Abstract
Chronic allograft nephropathy (CAN) represents progressive deterioration of renal allograft function with fibroinflammatory changes. CAN, recently reclassified as interstitial fibrosis (IF) and tubular atrophy (TA) with no known specific etiology, is a major cause of late renal allograft loss and remains a significant deleterious factor of successful renal transplantation. Carbon monoxide (CO), an effector byproduct of heme oxygenase pathway, is known to have potent anti-inflammatory and antifibrotic functions. We hypothesized that inhaled CO would inhibit fibroinflammatory process of CAN and restore renal allograft function, even when the treatment was initiated after CAN was established. Lewis rat kidney grafts were orthotopically transplanted into binephrectomized allogenic Brown Norway rats under brief tacrolimus (0.5 mg/kg im, days 0–6). At day 60, CO (20 ppm) inhalation was initiated to recipients and continued until day 150 or animal death. Development of CAN was confirmed at day 60 with decreased creatinine clearance (CCr), significant proteinuria, and histopathological findings of TA, IF, and intimal arteritis. Air-treated control recipients continued to deteriorate with further declines of CCr and increases of urinary protein excretion and died with a median survival of 82 days. In contrast, progression of CAN was decelerated when recipients received CO on days 60–150, showing markedly improved graft histopathology, restored renal function, and improved recipient survival to a median of >150 days. CO significantly reduced intragraft mRNA levels for IFN-γ and TNF-α at day 90. Expression of profibrotic TGF-β/Smad was significantly suppressed with CO, together with downregulation of ERK-MAPK pathways. Continuous CO (20 ppm) treatment for days 0–30, days 30–60, or days 0–90, or daily 1-h CO (250 ppm) treatment for days 0–90, also showed efficacy in inhibiting CAN. The study demonstrates that CO is able to inhibit progression of fibroinflammatory process of CAN, restore renal allograft function, and improve survival even when the treatment is started after CAN is diagnosed.
Keywords: kidney transplantation, renal fibrosis, anti-inflammatory effects, antifibrotic effects
although the frequency and severity of acute rejection episodes have been significantly improved, the half-life of renal allografts has stayed unchanged for over a decade (2, 31). There is a gradual loss of ∼5% renal allografts per year, resulting in 5- and 10-yr graft survival rates of ∼70 and 50%, respectively (7). The most common clinical correlate of late allograft loss is referred to nonspecifically as chronic allograft nephropathy (CAN), and more than 40% of kidney allograft recipients suffer from CAN during 5 yr after transplantation (3, 7). CAN is characterized by progressive renal allograft dysfunction with histopathological features of chronic interstitial fibrosis (IF), tubular atrophy (TA), vascular occlusive changes, and glomerulosclerosis. Thus, CAN is a clinicopathological classification caused by multiple factors, including alloantigen-dependent immune responses, as well as nonimmunological factors, such as ischemia-reperfusion (I/R) injury, calcineurin inhibitor nephrotoxicity, metabolic and cardiovascular disorders, and exacerbating preexisting donor disease (10, 22, 28). This has led the Banff group (26) to recommend eliminating the term CAN in those cases with no known etiology in favor of “interstitial fibrosis and tubular atrophy with no known specific cause” (IF/TA). However, since the term CAN is presently entrenched in the literature, it will be used as a synonym for IF/TA in its descriptive, generic sense in this report and is not meant to imply any specific etiology. There have been a number of approaches aiming at reducing the impact of CAN; however, no practical therapeutic strategies are currently available to prevent and treat CAN.
Recent studies showed that endogenously generated carbon monoxide (CO) by heme oxygenase (HO) system serves as a key mechanism to maintain the integrity of the physiological function of organs (15, 30). Numerous reports subsequently show that exogenously delivered CO provides cytoprotection in various injury conditions. These observations strongly indicate that CO is a biologically active gaseous molecule in the body (16). Earlier study in our laboratory demonstrated that treatment of kidney allograft recipients with inhaled low-dose CO (20 ppm) for the first 30 days of kidney transplant (KTx) effectively prevented CAN development by 1) inhibiting recruitment of inflammatory infiltrates, 2) downregulating proinflammatory mediators, cytokines, and chemokines, 3) promoting vasodilation and maintaining cortical blood flow, 4) preventing tissue fibrosis, and 5) regulating T cell alloreactivity (21).
Considering potent cytoprotective functions of CO observed in our previous study, we hypothesized in this study that CO could reverse established CAN and restore allograft function even when CO was given after CAN was developed. To test the hypothesis, rat kidney allograft recipients were kept without treatment for the first 60 days of KTx to develop CAN and then treated with 20 ppm CO. The result shows that low-dose CO is able to reverse CAN and restore renal allograft function through its anti-inflammatory and antifibrosis actions.
MATERIALS AND METHODS
Animals.
Inbred male Lewis (LEW, RT1l) and Brown Norway (BN, RT1n) rats weighing 200–250 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). Animals were maintained in laminar flow cages in a specific pathogen-free facility at the University of Pittsburgh and fed a standard diet and water ad libitum. All procedures were performed according to the guidelines of the National Research Council's Guide for the Humane Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Kidney transplantation.
Orthotopic KTx was performed using a previously described technique (21). In short, after intravenous heparinization (300 U), the donor's left kidney was removed with the left renal artery in continuity with a short aortic segment and the left renal vein with a patch of vena cava. The excised graft was flushed with 3 ml of UW solution (Viaspan, Du Pont, Wilmington, DE). The kidney graft was orthotopically transplanted into the recipient by end-to-side microvascular anastomoses between graft aorta and recipient infrarenal abdominal aorta, and between graft renal vein and recipient infrarenal vena cava with 10–0 suture. Both native kidneys were removed, and end-to-end ureteral anastomosis was performed using 10-0 suture. Recipients received prophylactic antibiotics (Cefotetan Disodium; 100 mg/kg im) for 3 days following KTx.
CO exposure.
Compressed 1% CO (Praxair, Danbury, CT) was mixed with air (21% oxygen) in a stainless steel mixing cylinder and then directed into a 3.70-ft3 glass exposure chamber at a flow rate of 12 l/min. A CO analyzer (Interscan, Chatsworth, CA) was used to continuously maintain the CO level at 20 ppm in the chamber. Animals were exposed to CO (20 ppm) by being housed in a CO chamber with a regular diet and water ad libitum (18, 19).
Experimental design.
In the first set of experiments, BN recipients of LEW allografts were treated with intramuscular tacrolimus (FK506, a gift from Astellas Pharmaceutical, Tokyo, Japan) at a daily dosage of 0.5 mg/kg for 7 days after KTx (days 0–6). At day 60, a group of recipients was housed in CO chamber for continuous CO (20 ppm) exposure, while air-treated control recipients were housed either in the regular laminar flow cage or in the identical chambers without CO. CO (20 ppm) treatment was continued until the day of death or for 150 days for survival study. LEW recipients with syngenic LEW kidney grafts without immunosuppression served as controls.
Recipients were killed at 60, 90, and 150 days after KTx, and blood and graft kidney samples were obtained. The half of kidney samples was snap-frozen and stored at −80°C until use. The one-fourth of the kidneys were fixed with 2% paraformaldehyde in PBS, cryoprotected in 2.3 M sucrose in PBS overnight, and frozen in optimal cold temperature (Sakura Finetek, Torrance, CA) with liquid nitrogen-cooled isopentane. The rest of the graft was fixed in 10% buffered formalin for routine histopathology.
In the second sets of experiments, efficacies of various timings of CO treatment were examined using renal allograft function at day 90 as endpoints. Under short tacrolimus (0.5 mg/kg on days 0–6), CO was administered to BN recipients of LEW kidney allografts for 90 days continuously (20 ppm on days 0–90), for 90 days intermittently (250 ppm for 1 h daily on days 0–90), for 30 days early continuously (20 ppm on days 0–30), or for 30 days late continuously (20 ppm on days 30–60). Renal functions of these recipients at day 90 were compared with those of air-treated controls and of CO-treated recipients in the first sets of experiments [CO (20 ppm) on days 60–90].
Assessment of graft functions.
Graft functions were evaluated by creatinine clearance (CCr), urinary protein excretion, and recipient survival. Urine samples were collected for 24 h using the metabolic cage system. The levels of creatinine in serum/urine and urinary protein were measured using an autoanalyzer (Beckman Instruments, Fullerton, CA) (18, 21).
Routine histopathological analysis.
Formalin-fixed graft tissues were paraffin embedded, cut into 5-μm sections, and stained with hematoxilin and eosin. Sections were blindly reviewed by a pathologist (MAN) without the knowledge of treatment groups. Severity of histopathological changes in the allograft was graded according to the Banff criteria as IF, TA, mononuclear cell interstitial inflammation, tubulitis, intimal arteritis, and glomerulitis (23).
Immunohistochemical staining.
Formalin-fixed, paraffin-embedded graft tissue was cut into 5-μm sections for immunohistochemical staining using the avidin-biotin-peroxidase complex method after antigen retrieval. Endogenous peroxidase activity was quenched using hydrogen peroxide (Sigma, St. Louis, MO). For the detection of macrophage, sections were incubated with monoclonal anti-rat CD68 (ED1, 1:200, Serotec, Raleigh, NC) or α-smooth muscle actin (α-SMA; DAKO, Carpinteria, CA), followed by LSAB+ horseradish peroxidase (DAKO). The immune complex was visualized with 3-amino-9-ethyl carbazole and hematoxylin counterstaining. Positively stained cells were counted in a blind fashion in five random cortical high-power fields (×400) per section.
SYBR green real-time RT-PCR.
The mRNAs for IL-6, IL-10, TNF-α, IFN-γ, intracellular adhesion molecule (ICAM)-1, monocyte chemoattractant protein (MCP)-1, TGF-β1, collagen I, and GAPDH were quantified in duplicate using SYBR green two-step, real-time RT-PCR, as previously described (17, 18).
Western blot.
Cytosolic protein was isolated from the kidney graft samples, and Western blot was performed as previously described (18, 20, 21). Fifty micrograms of cytosolic protein were separated by electrophoresis on 8–15% acrylamide sodium dodecyl sulfate gels and transferred to nitrocellulose membranes (Scleicher & Schuell, Keene, NH). For the blocking of nonspecific binding, 5% nonfat dry milk in PBS-Tween (0.1%) was added to the membrane for 1 h at room temperature. Membranes were washed in PBS-Tween and then incubated overnight with primary antibodies followed by incubation with secondary antibody for 45 min. After repeat washings with PBS-Tween, membranes were developed with the SuperSignal detection systems (Pierce Chemical, Rockford, IL) and exposed to film. The band intensities were quantified by NIH Image analysis software. The following primary antibodies were used: rabbit anti-human polyclonal antibodies for β-actin (Sigma), mouse monoclonal phosphorylated and total ERK 1/2 (Santa Cruz Biotechnology, Santa Cruz, CA), and Smad3 (Chemicon International, Temecula, CA).
IFN-γ protein levels.
Serum IFN-γ levels were measured by ELISA using a commercially available kit (R&D Systems, Minneapolis, MN).
Data analysis.
The results were expressed as means ± SD except the data of urinary protein secretion shown as means ± SE. Statistical analysis was performed using unpaired Student's t-test or ANOVA where appropriate. A probability level of P < 0.05 was considered to be statistically significant.
RESULTS
Development of CAN at day 60 after KTx.
The function of LEW kidney allografts in BN recipients under brief tacrolimus deteriorated with progressive declines of CCr and gradual increases of urinary protein excretion. CCr of allografts decreased to ∼40% of the pretransplant value by day 60, while isografts maintained normal CCr values (Fig. 1, A and B). Typical histopathological features of CAN, such as TA, IF, and infiltrates, were seen at day 60 (Fig. 2). These data confirmed the establishment of CAN by day 60 after KTx in this model.
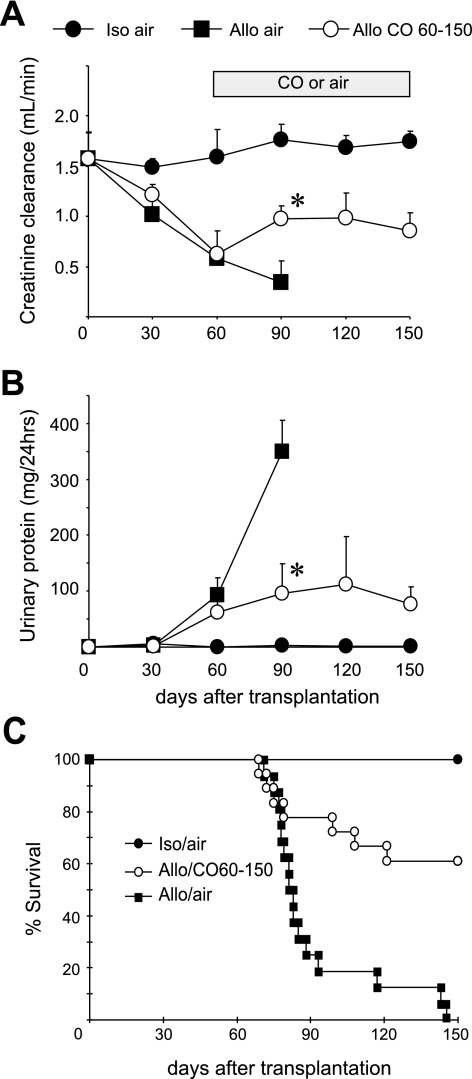
Fig. 1.
Long-term kidney graft functions with and without carbon monoxide (CO) treatment on days 60–150. Sequential analysis of creatinine clearance (CCr; A) and urinary protein amounts (B) revealed progressive deterioration of the allograft functions in air-treated controls (▪, n = 16). In contrast, treatment with inhaled CO (20 ppm on days 60–150) improved allograft functions (○, n = 18). Isograft maintained normal functions for 150 days (•, n = 7). *P < 0.05 air vs. CO. Recipient survival (C) suitably correlated to renal graft functions. While all isograft recipients survived for >150 days (n = 7), all air-treated control allograft recipients (n = 16) died of renal insufficiency within 150 days. CO inhalation treatment initiated at day 60 significantly improved recipient survival, and 11 of 18 recipients survived for >150 days [*P < 0.05, air-treated vs. CO (20 ppm) on days 60–150].
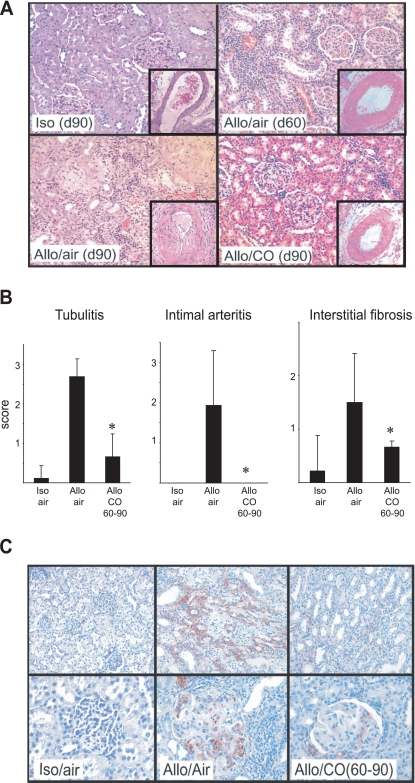
Fig. 2.
Histopathological analyses of allografts at day 90 (d90). A: isograft day 90 maintained normal renal architecture. Allografts at day 60 (d60), before treatment initiation, showed widespread interstitial infiltrates and tubular atrophy. At day 90, air-treated control grafts showed more prominent histopathological changes with significant parenchymal atrophy and interstitial fibrosis. CO-treated grafts had dramatically reduced cellular infiltrates and fibrosis by day 90. Hematoxylin and eosin, original magnification ×200. Intimal arteritis was less severe in CO-treated than in air-treated grafts (insets, original magnification ×400). B: histopathological changes at day 90 were quantified using Banff score; n = 9 for isografts, n = 8 for control allografts in air, and n = 5 for CO-treated allografts. *P < 0.05, air-treated vs. CO. C: immunohistochemistry for α-smooth muscle actin (α-SMA; red) revealed numerous α-SMA-positive myofibroblasts in air-treated allografts at day 90. CO-treated grafts had dramatically reduced numbers of α-SMA-positive cells. Original magnification, top: interstitium ×200; bottom: glomeruli ×400.
CO treatment initiated on day 60 prevented CAN progression and restored allograft function.
Air-treated control kidney allografts continued to show functional deterioration with a continual CCr decline and striking increase of proteinuria by day 90 of KTx. Recipient animals started to fail after day 70, and all died within the followup period of 150 days (Fig. 1, A–C). On the other hand, when CO inhalation (20 ppm) was initiated at day 60, renal allograft function started to show improvement by day 90, and CCr levels and urinary protein amounts were held at the same level until day 150. The restoration of renal allograft function resulted in significant improvement in recipient survival (Fig. 1, A–C). Isografts maintained renal functions at the comparable levels to those of normal LEW rats for 150 days.
CO improved histopathological changes.
At day 90, air-treated control grafts showed prominent interstitial mononuclear cell infiltration, tubulitis, fibrosis, and glomerulitis. On the other hand, CO-treated grafts at day 90 showed less severe changes compared with those of air-treated control grafts. Reduced degrees of intimal arteritis also were seen with CO treatment (Fig. 2, A and B). To further investigate the degree of IF, the kidney grafts were stained for α-SMA. Isografts at day 90 showed scarce interstitial α-SMA expression. Strong α-SMA expression was noted in both interstitial lesion and glomeruli in the air-treated control allografts. There was no definitive α-SMA stain on tubular epithelial cells. In contrast, there was a remarkable reduction of α-SMA-positive myofibroblast accumulation in the grafts treated with CO on days 60–90 (Fig. 2C).
CO diminished IF by downregulating TGF-β/Smad/ERK pathway.
Fibrotic response has been characterized by an increased accumulation of extracellular matrix, particularly collagen in the graft interstitial tissue. TGF-β has been considered to be a key mediator in the progression of fibrosis by activating fibroblasts for their differentiation into myofibroblasts to produce matrix proteins such as collagen I, III, IV, and fibronectin (11, 29). Both collagen I and TGF-β mRNA were significantly upregulated in air-treated allografts at day 90. CO treatment significantly reduced mRNA levels for these fibrosis-related molecules at day 90 (Fig. 3A). Isografts showed marginal increases of these mRNA, regardless of CO inhalation.
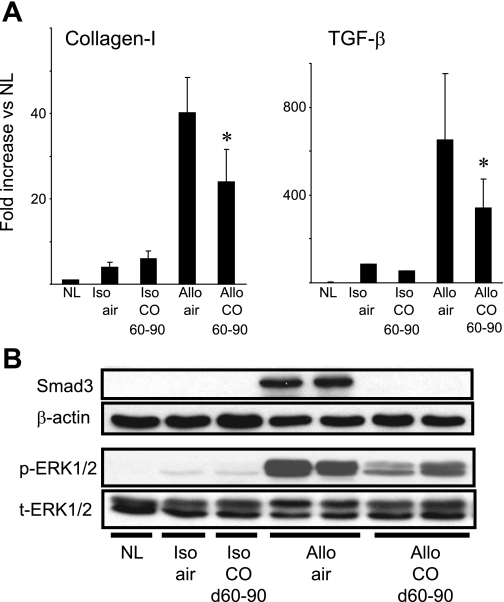
Fig. 3.
Expression of fibrosis-related molecules. A: real-time RT-PCR of kidney allografts at day 90 showed that both collagen I and TGF-β mRNA levels dramatically increased in air-treated control allografts. CO inhalation (20 ppm on days 60–90) significantly reduced mRNA levels of these molecules (n = 5–6, *P < 0.05 air-treated vs. CO). B: Western blot of kidney allografts at day 90 revealed strong expressions of Smad3 and phosphorylated (p)-ERK1/2 in air-treated control group. Inhaled CO (20 ppm) remarkably reduced expressions of these molecules. Lewis (LEW, RT1l) recipients of Brown Norway (BN, RT1n) kidney allografts were treated with CO (20 ppm) on days 60–90, and kidney grafts were obtained at day 90. Control recipients were exposed to air. Displayed pictures were representative from 3 independent experiments using 4–5 individual graft samples from each experimental group.
TGF-β exerts its biological functions mainly through its downstream signaling molecules Smad2/3 or Smad-independent signaling pathway by directly activating ERK and p38MAPK (13, 33, 34). Western blot analysis of kidney allografts at day 90 revealed strong expressions of Smad3 and phosphorylated (p) ERK1/2 in the air-treated allografts, whereas CO-treated allografts showed reduced Smad3 and p-ERK1/2 at day 90 (Fig. 3B).
CO decreased macrophage infiltration into the grafts.
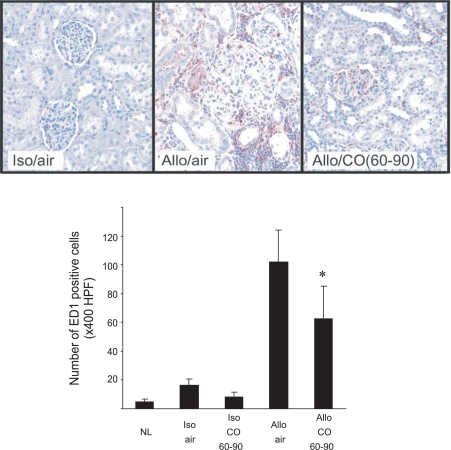
Infiltrating macrophages have been suggested to secret inflammatory mediators and contribute to fibroinflammatory responses of CAN process. We investigated ED1+ inflammatory macrophages in kidney allografts at day 90. ED1+ macrophages were seen in interstitial tissue and glomeruli of air-treated control grafts. CO treatment significantly inhibited inflammatory macrophage recruitment. In CO-treated grafts, the number of ED1-positive macrophage infiltration was significantly less and limited to the perivascular area (Fig. 4).
Fig. 4.
Macrophage infiltration into grafts. There was a number of infiltrating ED1+ macrophages in the air-treated allografts at day 90. The numbers of ED1+ cells in CO-treated recipients were significantly less than those of the grafts in air. Top: representative pictures from 5 individual animals from each group. The histogram revealed the number of the positive cells per ×400 high-power field (HPF). *P < 0.05 air-treated vs. CO, n = 4–5.
CO inhibited upregulation of inflammatory mediators in grafts.
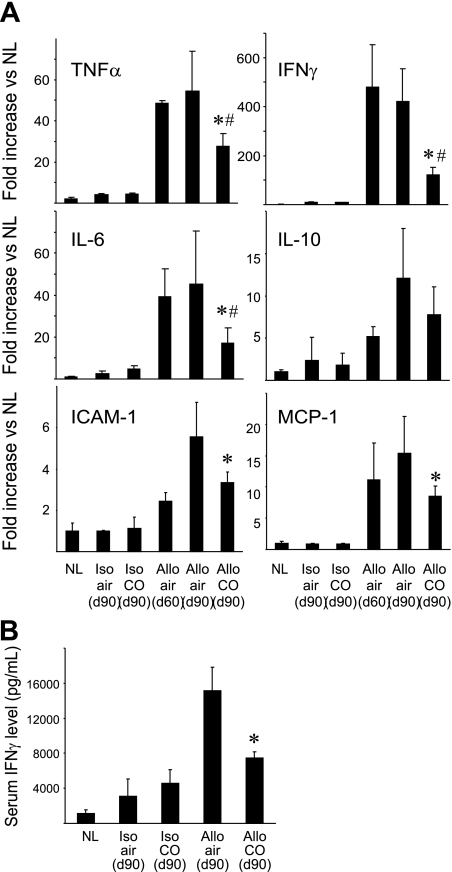
As inflammatory reaction is a critical component of CAN development, we assessed the effects of delayed CO treatment on mRNA levels for inflammation-related mediators and adhesion molecules. Real-time RT-PCR demonstrated that mRNA levels for TNF-α, IFN-γ, and IL-6 markedly increased by day 60. Air-treated control grafts continued to show high mRNA levels for these molecules at day 90. CO treatment initiated at day 60 significantly downregulated these cytokine mRNA levels at day 90 compared with those before the treatment at day 60, and to those in air-treated allograft at day 90. IL-10 mRNA levels, known to be an anti-inflammatory cytokine, tended to be increased in allografts after KTx; however, there was no significant difference between groups (Fig. 5A). The expression of adhesion molecule such as ICAM-1 contributes to fibroinflammatory events of CAN development (6). MCP-1 also plays a role by recruiting monocytes to the injury sites and is known to increase during CAN process (4). Real-time RT-PCR demonstrated that both ICAM-1 and MCP-1 mRNA levels elevated in allografts. CO treatment significantly reduced ICAM-1 and MCP-1 mRNA levels at day 90 compared with those in air-treated control group (Fig. 5A). In correlation to mRNA levels, serum IFN-γ protein levels were significantly low in CO-treated recipients (Fig. 5B).
Fig. 5.
Intragraft mRNA expression for inflammation-related mediators. A: intragraft cytokine mRNA expressions for TNF-α, IFN-γ, IL-6, and IL-10 were analyzed by real-time RT-PCR at day 60 (before CO treatment) and day 90 with and without CO (20 ppm; days 60–90). CO inhalation initiated at day 60 significantly reduced mRNA levels for TNF-α, IFN-γ, and IL-6 compared with those of allografts in air at day 60 and day 90. Similarly, CO significantly prevented ICAM-1 and MCP-1 mRNA upregulations at day 90. CO did not alter mRNA levels for IL-10 in the allografts. *P < 0.05 CO-treated vs. air-treated at day 90. #P < 0.05 CO-treated day 90 vs. before treatment day 60, n = 4–6. B: serum IFN-γ levels were determined at day 90. *P < 0.05 air-treated vs. CO, n = 4–6.
Different CO inhalation treatment strategies.
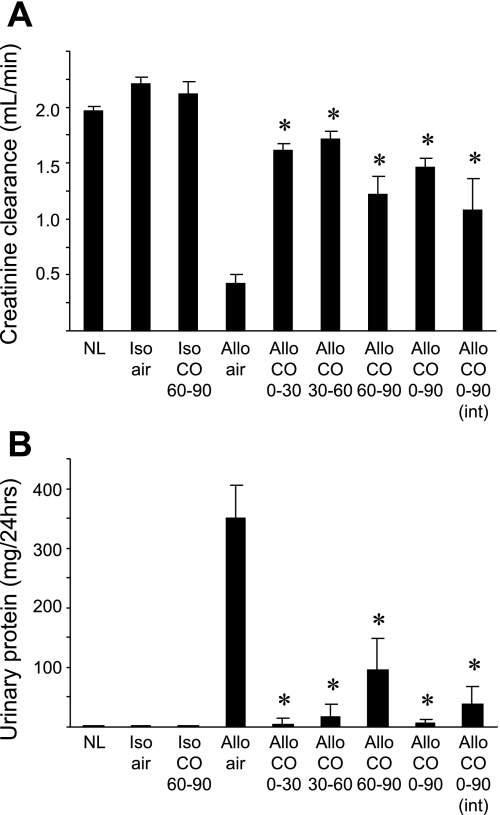
These data support that delayed low-dose CO treatment initiated on day 60 after KTx can reverse the severity of CAN and improve renal allograft function. To further investigate whether different CO treatment strategies can be effective to prevent/reverse CAN progression, we applied various durations and timings of CO exposure and compared the graft functions at day 90. CO exposure for 30 days starting earlier time points at day 0 (days 0–30) or day 30 (days 30–60) dramatically improved CCr and reduced proteinuria, indicating that the initiation of CO treatment before disease establishment effectively prevented CAN development. As expected, CO exposure throughout the experiments significantly inhibited CAN and showed good renal allograft function. The daily brief CO inhalation at 250 ppm for 1 h, a more practical CO inhalation protocol, also significantly improved the allograft functions and offered comparable efficacies to those seen in continuous (20 ppm) CO exposure (Fig. 6).
Fig. 6.
Allograft function at day 90 with various CO treatment protocols. Allograft functions at day 90 after kidney transplant were determined with various timings or doses of CO treatment protocols. CCr (A) and urinary protein excretion (B) were determined in groups of syngenic recipients without treatment (Iso/air, n = 4) and with 20 ppm CO for days 60–90 (Iso/CO 60–90, n = 4), and allograft recipients without treatment (Allo/air, n = 16), and 20 ppm CO for days 0–30 (Allo/CO 0–30, n = 7), 20 ppm CO for days 30–60 (Allo/CO 30–60, n = 5), 20 ppm CO for days 60–90 (Allo/CO 60–90, n = 18), 20 ppm CO for days 0–90 (Allo/CO 0–90, n = 10), and daily 250 ppm CO for 1 h for days 0–90 (Allo/CO 0–90 int, n = 5). Normal LEW rats (NL) were used as controls. *P < 0.05 vs. air-treated control allografts (Allo/air).
DISCUSSION
The current study demonstrates that delayed treatment with inhaled low-dose CO, started after CAN development, can improve histopathological lesions associated with CAN and restore renal allograft functions. In LEW to BN rat KTx model under a brief tacrolimus immunosuppression, renal allograft functions deteriorated by day 60 with reduced CCr levels and significant urinary protein excretion. Histopathological analysis revealed typical features of CAN in these allografts. Without any treatment, CAN further progressed and all recipients died of renal failure by day 150 with CCr <20% of normal values and >300 mg/24 h proteinuria. However, when continuous low-dose CO (20 ppm) was initiated at day 60, CO was able to halt CAN progression, restore renal allograft function, and improve animal survival to 61% at day 150. These results indicate that low-dose CO inhalation could be an effective therapeutic option to treat progressive deterioration of renal allografts with changes of CAN. To further investigate the efficacies of CO during the disease progression, we applied CO treatment at different time points after KTx using the same KTx model. All of 30-day continuous (20 ppm) CO treatments initiated at day 0, day 30, and day 60 resulted in improved renal allograft function at day 90 after KTx. Although earlier initiation of CO treatment tended to result in better renal function, we did not find statistically significant differences in CCr levels or urinary protein amounts among three treatment protocols. Interestingly, the efficacies of 30-day CO inhalation were relevant to those of 90-day CO inhalation with 20 ppm (continuous) or 250 ppm (1 h daily). Thus, CO could be used any time point of CAN to prevent, halt, and reverse the overall process. The study used extremely low-dose (20 ppm) CO, which marginally increased COHb levels to ∼6% from ∼2% in air (19, 21). The dose was chosen to avoid any concern regarding adverse events due to inhaled CO; however, it remains to be determined whether higher doses of CO have a greater control of CAN.
Fibrosis is a central feature of CAN, and activated tissue myofibroblasts and macrophages are believed to be key cellular players of fibrogenesis. Additionally, tubular epithelial cells have been shown to play important roles in CAN process through epithelial-mesenchymal transition, the acquisition by tubular epithelial cells of the phenotypic and functional properties of fibroblasts (24, 32). These cells promote fibrosis via the secretion of profibrotic cytokines, adhesion molecules, and growth factors. Among them, TGF-β has been considered to be a key mediator in the progression of fibrosis by activating fibroblast proliferation and production of matrix proteins such as collagen I, III, IV, and fibronectin (11, 29), mainly through its downstream signaling molecules Smad2/3 (5, 33). Low-dose CO in this study shows potent antifibrotic actions with significant downregulation of TGF-β and Smad3. CO also inhibited ERK-MAPK pathway activation associating with CAN. Several studies show the involvement of MAPK signaling pathways in renal fibrosis, and JNK, p38, and ERK-MAPKs can be activated directly by TGF-β (1, 13, 34). Conversely, the phosphorylation of ERK pathways has been shown to directly activate Smads (8). Thus, the complex interaction among TGF-β, Smad, and MAPK pathways is involved in renal fibrotic process, and CO appears to induce antifibrotic actions by modulating these pathways.
As interstitial and glomerular inflammation plays an important role in the initiation/progression of renal fibrosis, the downregulation of inflammatory reactions with CO may be one of mechanisms of CO's antifibrotic function in this study. With low-dose CO treatment, kidney allografts showed significantly decreased numbers of inflammatory infiltrates, in particular ED1+ monocyte/macrophage infiltration, which at least in some part was mediated through downregulation with CO of adhesion molecules and chemokines/chemokine receptors, such as MCP-1. CO in this study also notably downregulated various inflammatory cytokines in kidney allografts (e.g., TNF-α, IFN-γ, and IL-6). Less ED1+ macrophage infiltration might contribute to decreased proinflammatory mRNA levels; however, previous in vitro studies indicate that CO regulates RAW 264.7 macrophage activation due to oxidative stress (14), and we previously showed that CO is able to downregulate liver resident macrophages (27). CO also has been shown to inhibit proliferation of human fetal lung fibroblasts (MRC-5) and suppress fibronectin and collagen 1 production via the regulation of p21, and cyclins A and D (35). These findings suggest that CO might directly modulate macrophages, fibroblasts, activated mesangial cells, and/or endothelial cells to restore renal function.
Several recent studies showed that CO mediates cytoprotection through an induction of HO-1 (9, 12, 25). In this study, however, endogenous HO-1 upregulation was seen in air-treated control kidney grafts at day 90, whereas CO-treated allografts showed less HO-1 expression (data not shown). The results may indicate that endogenous HO-1 expression associates with renal graft injury, and effective treatments appear to inhibit HO-1 upregulation. Thus, CO in this study does not seem to involve an HO-1-dependent pathway in inhibiting CAN progression.
CAN is a clinicopathological description and often diagnosed after fibrosis is progressed. Due to its nonspecific nature, and the tendency to use the term as a specific disease entity, elimination of the term CAN and its replacement by the more accurate term “interstitial fibrosis and tubular atrophy, no evidence of any specific etiology” has been recommended (26). Specific disorders that may elicit some of the features of CAN include chronic hypertension, calcineurin toxicity, chronic obstruction, bacterial pyelonephritis, and polyomavirus infection. Although specific therapy in these cases as well as control of common risk factors (e.g., hypertension, hyperglycemia) might slow the disease progression, effective therapeutic strategies to stop the fibrotic process and restore renal function are limited. Further investigation and better understanding of the mechanisms that lead to kidney allograft injury and ultimately CAN will assist to develop effective treatment; however, CO appears to have potent antifibrotic properties to restore renal allograft function. Similar antifibrotic actions of CO and the reversal of pulmonary fibrosis/hypertension in mice have been shown by Zuckerbraun et al. (36).
In conclusion, the study demonstrates potent anti-inflammatory and antifibrotic effects of CO in the rat model of CAN. Low-dose inhaled CO was able to restore renal allograft function, even when CO treatment was initiated after significant fibrosis and deterioration of renal function were observed. CO reduced inflammatory reactions, inhibited renal fibrosis, and decreased infiltrates, in part through the downregulation of TGF-β and Smad pathways, as well as ERK-MAPK pathways. Our data show that CO may be used as a rescue therapy for restoring renal allograft function after CAN establishment, which may be more applicable for clinical patients rather than preventative CO administration before disease onset.
GRANTS
This work was supported by National Institutes of Health Grant DK-071753.
Acknowledgments
We thank M. Tabacek and L. Chedwick for excellent technical support and C. Forsythe for preparation and organization of the manuscript.
REFERENCES
- 1.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 Mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci 115: 3193–3206, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bicknell GR, Williams ST, Shaw JA, Pringle JH, Furness PN, Nicholson ML. Differential effects of cyclosporin and tacrolimus on the expression of fibrosis-associated genes in isolated glomeruli from renal transplants. Br J Surg 87: 1569–1575, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol 16: 3015–3026, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Corsi MM, Leone G, Fulgenzi A, Wasserman K, Leone F, Ferrero ME. RANTES and MCP-1 chemokine plasma levels in chronic renal transplant dysfunction and chronic renal failure. Clin Biochem 32: 455–460, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol 21: 659–693, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs P, Berkley LM, Bolton EM, Briggs JD, Bradley JA. Adhesion molecule expression (ICAM-1, VCAM-1, E-selectin and PECAM) in human kidney allografts. Transpl Immunol 1: 109–113, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Gourishankar S, Halloran PF. Late deterioration of organ transplants: a problem in injury and homeostasis. Curr Opin Immunol 14: 576–583, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J 17: 1576–1578, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 202: 1703–1713, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero-Fresneda I, Torras J, Cruzado JM, Condom E, Vidal A, Riera M, Lloberas N, Alsina J, Grinyo JM. Do alloreactivity and prolonged cold ischemia cause different elementary lesions in chronic allograft nephropathy? Am J Pathol 162: 127–137, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens 13: 279–284, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Lee BS, Heo J, Kim YM, Shim SM, Pae HO, Chung HT. Carbon monoxide mediates heme oxygenase 1 induction via Nrf2 activation in hepatoma cells. Biochem Biophys Res Commun 343: 965–972, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol 164: 1389–1397, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci USA 103: 5191–5196, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita T, Perrella MA, Lee ME, Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA 92: 1475–1479, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med 10: 650–671, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, Murase N. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol 163: 1587–1598, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant 5: 282–291, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A, Toyokawa H, Abe M, Kiyomoto T, Nakahira K, Choi AM, Nalesnik MA, Thomson AW, Murase N. Heart allograft protection with low-dose carbon monoxide inhalation: effects on inflammatory mediators and alloreactive T-cell responses. Transplantation 81: 220–230, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol 287: F979–F989, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Neto JS, Nakao A, Toyokawa H, Nalesnik MA, Romanosky AJ, Kimizuka K, Kaizu T, Hashimoto N, Azhipa O, Stolz DB, Choi AM, Murase N. Low-dose carbon monoxide inhalation prevents development of chronic allograft nephropathy. Am J Physiol Renal Physiol 290: F324–F334, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Pratschke J, Paz D, Wilhelm MJ, Laskowski I, Kofla G, Vergopoulos A, MacKenzie HJ, Tullius SG, Neuhaus P, Hancock WW, Volk HD, Tilney NL. Donor hypertension increases graft immunogenicity and intensifies chronic changes in long-surviving renal allografts. Transplantation 77: 43–48, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Robertson H, Ali S, McDonnell BJ, Burt AD, Kirby JA. Chronic renal allograft dysfunction: the role of T cell-mediated tubular epithelial to mesenchymal cell transition. J Am Soc Nephrol 15: 390–397, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Sawle P, Foresti R, Mann BE, Johnson TR, Green CJ, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol 145: 800–810, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (CAN). Am J Transplant 7: 518–526, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, Afrazi A, Gandhi C, Tokita D, Geller DA, Murase N. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology 48: 1608–1620, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Tullius SG, Heemann U, Hancock WW, Azuma H, Tilney NL. Long-term kidney isografts develop functional and morphologic changes that mimic those of chronic allograft rejection. Ann Surg 220: 425–432, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyler JR, Robertson H, Booth TA, Burt AD, Kirby JA. Chronic allograft nephropathy: intraepithelial signals generated by transforming growth factor-beta and bone morphogenetic protein-7. Am J Transplant 6: 1367–1376, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science 259: 381–384, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, Backman L, Burdick J. Interleukin-2 receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med 338: 161–165, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant 5: 1367–1374, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 10: 48–56, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Fraser D, Phillips A. ERK, p38, and Smad signaling pathways differentially regulate transforming growth factor-beta1 autoinduction in proximal tubular epithelial cells. Am J Pathol 169: 1282–1293, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z, Song R, Fattman CL, Greenhill S, Alber S, Oury TD, Choi AM, Morse D. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol 166: 27–37, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med 203: 2109–2119, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]