Abstract
Given that the physiology of heme oxygenase-1 (HO-1) encompasses mitochondrial biogenesis, we tested the hypothesis that the HO-1 product, carbon monoxide (CO), activates mitochondrial biogenesis in skeletal muscle and enhances maximal oxygen uptake (V̇o2max) in humans. In 10 healthy subjects, we biopsied the vastus lateralis and performed V̇o2max tests followed by blinded randomization to air or CO breathing (1 h/day at 100 parts/million for 5 days), a contralateral muscle biopsy on day 5, and repeat V̇o2max testing on day 8. Six independent subjects underwent CO breathing and two muscle biopsies without exercise testing. Molecular studies were performed by real-time RT-PCR, Western blot analysis, and immunochemistry. After V̇o2max testing plus CO breathing, significant increases were found in mRNA levels for nuclear respiratory factor-1, peroxisome proliferator-activated receptor-γ coactivator-1α, mitochondrial transcription factor-A (Tfam), and DNA polymerase γ (Polγ) with no change in mitochondrial DNA (mtDNA) copy number or V̇o2max. Levels of myosin heavy chain I and nuclear-encoded HO-1, superoxide dismutase-2, citrate synthase, mitofusin-1 and -2, and mitochondrial-encoded cytochrome oxidase subunit-I (COX-I) and ATPase-6 proteins increased significantly. None of these responses were reproduced by V̇o2max testing alone, whereas CO alone increased Tfam and Polγ mRNA, and COX-I, ATPase-6, mitofusin-2, HO-1, and superoxide dismutase protein. These findings provide evidence linking the HO/CO response involved in mitochondrial biogenesis in rodents to skeletal muscle in humans through a set of responses involving regulation of the mtDNA transcriptosome and mitochondrial fusion proteins autonomously of changes in exercise capacity.
Keywords: heme oxygenase-1, superoxide dismutase-2, peroxisome proliferator-activated receptor-γ coactivator-1α, nuclear respiratory factor-1, oxygen uptake
carbon monoxide (CO), as a toxic gas, causes tissue hypoxia (36), but direct cellular effects of CO also contribute to adaptive responses (24). Endogenous CO is detectable at nanomolar levels as a by-product of heme catabolism (8), which induces the expression of antioxidant enzymes, including heme oxygenase-1 (HO-1) and mitochondrial superoxide dismutase (SOD2). These responses depend on CO and protect against oxidative stress and thus raise the possibility that near-physiological concentrations of CO might be therapeutically useful (29).
CO also stimulates mitochondrial biogenesis, which is activated in part when CO binds to cytochrome c oxidase and boosts mitochondrial hydrogen peroxide production (32, 33). The functionality of this oxidant as a signaling molecule underscores the importance of understanding CO's physiological roles, as mitochondrial oxidative stress develops both during exercise and in disease states, and mitochondrial DNA (mtDNA) is open to attack by reactive oxygen species (3). Oxidative mtDNA damage contributes to the pathogenesis of multiple diseases, such as atherosclerosis (12) and inflammatory organ dysfunction (35), and, without bigenomic coordination of mitochondrial biogenesis, mitochondria cannot adapt to changing physiological conditions (17, 19, 31, 40). New insights into mitochondrial biogenesis have also generated interest in ways to ameliorate mitochondrial pathogenesis. For instance, mitochondrial damage is linked to outcome in sepsis where patients with poor outcomes have greater mitochondrial dysfunction and higher tissue Po2 than those who recover fully (4) and, in mice, where metabolic recovery from gram-positive sepsis occurs in conjunction with mitochondrial biogenesis (14). Also, interference with mitochondrial biogenesis in doxorubicin cardiac toxicity is abrogated by HO/CO (25, 32).
Mitochondrial biogenesis in various organs is activated by the gaseous signaling molecules nitric oxide (NO) (23) and CO (33). NO is involved in the heart (22) and the brain (13), whereas in the heart CO initiates mitochondrial biogenesis through transcriptional activation of the peroxisome proliferator-activated receptor-γ coactivator (PGC-1α), nuclear respiratory factors-1 and -2 (NRF-1 and -2), and mitochondrial transcription factor-A (Tfam) (32). Because CO is simple to administer by inhalation, if it exerts similar effects in humans, small amounts might be used to activate mitochondrial biogenesis during training or in rehabilitation from muscle diseases associated with dysfunction of previously healthy mitochondria.
Mitochondrial biogenesis is also clearly necessary for muscle conditioning (15, 16), but the degree to which mitochondrial content determines maximal aerobic exercise capacity is an interesting open question (9, 15). Maximal oxygen uptake (V̇o2max) may be limited by systemic factors like cardiac output (30) or by peripheral factors like microcirculatory O2 diffusion or mitochondrial oxidative capacity (37, 38). In rodents, inhibition of cytochrome c oxidase causes a dose-dependent decrease in O2 consumption (21), whereas exercise training increases mitochondrial activity and V̇o2max compared with controls, but those gains are eliminated by fixing the respiratory capacity (28).
On this basis, we tested the hypothesis that low-level inhaled CO activates mitochondrial biogenesis in human skeletal muscle. Changes in the nuclear regulation of mitochondrial biogenesis, antioxidant enzyme expression, and selected nuclear- and mitochondrial-encoded proteins were measured after brief, intermittent periods of CO inhalation. We also analyzed the effects of V̇o2max on these parameters and assessed the possibility that V̇o2max is affected by CO-induced changes in muscle mitochondrial capacity.
EXPERIMENTAL PROCEDURES
The study was approved by the Duke University Institutional Review Board, and, after informed consent, we enrolled 16 healthy nonsmoking volunteers (ages 19–39 yr, 4 females, 12 males) who agreed not to deviate from their regular routine of diet and activities. Baseline biopsies of the vastus lateralis muscle were performed with lidocaine anesthesia using a U.C.H. Muscle Biopsy Needle (Popper & Sons, New Hyde Park, NY). Two 30- to 50-mg muscle samples were obtained: one was snap-frozen at −80°C and the other fixed immediately in 10% neutral-buffered formalin.
Ten subjects were assigned in a double-blind fashion to breathe either 100 parts/million (ppm) CO or air from identical breathing systems for 1 h daily on five consecutive days after the first muscle biopsy. These subjects underwent radial arterial line placement and performed V̇o2max tests on a stationary bicycle using a standard graded-exercise protocol. Blood samples (2–3 ml) were drawn at rest, at 9 min exercise, end of exercise (V̇o2max), and 5 min postexercise. After the CO exposure on day 5, venous blood was drawn to measure carboxyhemoglobin (HbCO), and a second biopsy was performed on the contralateral vastus muscle. After the last CO exposure (3 days), the subjects performed a second V̇o2max test with radial arterial line placement and blood samples. To independently evaluate the CO response, six separate subjects underwent the CO exposures without exercise testing between the two muscle biopsies.
RNA extraction and real-time quantitative PCR.
Total RNA was prepared from muscle biopsies using Trizol reagent (Invitrogen, Carlsbad, CA). RNA (1 μg) was reverse-transcribed using Random Hexomer primers and a Superscript enzyme (Invitrogen). Real-time RT-PCR was performed using an ABI Prism 7000 and gene expression master mix (Applied Biosystems, Foster City, CA) (32). Primers and probes for human NRF-1, NRF-2, PGC-1α, and Tfam were from Applied Biosystems, and 18S rRNA was used as an internal control. ABI Prism 7000 SDS Software was used to quantify differences in gene expression. The threshold cycle (Ct) was determined in the exponential amplification phase. The amount of transcript was normalized to 18S RNA by subtracting the mean Ct values for each condition. Because of the exponential PCR reaction, a difference of n in Ct values represents a twofold difference in transcript levels. PCR was performed in triplicate.
Quantitative PCR for mtDNA copy number.
Total and mtDNA was isolated from muscle specimens using the mtDNA Extractor Kit (Wako Chemicals, Richmond, VA). DNA primers were designed to detect cytochrome oxidase (COX)-II and 18S rDNA at a maximum amplicon length of 150 bp: 18S rDNA forward, 5′-GAATTCCCAGTAAGTGCGGGTCA-3′, and reverse, 5′-TAATGATCCTTCCGCAGGTTC-3′; COX II forward, 5′-ATGGCACATGCAGCGCAAGTAGG-3′, and reverse, 5′-ATTAGTTAGTTTTGTTGTGAGTGT-3′. The PCR reaction mixture contained 1× platinum SYBR green qPCR SuperMix UDG (Invitrogen), 500 nM of each primer, and 10 ng of total genomic DNA or mtDNA. Real-time PCR conditions were 2 min at 50°C and 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 60 s at 60°C using the ABI Prism 7000 sequence detector system (Applied Biosystems 7000 SDS software). The Ct values in the linear exponential phase were used to measure the original DNA template copy numbers from a standard curve generated from five 10-fold dilutions of either pure mtDNA or pure nuclear 18S rDNA. Relative values for COX II and 18S rDNA within samples were used to obtain a ratio of mtDNA to nuclear DNA.
Western analysis.
Muscle proteins were separated by SDS-PAGE and identified by Western analysis (34). Membranes were incubated with validated polyclonal antibodies against human citrate synthase, SOD2, COX-I, ATPase-6, and protein kinase B (Akt)/phosphorylated Akt (Santa Cruz Biotechnology, Santa Cruz, CA), mitofusin-1 and -2, optic atrophy-1 (OPA-1), or β-actin (1:5,000; Sigma). After three washes in TBS-Tween, membranes were incubated at a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham). Blots were developed with enhanced chemiluminescence, and proteins were quantified by densitometry on digitized images from the middynamic range and expressed relative to β-actin.
Myosin heavy chain isoform analysis.
Muscle was homogenized in 250 mM sucrose, 100 mM KCl, 5 mM EDTA, and 20 mM Tris base, pH 6.8, using a glass tissue homogenizer, sonicated, and centrifuged at 1,000 g for 10 min (4°C). The pellet was resuspended in a wash buffer (175 mM KCl, 0.5% Triton X-100, 2 mM EDTA, and 20 mM Tris base, pH 6.8, 4°C) and centrifuged again at 1,000 g for 10 min (4°C). The pellet was resuspended in the buffer, centrifuged again at 1,000 g, and suspended in 150 mM KCl and 20 mM Tris base (pH 7.0). Myosin heavy chain (MHC) isoforms were separated via SDS-PAGE using stacking gels of 4% acrylamide-bis and separating gels of 8% acrylamide-bis. Gels were stained (Bio-Rad Silver Plus) and scanned using QuantityOne Software (Bio-Rad).
Immunofluorescence microscopy.
Paraffin-embedded tissue sections (4 μm thick) were processed for immunostaining by deparaffinization in xylene and rehydration through a descending series of alcohols, washed extensively in 0.1 M PBS, blocked with 10% normal goat serum with avidin, and incubated overnight at 4°C in primary antibody diluted in 10% normal goat serum and biotin. Primary succinate dehydrogenase (SDH) subunit A and COX-I antisera (Invitrogen) were used at a working dilution of 1:100. Sections were washed extensively in PBS and incubated in appropriate secondary IgG, Alexa Fluor 594 red (to detect COX-I), or Alexa Fluor 488 green (to detect SDH subunit A). Conventional fluorescence images were obtained using a Nikon Microphot-FXA fluorescence microscope.
Statistics.
Grouped data are reported as means ± SD. The values for the CO control group were compared using the paired t-test. Mean values from the V̇o2max groups were compared within and between groups using two-way ANOVA (SigmaStat; Systat Software). P ≤ 0.05 was accepted as statistically significant.
RESULTS
The characteristics of the subjects are shown in Table 1. HbCO levels measured at baseline and immediately after the last CO exposure in the V̇o2max group showed that mean HbCO levels increased from 1.6 ± 0.2 to 3.3 ± 0.60% (P = 0.002). In the CO only group, HbCO measurements showed a similar increase following CO exposure (1.2 ± 0.2 to 3.2 ± 0.4%; P = 0.002).
Table 1.
Experimental Groups: age, HbCO, and V̇o2max
| Group | Age | n | Exposure | Pre-HbCO | Pre-V̇o2max, ml·kg−1·min−1 | Post-HbCO | Post-V̇o2max, ml·kg−1·min−1 |
|---|---|---|---|---|---|---|---|
| CO + V̇o2max | 25.2 (19–32) | 5 | 100 ppm CO 1 h × 5 days | 1.6±0.2 | 40.9±4.18 | 3.3±0.6 | 41.8±4.79 |
| Air + V̇o2max | 24.2 (20–32) | 5 | Air 1 h × 5 days | 1.7±0.6 | 42.98±6.64 | 1.0±0.5 | 43.44±7.84 |
| CO only | 26.3 (24–30) | 6 | 100 ppm CO 1 h × 5 days | 1.2±0.2 | ND | 3.2±0.5 | ND |
Values are means ± SE with ranges in parentheses; n, no. of subjects. HbCO, carboxyhemoglobin; maximal oxygen uptake; CO, carbon monoxide; ppm, parts/million; ND, not determined.
Transcriptional activation of mitochondrial biogenesis after inhaled CO and V̇o2max testing.
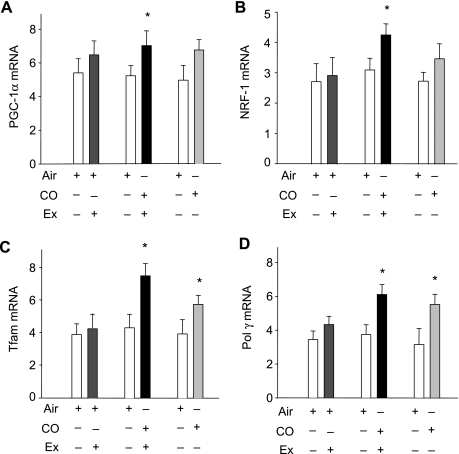
By real-time RT-PCR, mRNA for two of the major nuclear transcriptional regulators of mitochondrial biogenesis, PGC-1α and NRF-1, as well as for Tfam and the mtDNA polymerase (Polγ) was evaluated before and after air or CO with V̇o2max testing, or before and after CO without exercise testing. The summary data, by group pairings (Air + Ex, CO + Ex, or CO alone), are displayed in Fig. 1 for PGC-1α (A), NRF-1 (B), Tfam (C), and Polγ (D). V̇o2max testing did not significantly affect these four muscle mRNA measurements. In the CO plus V̇o2max group, the levels of all four mRNAs increased significantly, and Tfam expression nearly doubled (P = 0.02). In the CO only group, two of the four muscle mRNA values, Tfam and Polγ, increased significantly.
Fig. 1.
Carbon monoxide (CO) and bigenomic transcriptional activity in human skeletal muscle. Real-time RT-PCR was performed on muscle biopsy samples of healthy subjects before and after 5 days of inhaled CO or air exposure and a maximal oxygen uptake (V̇o2max) test, or before and after 5 days inhaled CO without exercise (Ex). Relative levels of mRNA for nuclear-encoded peroxisome proliferator-activated receptor-γ coactivator (PGC-1α) (A), nuclear respiratory factor (NRF)-1 (B), mitochondrial-encoded transcription factor-A (Tfam; C) and DNA polymerase γ (Polγ) (D) are shown before and after CO with or without a V̇o2max test. Values are means ± SD (*P < 0.05 for between-group comparisons).
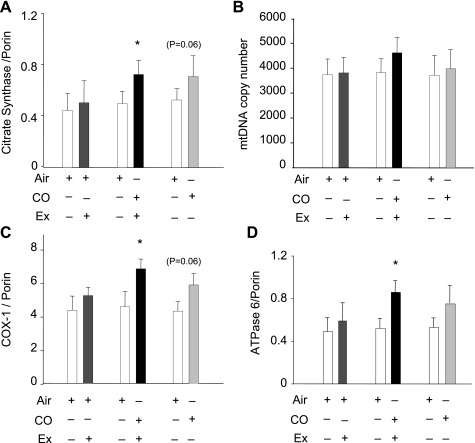
Figure 2 summarizes the data from the three groups of subjects for citrate synthase (A), mtDNA copy number (B), and two mitochondrial encoded proteins, COX-I (C) and ATPase-6 (D). None of these four markers increased in the air control group. In the CO plus V̇o2max group, the nuclear-encoded tricarboxylic acid cycle enzyme citrate synthase, a marker of muscle oxidative capacity, respiratory capacity, and mitochondrial volume density, increased significantly. However, the mtDNA copy number in all groups remained stable (13% increase in CO plus V̇o2max group; P = 0.072), indicating that mtDNA replication had not been activated at the time of the muscle biopsy. To determine if CO had activated mitochondrial protein synthesis, mitochondrial-encoded COX-I and ATPase-6 expression was examined, and both protein levels increased significantly in the CO plus V̇o2max group. After CO alone, the COX-I and ATPase-6 protein levels also increased significantly.
Fig. 2.
Nuclear and mitochondrial protein expression and mitochondrial DNA (mtDNA) copy number. A: expression of skeletal muscle citrate synthase determined by Western blot before and after CO with or without V̇o2max testing compared with porin, the loading control. B: mtDNA copy number in muscle before and after 5 daily exposures to air or CO and one V̇o2max test, or before and after CO without exercise. C and D: mitochondrial-encoded cytochrome oxidase (COX)-I (C) and ATPase-6 protein (D) relative to porin before and after CO with or without V̇o2max test. Values are means ± SD (*P < 0.05 between groups).
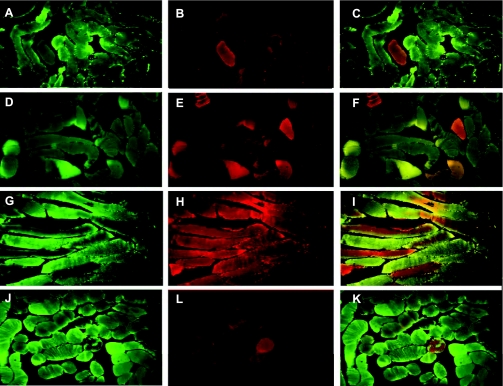
The distribution of specific mitochondrial proteins was assessed by immunofluorescence microscopy and staining for SDH subunit A, a nuclear-encoded mitochondrial Complex II protein (green fluorescence), and for the mtDNA-encoded electron transport protein COX-I (red fluorescence) (Fig. 3). COX-I staining is more significant in type I muscle fiber, whereas SDH stains both type I and type II fibers. The superimposed images allow assessment of colocalization and heterogeneity of these proteins. Figure 3, A–C, is representative of normal skeletal muscle in one subject before CO exposure or V̇o2max testing. Figure 3B shows modest COX-I expression and minimal SDH colocalization (Fig. 3C). Figure 3, D–F, shows typical staining in one subject in the air plus V̇o2max group. There is a minimal increase in COX-I staining but better colocalization with SDH than in the preexposure biopsy. At 3 days after the final CO exposure in the CO plus V̇o2max group, muscle SDH and COX-I expression are heterogeneously enhanced, and colocalization within fibers is also heterogeneous (Fig. 3, G–I). In the CO only group, primarily COX-I staining is enhanced (Fig. 3, J–L).
Fig. 3.
Immunofluorescence microscopy of skeletal muscle. COX-I (green fluorescence-mitochondrial encoding) and succinate dehydrogenase (SDH) subunit A (red fluorescence-nuclear encoding) were stained with specific antibodies, and the images were merged to assess colocalization. A–C: typical SDH (A) and COX-I (B) staining of control muscle and the overlay (C). D–F: typical stained muscle sections for SDH (D), COX-I (E), and the overlay (F) from the air plus V̇o2max group. G–I: muscle sections from the CO plus V̇o2max group stained for SDH (G), COX-I (H), and the overlay (I). J–K: SDH (J) and COX-I (L) staining and the overlay (K) in a sample of muscle after 5 days of CO breathing.
CO and skeletal muscle antioxidant enzymes.
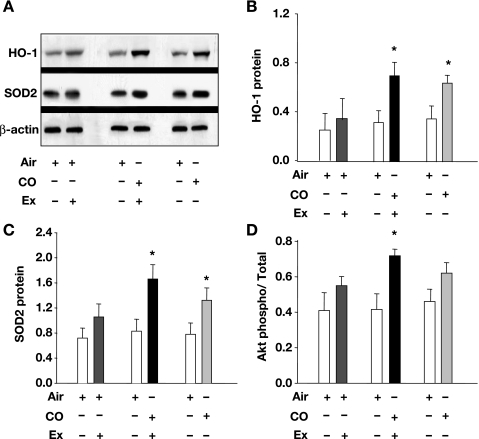
CO is known to induce the antioxidant enzymes HO-1 and SOD2; both are indicators of the oxidative stress response and are involved in mitochondrial biogenesis. Therefore, Western blots for HO-1 and SOD2 were performed in the muscle tissue before and after inhaled CO, with and without the exercise protocol. A representative Western blot and the mean densitometry data for HO-1 protein are shown in Fig. 4, A and B, respectively. Muscle HO-1 protein levels did not increase in the air V̇o2max group but more than doubled in the CO plus V̇o2max group and increased about twofold in the CO only group. Figure 4A also shows a representative Western blot and 4C the mean densitometry data for SOD2, which more than doubled after CO plus V̇o2max, and increased nearly twofold in the CO alone group (P < 0.05 for both). These data indicate that CO causes an oxidative stress response in skeletal muscle mitochondria, which is accentuated by V̇o2max testing.
Fig. 4.
Oxidative stress response and protein kinase B (Akt) activation in skeletal muscle after CO breathing with or without V̇o2max testing. Western blots for heme oxygenase (HO)-1, superoxide dismutase (SOD)-2, and phospho- and total Akt were performed on muscle samples before and after 5 days of inhaled CO, with or without the V̇o2max test. Relative protein expression was compared by normalizing HO-1 and SOD2 to β-actin. Akt activation was determined by the ratio of phospho- to total Akt. A: representative Western blots for HO-1 and SOD-2. B: densitometry for HO-1 expression relative to β-actin. C: densitometry for SOD-2 expression relative to β-actin. D: Akt activation before and after CO exposure. Values are means ± SD (*P < 0.05 between groups).
We have demonstrated in the mouse that mitochondrial oxidant generation activates Akt, which phosphorylates NRF-1 and stimulates mitochondrial biogenesis (33). To evaluate Akt in human muscle, we performed Western blots for phospho- and total Akt, and found a significant increase in phospho-Akt in the CO plus V̇o2max group (P < 0.05), and a trend toward Akt phosphorylation in the CO alone group (P = 0.05). Subjects in the air V̇o2max group showed no significant increase in Akt activation (Fig. 4D).
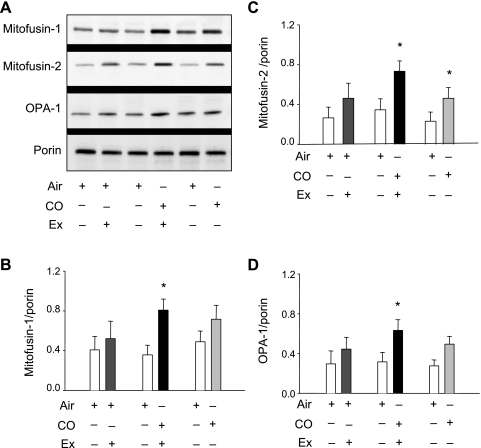
Mitochondrial fusion proteins.
The expressions of mitofusin-1, mitofusin-2, and OPA-1 proteins were measured because these proteins are integral to autonomous mitochondrial fusion that occurs in uniting contiguous units (6, 7). These data are displayed relative to the mitochondrial reference protein porin in Fig. 5. Figure 5A shows representative Western blots, and Fig. 5, B-D, shows the densitometry for each of the proteins. None of the levels changed significantly in the air plus V̇o2max group, but, in the CO plus V̇o2max group, the expression of all three proteins doubled (P = 0.032, 0.04, and 0.037). In the subjects breathing CO without exercise, the effects, except for mitofusin-2, were not statistically significant.
Fig. 5.
Mitochondrial fusion [mitofusin (mFn)] proteins and optic atrophy (OPA)-1 responses to inhaled CO with or without V̇o2max testing. A: Western blots of muscle tissue for mFn1, mFn2, and OPA-1 before and 5 days after inhaled CO with or without the V̇o2max test. Relative protein expression was derived by normalizing mFn1, mFn2, and OPA-1 to porin, a stable mitochondrial outer membrane protein. B: relative mFn1 expression. C: relative mFn2 expression. D: relative OPA-1 expression. Values are means ± SD (*P < 0.05 between groups).
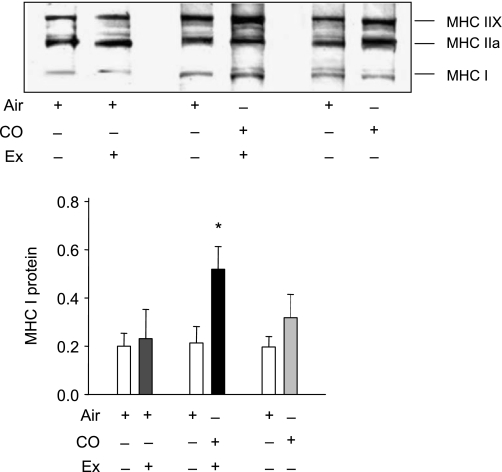
Exercise-induced MHC isotype switching.
We measured the ratio of MHC protein to determine if CO induces muscle fiber isotype switching from anaerobic to aerobic fibers. Figure 6 shows a Western blot and densitometry data for MHC class I and class II. MHC I protein expression increased significantly only in the CO plus V̇o2max group (P = 0.038). There was no significant change in MHC expression after CO alone. These data indicate that the myocyte fiber type switching after the exercise test is augmented by CO.
Fig. 6.
Myosin heavy chain (MHC) protein expression in skeletal muscle after CO breathing with or without V̇o2max testing. Gels of muscle samples for MHC isoforms from subjects before and 5 days after inhaled CO with or without the V̇o2max test. MHC I was expressed relative to β-actin, used as a loading control. Values are means ± SD (*P < 0.05 between groups).
Maximal oxygen uptake.
V̇o2max was measured before and 3 days after the last CO session, and no significant effect of CO was found (Table 1). There were also no differences detected in the exercise-induced changes in arterial pH or base deficit in the pre- to postexposure comparison for either the air or the CO group.
DISCUSSION
This is the first appraisal of the effects of inhaled CO on mtDNA transcription, replication, and mitochondrial biogenesis in humans. Using a daily regimen of 1 h of 100 ppm CO breathing for 5 days after a single V̇o2max test, we found increases in muscle mRNA for two key nuclear regulators of mitochondrial biogenesis, PGC-1α and NRF-1, as well as for Tfam and Polγ. Also, CO plus V̇o2max testing increased the muscle mitofusin and OPA-1 protein content coincident with COX-I and ATPase-6 protein expression; the latter is consistent with new mtDNA transcriptional and protein synthetic activity. This regimen also produced mitochondrial oxidative stress and activated the prosurvival kinase Akt. These responses are consistent with our mouse data, and the CO requirement, since they are not found in air-breathing subjects after V̇o2max testing. In six subjects breathing CO alone, however, not all of these markers increased, indicating that our protocol did not fully activate the mitochondrial biogenesis program in skeletal muscle (33).
A link between CO and mitochondrial biogenesis was first established in the mouse heart, where HO-1/CO enhances mitochondrial H2O2 production and activates Akt (33). Akt phosphorylates the NRF-1 transcription factor, which translocates to the nucleus and activates multiple genes of mitochondrial biogenesis (26). As in the mouse, CO exposures that doubled basal HbCO also increased HO-1 and SOD2 expression, due perhaps to mitochondrial heme release (8) and/or activation of the antioxidant response (25).
Although CO is clearly prooxidant and cytotoxic (27), it also induces protective or adaptive responses (10, 20). The CO concentration-time product employed here is well under that which produces overt acute or chronic health effects and is one-fourth of the Occupational Safety and Health Administration's maximum 8-h, time-weighted average. HbCO levels increased to only 3%, which does not produce tissue hypoxia, and our subjects were never symptomatic. Also, the cumulative inhibition of respiration is highly improbable because nearly five HbCO half-lives elapsed between each dose (36).
Originally, we had intended to test whether CO-related mitochondrial responses are associated with an increase in maximal aerobic exercise capacity but discovered an interaction between CO and the V̇o2max test. Because the main determinants of human V̇o2max are considered to be O2 uptake by the lungs, O2 delivery, O2 diffusion from capillary to mitochondria, and mitochondrial oxidative capacity (9), we had reasoned that, if muscle mitochondrial mass increased after CO, V̇o2max might improve. Rodent studies indicate that mitochondrial density is an important determinant of V̇o2max (21, 28), and mitochondria may play a larger role in governing V̇o2max at altitude (9) or in sustaining submaximal exercise (15). Although the transcriptional regulators of mitochondrial biogenesis were activated and mtDNA-encoded proteins increased in the CO plus V̇o2max group, mtDNA copy number did not increase significantly, and, 3 days later, no increase in V̇o2max was detected. This implies that our protocol was not sufficient to increase mitochondrial mass, that a minor increase in mitochondrial mass does not enhance maximal aerobic exercise capacity, or that other study limitations precluded detection of a performance improvement.
The relatively small size of this study also affords a low power to detect small effects. V̇o2max was measured at a single point after activation of the transcriptional events, and there may have been insufficient time to complete the assembly of new mitochondria. This could also account for an apparent discrepancy between increased citrate synthase expression, which is under nuclear control, and the stable mtDNA copy number. Some heterogeneity in nuclear-mitochondrial communication is also evident in the immunohistochemistry (Fig. 3), and may be related to the complex stimulus. Moreover, physiologically, CO would not be expected inhibit respiration for 3 days after the last exposure. Even though we could not ascertain whether mitochondrial mass limits aerobic capacity in healthy subjects, the approach is novel among human studies because it may be possible to address the question by stimulating mitochondrial biogenesis without exercise training.
It is noteworthy that no significant increases were found in air-control subjects in any of the measured parameters 5 days after a single exercise test, but earlier transcriptional responses for mitochondrial biogenesis are not precluded. For instance, a single swimming session can increase PGC-1α and NRF-1 levels (2), and endurance exercise will stimulate Tfam expression and mitochondrial proliferation (2, 16). There is also evidence that high AMP levels during V̇o2max testing induce mitochondrial enzymes (39) that contribute to endurance adaptation in skeletal muscle (1). However, the addition of CO to a single V̇o2max test activated a program that allows mitochondria to adjust to stress, and notably much of the effect is attributable to CO alone. These results may imply that CO and exercise recruit parallel transcriptional circuitry with synergy between the two pathways.
A change in mitochondrial fusion protein expression was clearly present in the V̇o2max plus CO group, but a relatively small shift toward a more aerobic muscle fiber type was found, implying that mitochondrial phenotype may be adjusted before muscle fiber phenotype. Although we did not directly evaluate mitochondrial fusion, mitochondria do exist in interconvertible forms: as small isolated organelles and as extended filaments, networks, or clusters connected by junctions. Mitochondria do fuse into electrically united systems that may facilitate ATP delivery from cell periphery to center and distribute antioxidant enzymes to clusters near the outer membrane where most of the O2 is consumed while distributing Δψ to the core network to distribute ATP production. Further characterization of mitochondrial function is thus desirable, but the small size of the muscle biopsies and the lack of any previous data on the CO effect precluded an extensive analysis of oxidative phosphorylation in this initial study.
In summary, intermittent CO breathing after a single V̇o2max test induces a mitochondrial oxidative stress response and conjunctively stimulates mitochondrial fusion protein expression and some aspects of mitochondrial biogenesis in human skeletal muscle. Despite the observed transcriptional responses and increases in each of a select group of mitochondrial proteins, maximal aerobic capacity did not change within the limits of a maximum exercise test. Subjects who underwent V̇o2max testing before CO showed a switch to a more aerobic fiber type that was not detected under either stimulus alone. CO alone did lead to significant increases in mRNA expression for Tfam and Polγ and protein expression for HO-1, SOD2, mitofusin-2, and mitochondrial-encoded COX-I and ATPase-6. These intriguing results raise the prospect that exogenous CO given in optimized, safe, regimens might offer a simple means to hasten recovery in diseases where mitochondrial dysfunction is acquired, for instance, in diabetes (18), sepsis (4), atherosclerosis (5), or chronic obstructive pulmonary disease (11).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant 1R01-HL-090679 and Gas Enabled Medical Innovations (GEMI) Fund No. 393-3140.
REFERENCES
- 1.Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. Faseb J 19: 786–788, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Faseb J 16: 1879–1886, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, Runge MS. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res 86: 960–966, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cakir Y, Yang Z, Knight CA, Pompilius M, Westbrook D, Bailey SM, Pinkerton KE, Ballinger SW. Effect of alcohol and tobacco smoke on mtDNA damage and atherogenesis. Free Radic Biol Med 43: 1279–1288, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chan DC Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Cronje FJ, Carraway MS, Freiberger JJ, Suliman HB, Piantadosi CA. Carbon monoxide actuates O(2)-limited heme degradation in the rat brain. Free Radic Biol Med 37: 1802–1812, 2004. [DOI] [PubMed] [Google Scholar]
- 9.di Prampero PE Factors limiting maximal performance in humans. Eur J Appl Physiol 90: 420–429, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med 7: 598–604, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Gosker HR, Hesselink MK, Duimel H, Ward KA, Schols AM. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J 30: 73–79, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol 280: L1300–L1308, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci 28: 2015–2024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haden DW, Suliman HB, Carraway MS, Welty-Wolf KE, Ali AS, Shitara H, Yonekawa H, Piantadosi CA. Mitochondrial Biogenesis Restores Oxidative Metabolism during Staphylococcus aureus Sepsis. Am J Respir Crit Care Med 176: 768–777, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209: 2265–2275, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18: 357–368, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res 102: 401–414, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18: 231–236, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 21.McAllister RM, Terjung RL. Acute inhibition of respiratory capacity of muscle reduces peak oxygen consumption. Am J Physiol Cell Physiol 259: C889–C896, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci 119: 2855–2862, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Piantadosi CA Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med 45: 562–569, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103: 1232–1240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem 281: 324–333, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Piantadosi CA, Zhang J, Demchenko IT. Production of hydroxyl radical in the hippocampus after CO hypoxia or hypoxic hypoxia in the rat. Free Radic Biol Med 22: 725–732, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Robinson DM, Ogilvie RW, Tullson PC, Terjung RL. Increased peak oxygen consumption of trained muscle requires increased electron flux capacity. J Appl Physiol 77: 1941–1952, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Saltin B Hemodynamic adaptations to exercise. Am J Cardiol 55: 42D–47D, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Shadel GS, Clayton DA. Mitochondrial DNA maintenance in vertebrates. Annu Rev Biochem 66: 409–435, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 117: 3730–3741, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci 120: 299–308, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Suliman HB, Carraway MS, Welty-Wolf KE, Whorton AR, Piantadosi CA. Lipopolysaccharide stimulates mitochondrial biogenesis via activation of nuclear respiratory factor-1. J Biol Chem 278: 41510–41518, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Suliman HB, Welty-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. Faseb J 19: 1531–1533, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Thom SR, Keim LW. Carbon monoxide poisoning: a review epidemiology, pathophysiology, clinical findings, and treatment options including hyperbaric oxygen therapy. J Toxicol Clin Toxicol 27: 141–156, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Wagner PD Diffusive resistance to O2 transport in muscle. Acta Physiol Scand 168: 609–614, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Wagner PD Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc 24: 54–58, 1992. [PubMed] [Google Scholar]
- 39.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. [DOI] [PubMed] [Google Scholar]