3.
Immunolocalization of HuR in kidney sections of sham-operated rats. Using double label immunohistochemistry, HuR (shown in brown) nuclear localization was observed in all renal tubules identified by segment-specific markers (shown in purple). The proximal tubules and the thin descending limbs of Henle were identified as immunoreactive cells for aquaporin-1 (AQ1) in the cortex (A) and medulla (B), respectively. The thin ascending limb of Henle (C) was distinguished in the medulla by the presence of chloride channel-K1 (CLC-K1). The thick ascending limb was identified as Tamm-Horsfall glycoprotein (THP)-positive cells in the medulla (D). The thiazide-sensitive Na+-Cl+ transporter (TSC) was used as a marker of distal convoluted tubules in the cortex (E). Aquaporin-2 (AQ2) antibodies were used to visualize collecting tubules (F). Insets: marker-positive cells in higher magnification.
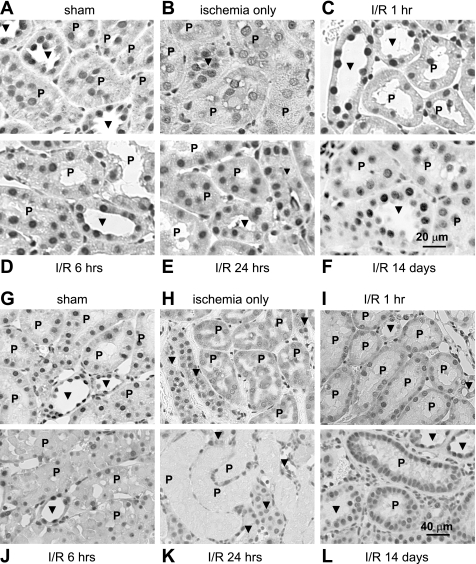
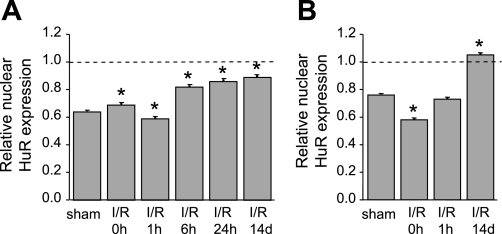
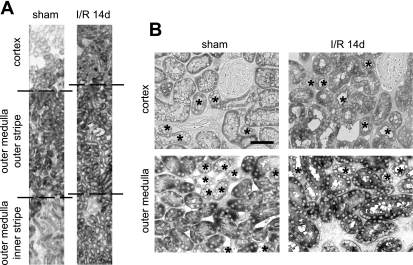
Figure 4 illustrates our examination of HuR expression and distribution in both cortical and outer medullary nephron segments subjected to sham operation or I/R injury. Analysis of adjacent paraffin-embedded sections stained with HuR- and nephron segment-specific markers was performed, although only HuR labeling is shown here. Initial observations of sham-operated kidneys showed that in both the cortex (Fig. 4A) and outer medulla (Fig. 4G), nuclear HuR staining of proximal tubule cells (indicated by “P”) was less robust than that of non-proximal tubule cells (indicated by arrowheads). These differences were quantified and expressed graphically in Fig. 5, A and B, which shows that cortical proximal tubule cells expressed ∼60% of the HuR compared with non-proximal tubule cells, with outer medullary proximal tubule cells expressing a slightly higher level. Furthermore, ischemic injury appeared to affect the intensity and distribution of HuR immunoreactivity primarily in proximal tubules. In the cortex, ischemia induced partial loss of HuR staining from the nuclei of proximal tubule cells (Fig. 4B). Based on other stress models of HuR distribution, including those in ATP-depleted LLC-PK1 cells (19, 20), this likely was due to transient redistribution of HuR to the cytosol; however, this translocation was not strong enough to produce an obvious increase in cytosolic HuR. After 1 h of reperfusion, HuR staining in the nuclei of cortical proximal tubule cells was recovered (Fig. 4C). During a further course of reperfusion (6–24 h), HuR immunoreactivity of cortical proximal tubules increased gradually and by day 14 became comparable to the level of HuR protein in other cortical segments (Fig. 4, D–F). Graphical representation of these changes in expression is shown in Fig. 5A.
Fig. 4.
I/R injury induced changes in HuR protein expression and distribution in proximal tubules. Kidney sections from rats subjected to sham operation, ischemia only, and I/R injury for 1, 6, or 24 h or 14 days were stained with anti-HuR antibodies. Proximal tubules (P) were identified by staining of adjacent sections with antibodies against AQ1 (not shown). Arrowheads indicate non-proximal tubules. A–F: sections from the renal cortex. G–L: sections from the outer medulla.
Fig. 5.
Relative increase in levels of HuR in nuclei of proximal tubules following I/R injury. The intensity of HuR staining in nuclei of proximal and non-proximal tubules in kidney sections was measured using image-analysis software, and values were normalized as the ratio of nuclear HuR staining in proximal vs. non-proximal tubules. A: cortex. B: medulla. The level of HuR immunoreactivity in nuclei of non-proximal tubules was assigned a value of 1. At least 50 nuclei were assessed for each group. Values are means ± SE. *Statistically significant differences from sham-operated control (P < 0.01).
Ischemia induced obvious cytosolic HuR staining in the pars recta (S3) segment of proximal tubules, which descends into the outer medulla. Immediately after ischemia, the HuR signal was distributed almost equally between cytoplasm and nuclei (Fig. 4H), indicating translocation of HuR out of the nucleus. After 1 h of reperfusion, HuR became relatively less abundant in cytoplasm and relatively increased in the nucleus (Fig. 4I). Evaluation of HuR expression and distribution at 6–24 h was limited due to intense damage of proximal tubules at these time points (Fig. 4. J and K). However, the outer medulla of 14-day postischemic rats was characterized by intense HuR nuclear staining in newly regenerated proximal tubules (Fig. 4L). These changes are represented graphically in Fig. 5B. No change in staining pattern or intensity was observed in cells of the inner medulla (data not shown). These data indicate that of all kidney tubule epithelia, cells of the proximal tubule effect the greatest changes in HuR distribution and expression, consistent with their highest sensitivity to injury from ischemia and reperfusion.
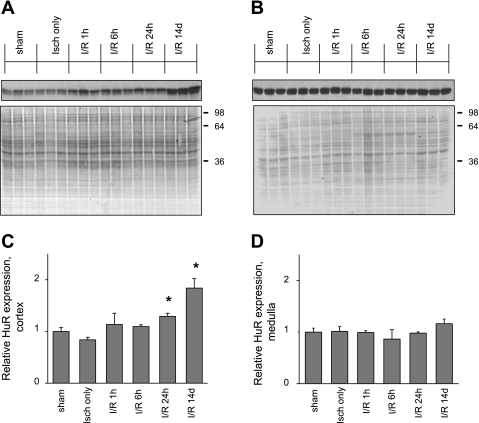
As further verification of these results, renal protein extracts from sham-operated and I/R-injured (ischemia only, 1, 6, or 24 h, or 14 days of reperfusion) rats were subjected to Western blot analysis. The amount of HuR protein in cortical fractions from sham-operated rats was noticeably less than that in the medullary preparation, consistent with the weak HuR staining seen in proximal tubule cells [compare Fig. 6, A (cortex) and B (medulla)]. HuR levels in the total medulla, where proximal tubule cells are a small minority of the total, were not significantly changed in the course of reperfusion (Fig. 6B). In contrast, in the cortex, elevation of signal was observed, and by day 14 of reperfusion HuR levels in the cortex and medulla became comparable (Fig. 6, A and B). This finding is also consistent with the increased intensity of HuR staining noted in proximal tubule cells. The changes in HuR protein levels in the cortex and medulla are represented graphically in Fig. 6, C and D, respectively.
Fig. 6.
Expression of HuR protein in renal cortex and medulla following I/R injury. Fifteen micrograms of total protein from kidney cortex (A) or whole medulla (B) homogenates were assayed with anti-HuR antibody by Western blot analysis. Shown are homogenates obtained from 3 sham-operated and from 3 postischemic rats at indicated times post-I/R injury. Ponceau S staining of nitrocellulose membranes of corresponding blots was used as a loading control. C and D: data in A and B were quantified and charted. Values are means of HuR expression level ± SE. *Statistically significant differences from sham-operated control (P < 0.05).
To examine segment-specific expression of HuR mRNA, we performed in situ hybridization on kidney sections from sham-operated rats and 14-day I/R injured rats with a specific antisense riboprobe corresponding to HuR. Figure 7A demonstrates that in sham-operated animals, intense mRNA labeling was present only in the outer stripe of the outer medulla, where S3 segments of the proximal tubule are located. The cortex, inner stripe of the outer medulla (Fig. 7A), and inner medulla (not shown) expressed lower levels of HuR mRNA. This is in contrast to the immunolocalization of HuR (Fig. 4), in which HuR protein of medullary proximal tubule cells was not notably higher than that of cortical proximal tubule cells. However, after 14 days of I/R injury, HuR mRNA levels increased in all parts of the nephron, including non-proximal tubule cells (Fig. 7A). Figure 7B shows that in the cortex, proximal tubule and non-proximal tubule cells (designated by asterisks) showed comparable levels of HuR mRNA in sham-operated animals, and all types of cortical cells increased their expression of HuR mRNA proportionately after I/R injury. In the outer medulla, however, proximal tubule cells expressed greater levels of mRNA than non-proximal tubule cells, and both appeared to express more mRNA after I/R injury. Additionally, cells of the inner medulla were found to increase their HuR mRNA expression after I/R (not shown). This finding is somewhat at odds with the expression of HuR protein levels, which increased most abundantly in proximal tubule cells in both the cortex and medulla. While the discrepancies between HuR protein and mRNA levels may at first seem counterintuitive, we and others have shown that levels of HuR protein and mRNA are often decoupled (20, 33, 35), and we have hypothesized that in proximal tubule cells, HuR expression may be under strong translational control (20). Indeed, binding of our riboprobe within proximal tubule cells required harsher denaturation of the tissue sections than did binding of the riboprobe within non-proximal tubule cells (data not shown), suggesting that the RNA may be bound in ribonucleoprotein complexes that modulate translational capacity.
Fig. 7.
In situ hybridization of kidney sections with rat-specific HuR probe. A: low-magnification images of sham-operated and 14-day I/R animals show changes in expression of HuR mRNA in the cortex and outer medulla. B: higher magnification images illustrate relative HuR mRNA expression levels in proximal tubule and non-proximal tubule cells (*). Scale bar = 50 μm.
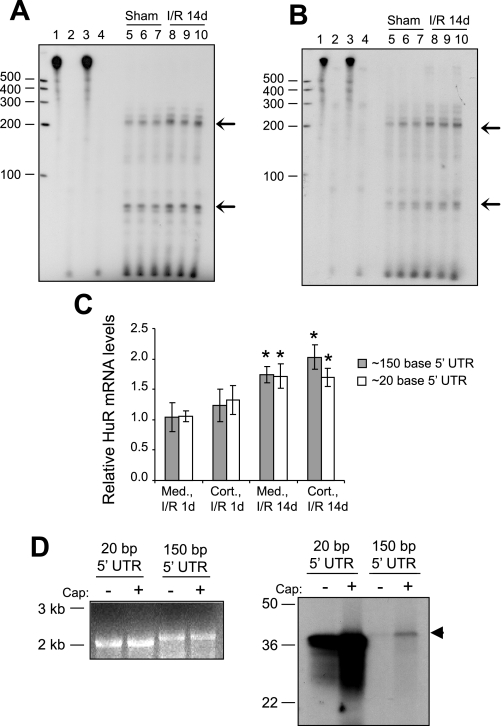
We then performed ribonuclease protection assays to examine overall HuR mRNA levels in cortical and total medullary fractions. Previous studies indicated that HuR mRNA is expressed in multiple forms with alternative transcriptional initiation sites (21). To detect any alternative forms present in rat kidney, a 572-base RNA probe homologous to rat HuR upstream sequences was allowed to hybridize with total mRNA from sham-operated and 14-day I/R-treated animals. Figure 8, A and B, shows that in rat kidneys two HuR mRNA forms are present that differ in the length of their 5′ UTRs. These forms, corresponding to mRNAs with 5′ UTRs of ∼150 and ∼20 bases in length are present in both cortical and medullary fractions. Ischemia, followed by 14 days of reperfusion, resulted in a parallel increase in both HuR mRNA transcripts in both the cortex and medulla. These increases are shown graphically in Fig. 8C, along with data from 1 day-I/R-treated animals. These findings are consistent with those in Fig. 7 in demonstrating increased HuR mRNA levels in both the cortex and medulla following I/R injury.
Fig. 8.
Expression of alternate transcripts of HuR in rat kidney. RNAse protection assays were performed to quantify changes in levels of HuR mRNA in renal cortex (A) and whole medulla (B) following I/R injury. Lane 1, probe only; lane 2, probe only digested by RNAse; lane 3, yeast tRNA+probe; lane 4, yeast tRNA+probe digested by RNAse; lanes 5–7, RNAse-protected fragments of HuR mRNA from sham-operated animals; lanes 8–10, RNAse-protected fragments of HuR mRNA from 14-day I/R-injured animals. The 2 major bands are indicated by arrows and correspond to HuR mRNAs with 5′ untranslated regions (UTRs) of ∼150 and ∼20 bases. C, top: data along with results from 1-day I/R gels not shown were quantified and charted. Values are means relative HuR mRNA expression ± SE. *Statistically different from sham controls (P < 0.05). D: capped or uncapped HuR RNAs corresponding to the alternately transcribed forms are shown (left), along with an in vitro translation reaction showing the variable translatability of the 2 forms (right).
Examination of the sequences of the two HuR forms reveals that the longer mRNA contains a highly G+C-rich 150-base 5′ UTR (70% G+C overall) with a secondary structure that may diminish its translatability relative to the shorter mRNA, which contains only 20 bases of A+T-rich sequence (20% G+C). To further explore our hypothesis that the two forms of HuR mRNA may be selectively translated, we performed in vitro translation reactions using rat HuR mRNAs that contained either 150 or 20 bases of 5′ UTR sequence. The mRNAs were first transcribed in vitro; then, equal amounts were translated in the presence of [35S]-methionine. Figure 8D (left) illustrates the two transcribed mRNA species, which were made either with or without a 5′ cap, while Fig. 8D (right) demonstrates their relative translatability in reticulocyte lysates. As expected, capped mRNAs were more ready translatable than their uncapped counterparts; however, more notable was the extreme difference in translatability between the mRNAs containing only 20 or 150 bases of 5′ UTR sequence. Thus, as predicted by the primary sequences and modeled secondary structure, HuR translation may be selectively regulated by expression of multiple mRNA forms.
DISCUSSION
Renal I/R injury leads to serious consequences in a variety of clinical contexts, representing a serious problem in medicine. I/R-induced changes include damage of renal tubular epithelial cells and especially cells of proximal tubules, known to be more susceptible to injury in this setting. Using an ATP-depletion model in the proximal tubule cell line LLC-PK1, we have suggested a role for HuR in protecting kidney epithelia from injury during ischemic stress (19, 20). ATP depletion resulted in detectable net movement of HuR out of nucleus, followed by net movement back into the nucleus on reversion to normal growth medium. Furthermore, ATP depletion resulted in both translational and transcriptional increases in HuR expression. Here, we show that HuR, which plays an antiapoptotic role in cultured renal cells, undergoes similar alterations in distribution and expression in vivo. In rat kidneys subjected to ischemia followed by varying periods of reperfusion, only proximal tubule cells demonstrated a reversible shift of HuR to the cytoplasm immediately after ischemia, followed by gradually increased expression of HuR during the course of reperfusion. Interestingly, the intensity of HuR cytoplasmic redistribution correlated with segment-dependent vulnerability of proximal tubules to I/R injury. Cytoplasmic HuR staining was most pronounced in the acutely sensitive straight segment (S3) of proximal tubules, which descend into the outer medulla, where relative hypoxia predisposes them to greater ischemic injury (40). The gradient in intensity along proximal tubules could be related to the protective role of a HuR cytoplasmic shift during ischemia. In the cytoplasm, HuR targets specific mRNAs, increasing their stability, inducing their translation, or performing both functions, and rarely, repressing mRNA translation (8, 22).
Based on pull-down of HuR-mRNA complexes followed by microarray analysis, HuR has been predicted to bind several thousand cellular mRNAs (27). Here, we have demonstrated that suppression of HuR in LLC-PK1 cells diminishes expression of at least two molecules key to cell survival following renal ischemia and reperfusion, Bcl-2 and Hsp70. A set of potential pathways of HuR involvement in cytoprotection during ischemia can be suggested from available data on HuR posttranscriptionally regulated targets. For example, it has been reported recently that HuR, together with polypyrimidine binding protein, binds hypoxia-inducible factor-1α (HIF-1α) mRNA and promotes HIF-1α translation under hypoxia-like conditions (14). Activation of HIF-1α has been identified as an important mechanism of cellular adaptation to low oxygen. Under a decreased oxygen level, one of the conditions ischemia resulted in at the cellular level, HIF-1α regulates a broad range of physiologically relevant genes involved in erythropoiesis, angiogenesis, apoptosis, and energy metabolism (28). It was shown that preconditional activation of HIF-1α protected against ischemic injury in rats (7). It is striking that many of HIF-1α target genes are also regulated posttranscriptionally by HuR, including VEGF, COX-2, IL-6, IL-8, and GLUT1 (16, 24, 32), and likely further contribute to the protective and adaptive role of HuR under ischemic conditions. HuR, which promotes Bcl-2 expression, has emerged as an elicitor of a broad antiapoptotic function through its influence on the expression of proteins directly (e.g., ProTα, SIRT, Bcl-2, Mcl-1) or indirectly (e.g., p21, p27, EGF, VEGF, IGF-IR), preventing apoptosis (1, 26). Intense nuclear staining in newly regenerated proximal tubules after I/R injury, observed in this study, is likely to be related to higher tolerance to injury in these tubules, indicating a role for HuR in renal preconditioning to consequent ischemic stress.
By acting upon various mRNA pools, HuR participates in numerous key cellular processes; thus it is of interest to know the mechanism of how HuR expression is regulated. Our data obtained previously in LLC-PK1 proximal tubules cells suggest regulation of HuR expression by multiple means during energy depletion, including translational mechanisms (20). We and others have found that levels of HuR mRNA and protein can be decoupled, suggesting regulation at the level of mRNA translation (20, 33, 35). In the current study, we found that HuR mRNA was particularly abundant in S3 segments of proximal tubules, and while I/R injury caused a nephron-wide increase in mRNA, HuR protein was selectively upregulated in the proximal tubules, both cortical and medullary. In other words, while I/R injury caused global upswings in HuR mRNA levels, increased translation was confined to those cells most susceptible to injury; i.e., the proximal tubule cells. Promiscuous overexpression of HuR protein is not desirable, given that increases in HuR of even a few-fold have been associated with oncogenicity (25). However, having ready pools of HuR mRNA in reserve may be one mechanism by which cells can rapidly and transiently synthesize new HuR protein under stressful conditions. Indeed, we found such a mechanism to be present in LLC-PK1 cells, where recovery from ATP depletion resulted in increased levels of HuR mRNA without its accompanying translation. Only upon further stress did new, rapid translation occur (20). Additional studies will be required to determine how translational control may affect HuR expression in native rat kidney. However, it is tempting to speculate that the high levels of HuR mRNA in 14-day I/R-treated animals, which are preconditioned against further injury, would allow rapid translation of HuR, and thus, protection against apoptosis.
In this study, we found HuR to be transcribed in two forms with alternate 5′ UTRs, and the relative ratios of these transcripts were maintained even as total HuR mRNA levels increased. Our results indicate that the longer form of HuR mRNA is significantly less translatable than the shorter form, due to the length and high G+C content of its 5′ UTR. However, our preliminary results in LLC-PK1 cells suggest that proximal tubule cells specifically upregulate only the shorter mRNA during ATP depletion and recovery (Ayupova DA, unpublished observations), which would result in cell-specific increases in translation. It is possible that this was not seen in the present studies since we used whole rat kidney cortex, where non-proximal tubule cells might mask specific responses in proximal tubules. Additional preliminary results suggest that in LLC-PK1 cells, the initiation site for the shorter HuR transcript is subject to control by BMP/Smad signaling (Jeyaraj S, unpublished observations), which plays an important role in kidney development and attenuation of cell death following ischemic injury (45, 46). Further analysis of HuR mRNA and protein expression is required to define the mechanisms by which HuR levels are modulated.
In summary, our data demonstrate that expression and distribution of HuR during I/R injury to the kidney are altered in a nephron segment-specific manner. Although expressed at lower levels in proximal tubules under sham conditions, HuR was noticeably increased in intensity in cortical and newly regenerated proximal tubule segments in reperfused and preconditioned rat kidneys. A transient shift of HuR to the cytoplasm, where it regulates target mRNA stability and translation, was observed only in proximal tubules immediately after ischemia. Taken together with our findings showing an antiapoptotic role for HuR in a proximal tubule cell line, these data suggest protective and preconditioning roles of HuR in renal ischemic injury. Furthermore, the nonparallel changes in HuR protein and mRNA during I/R injury, coupled with the existence of multiple forms of HuR transcripts, are an indication of posttranscriptional mechanisms involved in its expression.
GRANTS
This work was funded by National Institutes of Health Grants RO1 DK-0512131 and RO1 AR-051515 (to B. S. Lee).
Acknowledgments
The authors thank Dr. Tibor Nadasdy and Cherry Bott of The Ohio State University Department of Pathology for technical advice. The authors also thank Dr. Selvi Jeyaraj and Suman Govindaraju for discussions during these studies.
REFERENCES
- 1.Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle 6: 1288–1292, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agre P Homer W. Smith award lecture. Aquaporin water channels in kidney. J Am Soc Nephrol 11: 764–777, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Amadio M, Scapagnini G, Laforenza U, Intrieri M, Romeo L, Govoni S, Pascale A. Post-transcriptional regulation of HSP70 expression following oxidative stress in SH-SY5Y cells: the potential involvement of the RNA-binding protein HuR. Curr Pharm Des 14: 2651–2658, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Aufricht C Heat-shock protein 70: molecular supertool? Pediatr Nephrol 20: 707–713, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Basile DP, Liapis H, Hammerman MR. Expression of bcl-2 and bax in regenerating rat renal tubules following ischemic injury. Am J Physiol Renal Physiol 272: F640–F647, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bernhardt WM, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Gunzler V, Amann K, Willam C, Wiesener MS, Eckardt KU. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci 58: 266–277, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canfield PE, Geerdes AM, Molitoris BA. Effect of reversible ATP depletion on tight-junction integrity in LLC-PK1 cells. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1038–F1045, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Chiang-Ting C, Tzu-Ching C, Ching-Yi T, Song-Kuen S, Ming-Kuen L. Adenovirus-mediated bcl-2 gene transfer inhibits renal ischemia/reperfusion induced tubular oxidative stress and apoptosis. Am J Transplant 5: 1194–1203, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Chien CT, Shyue SK, Lai MK. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation 84: 1183–1190, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Devarajan P Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Galban S, Kuwano Y, Pullmann R Jr, Martindale JL, Kim HH, Lal A, Abdelmohsen K, Yang X, Dang Y, Liu JO, Lewis SM, Holcik M, Gorospe M. RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1alpha. Mol Cell Biol 28: 93–107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallouzi IE, Brennan CM, Steitz JA. Protein ligands mediate the CRM1-dependent export of HuR in response to heat shock. RNA 7: 1348–1361, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gantt KR, Cherry J, Richardson M, Karschner V, Atasoy U, Pekala PH. The regulation of glucose transporter (GLUT1) expression by the RNA binding protein HuR. J Cell Biochem 99: 565–574, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer JR, Sisson SP, Vernier RL. Tamm-Horsfall glycoprotein: ultrastructural immunoperoxidase localization in rat kidney. Lab Invest 41: 168–173, 1979. [PubMed] [Google Scholar]
- 18.Izquierdo JM Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem 283: 19077–19084, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Jeyaraj S, Dakhlallah D, Hill SR, Lee BS. HuR stabilizes vacuolar H+-translocating ATPase mRNA during cellular energy depletion. J Biol Chem 280: 37957–37964, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaraj SC, Dakhlallah D, Hill SR, Lee BS. Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells. Am J Physiol Renal Physiol 291: F1255–F1263, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King PH, Fuller JJ, Nabors LB, Detloff PJ. Analysis of the 5′ end of the mouse Elavl1 (mHuA) gene reveals a transcriptional regulatory element and evidence for conserved genomic organization. Gene 242: 125–131, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Kullmann M, Gopfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev 16: 3087–3099, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal A, Kawai T, Yang X, Mazan-Mamczarz K, Gorospe M. Antiapoptotic function of RNA-binding protein HuR effected through prothymosin alpha. EMBO J 24: 1852–1862, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy NS, Chung S, Furneaux H, Levy AP. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem 273: 6417–6423, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Lopez de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene 22: 7146–7154, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lopez de Silanes I, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol 2: 11–13, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lopez de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci USA 101: 2987–2992, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maxwell P HIF-1: an oxygen response system with special relevance to the kidney. J Am Soc Nephrol 14: 2712–2722, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Mazroui R, Di Marco S, Clair E, von Roretz C, Tenenbaum SA, Keene JD, Saleh M, Gallouzi IE. Caspase-mediated cleavage of HuR in the cytoplasm contributes to pp32/PHAP-I regulation of apoptosis. J Cell Biol 180: 113–127, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meldrum KK, Meldrum DR, Sezen SF, Crone JK, Burnett AL. Heat shock prevents simulated ischemia-induced apoptosis in renal tubular cells via a PKC-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 281: R359–R364, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Meng Z, King PH, Nabors LB, Jackson NL, Chen CY, Emanuel PD, Blume SW. The ELAV RNA-stability factor HuR binds the 5′-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res 33: 2962–2979, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, Nordling S, Ristimaki A, Haglund C. Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res 11: 7362–7368, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3′ untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res 61: 2154–2161, 2001. [PubMed] [Google Scholar]
- 34.Obermuller N, Bernstein P, Velazquez H, Reilly R, Moser D, Ellison DH, Bachmann S. Expression of the thiazide-sensitive Na-Cl cotransporter in rat and human kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F900–F910, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci 17: 3024–3037, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padanilam BJ, Lewington AJ. Molecular mechanisms of cell death and regeneration in acute ischemic renal injury. Curr Opin Nephrol Hypertens 8: 15–19, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem 277: 2040–2049, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Patel NS, Cuzzocrea S, Collino M, Chaterjee PK, Mazzon E, Britti D, Yaqoob MM, Thiemermann C. The role of cyclooxygenase-2 in the rodent kidney following ischaemia/reperfusion injury in vivo. Eur J Pharmacol 562: 148–154, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Rasband WS ImageJ. Bethesda, MD: US National Institutes of Health, 1997. –2008.
- 40.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 114: 5–14, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smoyer WE, Ransom R, Harris RC, Welsh MJ, Lutsch G, Benndorf R. Ischemic acute renal failure induces differential expression of small heat shock proteins. J Am Soc Nephrol 11: 211–221, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 293: F269–F278, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki C, Isaka Y, Shimizu S, Tsujimoto Y, Takabatake Y, Ito T, Takahara S, Imai E. Bcl-2 protects tubular epithelial cells from ischemia reperfusion injury by inhibiting apoptosis. Cell Transplant 17: 223–229, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Uchida S, Sasaki S, Nitta K, Uchida K, Horita S, Nihei H, Marumo F. Localization and functional characterization of rat kidney-specific chloride channel, ClC-K1. J Clin Invest 95: 104–113, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102: 202–214, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vukicevic S, Kopp JB, Luyten FP, Sampath TK. Induction of nephrogenic mesenchyme by osteogenic protein 1 (bone morphogenetic protein 7). Proc Natl Acad Sci U S A 93: 9021–9026, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Furneaux H, Cheng H, Caldwell MC, Hutter D, Liu Y, Holbrook N, Gorospe M. HuR regulates p21 mRNA stabilization by UV light. Mol Cell Biol 20: 760–769, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinberg JM The cell biology of ischemic renal injury. Kidney Int 39: 476–500, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Yoshikawa M, Uchida S, Yamauchi A, Miyai A, Tanaka Y, Sasaki S, Marumo F. Localization of rat CLC-K2 chloride channel mRNA in the kidney. Am J Physiol Renal Physiol 276: F552–F558, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Zhang PL, Lun M, Schworer CM, Blasick TM, Masker KK, Jones JB, Carey DJ. Heat shock protein expression is highly sensitive to ischemia-reperfusion injury in rat kidneys. Ann Clin Lab Sci 38: 57–64, 2008. [PubMed] [Google Scholar]
- 51.Zheng X, Zhang X, Sun H, Feng B, Li M, Chen G, Vladau C, Chen D, Suzuki M, Min L, Liu W, Zhong R, Garcia B, Jevnikar A, Min WP. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation 82: 1781–1786, 2006. [DOI] [PubMed] [Google Scholar]