Abstract
The ability of pharmacological preconditioning mimetics to confer long-lasting and sustained cardioprotection may be a logical criterion to develop a drug that can be used clinically for cardioprotection. We propose here that the use of long-acting phosphodiesterase-5 inhibitor, tadalafil, may confer sustained cardioprotection against ischemia. Tadalafil (5 mg/kg) was administered orally to male C57B/6J mice (n = 6 in each treatment subgroup at each time point studied). Hearts were isolated and subjected to 40 min of ischemia and 30 min of reperfusion on Langendorff's apparatus at 1, 12, 24, 36, 48, 60, 72, and 108 h after tadalafil administration. In 1- to 48-h subgroups, tadalafil was given once at 0 h only. In 60- and 72-h subgroups, tadalafil was given twice at 0 and 36 h. Similarly, in the 108-h subgroup, tadalafil was administered at 0, 36, and 72 h. In the same subgroups, wortmannin (15 μg/kg ip), an inhibitor of phosphatidylinositol 3-kinase or 5-hydroxydecanoic acid (5 mg/kg ip), an inhibitor of mitochondrial ATP-sensitive K+ channels, was given together with tadalafil, and the hearts were subjected to ischemia-reperfusion at 36 h to determine whether the effect of tadalafil on ischemia-reperfusion injury was abolished. As a result, tadalafil treatment reduced left ventricular end-diastolic pressure and increased left ventricular developed pressure as well as reduced lactate dehydrogenase release. This protection remained till 36–40 h, and thereafter it vanished. The readministration of tadalafil at 36 and 72 h restored the protection till 108 h. Tadalafil treatment accelerated Akt phosphorylation in cardiac tissue and decreased myocyte apoptosis. The administration of wortmannin abolished the beneficial effects of tadalafil on hemodynamic parameters and myocyte apoptosis, together with significantly reduced Akt phosphorylation. 5-Hydroxydecanoic acid also abolished the antiapoptotic effect of tadalafil. It is concluded that tadalafil treatment induces the long-term protection of ischemic myocardium via phosphatidylinositol 3-kinase/Akt signaling pathway.
Keywords: Akt, preconditioning, sustained cardiac protection
the effectiveness of myocardial protection after ischemic preconditioning (IPC) is well documented (12, 23). The fundamental concept of IPC is that a brief period of ischemia and reperfusion delimits myocardial cellular damage during a subsequent event of prolonged lethal ischemia. The effects of IPC have been reproduced by using preconditioning mimetics such as mitochondrial ATP-sensitive K+ (mitoKATP) channel openers (23). Unfortunately, the protective effects of IPC are short-lived. The search for pharmacological agents that can keep the heart in a sustainable state of protection will be a right step in the right direction. BMS-191095, a specific KATP channel opener, given at an interval of 20 h, kept the heart in protective state (23). The emerging concept of chronic intermittent preconditioning in which the heart can be constantly protected by chronic exposure to morphine (15) could be extended to the clinic by using promising pharmacological agents that can provide long-lasting protective effects to the heart before surgical procedures.
Phosphodiesterase inhibitors (PDIs) have important vascular and myocardial protective effects and have shown therapeutic usefulness in the clinical settings for treatment of patients with heart failure, pulmonary hypertension, and coronary artery disease (2, 7). PDIs selectively antagonize phosphodiesterase 5 (PDE5), which is found in high abundance in a variety of cells in humans (9), including canine and mouse cardiomyocytes (4, 18). The pharmacodynamics of PDIs in general and sildenafil in particular were consistent with the tissue distribution profile of PDE-5 and its isoform PDE-3. PDIs prevent the breakdown of nitric oxide (NO)-driven cGMP, primarily in vascular smooth muscle cells, and act as potent vasodilators. In vitro, PDIs have shown protection of adult cardiomyocytes during ischemia-reperfusion (I/R) injury with a possible role for cGMP-dependent protein kinase-1α (4). Sildenafil has also been shown to produce a marked preconditioning-like effect in the intact rabbit hearts (14). It appears that the protection incurred by sildenafil lasts for a limited period. Kukreja and colleagues (3) have also demonstrated that the potent effect of sildenafil-induced cardioprotection was due to the early translocation of protein kinase C (PKC) from cytosol to the membrane (3). These data provided direct evidence of an essential role of PKC in sildenafil-induced cardioprotection.
Since one of the PDE-5 inhibitors, tadalafil, has long-lasting effects, we reasoned that tadalafil can be used for longer-term protective effects. The present study was designed to demonstrate the persistent cardioprotective effects of tadalafil using an in situ mice model of myocardial infarction.
MATERIALS AND METHODS
The current study conforms to the guidelines established by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996), and the protocol was approved by the Institutional Animal Care and Use Committee. Adult male mice (C57B/6J) were obtained from Harlan. Tadalafil (Lilly), wortmannin (Wort) and 5-hydroxydecanoic acid (5-HD; Sigma), and Akt kit (Cell Signaling Technology) were purchased for use in the study.
Experimental Protocol
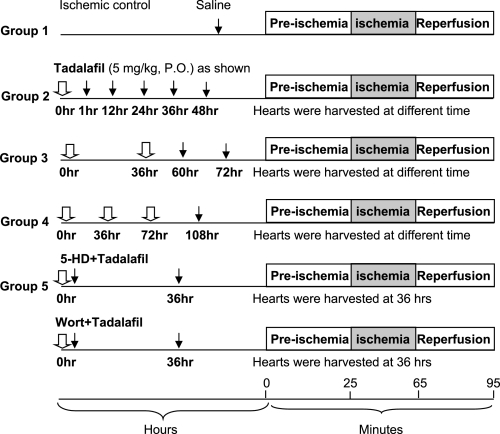
The experimental design has been summarized in Fig. 1. A total of 80 adult male mice C57B/6J mice, each weighing 25–30 g, were used in this study. Animals were randomized into five groups and multiple subgroups (n = 6 in each subgroup), which were harvested at the time points shown in Fig. 1. Group 1 consisted of ischemic control with no drug treatment; group 2, tadalafil treatment at 0 h. Hearts were isolated at 1, 12, 24, 36, and 48 h and subjected to I/R. Group 3 consisted of tadalafil treatment at 0 and 36 h. Hearts were isolated at 60 and 72 h for I/R. Group 4 was given tadalafil three times at 0, 36, and 72 h. Hearts were isolated at 108 h for I/R. Group 5 was given tadalafil + Wort (15 μg/kg ip) (5), an inhibitor of phosphatidylinositol 3-kinase (PI3K), or tadalafil + 5-HD ip, a specific inhibitor of mitoKATP channel. Tadalafil tablets were crushed in water and given to animals orally via gauge needle. Hearts were subjected to Langendorff perfusion at various time indicated. After an equilibration of 25 min, ischemia was induced for 40 min by shutting off the oxygenated buffer, followed by reperfusion for 30 min. Four hearts in each group were used for phosphorylated (p)Akt measurement by Western blot analysis.
Fig. 1.
Experimental groups and protocol. 5-HD, 5-hydroxydecanoic acid; Wort, wortmannin; PO, per os. Drug administration (white arrow) and heart removal (black arrow) are indicated.
Heart Preparation for Langendorff Perfusion
The animals were anesthetized with pentobarbital sodium (40 mg/kg ip) and heparinized (5,000 U/kg) to protect the heart against microthrombi. The chest was opened at the sternum, and the heart was quickly removed and cannulated with a 20-gauge phalanged stainless steel cannula. The heart was perfused on a noncirculating Langendorff apparatus with pH 7.4 Krebs-Henseleit buffer (in mM) consisting of 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 25 NaHCO3, and glucose (23). The buffer was saturated with 95% O2-5% CO2 at 37°C for 25 min. The heart was perfused at a constant pressure of 80 mmHg. A homemade water-filled balloon was inserted into left ventricle (LV) through left atrium and adjusted to a LV end-diastolic pressure (LVEDP) of 5–8 mmHg during the initial equilibration. The distal end of the catheter was connected to a DigiMed heart performance analyzer (model 210, version 1.01, Micro-Med) by way of a pressure transducer (Case, Lakewood). Coronary flow (CF) was measured by a transit time flowmeter (Transonic Systems). The heart was paced at 350 beats/min except during ischemia. The pacing was reinitiated after 3 min of reperfusion for 30 min in all groups. The index of myocardial function was determined as previously described (22).
Measurement of Lactate Dehydrogenase
Lactate dehydrogenase (LDH), an indicator of myocardial tissue injury, was determined in the coronary effluent by a coupled enzyme-spectrometric technique (DU Series 500 Spectrophotometer, Beckman Instruments) using LDH assays kit (MBL) as per the manufacturer's instructions. LDH was measured at 3, 5, 10, 20, and 30 min after reperfusion. The accumulated amount was obtained by integrating the area underneath the individual time-course curve for 30 min of reperfusion, and the mean value was calculated form six animals per group.
Western Blot Analysis for Akt Phosphorylation
Pretreated hearts (LVs) were harvested, weighed, and stored at −70°C until used for protein extraction. Each heart tissue sample was homogenized for six bursts (15 s each) at 4°C with a Polytron-PT homogenizer using lysis buffer containing (in mM) 0.1 NaCl, 10 Tris (pH 7.6), 1 EDTA, 2 Na pyrophosphate, 2 NaF, 2 β-glycerophosphate, 0.5 4-(2-aminoethyl)benzenesulfonyl fluoride, and a cocktail of protease inhibitors. After sonication for 5 s, the tissue lysate was centrifuged at 14,000 rpm for 5 min at 4°C. The protein contents were determined using Protein Assay Reagent kit (Pierce, Rockford, IL). Equal amounts of protein samples (30 μg/μl) were mixed with equal volume of sample buffer containing 2% SDS, 100 mM Tris, 0.2% bromophenol blue, 20% glycerol, and 200 mM dithiotheritol, boiled for 15 min, and electrophoresed on 10% polyacrylamide gels (Precast Gels, ISC Bioexpress). The electrophoresed proteins were transferred from the gel to nitrocellulose membranes (Bio-Rad) and immunodetected for total Akt and pAkt using anti-Akt and anti-pAkt antibodies (each 1:1,000 dilution) and horseradish-conjugated anti-rabbit secondary antibody (BD Pharmingen) as described earlier (13). The blots were developed with LumiGlo developing solutions for a visualization of the immune reaction. The amount of total and pAkt was quantified by densitometry (13) .
Terminal dUTP Nick End Labeling Assay
For apoptosis, additional hearts were reperfused for 2 h after ischemia to render deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive nuclei visible. TUNEL was performed on 5-μm-thick deparaffinized histological sections, using MEBSTAIN Apoptosis Kit II (Medical and Biological Laboratories). The sections were made from the mid-LV of the heart and were stained with fluorescent TUNEL using the method described previously (20). The sections were stained with 4,6-diamidino-2-phenylindole to visualize nuclei and photographed with an Olympus BX41 microscope (Olympus America, Melville, NY) equipped with a digital camera.
Statistical Analysis
All values are expressed as means ± SE. Group comparisons were analyzed by one-way ANOVA (StatView 4.0). All groups were analyzed simultaneously with a Bonferroni/Dunn test. A difference of P < 0.05 was considered as statistically significant.
RESULTS
Hemodynamic and Biochemical Assessment of LV Function
Preischemic LV functional parameters.
The mean values of LV developed pressure (LVDP), LVEDP, and CF were not significantly different between the groups during the equilibration period. Similarly, there was also no significant difference in the body or heart weight between the groups.
Postischemic LV functional parameters.
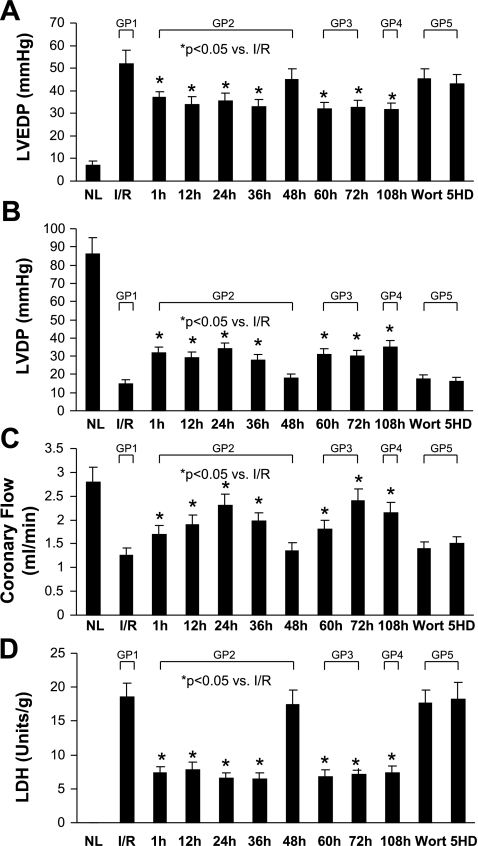
Cardiac function was assessed after each heart was subjected to ischemia (40 min) and reperfusion (30 min). LVEDP, LVDP, and CF were recorded and compared with the ischemic control group (Fig. 2, A–C). Heart function was significantly reduced after I/R. CF was also decreased, whereas LVEDP was increased after I/R (Fig. 2).
Fig. 2.
Effect of various interventions on left ventricular (LV) developed pressure (LVDP; A), LV end-diastolic pressure (LVEDP; B), coronary flow (C), and lactate dehydrogenase (LDH; D). NL, normal control; I/R, ischemic-reperfusion control; Wort, tadalafil + wortmannin; 5-HD, tadalafil + 5-HD; GP1, group 1. Values are means ± SE; n = 6 animals for each group. *P < 0.05 vs. I/R.
Protective effects of tadalafil on postischemic injury.
Following tadalafil pretreatment, hearts examined at 1, 12, 24, 36, and 48 h showed significantly increased LVDP and CF till 40 h after I/R compared with the control. On the other hand, LVEDP was decreased in tadalafil-treated hearts compared with the ischemic control animals. LDH release was greatly reduced from hearts treated at different time intervals compared with hearts from the ischemic control group (Fig. 2D). The cardioprotective effects of tadalafil persisted until 36–40 h after pretreatment (Fig. 2). However, by 48 h, the cardioprotective effects of tadalafil were dissipated as indicated by the reduced LVDP and CF and the elevated LVEDP and LDH release compared with those levels in the ischemic control group. Interestingly, the readministration of tadalafil at 36 h restored cardioprotection until 72 h. The readministration of tadalafil at 0, 36, and 72 h extended protection until 108 h.
Antiapoptotic Effect of Tadalafil on Ischemic Injury Through Akt Signaling Pathway
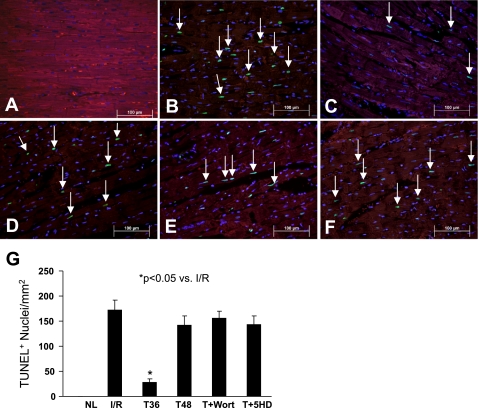
The mitoKATP channel is an important modulator of various events in a cell, especially those involved in cell-death signaling pathways. The activation of these channels by Akt prevents the release of cytochrome-c and Ca2+ influx into the cell and increase ATP synthesis. In the mice treated with tadalafil at different times, a significant reduction in the number of TUNEL-positive nuclei was observed compared with that in the ischemic control group (Fig. 3). In line with these results, apoptosis was significantly increased in tadalafil-treated hearts after the administration of either Wort or 5-HD (Fig. 3, E and F). The data further support the concept that the PI3K/Akt pathway plays a critical role in cardioprotection induced by tadalafil.
Fig. 3.
Representative photomicrographs showing terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive nuclei in various treatment groups. A: normal heart tissue. B: TUNEL-positive nuclei (green, white arrow) in I/R control group. C: tadalafil-treated group at 36 h (T36). TUNEL-positive nuclei were significantly decreased in tadalafil-treated hearts compared with those in the I/R group. D: tadalafil-treated group at 48 h (T48) showing reappearance of apoptosis. E: tadalafil + Wort treatment group. F: tadalafil + 5-HD treatment group. Apoptosis is similar to I/R group (B). All nuclei were stained blue by 4,6-diamidino-2-phenylindole, and heart tissue was stained with α-sarcomeric actin (red) for cardiomyocytes. Magnification, ×400; n = 4 animals for each group. Bars = 100 μm. G: quantitative estimate values of TUNEL-positive nuclei in data are means ± SE. *P < 0.05 vs. I/R Control; n = 4 animals for each group.
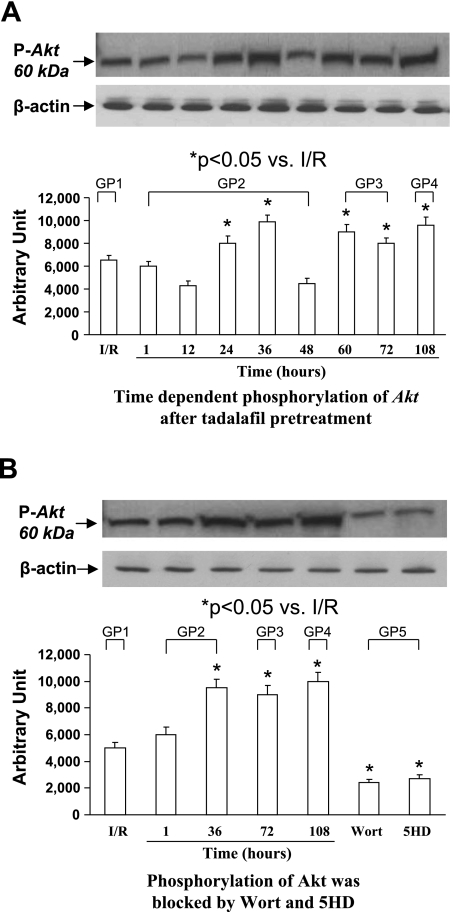
Phosphorylation of Akt by Tadalafil
Western blot analysis revealed that the phosphorylation of Akt was increased in tadalafil-treated hearts at different time points (Fig. 4, A and B). A significant increase in Akt phosphorylation was observed at 24 and 36 h (group 2), 60 and 72 h (group 3), and 108 h (group 4). The administration of Wort, a specific PI3K inhibitor, downregulated Akt phosphorylation. Similarly, the administration of 5-HD, which acts at the mitochondrial level, significantly reduced Akt phosphorylation (Fig. 4B).
Fig. 4.
Western blot analysis for Akt protein. A, top: time-dependent phosphorylation of Akt (pAkt; groups 1–4). A, bottom: quantitative measurement of pAkt. Data are means ± SE; n = 4 animals for each group. B: effect of Wort and 5-HD on phosphorylation of Akt. Data are means ± SE; n = 6 animals for each group. *P < 0.05 vs. control. β-Actin was used as equal-loading control.
DISCUSSION
The major findings of this study are that the heart is protected against ischemia with long-acting tadalafil for 40 h and that protection continues till 108 h if the heart is treated again with the drug at 36 and 72 h. The protection appears to be mediated by PI3K/Akt and mitoKATP channel pathways. Among the various pharmacological strategies for cardiac protection, the use of clinically relevant agents is more appealing. PDE-5 inhibitors have a potential use in cardiovascular diseases (7, 9). This class of drugs has myocardial and vascular protective effects. Moreover, these drugs are free of serious side effects except under conditions of hypotension in patients caused by the combined use of α-blockers and nitrates as reviewed by Gross et al. (6). Sildenafil has been shown to be cardioprotective in rabbits after myocardial infarction (14). There was an improved postischemic recovery of ventricular function, a reduced incidence of ventricular fibrillation, and a decreased myocardial infarction. Sesti et al. (19) also reported that tadalafil reduced infarct size in rats following coronary artery ligation. In the present study, we have demonstrated that tadalafil was significantly cytoprotective and resultantly preserved the LV contractile functions. Moreover, we have shown that 5-HD, a mitoKATP channel blocker, or the inhibition of PI3K/Akt signaling by Wort abrogated the effects of tadalafil, thus suggesting the protective role of mitoKATP channels via PI3K signaling.
Activation of PI3K Signaling Attenuated Cell Death
We hypothesized that activation of PI3K signaling pathway upstream of mitoKATP channel resulted in decreased cardiac cell death and apoptosis. Akt activation not only reduces the number of apoptotic cells but also substantially delimits infarct size and improves cardiac function (11, 20). Western blot analysis and TUNEL assay clearly indicated that tadalafil activated PI3K/Akt pathway for its antiapoptotic activity. The administration of Wort, which is a specific inhibitor of PI3K, abolished Akt activation when given with tadalafil. Similarly, 5-HD, when given together with tadalafil, also abolished the beneficial effects of tadalafil at 36 h by blocking the activation of mitoKATP channels, which are downstream targets of Akt. These results support the concept that a downstream target of PI3K system is the mitoKATP channel.
Apoptosis
There is growing evidence that cardiomyocytes undergo apoptosis primarily during reperfusion (25). Preconditioning activates PI3K and suppresses caspase-3 and DNA fragmentation, thus reducing apoptosis (8, 16). These studies highlight the role of caspase-3 as an important signaling component of apoptosis and cell death. Although apoptosis in the ischemic myocardium is controversial, reperfusion is known to accelerate cardiomyocyte death in the ischemic myocardium. TUNEL-positive cells were significantly increased in ischemic control hearts. Our data indicate that apoptosis was attenuated in hearts treated with tadalafil at various time intervals compared with ischemic control group. The increased number of TUNEL-positive cells was also observed in the tadalafil + Wort-treated group, thus suggesting that PI3K pathway is prosurvival. In the tadalafil + 5-HD group, an increased number of apoptotic nuclei were observed, which supports the concept that Akt is upstream of the mitoKATP channels (1). Another study by Kukreja's group (17) using mice reported that the protection by sildenafil was mediated by the upregulation of inducible and endothelial NO synthases.
This is the first report to show that PDE-5 inhibitor tadalafil, which has been clinically approved for erectile dysfunction, can provide long-lasting protection against ischemia compared with the other known pharmacological preconditioning agents. The selective activation of mitoKATP channels seems to play an important role in cardioprotection against ischemia via PI3K and Akt phosphorylation. Although the cardioprotective effect of tadalafil is abolished with the mitoKATP channel inhibition, it is also likely that protection may also be mediated by alternate pathways. Thus cross talk between PI3K/Akt and downstream targets, such as glycogen synthase 3 (21), or other prosurvival kinases remains to be investigated. The concept of sustained cardioprotection by long-acting PDE5A inhibitor tadalafil is novel and can be exploited under the clinical settings.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-087246 and HL-095375 (to M. Ashraf) and HL-089824 and HL-081859 (to Y. Wang).
REFERENCES
- 1.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, Ashraf M. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol 290: H2402–H2408, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Boswell-Smith V, Page CP. Roflumilast: a phosphodiesterase-4 inhibitor for the treatment of respiratory disease. Expert Opin Investig Drugs 15: 1105–1113, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Das A, Ockaili R, Salloum F, Kukreja RC. Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 286: H1455–H1460, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281: 38644–38652, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circ Res 94: 960–966, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Gross GJ Sildenafil and endothelial dysfunction in humans. Circulation 111: 721–723, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Guazzi M, Samaja M. The role of PDE5-inhibitors in cardiopulmonary disorders: from basic evidence to clinical development. Curr Med Chem 14: 2181–2191, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Han H, Wang H, Long H, Nattel S, Wang Z. Oxidative preconditioning and apoptosis in L-cells. Roles of protein kinase B and mitogen-activated protein kinases. J Biol Chem 276: 26357–26364, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 101: 1084–1095, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Kukreja RC, Ockaili R, Salloum F, Yin C, Hawkins J, Das A, Xi L. Cardioprotection with phosphodiesterase-5 inhibition—a novel preconditioning strategy. J Mol Cell Cardiol 36: 165–173, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Niagara MI, Haider HKh, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res 100: 447–449, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol 283: H1263–H1269, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Peart JN, Gross GJ. Morphine-tolerant mice exhibit a profound and persistent cardioprotective phenotype. Circulation 109: 1219–1222, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther 318: 186–194, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–597, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Senzaki H, Smith CJ, Juang GJ, Isoda T, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J 15: 1718–1726, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Sesti C, Florio V, Johnson EG, Kloner RA. The phosphodiesterase-5 inhibitor tadalafil reduces myocardial infarct size. Int J Impot Res 19: 55–61, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol 290: H441–H449, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Gang Zhang Z, Lan Zhang R, Chopp M. Activation of the PI3-K/AKT pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab 25: 1150–1158, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Ahmad N, Kudo M, Ashraf M. Contribution of Akt and endothelial nitric oxide synthase to diazoxide-induced late preconditioning. Am J Physiol Heart Circ Physiol 287: H1125–H1131, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Ahmad N, Wang B, Ashraf M. Chronic preconditioning: a novel approach for cardiac protection. Am J Physiol Heart Circ Physiol 292: H2300–H2305, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Haider HK, Ahmad N, Ashraf M. Mechanisms by which KATP channel openers produce acute and delayed cardioprotection. Vascul Pharmacol 42: 253–264, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Zhao ZQ, Nakamura M, Wang NP, Wilcox JN, Shearer S, Ronson RS, Guyton RA, Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res 45: 651–660, 2000. [DOI] [PubMed] [Google Scholar]