Abstract
Exposure to prolonged hypoxia can result in pulmonary vascular remodeling and pulmonary hypertension. Hypoxia induces pulmonary vascular smooth muscle cell (PVSMC) proliferation and vascular remodeling by affecting cell adhesion and migration and secretion of extracellular matrix proteins. We previously showed that acute hypoxia decreases cGMP-dependent protein kinase (PKG) activity in PVSMC and that PKG plays a role in maintaining the differentiated contractile phenotype in normoxia. In this study, we investigated the effect of hypoxia on PVSMC adhesion and migration and the role of PKG in these functions. Ovine fetal pulmonary artery SMC were incubated in normoxia (Po2 ∼100 Torr) or hypoxia (Po2 ∼30–40 Torr) or treated with the PKG inhibitor DT-3 for 24 h in normoxia. To further study the role of PKG in the modulation of adhesion and migration, PVSMC were transiently transfected with a full-length PKG1α [PKG-green fluorescent protein (GFP)] or a dominant-negative construct (G1αR-GFP). Cell adhesion to extracellular matrix proteins was determined, and integrin-mediated adhesion was assessed by α/β-integrin-mediated cell adhesion array. Exposure to hypoxia (24 h) and pharmacological inhibition of PKG1 by DT-3 significantly promoted adhesion mediated by α4-, β1-, and α5β1-integrins to fibronectin, laminin, and tenacin and also resulted in increased cell migration. Likewise, inhibition of PKG by expression of a dominant-negative PKG1α construct increased cell adhesion and migration, comparable to that induced by hypoxia. Dynamic actin reorganization associated with integrin-mediated cell adhesion is partly regulated by the actin-binding protein cofilin, the (Ser3) phosphorylation of which inhibits its actin-severing activity. We found that increased PKG expression and activity is associated with decreased cofilin (Ser3) phosphorylation, implying a role for PKG in the modulation of cofilin activity and actin dynamics. Together, these findings identify cGMP/PKG1 signaling as central to the functional differences between PVSMC exposed to normoxia versus hypoxia.
Keywords: pulmonary artery, vascular remodeling
chronic hypoxia can induce pulmonary vascular remodeling with thickening of the vascular smooth muscle coat and altered vasoreactivity, changes that result in pulmonary hypertension (7, 21). Vascular smooth muscle cell (VSMC) responses to environmental stresses such as chronic hypoxia are characterized by alterations in the smooth muscle cell (SMC) to the synthetic phenotype, demonstrating increased proliferation, increased secretion of extracellular matrix (ECM) proteins, and enhanced motility of SMC (15).
Our laboratory showed previously (8) that cGMP-dependent protein kinase (PKG) plays an important role in nitric oxide-cGMP-mediated relaxation in fetal pulmonary arteries and veins under normoxic conditions. Earlier, we reported that acute hypoxia decreases PKG activity in fetal pulmonary vascular smooth muscle. In acute hypoxia, PKG activity is significantly lower than in normoxia, in both the arteries and veins, although the effect of hypoxia is greater in veins (9). PKG has also been found to have other functions in vascular smooth muscle, including in the systemic circulation. Lincoln et al. (12, 13) reported that PKG is essential for maintaining the contractile phenotype of aortic smooth muscle. We also showed previously (28) that hypoxia-induced decreases in PKG activity in fetal pulmonary vascular smooth muscle are associated with loss of the contractile properties and the differentiated phenotype of pulmonary VSMC.
Integrins interact with ECM proteins to facilitate adherence of the contractile SMC to the ECM (15). Integrins regulate SMC functions such as adhesion, migration, and proliferation. When the SMC becomes dedifferentiated, a different set of adhesion receptors are expressed in the motile, synthetic, noncontractile SMC. Disengagement of adhesion receptors is required for the synthetic and migratory activities of motile SMC (15). For cells to migrate, there must be initial transient attachments at the leading edge occurring concurrently with the release of the receding edge. There is a delicate balance that determines the extent of migration. Adhesion must not be too strong to prevent movement, yet adhesion must not be too weak to provide traction (1).
In this study, we investigated the effect of hypoxia on the adhesion and migration properties of ovine fetal pulmonary artery SMC (FPASMC). We also explored the role of PKG in regulating cell adhesion and migration by studying the effects of inhibition of PKG expression and function.
METHODS
Reagents.
Rabbit polyclonal anti-green fluorescent protein (GFP) antibody was obtained from Abcam (Cambridge, MA). Rabbit polyclonal antibodies against cofilin, phospho-cofilin (Ser3), vasodilator-stimulated phosphoprotein (VASP) (A290), and phospho-VASP (Ser239) were obtained from Cell Signaling Technologies. Anti-PKG (CT) antibody was obtained from StressGen Bioreagents. All other reagents were obtained from Sigma unless otherwise specified.
Cell culture.
Ovine FPASMC were isolated and prepared as described previously (17). The cells were cultured in DMEM containing 10% heat-inactivated fetal bovine serum (FBS), 400 ng/ml amphotericin B, and 160 U/ml of penicillin and streptomycin (Invitrogen). Cells were cultured in a humidified incubator with constant supply of 5% CO2 at 37°C. For all experiments, subconfluent (50–70%) FPASMC (passages 3–6) were growth arrested in 0.3% FBS starvation medium for 24 h before treatment/assay.
Hypoxia and normoxia treatments.
A humidified box for cell culture (C-Chamber Series, BioSpherix) was used as a hypoxia chamber. A gas mixture of 5% CO2, balance N2 was used to maintain the chamber at 2% oxygen for hypoxia (Po2 ∼30–40 Torr in the medium), and the oxygen concentration was monitored with an oxygen sensor (ProOxC, BioSpherix). For normoxia experiments, cells were incubated in a humidified incubator with constant supply of 5% CO2 at 37°C (21% oxygen, Po2 ∼100 Torr in the medium). The Po2 in the cell medium was 30–40 Torr during hypoxia and ∼100 Torr during normoxia. Cells were exposed to hypoxia or normoxia for 24 h before the experiments were performed.
Treatment of FPASMC with PKG inhibitor in normoxia.
Subconfluent cells were starved for 24 h in 0.3% FBS-containing DMEM and treated with DT-3 (3 × 10−6 M, Calbiochem), a membrane-permeant peptide inhibitor of PKG1α, for 24 h, and then the cells were resuspended in the presence or absence of DT-3 before use in adhesion assays and the Boyden chamber method for migration assay.
Transient transfection of FPASMC.
Plasmids encoding full-length PKG1α tagged with GFP (PKG-GFP) or a dominant-negative construct consisting of a truncation encoding the regulatory domain of PKG1α (PKG G1αR-GFP) in pEGFP-N1 vector (Clontech) were kindly provided by Dr. Darren D. Browning (Medical College of Georgia, Augusta, GA). The PKG G1αR-GFP truncated protein was found to dimerize with endogenous type 1 PKG and behave in a dominant-negative manner (3). FPASMC were transfected with PKG-GFP, PKG G1αR-GFP, or vector alone (pEGFP-N1) with Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer's protocol. Briefly, cells were seeded at a density of ∼6.3 × 104/cm2 in DMEM with 10% FBS and allowed to attach overnight. Transfection was performed 1 day after seeding with 2.5 μg of DNA, 2.5 μl of PLUS reagent, and 6.25 μl of Lipofectamine LTX per well. Cells were trypsinized and seeded onto P100 tissue culture plates 48 h after transfection. The next day, cells were starved overnight and treated with hypoxia or normoxia for 24 h. Transfection efficiency was confirmed by Western blot analysis of PKG expression and by assaying PKG kinase activity. Because of the similarity in size of GFP (∼30 kDa) to the deleted portion of PKG, the molecular mass of the fusion protein is 85 kDa (3).
Western immunoblot analysis.
Cell lysates were prepared from treated and untreated cells. After appropriate treatment, cells were washed with PBS, lysed with RIPA buffer (50 mM Tris·HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS; Boston BioProducts) containing protease inhibitor cocktail (Roche Diagnostics) and PMSF (1 mM), sonicated twice on ice for 5 s each, and centrifuged (12,000 g, 10 min, 4°C), and supernatant was stored at −80°C. Protein concentration was determined with the BCA Protein Assay Kit (Pierce). Cell extracts were separated by SDS-PAGE, transferred onto nitrocellulose membranes, blocked with 5% nonfat milk in TBS-T (20 mM Tris pH 7.4, 137 mM NaCl, 0.1% Tween 20), and incubated at 4°C overnight with primary antibodies that were diluted in 5% BSA in TBS-T. Membranes were then washed with TBS-T and incubated with diluted secondary antibody for 1 h at room temperature. SuperSignal West Pico chemiluminescent substrate (Pierce) was used for signal generation. The primary antibodies used were anti-PKG type I (catalog no. KAS-PK005, polyclonal, dilution 1:5,000; Stressgen, Victoria, BC, Canada); anti-VASP (catalog no. 3120, polyclonal, dilution 1:1,000; Cell Signaling Technology, Danvers, MA); anti-phospho-VASP (Ser239) (catalog no. 3114, polyclonal, dilution 1:1,000; Cell Signaling Technology); anti-cofilin (catalog no. 3318, polyclonal, dilution 1:1,000; Cell Signaling Technology); anti-phospho-cofilin (Ser3) (catalog no. 3313, polyclonal, dilution 1:1,000; Cell Signaling Technology), anti-actin antibody (catalog no. CP01, monoclonal, dilution 1:10,000; EMD Chemicals, La Jolla, CA), and anti-GFP (catalog no. ab6556, polyclonal, dilution 1:2,000; Abcam). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies were used at 1:2,000 dilution [for VASP, phospho-VASP(Ser239), cofilin, phospho-cofilin(Ser3)], 1:5,000 (for PKG1), and 1:20,000 (for actin).
PKG kinase assay.
Cultured FPASMC were rinsed with ice-cold PBS, harvested and sonicated in a buffer containing 50 mM Tris·HCl (pH 7.4), 10 mM EDTA, 2 mM dithiothreitol, 1 mM 3-isobutyl-1-methylxanthine, 100 μM nitro-l-arginine, and 10 μM indomethacin, and centrifuged at 13,000 g for 10 min at 4°C. Supernatants were assayed for PKG activity by measuring the incorporation of 32P from [γ-32P]ATP into a specific PKG substrate, BPDEtide (Bachem, King of Prussia, PA), as described by us previously (9). Aliquots (20 μl) of tissue extract were added to a mixture (total volume 50 μl) containing 50 mM Tris·HCl (pH 7.4), 20 mM MgCl2, 0.1 mM 3-isobutyl-1-methylxanthine, 10 μM indomethacin, 100 μM nitro-l-arginine, 150 μM BPDEtide, 1 μM PKI (a synthetic PKA inhibitor; Peninsula Laboratories, Belmont, CA), and 0.2 mM [γ-32P]ATP (specific activity 3,000 Ci/mmol). The mixture was incubated at 30°C for 10 min in the presence or absence of 3 μM exogenous cGMP. In the absence of added cGMP, the constitutive kinase activity is measured and the addition of cGMP elicits the cGMP-stimulated PKG kinase activity. The reaction was terminated by spotting 40-μl aliquots of mixture on phosphocellulose papers (2 × 2 cm; P81, Whatman) and placing them in ice-cold 75 mM phosphoric acid. The filter papers were washed, dried, and counted in a liquid scintillation counter. Assays were performed in triplicate with appropriate controls. After control counts are subtracted, the counts obtained indicate PKG activity, which was expressed as picomoles of 32P incorporated into PKG substrate per minute per milligram of protein.
Adhesion assay.
To study cell adhesion to ECM proteins, adhesion assays were performed on ECM Array Plates (Chemicon/Millipore). Each eight-well strip consists of seven different human ECM protein-coated wells (collagen I, collagen II, collagen IV, fibronectin, laminin, tenascin, vitronectin) and one BSA-coated well (negative control). Briefly, wells were rehydrated with PBS for 15 min at room temperature, and the PBS was removed before seeding of cells. Treated FPASMC were then harvested and plated at 3 × 105 cells per well in serum-free culture medium and incubated at 37°C for 1 h. Medium and nonadherent cells were then removed from the wells and rinsed gently with PBS, and adherent cells were fixed and stained with 0.2% crystal violet in 10% acetic acid. The dye was solubilized with a buffer of 0.1 M NaH2PO4 and 50% ethanol, and absorbance (570 nm) was measured.
Integrin-mediated binding assays.
Integrin-mediated adhesion was examined with α/β Integrin-Mediated Cell Adhesion Array Combo Kits (Chemicon/Millipore). These kits use mouse monoclonal antibodies generated against human α (α1, α2, α3, α4, α5, αV, and αVβ3)- and β (β1, β2, β3, β4, β6, αVβ5, and α5β1)-integrins/subunits that are immobilized onto a goat anti-mouse antibody-coated microtiter plate. The plate is then used to capture cells expressing these integrins on their cell surface. Cells were exposed to normoxia, hypoxia, or normoxia in the presence of DT-3 (3 μM) for 24 h, harvested, plated at 3 × 105 cells per well in triplicate in serum-free culture medium, and incubated at 37°C for 1 h. Medium and unbound cells were removed from the wells and rinsed gently twice with 200 μl of assay buffer. One hundred microliters of cell stain solution was added to each well and incubated for 5 min at room temperature. The plates were washed three times with deionized water and allowed to air dry. One hundred microliters of extraction buffer was added to each well, and absorbance (570 nm) was measured.
Wound healing migration assay.
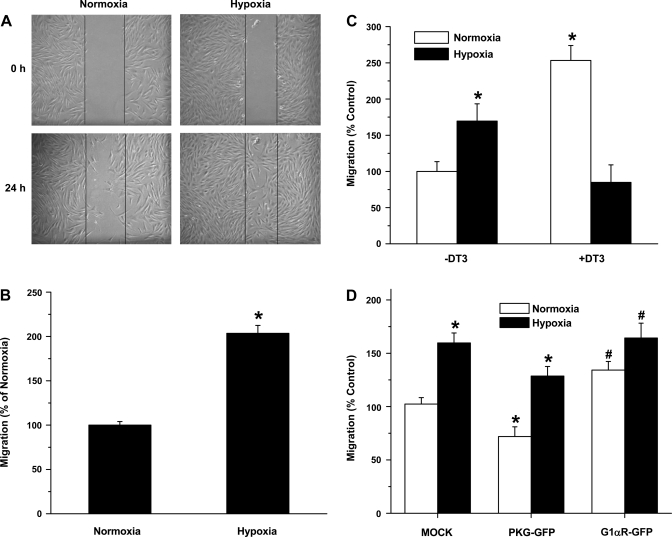
To study cell migration, a wound healing method was used. FPASMC were grown to confluence on 60-mm dishes. Cells were then growth arrested for 24 h, and 5 mM hydroxyurea was added to prevent cell proliferation. A scratch was made in the cell monolayer, baseline images were captured, and the dishes were placed in hypoxia or normoxia. Migration was assessed at 24 h by counting the number of cells that migrated across the scratch and normalized to scratch area. Differences among control and treated groups were expressed as percentage of control (assigned 100%) and shown in Fig. 5 as means ± SE. Migration data were obtained from three independent wound healing experiments.
Boyden chamber migration assay.
Cell migration was also studied with an 8-μm-pore size Transwell chemotaxis cell migration assay kit (Chemicon International) according to the manufacturer's protocol. Briefly, FPASMC were first treated with normoxia, hypoxia, or normoxia with PKG inhibitor and harvested, and 5 × 105 cells were plated into each well of the migration chamber, with each condition performed in triplicate. DMEM without FBS was added to the bottom wells and incubated for 24 h at 37°C in normoxia or hypoxia. Migration assay was also performed with the transfected cells. Cells that did not migrate were gently discarded from the top; the migration chamber plate then placed onto a new feeder tray containing prewarmed cell detachment solution and incubated for 30 min at 37°C. Cells that migrated were lysed with a lysis buffer containing the patented CyQuant green-fluorescent dye (Molecular Probes), and fluorescence was determined at 480/520 nm. Differences among control and treated groups were expressed as percentage of control (assigned 100%) and shown in Fig. 5 as means ± SE.
Data analysis.
Student's t-test for unpaired observations was used to compare the mean values of two groups. Comparison of mean values of more than two groups was made with one-way ANOVA test with Student-Newman-Keuls test for post hoc testing of multiple comparisons. Statistical significance was accepted when the P value (2 tailed) was <0.05.
RESULTS
Hypoxia increases cell adhesion to matrix proteins.
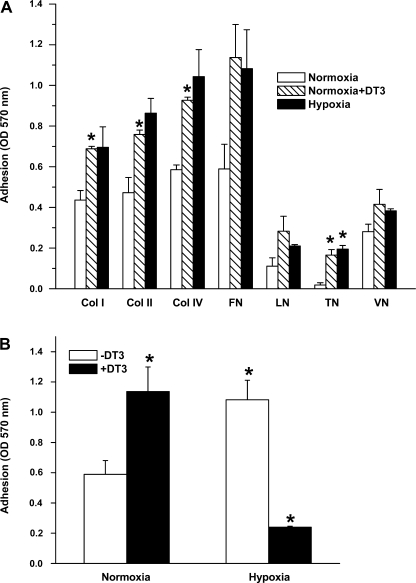
The effect of hypoxia on PASMC adhesion was studied after 24 h of treatment (Fig. 1A). We explored cell adhesion to different matrix proteins (collagen I, collagen II, collagen IV, fibronectin, laminin, and vitronectin) and found that cell adhesion to the four matrix proteins increased by 25–50% after 24 h of hypoxia. The increase noted was statistically significant for collagen I, collagen IV, and tenacin, with a trend toward significance for cell adhesion to fibronectin and laminin. The role of PKG in the modulation of cell adhesion properties in hypoxia was studied by treating cells with the PKG1-specific inhibitor DT-3 for 24 h under normoxic conditions. PKG inhibition resulted in increased cell adhesion, a finding similar to that in cells treated with hypoxia. The effect of PKG1 inhibition with DT-3 in hypoxia was examined by monitoring adhesion properties of cells exposed to hypoxia in the presence of DT-3 (Fig. 1B). We found that in hypoxia DT-3 leads to a significant decrease in adhesion, below that observed in normoxia, and speculate that this may be due to additive effects of hypoxia and DT-3 on PKG activity or perhaps due to nonspecific effects.
Fig. 1.
A: hypoxia-induced increased adhesion of fetal pulmonary artery smooth muscle cells (FPASMC) to extracellular matrix proteins and effect of pharmacological inhibition of cGMP-dependent protein kinase (PKG) by DT-3. col I, col II, col IV, FN, LN, TN, and VN correspond to collagen I-, collagen II-, collagen IV-, fibronectin-, laminin-, and vitronectin-coated wells, respectively. FPASMC were exposed to normoxia, hypoxia, or normoxia in the presence of DT-3 (3 μM) for 24 h, harvested, and incubated for 1 h at 37°C in wells coated with extracellular matrix proteins. Values are means ± SE (n = 4). *Significantly different from respective values observed in control (P < 0.05). OD, optical density. B: effect of DT-3 on adhesion of FPASMC exposed to hypoxia to fibronectin. Cells were incubated in normoxia or hypoxia in the presence or absence of DT-3 for 24 h. Values are means ± SE (n = 4). *Significantly different from normoxia (−DT-3) (P < 0.05).
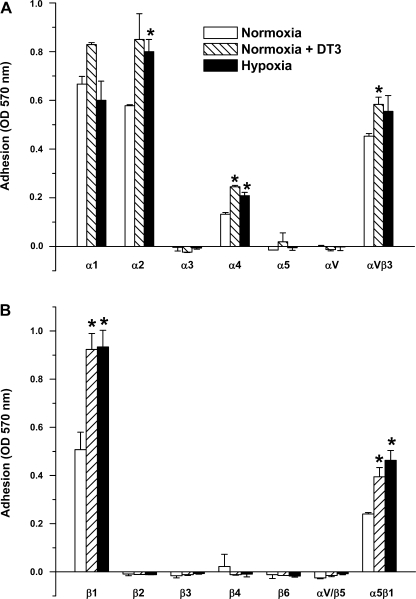
Effect of hypoxia on integrin binding.
Since ECM protein adhesion is mediated by integrins, we examined the effects of hypoxia treatment on α (Fig. 2A) - and β (Fig. 2B)-integrin binding in FPASMC. FPASMC were exposed to normoxia, hypoxia, or DT-3 in normoxia for 24 h. Under normoxia, significant binding to α1, α2, αVβ3, β1, and, to a lesser extent, to α4 and α5β1 was observed. Treatment with hypoxia or with DT-3 increased adhesion to these integrins, with the most significant effect being on α4, β1, and α5β1.
Fig. 2.
α-Integrin (A)- and β-integrin (B)-mediated binding profile of FPASMC exposed to normoxia, hypoxia, or normoxia in the presence of PKG inhibitor DT-3 (3 μM) for 24 h, harvested, and incubated for 1 h at 37°C in wells coated with anti-α- or anti-β-integrin monoclonal antibodies. Values are means ± SE (n = 3). *Significantly different from respective values observed in control (P < 0.05).
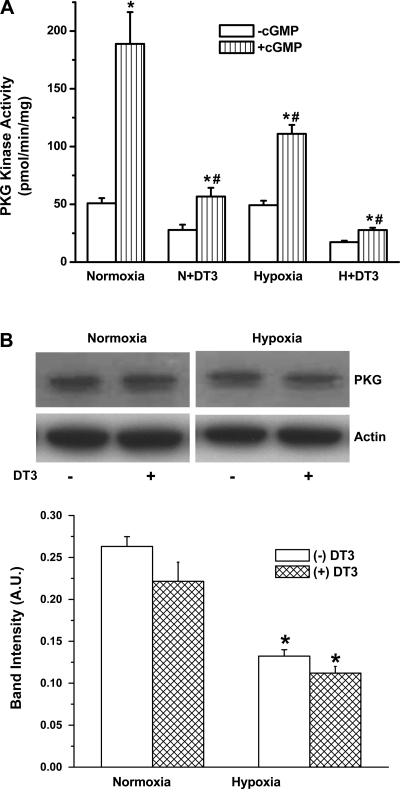
Hypoxia decreases PKG expression and kinase activity.
Constitutive PKG kinase activity, in the absence of exogenous cGMP, in FPASMC under normoxia or hypoxia was significantly lower than PKG kinase activity in the presence of exogenous cGMP (Fig. 3A). This observation is consistent with the ability of cGMP to bind and activate PKG (14). Constitutive and cGMP-stimulated PKG kinase activities were reduced by treatment with DT-3 in normoxia or hypoxia compared with control, while exposure to hypoxia alone inhibited the cGMP-stimulated kinase activity. Immunoblot analyses indicate that, under similar conditions, PKG protein expression is decreased by exposure to hypoxia or DT-3 (Fig. 3B).
Fig. 3.
Downregulation of PKG kinase activity (A) and protein expression (B) in FPASMC after exposure to normoxia or hypoxia for 24 h in the presence or absence of 3 μM DT-3. (−)cGMP, without cGMP; (+)cGMP, with 3 × 10−6 M cGMP. Values are means ± SE (n = 4). *Significantly different from respective (−)cGMP values (P < 0.05); #significantly different from normoxia (+)cGMP value (P < 0.05). B: PKG and actin expression in cell extracts of FPASMC exposed to 24-h normoxia or hypoxia in the presence or absence of DT-3. Top: representative blots of PKG and actin expression in homogenates exposed to normoxia (control), normoxia + DT-3, hypoxia, or hypoxia + DT-3. Bottom: densitometric quantification of PKG protein normalized to actin. AU, arbitrary units. Values are means ± SE (n = 4). *Significantly different from normoxia (−DT-3) (P < 0.05).
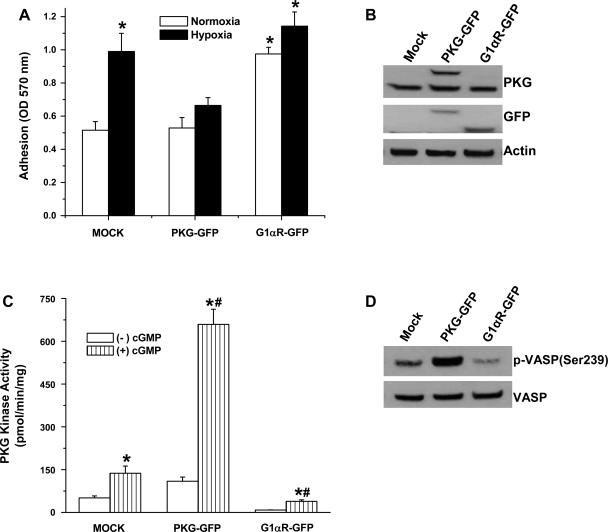
PKG inhibition with expression of dominant-negative PKG construct mimics the effect of hypoxia on cell adhesion.
To further study the role of PKG in modulating cell adhesion properties, we transiently transfected FPASMC with plasmids encoding full-length PKG1α tagged with GFP (PKG-GFP), a dominant-negative construct consisting of a truncation encoding the regulatory domain of PKG1α (PKG G1αR-GFP), or vector alone (pEGFP-N1). Figure 4A shows that there is a significant increase in cell adhesion to fibronectin in PKG G1αR-GFP-transfected cells compared with mock (pEGFP-N1 vector)-transected or PKG-GFP-transfected cells. Hypoxia induced a significant increase in adhesion in cells transfected with vector alone, comparable to that of cells treated with hypoxia and cells treated with DT-3 in the presence of normoxia (Fig. 1A), whereas PKG overexpression blunted this effect. A slight but insignificant increase in adhesion was observed upon exposure to hypoxia of cells expressing the dominant-negative construct (PKG G1αR-GFP). Transfection efficiency in these cells was confirmed by analysis of PKG protein expression (Fig. 4B) and kinase activity (Fig. 4C). Expression of wild-type PKG induces a significant increase in constitutive and cGMP-stimulated kinase activities, while the dominant-negative construct inhibits both. The effect of PKG expression on VASP phosphorylation levels was examined by immunoblot detection of VASP(Ser239). PKG-mediated phosphorylation of VASP, a ubiquitously expressed endogenous substrate for PKG and biomarker for activation of the cGMP/PKG pathway, was markedly elevated in cells expressing PKG (Fig. 4D).
Fig. 4.
Increased cell adhesion to fibronectin by PKG inhibition with transient transfection of FPASMC with a green fluorescent protein (GFP)-tagged dominant-negative PKG construct. A: FPASMC transfected with pEGFP-N1 vector (mock), wild-type PKG (PKG-GFP), or PKG 1αR-GFP (dominant negative) were exposed to 24-h normoxia or hypoxia, harvested, and allowed to adhere to fibronectin-coated wells for 1 h at 37°C. Values are means ± SE (n = 3). *Significantly different from mock, normoxia value (P < 0.05). B and C: immunoblot detection of PKG expression (B) using anti-PKG antibody and measurement of kinase activity (C) in the presence or absence of 3 μM cGMP in FPASMC transiently transfected with vector alone (mock), wild-type PKG (PKG-GFP), and a dominant-negative PKG construct (G1αR-GFP). Because of the similarity in size of GFP (∼30 kDa) to the deleted portion of PKG, the molecular mass of the fusion protein is 85 kDa. *Significantly different from respective (−)cGMP values (P < 0.05); #significantly different from normoxia (+)cGMP value (P < 0.05). D: expression of endogenous PKG substrate vasodilator-stimulated phosphoprotein (VASP) and immunodetection of phospho (p)-VASP (Ser239) in transfected cells.
Hypoxia and PKG inhibition with DT-3 or by expression of dominant-negative construct increase cell migration.
The effect of hypoxia on cell migration was studied with the wound healing model (Fig. 5A). There was a significant increase in cell migration at 24 h of hypoxia. To confirm the findings seen with the wound healing model, we studied cell migration with the Boyden chamber method. We found a statistically significant increase in cell migration in cells exposed to hypoxia (Fig. 5B). There was a statistically significant increase in cell migration when cells were treated with DT-3 (Fig. 5C), as was seen in cells treated with hypoxia. The role of PKG in cell migration was further evaluated with FPASMC transiently transected with plasmids encoding full-length PKG1α (PKG-GFP) or a dominant-negative PKG1α construct (PKG G1αR-GFP) or vector alone. Figure 5D shows that there was a small, but significant, decrease in cell migration in PKG1α-expressing cells, while PKG G1αR-GFP transfection induced an increase in cell migration compared with vector only-transfected cells. In all cases, hypoxia induced increased cell migration, although the effect observed with wild-type PKG-expressing cells was blunted. These findings are comparable to those in cells treated with hypoxia and cells treated with DT-3 in the presence of normoxia.
Fig. 5.
Downregulation of PKG in hypoxia or inhibition of PKG in normoxia with a dominant- negative PKG construct (G1αR-GFP) or PKG1 inhibitor DT-3 (3 μM) leads to increased FPASMC migration. A: hypoxia-induced increase in migration of FPASMC by wound healing model. Scratch on cell monolayer (0 h) and migration at 24-h normoxia and hypoxia. B: migration was assessed at 24 h by counting the number of cells that migrated across the scratch, normalized to scratch area and presented as %, with normoxia set at 100%. Values are means ± SE (n = 4). C: Boyden chamber migration assay of FPASMC treated with normoxia, hypoxia, or normoxia in the presence or absence of PKG1 inhibitor (DT3, 3 μM). Migration is presented as %, with normoxia (−DT3) set at 100%. Values are means ± SE (n = 3). *Significantly different from normoxia (−DT3) value (P < 0.05). D: Boyden chamber migration assay of FPASMC transfected with vector alone (pEGFP-N1, mock), PKG-GFP, or PKG 1αR-GFP (dominant negative) constructs. Migration is presented as %, with vector set at 100%. Values are means ± SE (n = 3). *Significantly different from respective normoxia values (P < 0.05); #significantly different from mock, normoxia value (P < 0.05).
Modulation of cofilin phosphorylation by PKG.
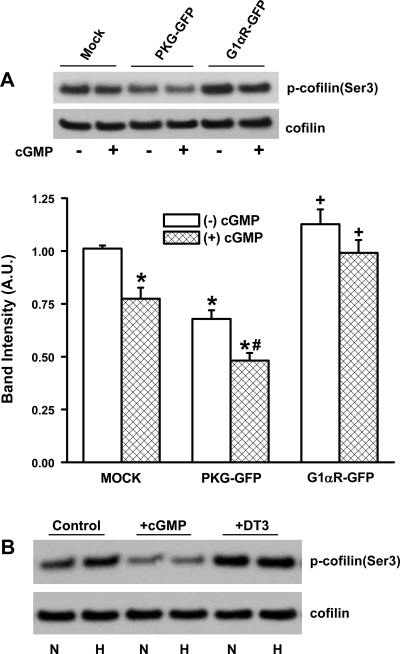
Integrin-mediated actin reorganization and focal adhesion formation, critical components of cell adhesion and migration, are regulated by actin-binding proteins such as cofilin (23). The activity of cofilin is reversibly regulated by phosphorylation and dephosphorylation at Ser3, with the phosphorylated form being inactive. To assess the possible role of PKG in modulating cofilin activation, we determined cofilin expression and phospho-cofilin(Ser3) levels in FPASMC transiently transfected with vector alone (pEGFP-N1, mock), PKG-GFP, or PKG 1αR-GFP (dominant negative) constructs (Fig. 6A). Cofilin expression was unaltered, while cofilin phosphorylation changed depending on the expression and activity level of PKG. Cofilin phosphorylation decreased in PKG-GFP-expressing cells and increased in PKG G1αR-GFP-expressing cells. Treatment with cGMP, in all three cases, led to a slight decrease in cofilin phosphorylation. The role of hypoxia in modulating cofilin phosphorylation was further assessed by exposing cells to normoxia or hypoxia under conditions of PKG activation or inhibition (Fig. 6B). Upon exposure to hypoxia, cellular phospho-cofilin(Ser3) levels were elevated, and this increase was eliminated when PKG was activated by the addition of cGMP. The inhibition of PKG with DT-3 led to increased cofilin phosphorylation in both normoxia and hypoxia. These data suggest that PKG may play a role in the modulation of upstream signaling pathways that phosphorylate and dephosphorylate cofilin.
Fig. 6.
Modulation of cofilin phosphorylation by PKG. A: cofilin expression and phospho-cofilin(Ser3) levels were determined in cell extracts prepared from FPASMC transfected with vector alone (pEGFP-N1, mock), PKG-GFP, or PKG1αR-GFP (dominant negative) constructs and incubated in the presence or absence of cGMP (3 μM, 10 min, 37°C). Top: representative blots of phospho-cofilin(Ser3) and cofilin expression. Bottom: densitometric quantification of phospho-cofilin levels normalized to cofilin expression in each sample. Values are means ± SE (n = 4). *Significantly different from respective −cGMP values (P < 0.05); #significantly different from PKG-GFP, −cGMP value (P < 0.05); +significantly different from PKG-GFP −cGMP and +cGMP values (P < 0.05). B: cofilin expression and phospho-cofilin(Ser3) levels were determined in FPASMC exposed to normoxia (N) or hypoxia (H) in the presence of cGMP or DT-3. FPASMC were exposed to normoxia, hypoxia, or normoxia in the presence or absence of DT-3 (3 μM) for 24 h. cGMP (3 μM, 10 min, 37°C) was added to cells 10 min before the end of a 24-h incubation period.
DISCUSSION
This is the first study to link PKG to hypoxia-induced changes in adhesion and migration properties of PASMC. We previously reported that hypoxia downregulates PKG (9, 28) and that this downregulation is associated with altered SMC phenotype characterized by changes in the expression of SMC marker proteins (28). Here we demonstrate that cell adhesion to matrix proteins increases when PKG is inhibited, findings that mimic the effects of hypoxia. We also show that cell migration is increased both with hypoxia and with PKG inhibition in normoxia. These findings suggest that the downregulation of PKG with hypoxia may be the mechanism by which hypoxia alters cell adhesion and migration.
PKG plays a key role in nitric oxide-cGMP-mediated pulmonary vascular relaxation (8). We chose to study PKG since we showed previously (28) that PKG activity is decreased in hypoxia and that it is involved in SMC phenotype modulation. PKG expression has also been shown to be decreased in PASMC cultured from an ovine fetal model of chronic intrauterine pulmonary hypertension (19). Murphy-Ullrich et al. (16) showed that PKG is necessary to mediate focal adhesion disassembly triggered by the counteradhesive ECM protein thrombospondin and tenascin in rat aortic SMC. The present study is the first to establish the role of PKG in hypoxia-induced changes in pulmonary VSMC adhesion and cell migration. Our data show that inhibition of PKG leads to an increase in cell adhesion and migration. This would suggest that the decrease in PKG activity associated with hypoxia may be the mechanism by which hypoxia alters cell adhesion and migration. Therefore, we can propose that PKG may be a regulator of cell adhesion and migration in hypoxia.
Exposure to hypoxia can lead to pulmonary hypertension and is accompanied by pulmonary vascular cell proliferation and vascular remodeling (18). Environmental stressors such as hypoxia induce multiple effects including phenotypic change of VSMC from the quiescent contractile state to the active synthetic state, demonstrating increased motility and proliferation (27). Many studies of cell adhesion and migration in hypoxia have been limited to circulating blood cells, where an increase in adhesion has been shown in response to hypoxia (6, 22, 26). Corley et al. (5), however, reported a decrease in adhesion of VSMC from bovine coronary arteries when treated with cobalt chloride, an agent that simulates hypoxia. In our study, we show a significant increase (25–50%) in VSMC adhesion in response to hypoxia. The differential response may be attributed to the different sources of cell type.
The regulation of VSMC adhesion and migration in hypoxia is poorly understood; however, several groups have studied different pathways. There have been reports of specific proteins involved in promoting cell migration in hypoxia in different cell types, such as hypoxia-induced mitogenic factor in endothelial cells (24) and EGF receptor (EGFR) in adenocarcinoma cells (25). There have been reports showing the regulation of migration of VSMC through the mitogen-activated protein kinase ERK1/2 (2, 14). Corley et al. (5) reported the role of hypoxia-inducible factor in regulating VSMC adhesion and migration. However, they report a decrease in cell adhesion in VSMC when treated with cobalt chloride. Li et al. (11) found an increase in the expression of the novel adhesion molecule periostin and osteopontin expression in hypoxia and demonstrated the role of the phosphatidylinositol 3-kinase and Ras signaling pathways. These observed changes in adhesion and migration are important in understanding the steps leading to increased proliferation seen in hypoxia. The present study is the first to link PKG to the hypoxia-induced changes in VSMC adhesion and migration.
Pulmonary artery remodeling is a complex pathological process involving all three layers of the vascular wall, including SMC proliferation (21). Cell adhesion and migration are important factors involved in the regulation of cell proliferation, and alterations in cell adhesion and migration properties are necessary for cells to proliferate. Migrating cells extend protrusions in the direction of migration and are stabilized by adhering to the ECM (20). These adhesion sites provide traction for the migrating cells as the cell moves forward, while they are disassembled at the cell rear, allowing the cell to detach. Protein kinases have been shown to be central in the regulation of adhesion stability and turnover (20). Since hypoxia leads to PASMC proliferation, as evidenced by vascular remodeling, we decided to focus our investigation on the effects of hypoxia on cell adhesion and migration and the role of PKG. Others have shown the role of kinases in the regulation of migration in normoxia. Several groups have demonstrated that PKCδ regulates VSMC adhesion and migration (10, 14). Here we show that hypoxia increases cell adhesion to matrix proteins and migration in PASMC and that PKG seems to be mediating this process in hypoxia. Our findings agree with previous data suggesting that VSMC adhesion to matrix proteins is increased in hypoxia (2). The discrepancy in our finding that DT-3 can augment VSMC adhesion and migration under hypoxia while inhibiting hypoxic VSMC adhesion and migration suggests that DT-3 may have nonspecific effects in hypoxia. As we reported previously (17), hypoxia induces increased generation of cellular reactive oxygen and nitrogen species. Under such conditions, various target proteins, including kinases involved in the regulation of VSMC adhesion and migration, could be modified by reactive oxygen and nitrogen species and their structures altered, possibly becoming more susceptible to inhibition by DT-3 at the concentration used under our experimental conditions.
The actin cytoskeleton is a dynamic structure that plays an integral role in regulating cellular function and architectural integrity. The dynamics of polymerization/depolymerization of actin filaments, essential for cell movement, adhesion, and division (23), are coordinately regulated by a group of actin-binding proteins including cofilin (4). Cofilin, in turn, is negatively regulated by Ser3 phosphorylation. The present study demonstrates, for the first time, that PKG is a critical modulator of cofilin (Ser3) phosphorylation and thus actin cytoskeletal dynamics in VSMC.
In summary, we report that hypoxia increases both VSMC adhesion and migration and the response seen with hypoxia is mediated by PKG, as hypoxia downregulates PKG. Inhibitors to PKG also increase cell-ECM protein interactions and cell migration, results that are comparable to the effects of hypoxia. Our data suggest that PKG plays a critical role in regulating VSMC adhesion and migration in hypoxia, in part through the regulation of cofilin (Ser3) phosphorylation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-59435-05, R01-HL-075187, and R01/MIRS-HL-75187-01.
REFERENCES
- 1.Abedi H, Zachary I. Signaling mechanisms in the regulation of vascular cell migration. Cardiovasc Res 30: 544–556, 1995. [PubMed] [Google Scholar]
- 2.Blaschke F, Stawowy P, Goetze S, Hintz O, Grafe M, Kintscher U, Fleck E, Graf K. Hypoxia activates beta1-integrin via ERK 1/2 and p38 MAP kinase in human vascular smooth muscle cells. Biochem Biophys Res Commun 296: 890–896, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Browning DD, McShane M, Marty C, Ye RD. Functional analysis of type 1alpha cGMP-dependent protein kinase using green fluorescent fusion proteins. J Biol Chem 276: 13039–13048, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Bernstein BW, Bamburg JR. Regulating actin-filament dynamics in vivo. Trends Biochem Sci 25: 19–23, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem 96: 971–985, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Friedman GB, Taylor CT, Parkos CA, Colgan SP. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: role of intracellular cAMP. J Cell Physiol 176: 76–84, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Fukumoto Y, Tawara S, Shimokawa H. Recent progress in the treatment of pulmonary arterial hypertension: expectation for rho-kinase inhibitors. Tohoku J Exp Med 211: 309–320, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Dhanakoti S, Tolsa JF, Raj JU. Role of protein kinase G in nitric oxide- and cGMP-induced relaxation of newborn ovine pulmonary veins. J Appl Physiol 87: 993–998, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Dhanakoti S, Trevino EM, Sander FC, Portugal AM, Raj JU. Effect of oxygen on cyclic GMP-dependent protein kinase-mediated relaxation in ovine fetal pulmonary arteries and veins. Am J Physiol Lung Cell Mol Physiol 285: L611–L618, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya K, Ryer E, Sakakibara K, Zohlman A, Kent KC, Liu B. Protein kinase C delta activated adhesion regulates vascular smooth muscle cell migration. J Surg Res 141: 91–96, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Li P, Oparil S, Feng W, Chen YF. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol 97: 1550–1558, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Lincoln TM, Dey NB, Boerth NJ, Cornwell TL, Soff GA. Nitric oxide-cyclic GMP pathway regulates vascular smooth muscle cell phenotypic modulation: implications in vascular diseases. Acta Physiol Scand 164: 507–515, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11: 356–367, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Ryer EJ, Kundi R, Kamiya K, Itoh H, Faries PL, Sakakibara K, Kent KC. Protein kinase C-delta regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. J Vasc Surg 45: 160–168, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moiseeva EP Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res 52: 372–386, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Murphy-Ullrich JE, Pallero MA, Boerth N, Greenwood JA, Lincoln TM, Cornwell TL. Cyclic GMP-dependent protein kinase is required for thrombospondin and tenascin mediated focal adhesion disassembly. J Cell Sci 109: 2499–2508, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Negash S, Gao Y, Zhou W, Liu J, Chinta S, Raj JU. Regulation of cGMP-dependent protein kinase-mediated vasodilation by hypoxia-induced reactive species in ovine fetal pulmonary veins. Am J Physiol Lung Cell Mol Physiol 293: L1012–L1020, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 30: 364–372, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Resnik E, Herron J, Keck M, Sukovich D, Linden B, Cornfield DN. Chronic intrauterine pulmonary hypertension selectively modifies pulmonary artery smooth muscle cell gene expression. Am J Physiol Lung Cell Mol Physiol 290: L426–L433, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 59: 89–144, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Sultana C, Shen Y, Johnson C, Kalra VK. Cobalt chloride-induced signaling in endothelium leading to the augmented adherence of sickle red blood cells and transendothelial migration of monocyte-like HL-60 cells is blocked by PAF-receptor antagonist. J Cell Physiol 179: 67–78, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Therriot JA Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton. J Cell Biol 136: 1165–1168, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong Q, Zheng L, Li B, Wang D, Huang C, Matuschak GM, Li D. Hypoxia-induced mitogenic factor enhances angiogenesis by promoting proliferation and migration of endothelial cells. Exp Cell Res 312: 3559–3569, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Niki T, Goto A, Ota S, Morikawa T, Nakamura Y, Ohara E, Ishikawa S, Aburatani H, Nakajima J, Fukayama M. Hypoxia increases the motility of lung adenocarcinoma cell line A549 via activation of the epidermal growth factor receptor pathway. Cancer Sci 98: 506–511, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weis M, Schlichting CL, Engleman EG, Cooke JP. Endothelial determinants of dendritic cell adhesion and migration: new implications for vascular diseases. Arterioscler Thromb Vasc Biol 22: 1817–1823, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Willis AI, Pierre-Paul D, Sumpio BE, Gahtan V. Vascular smooth muscle cell migration: current research and clinical implications. Vasc Endovascular Surg 38: 11–23, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L1459–L1466, 2007. [DOI] [PubMed] [Google Scholar]