Abstract
Cardiac L-type voltage-dependent Ca2+ channels are heteromultimeric polypeptide complexes of α1-, α2/δ-, and β-subunits. The α2/δ-1-subunit possesses a stereoselective, high-affinity binding site for gabapentin, widely used to treat epilepsy and postherpetic neuralgic pain as well as sleep disorders. Mutations in α2/δ-subunits of voltage-dependent Ca2+ channels have been associated with different diseases, including epilepsy. Multiple heterologous coexpression systems have been used to study the effects of the deletion of the α2/δ-1-subunit, but attempts at a conventional knockout animal model have been ineffective. We report the development of a viable conventional knockout mouse using a construct targeting exon 2 of α2/δ-1. While the deletion of the subunit is not lethal, these animals lack high-affinity gabapentin binding sites and demonstrate a significantly decreased basal myocardial contractility and relaxation and a decreased L-type Ca2+ current peak current amplitude. This is a novel model for studying the function of the α2/δ-1-subunit and will be of importance in the development of new pharmacological therapies.
Keywords: cardiac calcium channel, murine knockout model, gabapentin binding, myocardial contractility
cardiac L-type voltage-dependent Ca2+ channels (L-VDCCs) are heteromultimeric polypeptide complexes of α1-, α2/δ-, and β-subunits. The α1-subunit is autoregulatory and harbors the channel pore, gating machinery, and modulatory drug binding sites (30). The accessory subunits (α2/δ and β) affect channel kinetics and are involved in the trafficking and insertion of the α1-subunit into the membrane. The α2-subunit is closely associated with an extracellular loop of the α1-subunit (15) and linked to a small protein called δ (2, 9). Both the α2 and δ are encoded by the same gene, separated by proteolytic cleavage, and extracellularly linked through a disulfide bridge (9). Currently, four α2/δ-subunits, each encoded by separate genes, have been identified (4). The α2/δ-1, originally cloned from skeletal muscle (10), is ubiquitously distributed (18), with high levels of protein expression in brain, heart, skeletal, and smooth muscle (13). It possesses a stereoselective, high-affinity binding site for gabapentin (GBP) (1-aminomethylcyclohexane acetic acid; Neurontin), widely used to treat epilepsy, postherpetic neuralgic pain, and sleep disorders (8, 13). Of the remaining α2/δ-subunits, only α2/δ-2 has also shown a propensity for GBP binding, but the affinity is much lower than α2/δ-1 (18, 24).
It is clear that the α2/δ-subunits of VDCCs are associated with some disease states. A spontaneous mutation in the α2/δ-2 murine gene resulted in the Ducky mouse, demonstrating clinical signs of epilepsy (1, 3). Similar results to those obtained for the Ducky mouse were found in an α2/δ-2 knockout mouse (17). These results included ataxic gait, seizures, signs of cerebellar degeneration, and premature death. There were some differences noted in the pathology of the cerebellar region for these two mice. There were no pathological abnormalities noted in the hearts of the α2/δ-2 null mice. In conscious mice, there was a trend toward bradycardia observed during ECG and transthoracic echo. However, while under the influence of isoflurane, the null mice showed a significantly less-pronounced decrease in heart rate. A mutation in the α2/δ-4 gene introduced a premature stop codon that truncated one third of the protein and led to a cone-rod dysfunction in the visual system of mice (35). In addition, a mutation of the α2/δ gene (unc-36) in Caenorhabditis elegans has been identified, manifesting in a lack of adaptation (desensitization) to dopamine and serotonin, a phenotype indistinguishable from the unc-2 phenotype that is caused by lack of the α1-subunit (25). Localization analysis of a gene encoding the polymorphous α2/δ-1-subunit to chromosome 7q provided some evidence of the segregation of flanking markers in families susceptible to malignant hyperthermia (16). The gene was localized to chromosome 5 in the mouse in an earlier study (7). In addition, the α2/δ-1-subunit has been shown to be upregulated in response to spinal injury and neuropathic pain (20, 36, 37). However, the effect of a knockout of the α2/δ-1-subunit in an in vivo model has not been accomplished.
We have developed a viable conventional α2/δ-1 knockout mouse using a construct targeting exon 2 of α2/δ-1. As we expected, the α2/δ-1-subunit knockout mice display a lack of the high-affinity GBP binding site and altered L-type Ca2+ current in cardiomyocytes, without causing a significant change in the expression of other Ca2+ channel subunits.
METHODS
The investigation conforms to the Guide for the Care and Use of Laboratory Animals, published by The National Institutes of Health. The animals in the present study were handled according to approved protocols and animal care regulations at the University of Cincinnati. With the exception of the mice used for genotyping, the animals used for experimentation, including binding, Western blot analysis, histopathology, ex vivo analysis, and electrophysiology were all between 2 and 8 mo of age.
Generation of the α2/δ-1 knockout mouse.
A 12-Kb genomic fragment containing exon 2 of the α2/δ-1 gene encoding residues I33-D59 [82 base pairs (bp)] was isolated and sequenced from a lambda mouse genomic DNA library (129 SVJ, Stratagene; La Jolla, CA). Mutations were introduced into exon 2 to include XhoI and NotI sites. The XhoI site was inserted using the nucleotides from L44 and V45 and the NotI site used L47, A48, and K49. Similarly, a NcoI site was introduced into the long arm (5′; 4.0 Kb) and an NdeI site into the short arm (3′; 2.3 Kb). The fragment between sites NcoI and NdeI was placed in the vector thyamine kinase gene elegans (pMCTK), and the neomysin resistance gene was introduced into the XhoI-NotI of exon 2. Figure 1A represents a schematic of the gene-targeting construct. This construct was electroporated into murine embryonic stem cells. After positive (G-418) and negative selection (gancyclovir), genomic DNA was isolated from 288 embryonic stem cell lines. These cell lines were screened by PCR to identify the cell lines with an integration of the full-length targeting region of the knockout construct. Following the testing of 10 of the positive cell lines, one cell line was found in which site-specific targeting occurred. This positive line was microinjected into C57Bl/6 blastocysts and implanted into F1 (C57Bl/6X129J) pseudopregnant females. Two separate injections into blastocysts yielded 12 pups. From these two litters, seven chimeric animals were identified (5 of them at the level of 100% chimerism and 2 at 80% chimerism). The 100% chimeras were bred with Black Swiss females. The resulting offspring were genotyped via PCR to determine which male chimeras had gametes capable of passing the mutant allele to progeny. Screening of pups using PCR genotyping confirmed heterozygous (+/−) and null (−/−) animals.
Fig. 1.
Conventional knockout (KO) of the α2/δ-1 gene. A: schematic of the α2/δ-1 ablation construct, the location of the α2/δ-1 gene (chromosome 5), and the resultant homologous recombination. B: diagram of the PCR for confirmation of the α2/δ-1 gene-targeting event. Approximate primer locations are depicted by small black lines and either P1, P2, or P3. The 15 bp (V45–K49) of the wild-type (WT) that was subsequently deleted in the targeted allele is located at the line indicating P2. C: agarose gel electrophoresis of the PCR products. The WT allele yields an 83-bp product, and the targeted allele yields a 459-bp product. The PCR control consisted of a 1:1 mixture of WT and (−/−) tail DNA. D: diagram for the tail-prep PCR genotyping. The PCR primers are shown in their approximate location and are listed as A, B, and C. E: representative DNA agarose gel of the PCR genotyping results. The PCR products were 346 [WT and heterozygous (Het)] and 635 (Het and KO) bp. pMCTK, thymidine kinase gene cassette; Ctr, control; Ex2, exon 2; Neo, neomysin; M, marker.
PCR genotyping for wild-type, +/−, and −/− α2/δ-1 murine pups.
DNA was isolated from tail preps of 3-wk-old murine pups. The DNA was then added to a cocktail containing Taq Polymerase (NEB), Taq buffer, dNTPs and three primers: primer A (5′-CATGGGTGGACAAGATGCAAG-3′), primer B (5′-CTGCACGAGACTAGTGAGACG-3′), and primer C (5′-CATTCTCAAGACTGTAGG-3′). Using the first nucleotide of exon 2 as the initial bp, the primers were located as follows: primer A begins at 9, primer B begins at 1,448, and primer C begins at 2,065 (Fig. 1D). The PCR conditions were 35 cycles of 62°C annealing for 1 min, 72°C extension for 1 min, and 94°C denaturing for 1 min. PCR products were run on a 2.5% agarose gel. The expected bands were 346 bp for the wild-type (WT) and 635 bp for the −/− (Fig. 1E). The heterozygous mice had both bands.
Determination of the targeting construct integration and knockout of the α2/δ-1-subunit.
To confirm that the targeting construct integrated at the homologous genomic site (i.e., exon 2 of the α2/δ-1 VDCC subunit-encoding gene), we attempted Southern blot analysis using probes from flanking regions of the construct. However, four independent attempts at this failed, due to the highly repetitive nature of the DNA in the intronic sequences flanking exon 2. We then took advantage of the fact that 15 bp of sequence were deleted from the second exon (Fig. 1B, small line indicating P2) during construction of the gene-targeting construct. This deletion consists of the bp corresponding to amino acids V45–K49. We designed a PCR primer that is homologous to this region (Fig. 1B, P2, 5′-GTTTTTGCCAGTGTGACAAGG-3′). An additional 3′ primer was designed within the neomycin resistance cassette of the construct (P3, 5′-CGCCTTCTATCGCCTTCTTGAC-3′), and both were designed to be compatible with a common 5′ primer (P1, 5′-TGGAGCTTTCTTTCTTCTGATTCC-3′). These primers are located at −28 (P1), 34 (P2), and 403 (P3) with respect to the first nucleotide of exon 2. The PCR was designed such that the WT allele resulted in a product of 83 bp in length, whereas the targeted allele produced a 459-bp band (Fig. 1B). Importantly, P2 will not bind to the targeted allele, since 15 of its 21 bp are missing (3 bp 5′ and 3 bp 3′ of the deletion). However, if the targeting construct is integrated elsewhere in the murine genome, both the 453- and the 82-bp products would be generated. Thus this experimental procedure clearly demonstrates that the targeting construct successfully replaced exon 2 of the α2/δ-1 gene.
GBP binding.
The [3H]GBP (American Radiolabeled Chemicals, St. Louis, MO) binding assay was performed as described by Gee et al. (12). Membranes (20 μg/ml) of mouse tissues were incubated with [3H]GBP at room temperature for 60 min. A separation of bound from free ligand was effected by filtration through 0.3% polyethyleneimine-soaked GF/B filters. The filters were washed with 3 × 4 ml of 10 mM HEPES (pH 7.4). Radioactivity retained by the filters was determined by scintillation counting. Nonspecific binding was defined in the presence of 100 μM unlabeled GBP.
Western blots for the Ca2+ channel subunits.
Total protein, isolated as described previously (14), was separated on SDS-PAGE gel, transferred to nitrocellulose, and analyzed with the following primary antibodies: voltage-dependent L-type Ca2+ channel (Cav1.2) (ACC-003, 1:1,000, Alomone), calsequestrin (PAI-913, 1:2,500, ABR), β2a [1:2,500; generous gift from M. M. Hosey (6)], α2/δ-1 (D219, 1:1,000; Sigma), α2/δ-2 (Ac-REIYKDNRNLFEVQENEPC-amide, 1:500; custom-made antibody by Johnson & Johnson, not published), α2/δ-3 [1:200; custom-made antibody by Johnson & Johnson (24)], α2/δ-4 [1:100; custom-made antibody by Johnson & Johnson (24)], Cav2.1 (C1353, 1:200; Sigma), Cav2.2 (AB5154, 1:100; Millipore), β3 (C1978, 1:500; Sigma), and the protein loading normalized to GAPDH (4300, 1:4,000; Ambion).
Histopathology.
Following anesthesia with ketamine (100 mg/kg)-xylazine (10 mg/kg), body weight (BW) measurements were taken. Hearts were removed via bilateral thoracotomy, weighed, flushed with PBS (pH 7.4), fixed in 10% buffered formalin, and processed for histopathology following routine methods.
Ex vivo analyses, working heart.
Control and α2/δ-1 (−/−) age-matched mice of either sex were anesthetized with ketamine (100 mg/kg)-xylazine (10 mg/kg) and injected with 1.5 IU of heparin intraperitoneally to prevent intracardiac blood coagulation. Isolated hearts were placed in the working heart mode as previously reported (27). Positive and negative first derivatives of intraventricular pressure (dP/dt), duration of contraction (time to peak pressure), and relaxation (time to half relaxation) were calculated. Data are presented as means ± SD. Statistical analysis was carried out using Student's t-test for unpaired observations. Values of P < 0.05 were regarded as statistically significant.
Isolation of cardiomyocytes.
Single ventricular myocytes were enzymatically dissociated from isolated hearts of WT and α2/δ-1 (−/−) age-matched mice of either sex, as previously reported (40), with modifications. In brief, the heart was rapidly excised and placed in Tyrode solution containing (in mM) 120 NaCl, 5.4 KCl, 1.2 NaH2PO4, 5.6 glucose, 20 NaHCO3, 1.6 MgCl2, 10 2,3-butanedione monoxime, and 5 taurine, infused with 95% O2-5% CO2. All solutions were filtered and equilibrated with 95% O2-5% CO2 for at least 20 min before use. The heart was perfused in retrograde mode for 4 to 5 min, followed by perfusion with buffer containing 1 mg/ml collagenase Type II (Worthington) at 37°C. After 2 min of enzyme perfusion, 50 μM CaCl2 was added to the solution. After ∼5 min, when the heart was demonstrating characteristics of global digestion, the enzyme was recirculated. The heart was perfused for an additional 8–12 min or until flow rate surpassed preenzyme flow rate. After perfusion, the ventricles were separated from the atria and minced. Only Ca2+-tolerant cells with clear cross striations and without spontaneous contractions or significant granulation were selected for experimental studies.
Electrophysiological measurements.
The electrophysiological studies were performed as previously reported (22). The following protocol was used to obtain steady-state inactivation parameters of L-type Ca2+ current (ICa). From a holding potential of −80 mV, a series of 1-s prepulses (from −80 to +50 mV in 10-mV increments) were applied, followed by a 6-ms repolarization to the holding potential and a subsequent test depolarization to +10 (WT) or +20 mV [α2/δ-1 (−/−)].
Peak currents at the test pulse were normalized to the peak current amplitude elicited after the −80-mV prepulse and plotted as a function of the prepulse voltage. All recordings were made after 3 min of membrane rupture at room temperature (22–24°C). These experiments were carried out in 1.8 mM Ca2+, using Ca2+ as the charge carrier.
Data analysis.
The individual activation data [current-voltage (I-V) curves] were fitted to standard Boltzmann equation in the form ICa = Gmax(Vm − Vrev)/{1 + exp[−(Vm − V0.5)/k]}, where Gmax is the maximal conductance, Vm is the membrane voltage, Vrev is the ICa reversal potential, V0.5 is the half-activation potential, and k is the slope factor. The obtained parameters of Gmax and Vrev were then used to calculate fractional conductance at each Vm, G/Gmax, using the equation: G/Gmax = ICa[Gmax(Vm − Vrev)], where G is the total macroscopic conductance at Vm. The G-V curves were plotted with the values obtained from the fit of the I-V curves, using the following form of Boltzmann equation: G/Gmax = 1/{1 + exp[−(Vm − V0.5)/k]}.
The time course of decay of ICa was fitted to a two-exponential equation by Chebyshev method on Clampfit version 6.03 (Axon Instruments). The fit interval started at 60 ms and ended at 440 ms.
For the steady-state inactivation curves, the normalized amplitude of the inward Ca2+ current evoked by the test pulse (+10 or +20 mV) is plotted as a function of prepulse voltage.
RESULTS
Confirmation of the integration of the targeting construct and knockout of the subunit.
Tail DNA isolated from homozygous mice (Fig. 1C, lanes 1–4) produced only the 459-bp PCR product, whereas the WT DNA produced only the 83-bp product (Fig. 1C, lanes 9–12). PCR from heterozygous mouse DNA (Fig. 1C, lanes 5–8) produced both bands. We performed a PCR control that consisted of a 1:1 mixture of homozygous and WT tail DNA. PCR of this DNA resulted in both PCR products, confirming that the homozygous DNA does not inhibit the production of the 83-bp product. These results demonstrate that the α2/δ-1 knockout gene-targeting event occurred precisely as predicted and indeed does replace the normal endogenous allele. The genomic-targeting PCR confirmed the results of the PCR genotyping for WT, +/−, and −/− mice.
Loss of the high-affinity GBP binding site.
The VDCC α2/δ-1-subunit possesses a high-affinity binding site for GBP. To determine the differences in protein expression in α2/δ-1 (−/−) mice, a GBP radioligand binding experiment was performed with skeletal muscle, brain, and heart tissue. As shown in Fig. 2 and Table 1, the α2/δ-1 (−/−) animals displayed no measurable or very low levels of 3H[GBP] binding in skeletal muscle and brain. The density of GBP binding in the α2/δ-1 (+/−) animals was also reduced compared with WT in either brain or skeletal muscle. Though the α2/δ-1 binding site in WT heart tissue was very low (713 fmol/mg), approximately fivefold lower than that in WT skeletal muscle and brain tissues, the deletion of the α2/δ-1-subunit further reduced the GBP binding sites in heart.
Fig. 2.
Comparison of [3H]gabapentin (GBP) binding sites in different tissues from α2/δ-1 (−/−) mice. Membranes (20 mg/ml) of brain (left), skeletal muscle (middle), and heart (right) from WT, +/−, and −/− mice were incubated with varying concentrations of [3H]GBP at room temperature for 60 min. The results are representative of 3 experiments, with each point assayed in duplicate. The data were analyzed using Graphpad PRISM. The computer-drawn curves represent the best fit to the data by a 1-site binding model. cpm, Counts per minute.
Table 1.
Comparison of [3H]GBP binding sites in different tissues from α2/-δ1 WT (+/+), heterozygous (+/−), and knockout (−/−) mice
| Bmax, fmol/mg | Kd, nM | |
|---|---|---|
| Brain | ||
| WT | 3,359±29 | 25±0.5 |
| +/− | 2,757±390 | 36±8.9 |
| −/− | 570±81 | 25±5 |
| Skeletal muscle | ||
| WT | 3,343±961 | 21±3 |
| +/− | 2,268±370 | 20±4 |
| −/− | 145±18* | 11±4 |
| Heart | ||
| WT | 713±227* | 27±12 |
| +/− | 423±202* | 27±18 |
| −/− | 201±193* | 20±7 |
Values are means ± SD. GBP, gabapentin; Bmax, binding maximum; Kd, dissociation equilibrium constant; WT, wild-type.
Value is out of detectable range.
Western blot analyses of the Ca2+ channel subunits.
The murine cardiac L-type VDCC complex is composed of Cav1.2 (α1C), α2/δ-1-, and β2-subunits. To determine whether an ablation of α2/δ-1 expression altered the expression of other subunits in the complex, we first performed Western blot analyses of Cav1.2 and β2a-subunit protein levels on total protein from heart tissue from WT and −/− mice. No significant changes were observed (Fig. 3A). Since four α2/δ-isoforms have been identified to date, we wanted to evaluate whether the deletion of the α2/δ-1-isoform may result in a compensatory increase in expression of the other isoforms. In addition, since it is well known that the α2/δ-1-subunit also associates with other Ca2+ channel pore-forming α1-subunits, such as Cav2.1, Cav2.2, and Cav2.3, to form P/Q-, N-, and R-type Ca2+ channels, respectively, the expression of these subunits was also investigated. To quantify the impact on expression of these proteins, Western blot analyses of total protein, obtained from heart, brain, and skeletal muscle from WT, +/−, and −/− α2/δ-1 mice, were performed for each Ca2+ channel isoform. Consistent with the GBP binding data, α2/δ-1 protein expression was not detected at all in the α2/δ-1 (−/−) mouse heart, brain, or skeletal muscle. In contrast, no significant changes in protein expression were observed for any of the other Ca2+ channel subunit isoforms tested (Fig. 3B).
Fig. 3.
Expression of Ca2+ channels in α2/δ-1-deficient mice. A: Western blot analyses of Ca2+ channel isoform voltage-dependent L-type Ca2+ channel (Cav1.2) and β2a-subunits in heart tissue normalized to calsequestrin. B: Western blot analyses of α2/δ-isoforms (1, 2, 3, and 4), Cav2.1, Cav2.2, and β3-subunit normalized to GAPDH in brain, heart, and skeletal muscle (SM) from α2/δ-1 WT, +/−, and −/− mice. The numbers reported as molecular mass are in kilodaltons.
Clinical characteristics and morphology.
The α2/δ-1 (−/−) animals clinically appear normal, although males display a tendency for bladder dilatation. Grossly, the α2/δ-1 (−/−) hearts appear normal with no obvious cardiac hypertrophy or dilatation (Fig. 4). At 8 mo, the average heart weight (HW) and BW were not significantly different between the two groups; α2/δ-1 (−/−) HW was 172.5 ± 18.9 mg, and BW was 29.3 ± 2.7 g (n = 4) compared with the WT HW of 154.3 ± 12.9 mg and BW of 28.8 ± 1.8 g (n = 7), respectively. HW-to-BW ratios also were not significantly different between α2/δ-1 (−/−) and WT (5.9 ± 0.42 and 5.37 ± 0.28 mg/g, respectively). The majority of the animals were female. When analyzed by sex, HW [163.3 ± 20.2 (n = 3) vs. 140 ± 9.1 mg (n = 5)], BW (28.1 ± 3.02 vs. 25.5 ± 1.4 g), and HW-to-BW ratios (5.83 ± 0.51 vs. 5.49 ± 0.011) for α2/δ-1 (−/−) and WT, respectively, were not significantly different.
Fig. 4.
Hematoxylin and eosin slides of WT and −/− hearts.
Diminished cardiac contractility.
We performed ex vivo working heart studies in WT (n = 6) mice and α2/δ-1 (−/−) (n = 7) littermates (Table 2), hypothesizing that the deletion of the α2/δ-1-subunit would result in a diminished contractility that was not dependent on altered vascular resistance or loading conditions. Indeed, when compared with WT, basal contractility and relaxation (+dP/dt and −dP/dt) were significantly lower in α2/δ-1 (−/−) hearts.
Table 2.
Contractile parameters in WT (+/+) and α2/δ-1 (−/−) hearts from isolated working heart preparations
| Parameters | WT | α2/δ-1 (−/−) |
|---|---|---|
| n | 6 | 7 |
| LVSP, mmHg | 119.7±31.4 | 95.7±8.1 |
| LVDP, mmHG | −17.5±6.8 | −7.3±9.2 |
| LVEDP, mmHg | 2.4±4.5 | 13.1±10.1 |
| +dP/dt, mmHg/s | 4,159±893 | 3,134±618* |
| −dP/dt, mmHg/s | 3,727±515 | 2,934±695* |
| Heart rate, beats/min | 352±16.5 | 348±66.7 |
| TPP, ms/mmHg | 0.47±0.07 | 0.52±0.14 |
| TR1/2, ms/mmHg | 0.70±0.15 | 0.70±0.17 |
Values are means ± SD; n, number of hearts. LVSP, left ventricular systolic pressure; LVDP, left ventricular diastolic pressure; LVEDP, left ventricular end-diastolic pressure; +dP/dt, maximal rate of pressure development; −dP/dt, maximal rate of pressure decline; TPP, time to peak pressure, normalized to peak pressure; TR1/2, time to half relaxation pressure, normalized to half relaxation pressure.
P < 0.05.
Ca2+ channel properties.
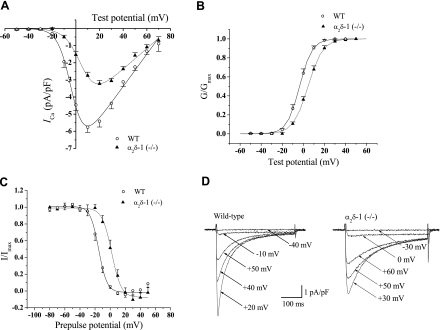
In whole cell patch-clamp measurements (I-V curves) on cardiomyocytes derived from WT and α2/δ-1 (−/−) mice, the L-type VDCC ICa peak current density was significantly decreased by ∼45%, compared with WT (ICa density; Fig. 5A, and Table 3). There was a significant increase in the half-activation potential (V0.5, more than 4-fold) and the slope factor (k, 28%) between the WT and α2/δ-1 (−/−) cardiomyocytes. Depolarizing pulses of different amplitudes were applied from a holding membrane potential of −50 mV. The inward currents reached their peak values at around +20 mV in the α2/δ-1 (−/−) and +10 mV for WT (steady state activation) mice. There was a hypopolarizing shift in the voltage for half-maximal activation (V0.5, right shift ∼8.1 mV; Fig. 5B, and Table 3). The slope parameter (kactivation) value was not statistically different between the two groups. For the steady-state inactivation curves, the normalized amplitude of the inward Ca2+ current evoked by the test pulse (+10 or +20 mV) was plotted as a function of prepulse voltage. The voltage of V0.5 was shifted to a hypopolarizing direction by ∼13 mV (Fig. 5C, and Table 3). The steepness of the inactivation curve (kinactivation) did not change significantly between groups. The time course of the decay of ICa was analyzed by fitting a double exponential equation by Chebyshev. The fit interval started at 60 ms and ended at 440 ms. The time constant of the rapid component (τfast) was significantly different depending on the pulse (+10 or +20 mV), suggesting the interference of a Ca2+-dependent inactivation process, since Ca2+ was used as a charge carrier. The slow time constant (τslow), representing voltage-dependent inactivation independent of current amplitude, was slowed in α2/δ-1-deficient cardiomyocytes (Table 3). Cell capacitance was significantly decreased in α2/δ-1 (−/−) cardiomyocytes (Table 3).
Fig. 5.
Voltage dependence and kinetics of L-type Ca2+ current (ICa) activation. A: voltage dependence of peak inward current densities [current-voltage (I-V) curve]. B: steady-state activation curves. Means and means ± SE are from 27–44 experiments for each group [WT and α2/δ-1 (−/−), respectively]. C: steady-state inactivation curves. Inactivation fraction was determined by using a 2-pulse protocol. Data are means ± SE from 8 experiments for each group. Lines represent Boltzmann fits. D: representative Ca2+ current traces. Currents were elicited by 380-ms depolarizing pulses between −40 and +50 mV or between −30 and +60 mV from a holding potential −60 mV at 0.2 Hz. G, conductance.
Table 3.
Comparison of WT and α2/δ-1 (−/−) mice on ICa
| WT | n | α2/δ-1 (−/−) | n | |
|---|---|---|---|---|
| ICa density, pA/pF | 5.76±0.30 (+10 mV) | 35/5 | 3.17±0.19* (+20 mV) | 47/7 |
| V0.5, mV (I-V curve) | −2.32±1.18 | 8.56±1.12† | ||
| k, mV (I-V curve) | 5.29±0.17 | 6.79±0.18† | ||
| V0.5, mV (steady-state activation) | −3.50±1.15 | 27/5 | 4.66±1.14† | 38/7 |
| k, mV (steady-state activation) | 4.87±0.14 | 5.88±0.12 | ||
| V0.5, mV (steady-state inactivation) | −15.15±1.71 | 8/3 | −2.18±2.59† | 8/6 |
| k, mV (steady-state inactivation) | 5.54±0.42 | 6.14±0.43 | ||
| τfast, ms | 19.90±1.25 | 23/5 | 44.74±3.67† | 28/7 |
| τslow, ms | 83.73±2.40 | 158.92±13.39† | ||
| Cell capacitance, pF | 222.25±11.84 | 171.78±6.77† |
Values are means ± SD; n, number of experiments performed (number of animals used to generate the myocytes/number of myocytes measured). ICa density, peak current density; I-V, current-voltage; V0.5 (I-V curve), half-activation potential; V0.5 (steady-state activation), half-maximal activation; V0.5 (steady-state inactivation), half-maximal inactivation; k, slope factor; τfast, time constant of the rapid component; τslow, slow time constant.
P < 0.05;
P < 0.001.
DISCUSSION
The role of the accessory subunits on VDCC kinetics has been closely studied using heterologous coexpression systems. From these studies, it has been shown that both α2/δ- and β-subunits increase membrane expression of the α1-subunit. The accessory subunits enhance current amplitude and density, as well as regulating the kinetics of activation and inactivation (5, 34, 38, 39). The coexpression of both accessory subunits appears synergistic, rather than additive, with respect to membrane expression of the α1C-subunit and additive for the voltage-dependence of activation of the channel. In addition, whereas the α2/δ-subunit increases Ca2+ channel density, the β-subunit both increases channel density and facilitates an opening of the channel (38). However, since uninjected oocytes express endogenous barium currents (28), as well as endogenous α1-, β-, and α2/δ-subunits (4, 29, 32), the studies on regulatory functions of α2/δ- and β-subunits are inevitably “contaminated” by these endogenous channels.
This is the first successful attempt at knocking out the α2/δ-1-subunit in vivo. The genomic DNA PCR verified that the integration of the targeting construct was at exon 2 of the gene. This confirmed the results of the genotyping PCR. Furthermore, the Western blot analysis and the binding data clearly demonstrate the lack of α2/δ-1 protein in these animals. The ablation of the α2/δ-1-subunit did not have any effect on the expression levels of the other Ca2+ channel subunits. This makes an ideal system for studying the α2/δ-1-subunit since there are no compensatory mechanisms that could possibly confuse the relevance of the data.
A comparison of the electrophysiological properties in isolated cardiomyocytes from the α2/δ-1 (−/−) and WT mice clearly showed that the absence of the α2/δ-1 gene in the heart results in an attenuated ICa amplitude, a decrease in ICa density, an increase in the time constants (fast and slow), and a hypopolarizing shift in activation and inactivation. Thus, recapitulating studies on heterologous systems (5, 34, 38, 39), the knockout of the α2/δ-1 gene either reduces the number of channels targeted to the membrane and/or changes the kinetics of the existing channels, as shown by the increased membrane potential necessary to achieve half-maximal activation and inactivation, changing the open/closed status of the channels. In other words, the results demonstrate that the channels are slower to activate as well as inactivate, thus decreasing Ca2+ current. Single-channel analyses are planned to decipher this mechanism. Basal contractility and relaxation were also significantly decreased in ex vivo working heart experiments, further indicating decreased Ca2+ load.
Several studies have been performed using small interfering RNAs to disrupt the α2/δ-1 in muscle cell lines (11, 21, 23, 33), although most have been aimed at skeletal muscle, measuring the effects of α2/δ-1 on the α1S-subunit (11, 21, 23). One of these systems involves a dysgenic muscle cell line, GLT, which lacks the α1-subunit (31) and has the α2/δ-1-subunit targeted to the cytoplasm and plasma cell membrane (23). Investigating this system revealed a major role for the α2/δ-1 subunit in excitation-contraction coupling in both skeletal (21) and cardiac tissue (33) but did not demonstrate a function in targeting of the α1-pore subunit to the membrane. Reconstitution with the α1C-pore subunit, mimicking cardiac muscle, and silencing of the α2/δ-1-subunit resulted in a decreased current amplitude, with a depolarizing shift in voltage dependence of activation as well as decreased activation and inactivation kinetics (33). Interestingly, in a recent study examining skeletal myotubes in C2C12 cells, in which the α2/δ-1-subunit had been silenced, Ca2+ current was similar to that of WT cells. However, a potential role for the α2/δ-1-subunit in extracellular signaling involving migration and spreading of skeletal myocytes was reported (11).
Homozygous knockout of the α1C-subunit is embryologically lethal before 14.5 days of development (26). Conditional smooth muscle-specific knockout of the Cav1.2 (SMACKO mouse) results in a complete loss of L-type current in single smooth muscle electrophysiology (19). The life span of these animals ranges 21–28 days following tamoxifen-induced inactivation of the Cav1.2 gene. Protein expression of Cav1.2 was <10% in these mice (19). In contrast to the α2/δ-2 knockout mouse, the α2/δ-1 knockout does not show obvious signs of seizures, which is most likely due to the different localizations of the individual isoforms. The α2/δ-2 isoform is predominantly located in the central nervous system, explaining the manifestation of ataxia and seizures (17).
We have developed a knockout model that, while obviously having a profound affect on the regulation of the α1C-subunit, is not lethal. It represents a novel model for studying the function of the α2/δ-1-subunit and characterizing its effects, particularly with respect to cardiovascular function, epileptogenesis, and nociception, potentially leading to new pharmacological therapies.
GRANTS
This work was supported by US National Heart, Lung, and Blood Institute Grants NIH-T32-HL-07382 (to A. Schwartz), R01-HL-079599 (to A. Schwartz), and P01-HL-22619 (to A. Schwartz).
Acknowledgments
Appreciation is expressed to Dr. Adolfo Cuadra (Dr. M. Hosey) for the generous gift of the β2a-antibody (Department of Physiology & Functional Genomics, University of Florida, College of Medicine, Gainesville, FL).
REFERENCES
- 1.Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci 21: 6095–6104, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brickley K, Campbell V, Berrow N, Leach R, Norman RI, Wray D, Dolphin AC, Baldwin SA. Use of site-directed antibodies to probe the topography of the alpha 2 subunit of voltage-gated Ca2+ channels. FEBS Lett 364: 129–133, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Brill J, Klocke R, Paul D, Boison D, Gouder N, Klugbauer N, Hofmann F, Becker CM, Becker K. entla, a novel epileptic and ataxic Cacna2d2 mutant of the mouse. J Biol Chem 279: 7322–7330, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Canti C, Davies A, Dolphin A. Calcium channel α2δ subunits: structure, functions and target site for drugs. Curr Neuropharm 1: 209–217, 2003. [Google Scholar]
- 5.Catterall W Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16: 521–555, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun E, Rios E, Hosey MM. Roles of a membrane-localized β-subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem 270: 30036–30044, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Chin H, Mock B, Kim HL, Kim H, Kozak CA. The gene for the dihydropyridine-sensitive calcium channel alpha 2 subunit (CCHL2A) maps to the proximal region of mouse chromosome 5. Genomics 13: 1325–1327, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Chouinard G The search for new off-label indications for antidepressant, antianxiety, antipsychotic and anticonvulsant drugs. J Psychiatry Neurosci 31: 168–176, 2006. [PMC free article] [PubMed] [Google Scholar]
- 9.De Jongh KS, Warner C, Catterall WA. Subunits of purified calcium channels. Alpha 2 and delta are encoded by the same gene. J Biol Chem 265: 14738–14741, 1990. [PubMed] [Google Scholar]
- 10.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science 241: 1661–1664, 1988. [DOI] [PubMed] [Google Scholar]
- 11.García K, Nabhani T, García J. The calcium channel alpha2/delta1 subunit is involved in extracellular signalling. J Physiol 586: 727–738, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem 271: 5768–5776, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Gong HC, Hang J, Kohler W, Li L, Su TZ. Tissue-specific expression and gabapentin-binding properties of calcium channel alpha2delta subunit subtypes. J Membr Biol 184: 35–43, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Groner F, Rubio M, Schulte-Euler P, Matthes J, Khan IF, Bodi I, Koch SE, Schwartz A, Herzig S. Single-channel gating and regulation of human L-type calcium channels in cardiomyocytes of transgenic mice. Biochem Biophys Res Commun 314: 878–884, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Gurnett CA, Felix R, Campbell KP. Extracellular interaction of the voltage-dependent Ca2+ channel alpha2delta and alpha1 subunits. J Biol Chem 272: 18508–18512, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Iles DE, Lehmann-Horn F, Scherer SW, Tsui LC, Olde Weghuis D, Suijkerbuijk RF, Heytens L, Mikala G, Schwartz A, Ellis FR, Stewart AD, Deufel T, Wieringa B. Localization of the gene encoding the alpha 2/delta-subunits of the L-type voltage-dependent calcium channel to chromosome 7q and analysis of the segregation of flanking markers in malignant hyperthermia susceptible families. Hum Mol Genet 3: 969–975, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov SV, Ward JM, Tessarollo L, McAreavey D, Sachdev V, Fananapazir L, Banks MK, Morris N, Djurickovic D, Devor-Henneman DE, Wei MH, Alvord GW, Gao B, Richardson JA, Minna JD, Rogawski MA, Lerman MI. Cerebellar ataxia, seizures, premature death and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am J Pathol 165: 1007–1018, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr 35: 639–647, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22: 6027–6034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res 95: 1–8, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J Biol Chem 280: 2229–2237, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Petrashevskaya NN, Bodi I, Rubio M, Molkentin JD, Schwartz A. Cardiac function and electrical remodeling of the calcineurin-overexpressed transgenic mouse. Cardiovasc Res 54: 117–132, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Powell JA, Petherbridge L, Flucher BE. Formation of triads without the dihydropyridine receptor alpha subunits in cell lines from dysgenic skeletal muscle. J Cell Biol 134: 375–387, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin N, Yagel S, Momplaisir ML, Codd EE, D'Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol Pharmacol 62: 485–496, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Schafer WR, Sanchez BM, Kenyon CJ. Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics 143: 1219–1230, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kühbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 275: 39193–39199, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Serikov VB, Petrashevskaya NN, Canning AM, Schwartz A. Reduction of [Ca2+]i restores uncoupled beta-adrenergic signaling in isolated perfused transgenic mouse hearts. Circ Res 88: 9–11, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science 253: 1553–1557, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Singer-Lahat D, Lotan I, Itagaki K, Schwartz A, Dascal N. Evidence for the existence of RNA of Ca2+-channel alpha 2/delta subunit in Xenopus oocytes. Biochim Biophys Acta 1137: 39–44, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Striessnig J, Scheffauer F, Mitterdorfer J, Schirmer M, Glossmann H. Identification of the benzothiazepine-binding polypeptide of skeletal muscle calcium channels with (+)-cis-azidodiltiazem and anti-ligand antibodies. J Biol Chem 265: 363–370, 1990. [PubMed] [Google Scholar]
- 31.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 336: 134–139, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Tareilus E, Roux M, Qin N, Olcese R, Zhou J, Stefani E, Birnbaumer L. A Xenopus oocyte beta subunit: evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc Natl Acad Sci USA 94: 1703–1708, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc Natl Acad Sci USA 104: 11091–11096, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varadi G, Lory P, Schultz D, Varadi M, Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature 352: 159–162, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci 47: 3523–3530, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA 99: 8360–8365, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaksh TL Calcium channels as therapeutic targets in neuropathic pain. J Pain 7: S13–S30, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi H, Okuda M, Mikala G, Fukasawa K, Varadi G. Cloning of the beta(2a) subunit of the voltage-dependent calcium channel from human heart: cooperative effect of alpha(2)/delta and beta(2a) on the membrane expression of the alpha(1C) subunit. Biochem Biophys Res Commun 267: 156–163, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Yasuda T, Chen L, Barr W, McRory JE, Lewis RJ, Adams DJ, Zamponi GW. Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells. Eur J Neurosci 20: 1–13, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000. [DOI] [PubMed] [Google Scholar]