Abstract
Expression of connexin 40 (Cx40) and Cx43 in cardiovascular tissues varies as a function of age, injury, and development with unknown consequences on the selectivity of junctional communication and its acute regulation. We investigated the PKC-dependent regulation of charge selectivity in junctions composed of Cx43, Cx40, or both by simultaneous assessment of junctional permeance rate constants (Bdye) for dyes of similar size but opposite charge, N,N,N-trimethyl-2-[methyl-(7-nitro-2,1,3-benzoxadiol-4-yl)amino]ethanaminium (NBD-M-TMA; +1) and Alexa 350 (−1). The ratio of dye rate constants (BNBD-M-TMA/BAlexa 350) indicated that Cx40 junctions are cation selective (10.7 ± 0.5), whereas Cx43 junction are nonselective (1.22 ± 0.14). In coexpressing cells, a broad range of junctional selectivities was observed with mean cation selectivity increasing as the Cx40 to Cx43 expression ratio increased. PKC activation reduced or eliminated dye permeability of Cx43 junctions without altering their charge selectivity, had no effect on either permeability or charge selectivity of Cx40 junctions, and significantly increased the cation selectivity of junctions formed by coexpressing cells (approaching charge selectivity of Cx40 junctions). Junctions composed of Cx43 truncated at residue 257 (Cx43tr) were also not charge selective, but when Cx43tr was coexpressed with Cx40, a broad range of junctional selectivities that was unaffected by PKC activation was observed. Thus, whereas the charge selectivities of homomeric/homotypic Cx43 and Cx40 junctions appear invariant, the selectivities of junctions formed by cells coexpressing Cx40 and Cx43 vary considerably, reflecting both their relative expression levels and phosphorylation-dependent regulation. Such regulation could represent a mechanism by which coexpressing cells such as vascular endothelium and atrial cells regulate acutely the selective intercellular communication mediated by their gap junctions.
Keywords: heteromeric channel, homomeric channel, carboxy-terminal domain, PKC-dependent regulation
gap junctions are aggregates of intercellular channels that form low-resistance pathways for direct communication of electrical and chemical signals between neighboring cells. Gap junction channels are composed of protein subunits termed connexins, for which there are 21 genes in the human genome (37). The importance of gap junctions in development and proper function of cells, tissues, organs, and organ systems is increasingly supported by connexin-linked hereditary diseases and the results of targeted gene deletion and replacement studies (18). The large number of connexin isoforms and their controlled expression and coexpression as a function of location, developmental stage, and both physiological and pathophysiological state suggest connexin-specific functional roles (13, 21). Connexin 43 (Cx43) and Cx40 are coexpressed in multiple tissues of the cardiovascular system, including prominent expression in atrial and endothelial cells (13, 25, 40), yet the physiological consequences of this coexpression and of variations in the amount and/or functional availability of either protein have not been thoroughly explored.
An important functional role for connexin proteins is their ability to form intercellular channels capable of supporting intercellular exchange of molecules up to ∼1,000 Da (22). The potential to form channels that in a connexin-specific manner differentially discriminate amongst available permeant molecules (i.e., display different selectivities) and the potential for connexin-specific regulation of junctional selectivity represent possible benefits of expressing multiple connexin isoforms at the cell and tissue level. Different connexins form channels and junctions with distinct permeability, selectivity, and conductance properties (22). That junctions formed of a single connexin display variable permselectivity (defined as dye permeability relative to conductance) suggests possible regulation of channel permeability (16, 17). Connexin-specific differences in junctional selectivity and regulated permselectivity for specific connexin-comprised junctions could provide for acute and long-term control of the specificity of intercellular communication, supporting or not the exchange of small signaling molecules and metabolites known to permeate gap junction channels (20, 22). Although these possibilities have earlier (and often) been suggested in the literature, few studies have successfully quantified such regulation (17, 19, 23, 44).
The aims of the present study were to determine the extent to which junctional charge selectivity could be varied by two mechanisms: 1) coexpression of multiple connexins (Cx43 and Cx40) and 2) protein kinase C (PKC)-dependent regulation. Our approach involved measuring the charge selectivity of individual junctions by simultaneous comparison of junctional permeance to two dyes differing in charge (23), which allows for both the specific measurement of the junctional selectivity for a large number of individual gap junctions as well as for valid comparison of selectivity between different gap junctions. We show that dye-charge selectivity is a fixed parameter for junctions formed of either Cx43 or Cx40, whereas the dye-charge selectivity of junctions formed by cells coexpressing these proteins varies between that of Cx43 and Cx40 junctions with an intermediate average charge selectivity that relates to their relative expression levels. Treating Cx40- and Cx43-coexpressing cells with phorbol 12-myristate 13-acetate (TPA) to activate PKC shifts junctional dye-charge selectivity toward the cationic charge selectivity of Cx40 junctions in a manner that requires the carboxy terminus (CT) of Cx43. Thus, by coexpressing variable ratios of two connexins, cells gain long-term control of junctional charge selectivity as well as short-term control through kinase-dependent regulatory strategies.
MATERIALS AND METHODS
Cells.
Rat insulinoma (Rin) cells stably transfected with wild-type (WT) rat Cx43 (Rin43) (from Dr. Paolo Meda) (43) or WT rat Cx40 (Rin40) (8) were used for single connexin isoform experiments. Rin and Rin40 cells transiently transfected [by using Nucleofector II (Amaxa Biosystems) or Lipofectamine (Invitrogen) per instructions] with rat Cx43 truncated at amino acid 257 (Cx43tr) (15) were used for the Cx43tr and Cx40:Cx43tr experiments, respectively. It should be noted that the Rin cells express no endogenous connexins; consequently, in the absence of successful transfection the cells are not coupled, even at the single channel level. All types of Rin cells were maintained in RPMI with 10% FBS; medium for Rin43 and Rin40 cells also contained 300 μg/ml G418. Transiently transfected Rin cells were used 12–48 h after transfection. Normal rat kidney epithelial (NRK) cells transfected for inducible expression of the CT (residues 235–382) of Cx43 were used for the Cx43+CT experiments (36). NRK+CT cells were grown in DMEM (Sigma D-1152 with 4.5 mg/ml glucose) with 500 μg/ml G418 and hygromycin; CT expression was induced in the NRK+CT cells with 1 μg/ml doxycycline for 24–48 h (17, 36). A7r5 cells, an embryonic rat aortic smooth muscle cell line that naturally coexpresses Cx40 and Cx43 (American Type Culture Collection, Manassas, VA), were grown in DMEM with 10% FBS. A7r5C3 cells, engineered to express higher levels of Cx40 than the A7r5 parental cells (11), were grown in DMEM (Sigma D-1152) with 10% FBS and 300 μg/ml G418.
TPA treatments.
Rin40, Rin43, and A7r5 cells in dishes or on coverslips were incubated in culture medium containing 100 ng/ml phorbol 12-myristate 13-acetate (TPA; Alexis Biochemicals) for 0, 15, 30, or 60 min at 37°C prior to isolation of total protein for Western blot analysis. Since changes in Cx43 mobility were already maximal at 15 min, dye injection experiments were carried out on these cell types pretreated for 15 min at 37°C with 100 ng/ml TPA and subsequently, throughout the dye injection period (at room temperature), in 100 ng/ml TPA in external solution (in mmol/l: 142.5 NaCl, 4 KCl, 1 MgCl2, 5 glucose, 2 sodium pyruvate, 10 HEPES, 1 BaCl2, 1 CaCl2, 15 CsCl, and 10 TEACl, pH 7.2 and osmolarity 315 mosM). Because this treatment paradigm reduced the incidence of coupling to zero in the Rin43 cells, thereby precluding assessment of TPA's effect on junctional selectivity, a second set of experiments was performed using a strategy similar to that used previously to examine the effects of TPA on junctional conductance and channel properties (28). Specifically, cells on coverslips were exposed at room temperature to 50 ng/ml TPA (in external solution) and dye injection experiments were performed over the ensuing 5–70 min.
Western blot.
Total protein was isolated from cells treated as described above using Laemmli buffer. Protein content of the samples was measured by the BCA assay (Pierce Chemical; Rockford, IL). The samples were loaded (equal amounts of total protein) onto polyacrylamide gels (12%), electrophoresed at 200 V and transferred to a nitrocellulose membrane. The membrane was subsequently probed overnight with antibody against Cx43 (Sigma antibody C6219; 1:4,000 dilution) or against Cx40 [affinity purified, rabbit anti-rat Cx40 (residues 234–332)-glutathione-S-transferase fusion protein; 1:4,000 dilution]. These primary antibodies were visualized with a horseradish peroxidase conjugated secondary antibody and enhanced chemiluminescence strategies [SuperSignal West Pico System (Pierce cat. no. 32106) or SuperSignal West Femto System (Pierce cat. no. 34095)].
Dye selectivity measurement.
The approach used to assess charge selectivity was previously described in detail (23) and briefly here.
Filters and dyes.
Junctional permeant Alexa 350 (hydrazide sodium salt; Molecular Probes; Invitrogen, Carlsbad, CA), an anionic dye, and N,N,N-trimethyl-2-[methyl-(7-nitro-2,1,3-benzoxadiol-4-yl)amino]ethanaminium (NBD-M-TMA), a cationic dye (1), were used to probe junctional charge selectivity (see Table 1 for dye properties). Tetramethylrhodamine dextran (molecular weight 3,000: Molecular Probes), which does not permeate gap junctions, was used to distinguish cells connected by cytoplasmic bridges. The filter sets employed to visualize each dye exhibited no overlap between dyes (23).
Table 1.
Physical properties of permeants
| Molecular Weight (no counter ion) | DAqueous (25°C), 10−6 cm2/s | Stokes-Einstein Radius, Å | λex/λem, nM | Net Charge | |
|---|---|---|---|---|---|
| NBD-M-TMA | 280 | 5.8* | 4.3 | 458/580 | 1+ |
| Alexa 350 | 326 | 5.6* | 4.4 | 345/445 | 1− |
NBD-M-TMA, N,N,N-trimethyl-2-[methyl-(7-nitro-2,1,3-benzoxadiol-4-yl)amino]ethanaminium.
Calculated by Wilke-Chang (45) correlation (see materials and methods). DAqueous, diffusion constant measured in aqueous solutions; ex/em, excitation/emission.
Data collection.
Cells were trypsinized and replated on glass coverslips 4–48 h prior to study. The coverslips were then placed in a chamber containing external solution and cell pairs were visualized with an Olympus IX71 fluorescence microscope. One cell of a pair was injected (via a 1.0-mm thin-walled microelectrode) with a mixture of the two junctionally permeant dyes as well as the impermeant dye. After overcompensating electrode capacitance to facilitate dye injection and subsequent removal of the electrode, images were taken [CoolSnap ES camera (Photometrics) driven by V++ software (Digital Optics)] at 0, 10, 20, 30, and 60 s, and at 1-min intervals thereafter up to visual equilibrium or 10 min for Cx43 junctions and at 0, 0.5, 1, and 2 min, and at 2-min intervals thereafter for Cx40-containing junctions to document transjunctional diffusion of each dye as a function of time. These digital images were stored for subsequent analysis.
Data processing and analysis.
Total fluorescence intensity of the donor and recipient cells were measured independently at each time point with ImageJ software. A selection area outside of but near the cell pair was similarly analyzed for background fluorescence. This was repeated for all images in the sequence (which included both dyes). The resulting image sequences were analyzed to obtain fluorescence intensity over time for both donor (injected) and recipient cells. These fluorescence intensity data were then fit with an exponential decay model (23) yielding a rate constant that is proportional to the permeance (permeability × cross-sectional area) of channels between the cells to each of the dyes measured. Cell pairs for which no increase in fluorescence in the recipient cell could be detected were regarded as not coupled. Incidence of dye coupling is reported as the number of detectably coupled cell pairs relative to the total number of injected cell pairs.
Statistics.
Rate constants for NBD-M-TMA and Alexa 350 for each cell pair of a specific cell type and treatment group were plotted and the correlation coefficient for changes in BNBD-M-TMA vs. BAlexa 350 was determined. The mean of the ratio of rate constants (junctional charge selectivity) was also calculated. Differences in mean junctional charge selectivity between cell types or treatment groups was determined with a t-test (unpaired, two sample, assuming unequal variance). The coefficient of variation (CV) of a population was calculated as the standard deviation of the population relative to its mean and expressed as a percentage of the mean. Differences in variability of two populations were approximated as the CV12/CV22 by use of the F statistic. In all comparisons, significance was assumed at P ≤ 0.05.
RESULTS
Charge selectivity of Cx43 and Cx40 junctions.
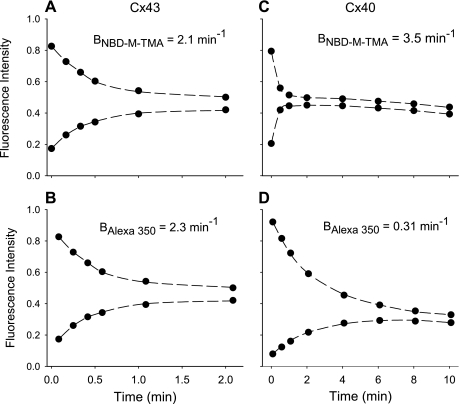
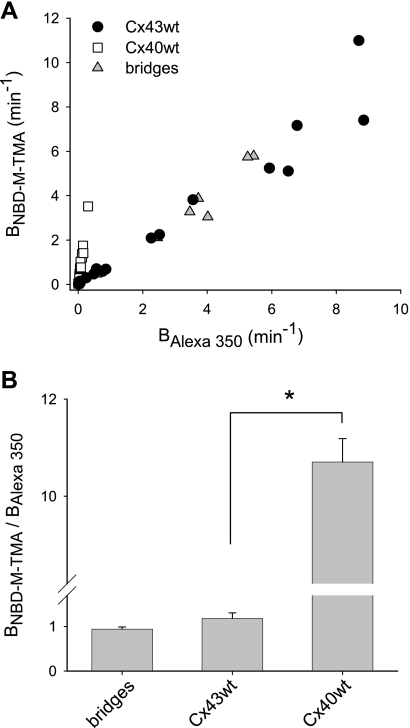
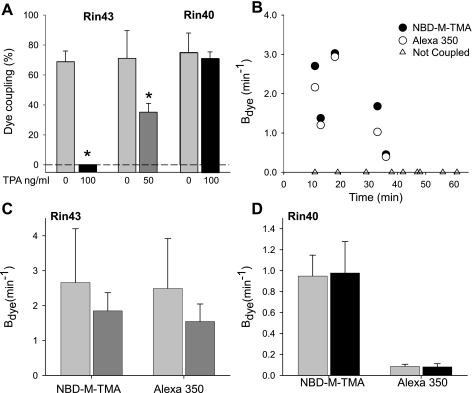
The charge selectivity of Cx43 and Cx40 homomeric/homotypic junctions was investigated by comparing the simultaneously measured permeances of individual Rin43 or Rin40 cell junctions with dyes of similar size but differing charge (23). Figure 1 shows data acquired during single experiments performed on a pair of Rin43 (Fig. 1, A and B) or Rin40 (Fig. 1, C and D) cells. The data for both NBD-M-TMA (Fig. 1, A and C) and Alexa 350 (Fig. 1, B and D) in both recipient and donor cells were well fit (dashed lines) and yielded comparable rate constants (Bdye) for the Rin43 junction but rate constants that differed by ∼10-fold for the Rin40 junction. The results of multiple such experiments are shown in Fig. 2, both as a plot of BNBD-M-TMA vs. BAlexa 350 for each junction (Fig. 2A) and as bar graphs in which the means for all pairs are compared (Fig. 2B). Data for cells connected by a cytoplasmic bridge (permeable to rhodamine-tagged dextran) rather than a gap junction are also shown. For all three data sets BNBD-M-TMA and BAlexa 350 were strongly and linearly correlated with slopes near 1 for Cx43 and cytoplasmic bridges and near 11 for Cx40, indicating an overall lack of charge preference or selectivity for Cx43 junctions and cytoplasmic bridges and an approximate 11:1 preference for permeation by cations over anions for Cx40 junctions.
Fig. 1.
Representative results and calculated fits from connexin 43 (Cx43) and Cx40 junctional dye-charge selectivity experiments. Shown are the results of the analysis of 2 dye-charge selectivity experiments. Total fluorescence intensity for N,N,N-trimethyl-2-[methyl-(7-nitro-2,1,3-benzoxadiol-4-yl)amino]ethanaminium (NBD-M-TMA; A and C) or Alexa 350 (B and D) for both donor (injected cell; top trace in each panel) and recipient (bottom trace in each panel) cells as a function of time for a Rin43 cell pair (A and B) and a Rin40 cell pair (C and D). Dashed lines represent the best fit of the data [using an exponential decay model (23)] that yielded the indicated junctional permeance rate constants (Bdye) and a junctional charge selectivity ratio (BNBD-M-TMA/BAlexa 350) of 0.91 for the Rin43 cell pair and 11.3 for the Rin40 cell pair.
Fig. 2.
Selectivity of Cx43 wild-type (Cx43wt) and Cx40wt junctions is invariant. Shown are the dye-charge selectivity results for Cx43 junctions (Rin43 cells), Cx40 junctions (Rin40 cells), and cytoplasmic bridges (incompletely divided cells). A: plot of permeance rate constants of NBD-M-TMA vs. Alexa 350 for Cx43 junctions (•), Cx40 junctions (□), and cytoplasmic bridges (▴). These parameters were significantly correlated for all 3 groups with correlation coefficients of 0.98 (n = 17) for Cx43 junctions, 0.99 (n = 15) for Cx40 junctions, 0.97 (n = 5) for bridges. B: average charge selectivities (BNBD-M-TMA/BAlexa 350 ratio) for the experiments plotted in A for bridges 0.91 ± 0.07 (n = 5), Rin43 1.22 ± 0.14 (n = 17), and Rin40 10.7 ± 0.5 (n = 15). *Selectivity of Cx40 junctions was significantly different from Cx43 junctions (Student's t-test).
Influence of the CT on junctional charge selectivity.
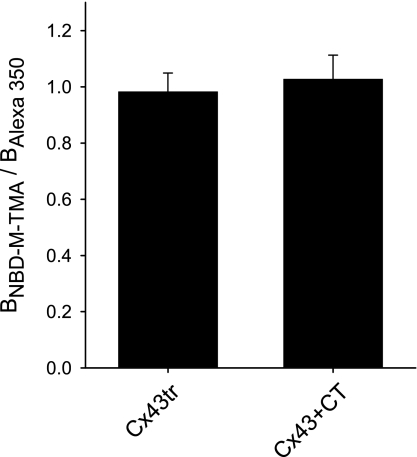
It is generally thought that the CT of Cx43 participates in regulation (voltage and chemical gating) of Cx43 channel function; certainly removal of the CT results in the loss of specific conductance states, which is widely regarded as evidence of such regulation (12, 24, 33). To determine whether the selectivity of Cx43 junctions to dyes of opposite charge might differ when the CT was absent or present in excess, we measured the dye-charge selectivity of junctions formed by only the pore-forming domain of Cx43 (Cx43tr) and of Cx43wt channels functioning in the presence of excess CT (17, 36). It should be noted that excess CT limits the extent of phosphorylation of the full-length coexpressed Cx43 (36). Figure 3 shows that charge selectivity of junctions composed of Cx43tr and of Cx43wt functioning in the presence of excess CT were not different. In both cases, the junctions showed no evidence of charge selectivity. The absence of any measurable difference in charge selective properties of junctions formed by truncated Cx43 and Cx43wt with excess CT suggests either that the domain does not regulate charge selectivity of Cx43 channels and junctions or that such regulation is not prevalent under control, nonstimulated conditions in these cell types.
Fig. 3.
Cx43 dye-charge selectivity is not affected by the carboxy terminal (CT). The average junctional charge selectivity (BNBD-M-TMA/BAlexa 350) for cells expressing Cx43 truncated at residue 257 (Cx43tr) (0.98 ± 0.07; n = 5) was not different from Cx43 functioning in the presence of excess CT (Cx43+CT) (1.03 ± 0.08; n = 6).
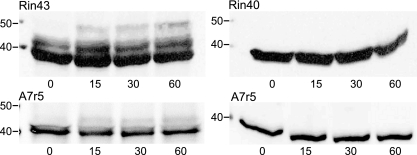
Despite the absence of obvious change in junctional charge selectivity upon CT truncation or with excess CT (dephosphorylation of full-length Cx43) demonstrated by the above data, numerous studies have shown regulation of Cx43 junctional function by kinases that directly target the CT for phosphorylation (27). To determine whether Cx43 charge selectivity might be regulated by phosphorylation, for example by increasing cationic dye permeability as suggested by Eckert (16), we assessed the effects of PKC activation (TPA treatment) on the dye-charge selectivity of Cx43 comprised junctions. Previous studies (28) demonstrated that (38) PKC activation reduces the electrophoretic mobility of Cx43, an effect resulting from phosphorylation of the Cx43 protein. Figure 4 shows that TPA treatment resulted in a similar reduction in electrophoretic mobility of Cx43 in both Rin43 and A7r5 cells but had no effect on the mobility of Cx40 in these cell types. The effect of TPA (100 ng/ml) on Cx43 was maximal after only 15 min of treatment at 37°C.
Fig. 4.
Electrophoretic mobility of Cx43, but not Cx40, is altered by phorbol 12-myristate 13-acetate (TPA) treatment of Rin43, Rin40, or A7r5 cells. Total protein was isolated from Rin43, Rin40, and A7r5 cells treated for 0, 15, 30, or 60 min with 100 ng/ml TPA at 37°C. Mobility of Cx43 (left) was altered in both Cx43-expressing cell types, consistent with PKC-induced phosphorylation of Cx43. No changes in the mobility of Cx40 were observed (right).
TPA treatment decreased the incidence of coupling in Rin43 but had no effect on coupling incidence in Rin40 cells. Whereas 69 ± 6% of untreated Rin43 cells (n = 50, nine groups) were dye coupled (Fig. 5A), dye coupling was significantly (P < 0.001) reduced to zero percentage in Rin43 cells pretreated for 15 min at 37°C with 100 ng/ml TPA (n = 18, 3 treatment groups). Since our ultimate goal was to assess the effect of TPA treatment on junctional selectivity, we also treated the Rin43 cells with 50 ng/ml at room temperature and found that only 35 ± 6% (n = 14, 4 groups) of Rin43 cell pairs remained dye coupled over the 65-min treatment period (compared to 86 ± 9% in untreated cell pairs, n = 15 in 3 groups). Both the incidence and magnitude of coupling decreased with treatment time (Fig. 5B). Although NBD-M-TMA and Alexa 350 permeances were reduced in the Rin43 cells that remained coupled during treatment with 50 ng/ml TPA (Fig. 5, B and C), their relative permeabilities were unaffected (Fig. 6), indicating that junctional charge selectivity was unaffected by PKC-dependent phosphorylation.
Fig. 5.
Incidence and extent of dye coupling are reduced following PKC activation in Rin43 but not Rin40 cells. A: the percentage of dye-coupled cell pairs for untreated (light shaded bars) vs. TPA-treated (dark shaded bar 50 ng/ml; solid bars 100 ng/ml) Rin43 and Rin40 cells. Coupling in treated pairs is compared with untreated pairs from cells plated simultaneously on the same day; the percentage of coupled pairs on a coverslip was determined, and the mean across multiple coverslips was determined. TPA reduced dye coupling in the Rin43 cells (untreated: 6 coverslips, 37 cell pairs; treated: 3 coverslips, 18 cell pairs) but had no effect at the highest concentration on Rin40 junctions (untreated: 5 coverslips, 31 cell pairs; treated: 5 coverslips, 18 cell pairs). B: NBD-M-TMA and Alexa 350 permeances decreased in parallel as a function of TPA (50 ng/ml) treatment duration in treated Rin43 cells. C: there was a downward trend in average permeance (Bdye) for NBD-M-TMA and Alexa 350 for Rin43 cells that were TPA treated (50 ng/ml, dark shaded bars; untreated controls, light shaded bars). D: no differences were detected in the average permeances for NBD-M-TMA and Alexa 350 (Bdye) in Rin40 cells untreated (light shaded bars) or treated with TPA (100 ng/ml; solid bars). *Dye coupling in TPA-treated cells significantly different from untreated cells (Student's t-test).
Fig. 6.
Charge selectivity of Cx43 and Cx40 junctions is unaffected by PKC activation. The dye-charge selectivity results for TPA-treated Rin43 and Rin40 cells are shown as a plot of permeance rate constants for NBD-M-TMA vs. Alexa 350 (only TPA treated shown) (A) and as the average charge selectivities (BNBD-M-TMA/BAlexa 350, treated and untreated shown) (B). For the experiments plotted in A, the parameters were significantly correlated [Cx43: r = 0.98 (n = 5); Cx40: r = 0.88 (n = 4)]. TPA had no detectable effect on selectivity of either the Rin43 or Rin40 junctions.
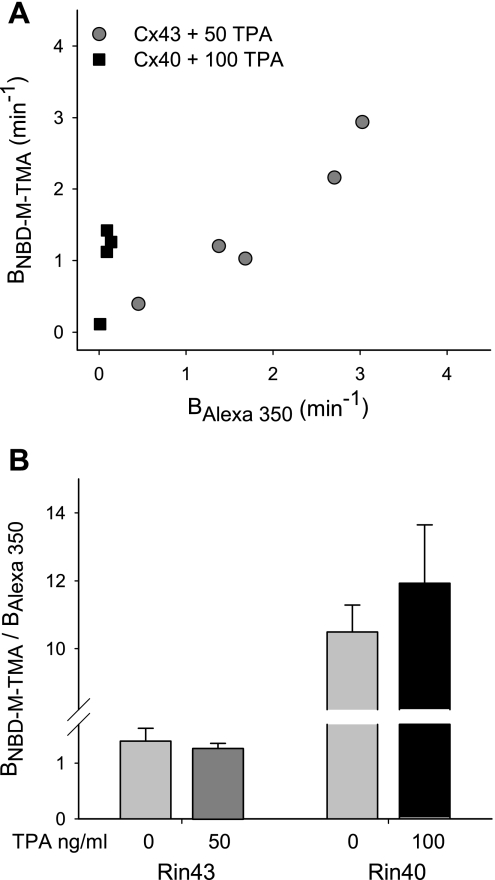
The above data indicate that Cx43 junctions are nonselective with respect to permeant charge and that the CT domain of the protein, despite affecting other parameters of channel function, has little if any influence on junctional charge selectivity. Like Cx43 junctions, charge selectivity of Cx40 junctions was invariant under control conditions (Fig. 2); but Cx40 junctions displayed an approximate 10-fold preference for cationic vs. anionic permeants. To determine whether the charge selectivity of Cx40 junctions might be regulated by PKC activation, we treated Rin40 cells with TPA (preincubation protocol, 100 ng/ml, 37°C, 15 min) and measured charge selectivity using the dual-dye technique. Both the incidence of dye coupling and the NBD-M-TMA and Alexa 350 permeances were unaffected by TPA treatment (Fig. 5, A and D), as was junctional charge selectivity (Fig. 6). These results indicate that the loss of dye coupling observed in 100 ng/ml TPA-treated Rin43 cells was not due to a nonspecific effect of TPA treatment on Rin cells and, importantly, further suggest that the incidence of functional Cx40 junctions and their overall dye charge selectivity are unaffected by TPA treatment.
Variable junctional charge selectivity in Cx40- and Cx43-coexpressing cells.
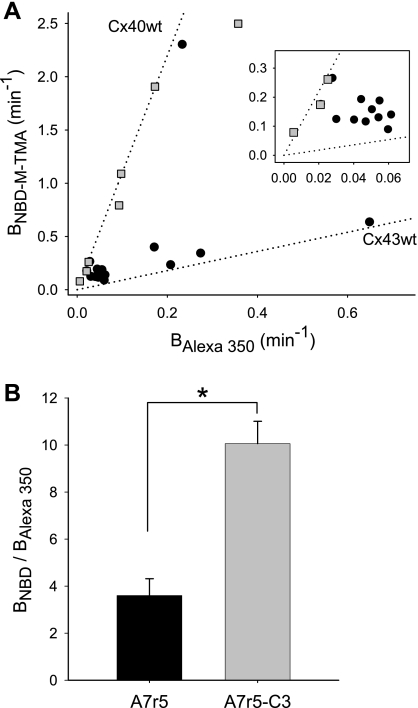
Since the selective properties of both Cx43 and Cx40 junctions were invariant, with Cx43 junctions being nonselective and Cx40 junctions being cation selective, we hypothesized that in coexpressing cells junctional charge selectivity would vary between the boundaries set by the homomeric/homotypic junctions in a manner reflecting the relative expression of Cx40 and Cx43. This hypothesis was tested by the dual-dye technique on cells that coexpress Cx40 and Cx43 at ratios (Cx40:Cx43) of ∼3:1 (A7r5) and 10:1 (A7r5C3) (6, 11). For A7r5 cells, in which junctional channels are predicted to be variably comprised with greater than 80% containing both connexins (11), junctional charge selectivity was quite variable (Fig. 7A) and BNBD-M-TMA vs. BAlexa 350 were poorly correlated (r = 0.34). For A7r5C3 cells, in which ∼60% of channels are predicted to contain only Cx40 (11), junctional charge selectivity was considerably less variable (Fig. 7A) and BNBD-M-TMA vs. BAlexa 350 were well correlated (r = 0.96). These data thus indicate that the individual junctions formed by cells that coexpress Cx40 and Cx43 can vary in their charge selectivity from that of homomeric/homotypic Cx40 to that of homomeric/homotypic Cx43 and further that average charge selectivity increases as the contribution of the connexin forming selective (Cx40) vs. nonselective (Cx43) channels increases.
Fig. 7.
Charge selectivity of junctions formed by cells coexpressing Cx43 and Cx40 at different expression ratios. A: plot of BNBD-M-TMA vs. BAlexa 350 for A7r5C3 ( ) and A7r5 (•) cell junctions (dotted lines are the linear regression fits for Cx40 and Cx43 junctions as shown in Fig. 2A). Inset shows data near graph origin at expanded scaling of both axes. B: the average charge selectivities (BNBD-M-TMA/BAlexa 350) of A7r5 and A7r5-C3 junctions are significantly different, P < <0.001. *Selectivity of A7r5-C3 junctions significantly greater than A7r5 junctions (Student's t-test).
) and A7r5 (•) cell junctions (dotted lines are the linear regression fits for Cx40 and Cx43 junctions as shown in Fig. 2A). Inset shows data near graph origin at expanded scaling of both axes. B: the average charge selectivities (BNBD-M-TMA/BAlexa 350) of A7r5 and A7r5-C3 junctions are significantly different, P < <0.001. *Selectivity of A7r5-C3 junctions significantly greater than A7r5 junctions (Student's t-test).
Regulation of junctional charge selectivity in Cx43- and Cx40-coexpressing cells.
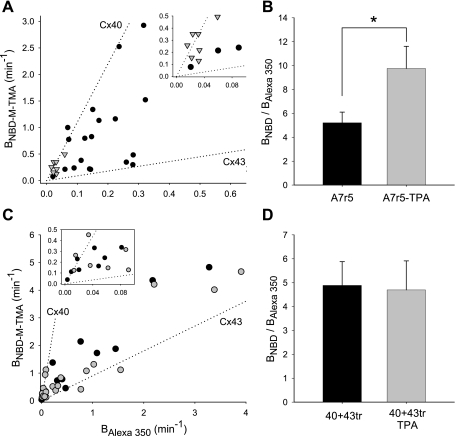
Since Cx43-, but not Cx40-, mediated dye coupling was significantly compromised following PKC activation, we hypothesized that such differential, PKC-dependent regulation of these nonselective vs. cation selective channels would provide a mechanism whereby junctional charge selectivity in coexpressing cells could be regulated acutely toward greater cation selectivity. To test this hypothesis the charge selectivity of junctions in TPA-treated A7r5 cells was determined and compared with junctions in untreated cells. TPA significantly increased average junctional charge selectivity of A7r5 cell junctions (BNBD-M-TMA/BAlexa 350 treated: 9.8 ± 1.9 vs. untreated: 5.2 ± 0.9, P = 0.043; Fig. 8, A and B). These data indicate that coexpression of a connexin subject to regulation by a specific signaling cascade with one that is not provides cells with an acute (as little as 15 min) mechanism for regulation of junctional charge selectivity.
Fig. 8.
Charge selectivity of junctions formed by cells coexpressing Cx43 and Cx40 is regulated by PKC in a manner requiring the CT of Cx43. A: plot of BNBD-M-TMA vs. BAlexa 350 for A7r5 (solid circle) and A7r5 cells treated with TPA (shaded triangle). Dotted lines are linear regression fits to comparable data for Cx40 and Cx43 junctions (from the data in Fig. 2A). Inset shows data near graph origin at expanded scaling of both axes. B: average charge selectivity (BNBD-M-TMA/BAlexa 350) of A7r5 cell junctions treated (shaded bars) or not (solid bars) with TPA (100 ng/ml, 15 min at 37°C followed by up to 80 min at room temperature). Selectivity of treated A7r5 cells was significantly increased, P < 0.045. C: plot of BNBD-M-TMA vs. BAlexa 350 for Rin40 coexpressing Cx43tr treated (shaded circles) or not (solid circles) with TPA. Dotted lines are linear regression fits to comparable data for Cx40 and Cx43 junctions (from the data in Fig. 2A). Inset shows data near graph origin at expanded scaling of both axes. D: average charge selectivity (BNBD-M-TMA/BAlexa 350) of Cx40+Cx43tr (40+43tr) junctions treated (shaded bars) or not (solid bars) with TPA (100 ng/ml, 15 min at 37°C followed by up to 80 min at room temperature). Selectivity of treated and untreated cells was not different. *Selectivity of A7r5 cell junctions significantly increased following TPA treatment.
Influence of Cx43 CT on regulation of junctional charge selectivity in coexpressing cells.
Since TPA increases the cationic selectivity of junctions formed by cells coexpressing Cx40 and Cx43, likely due to closure of channels containing (principally) Cx43, we hypothesized that coexpression of Cx40 with Cx43tr, which lacks the residues targeted by PKC, would result in TPA-insensitive junctions with variable charge selectivity intermediate to homomeric/homotypic Cx43 and Cx40 junctions. As expected from the A7r5 cell data, transient introduction of Cx43tr into Rin40 cells resulted in junctions with an average charge selectivity of 4.9 ± 1 (n = 17), a value intermediate to that of Cx40 or Cx43 comprised junctions (Fig. 8, C and D). TPA treatment of these Cx43tr expressing Rin40 cells did not alter their average charge selectivity (4.7 ± 1.2, n = 19) (Fig. 8, C and D). These data indicate that the increase in cation selectivity observed for TPA treated A7r5 cell junctions results from TPA-induced (PKC-dependent phosphorylation) regulation of channels containing Cx43, regulation that requires the CT of Cx43.
Regulated charge selectivity: channels.
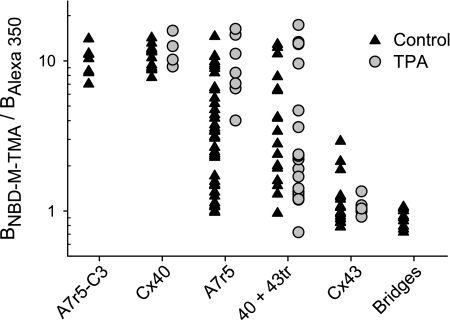
The range of junctional charge selectivity (BNBD-M-TMA/BAlexa 350 ratio) values for the Rin43 and Rin40 data sets was always less than twofold and never differed from the mean of the respective data set by more than 33%. Comparable small coefficients of variation (F test: P > 0.5) for the Rin43, Rin40, and cytoplasmic bridge data sets (17, 16, and 15%, respectively) suggest that this range of variability stems from limitations of the dual-dye measurement technique and further that charge selectivity is essentially a fixed, invariant property of Cx43 and Cx40 junctions and thus of the channels comprising those junctions, as was previously concluded (23). In contrast, the individual values for charge selectivity of A7r5 junctions varied over a 15-fold range and differed from the mean value by as much as 225% (Fig. 9). Similarly, the individual charge selectivity values of the Cx43tr expressing Rin40 junctions varied over a 13-fold range, differing from the mean value by as much as 160% (CV: 81%). This variability was much greater than for either Cx43 or Cx40 junctions and resulted in an approximately fivefold larger CV (78% for A7r5, 81% for Rin40+Cx43tr vs. 17 and 16% for Cx40 and Cx43 junctions; F tests: P < 0.001). These data strongly suggest that the channels formed by cells coexpressing Cx40 and Cx43 are not homogeneously selective, consistent with their mixed unitary conductances and variable voltage-dependent regulation (9–11).
Fig. 9.
Variable charge selectivity and its regulation in Cx43- and Cx40-coexpressing cells. Individual charge selectivities (BNBD-M-TMA/BAlexa 350) of the differently comprised junctions studied herein treated (shaded symbols) or not (solid symbols) with TPA (as described in previous figures) and plotted on a logarithmic scale to emphasize the ∼15-fold range in junctional selectivity of coexpressing cells. Note the decreased range of selectivity values in TPA-treated A7r5 cells and absence of similar decrease in Rin40+Cx43tr cells, indicating the necessity for the CT domain for such regulation.
As shown above, TPA treatment of A7r5 cells increased the mean selectivity of A7r5 cell junctions toward a Cx40-like selectivity; however, the variability in junctional selectivity in TPA-treated A7r5 cells, although reduced relative to nontreated A7r5 cells, was still greater (P < 0.05) than that of Cx40-only junctions (range of junctional charge selectivity values was ∼4-fold, compared with 15-fold in untreated A7r5; CV was 45% compared with 225% in untreated A7r5; individual values differed from the mean by as much as 66% compared with 160% in untreated A7r5). These data suggest that the channels comprising the TPA-treated A7r5 cell junction remain heterogeneously charge selective. Finally, the changes in junctional properties induced by TPA treatment of A7r5 cells depend entirely on the presence of the CT domain of Cx43 in those junctions. Thus the selectivity of junctions formed by cells coexpressing Cx40 and Cx43tr was unaffected by TPA treatment; the variability in the individual values of junctional selectivity varied over a 24-fold range, with a maximum difference from the mean of 250% and a CV of 104%. None of these values differed significantly from untreated cells coexpressing these proteins.
DISCUSSION
In the present study we investigated the long- and short-term consequences on junctional charge selectivity of coexpressing Cx40 with Cx43 at different expression ratios (long-term regulation) and different acute stimulatory conditions (short-term regulation). Comparison of results from coexpressing cells to cells expressing a single connexin provided evidence for both short- and long-term regulation of junctional charge selectivity in the coexpression setting that was absent in cells expressing a single connexin.
The dual-dye approach used in the present study for assessing junctional charge selectivity is well suited to quantifying the extent to which junctional charge selectivity and therefore the combined selectivities of the comprising channels vary from one junction to the next. Such an evaluation is not possible by using traditional approaches in which the permeability of each dye is determined in separate experiments across different junctions (and comprising channels). In addition, the dual-dye approach facilitates acquisition of highly quantitative selectivity measurements from large numbers of junctions, allowing for accurate assessment of average junctional selectivity properties that are independent of the variations in channel number that can be expected to occur between different cell pairs, cell types, and connexin isoforms.
Invariant charge selectivity of Cx43 and Cx40 junctions.
The dual-dye technique yields a quantitative measure of the charge selectivity of a junction, which reflects the combined charge selectivities of the channels therein. Variation in charge selectivity from one junction to the next thus indicates variation in the charge selectivity of the comprising channels. Thus comparison of charge selectivity data obtained from multiple junctions reveals the extent to which the selectivity of the comprising channels varies under similar or different experimental conditions. The excellent correlation between permeance to NBD-M-TMA and Alexa 350 across junctions formed of either Cx40 or Cx43 channels (Fig. 2A) and the fact that the less than twofold range in variability in these selectivities is similar to that found for cytoplasmic bridges (Fig. 9) suggest that the charge selectivity of junctions and therefore channels formed of either of these two connexins did not vary significantly from one cell pair to the next under control, nonstimulated conditions.
Charge selectivity of junctions composed solely of Cx43 or Cx40 is not obviously regulated.
It has been suggested that charge selectivity of Cx43 channels could be altered by the extent of resident negative charge resulting from variable phosphorylation of the CT (16). If this were true, then phosphorylation could provide a mechanism whereby gap junctions composed of a single connexin could display a broad range of charge selectivity, a range that would reflect the variable phosphorylation of the CT of each of the 12 connexin subunits. Certainly previously published results suggest involvement of the CT in phosphorylation-dependent gating (28), regulation of channel permeability (17), and charge selectivity (2, 16, 34), as well as in targeting to and removal of the protein and channel from the membrane (30, 35). In the present study we show that truncation of the CT did not significantly alter, relative to the wild-type channel under control conditions, the dye-charge selectivity of dye-permeable Cx43 channels. We also show that strategies previously shown to reduce [expression of excess CT (36)] or maximize [through TPA-mediated activation of PKC (28)] phosphorylation of Cx43 did not obviously change the charge selectivity of Cx43 junctions despite, with TPA treatment, reducing both the incidence of dye coupling and the average permeance values for both dyes. These results suggest that the CT does not regulate the charge selectivity of the Cx43 pore (by altering the electrostatic environment of the channel). This conclusion is consistent with conclusions of other studies involving different connexins showing that the primary determinants of conductance, permeability, and selectivity do not reside in the residues of the CT but elsewhere in the connexin protein (14, 32, 39). It is noteworthy that, although data from the present study suggest a lack of involvement of the Cx43 CT in determining the selective properties of dye-permeable channels, the data do not preclude a role for the CT (and phosphorylation therein) in regulating channel configuration to one that is not readily permeated by dyes (and other molecules of comparable size) (2, 5) but possibly still permeated by current-carrying ions (2, 17).
Although Cx40 has not been demonstrated to be a direct target for PKC-dependent phosphorylation, we nevertheless tested Cx40 comprised junctions for possible (direct or indirect) regulation of charge selectivity following PKC activation. Our data demonstrate that unlike Cx43, the incidence of coupling in Cx40 expressing cells and the permeances of Cx40 junctions to both NBD-M-TMA and Alexa 350 were unaffected by PKC activation. Thus, like Cx43 comprised junctions, the charge selectivity of Cx40 junctions was unaffected by PKC activation.
Long-term regulation of junctional charge selectivity in cells coexpressing Cx43 and Cx40.
Given that Cx43 vs. Cx40 junctions and channels display different charge selectivities that appear to be essentially fixed parameters of channel function (i.e., not acutely regulated), one would predict that junctions containing both connexins would display a charge selectivity value intermediate to homomeric/homotypic Cx40 and Cx43 junctions. If the contribution of Cx40 and Cx43 to the channels comprising the junction varied from one cell pair to the next [as is predicted by probability theory (11)], then junctional charge selectivity would be expected to vary in parallel with their relative expression, with cells expressing more Cx40 showing higher cationic charge selectivity and cells with more Cx43 showing lower charge selectivity. Two experimental groups in the present data support this expectation. First, A7r5C3 cells (10:1 Cx40:Cx43) showed significantly higher average cationic charge selectivity than A7r5 cells (3:1 Cx40:Cx43) (Fig. 7). Second, the range of junctional selectivities measured in A7r5 cells and in Rin40 cells coexpressing Cx43tr varied from nonselective, like Cx43 junctions, to strongly cation selective, like Cx40 junctions (Fig. 9A). These results indicate that although the average Cx40:Cx43 protein expression ratio in these cells may be predictive of average junctional connexin composition, it is not predictive of the junctional composition of every cell pair, as previously concluded (6, 11). The significance of this observation is potentially large when it is recognized that heterogeneity of coupling, arising from nonhomogeneous expression or regulation, contributes to abnormalities of function in the heart (29, 31), brain (46), and potentially other tissues. The data also indicate that charge selectivity of the junctions formed by coexpressing cells depends on the selective properties and relative permeability of each of the comprising channels whose compositions are expected to reflect the relative contribution of each connexin to the junction. Thus, by regulating the relative expression of the two proteins (long-term regulation), cells regulate the selectivity of the intercellular communication pathway. However, such long-term regulation does not preclude acute, short-term regulation, as discussed next.
Short-term regulation of junctional charge selectivity in cells coexpressing Cx43 and Cx40.
Increased phosphorylation of Cx43, particularly by PKC, has been shown to reduce dye permeability of Cx43 junctions (26, 35) and hemichannels (2) to molecules similar in size to the dyes used in the present study. Increased phosphorylation of Cx43, through PKC activation, has also been shown to reduce both macroscopic and unitary conductance of Cx43 junctions and channels (7, 28, 34), while having no effect on (7) or reducing (3, 4) the electrical communication properties of Cx40 junctions. These results were confirmed and extended in the present study. Following TPA exposure, dye permeability of Cx43 junctions was reduced without affecting charge selectivity; neither charge selectivity or dye permeability of Cx40 junctions was altered by TPA treatment. The significant increase in charge selectivity of TPA-treated A7r5 cells is consistent with elimination or reduction of permeability through channels that were predominantly composed of Cx43. The lack of sensitivity of Cx40:Cx43tr junctions to TPA indicates a requirement for the Cx43-CT domain in the TPA-induced increase in charge selectivity of A7r5 cell junctions. The extent to which channel removal or gating to a dye-impermeable configuration are involved in the decrease in dye permeability of these junctions cannot be evaluated. However, the data do suggest that PKC activation preferentially reduces permeability through Cx43-containing channels and results in increased cationic charge selectivity of junctions formed by Cx40- and Cx43-coexpressing cells.
Significance.
Although junctional selectivity properties for permeants of the size and charge of the dyes used in the present study do not appear to be variable (regulated) parameters of junctions composed solely of either Cx43 or Cx40, data presented herein suggest that coexpression of these connexin isoforms provides cells with a strategy for both long- and short-term regulation of junctional charge selectivity and permeability, in a manner that is predictable from the properties of the channels formed by the individual connexins. Such long- and short-term regulation strategies could represent a general mechanism by which the selective diffusion of signaling molecules or metabolites through gap junctions could be controlled at different times, locations, or physiological and pathophysiological conditions, where the ability to differentially discriminate between possible junctional permeants could be of great importance (41, 42). Understanding the selectivity and permeability properties of junctions and channels formed of each connexin isoform and the potential ability to modulate these parameters could lead to reasonable predictions of the selective properties of permeation through junctions of mixed connexin composition under different conditions. The capacity of different connexin isoforms to respond uniquely to similar stimuli and thereby alter the selectivity and permeability properties of gap junctions of mixed connexin composition could prove to be a key concept in understanding connexin-specific functional roles and to be an important reason for the large number of connexin isoforms and their coexpression.
GRANTS
This work was supported by the National Institutes of Health (RO1 HL058732 and T32 HL007249, to J. M. Burt; NRSA award to N. S. Heyman).
Acknowledgments
The authors acknowledge the technical assistance of Tasha K. Nelson.
REFERENCES
- 1.Aavula BR, Ahad Ali M, Mash EA, Bednarczyk D, Wright SH. Synthesis and fluorescence of N,N,N-trimethyl-2-[methyl(7-nitrobenzo[c][l,2,5]oxadiazol-4-yl) amino]ethanaminium iodide, a pH-insensitive reporter of organic cation transport. Synth Commun 36: 701–705, 2006. [Google Scholar]
- 2.Bao X, Lee SC, Reuss L, Altenberg GA. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junctional hemichannels by PKC. Proc Natl Acad Sci USA 104: 4919–4924, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolon ML, Kidder GM, Simon AM, Tyml K. Lipopolysaccharide reduces electrical coupling in microvascular endothelial cells by targeting connexin40 in a tyrosine-, ERK1/2-, PKA-, and PKC-dependent manner. J Cell Physiol 211: 159–166, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bolon ML, Peng T, Kidder GM, Tyml K. Lipopolysaccharide plus hypoxia and reoxygenation synergistically reduce electrical coupling between microvascular endothelial cells by dephosphorylating connexin40. J Cell Physiol 217: 350–359, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol 119: 171–186, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burt JM, Fletcher AM, Steele TD, Wu Y, Cottrell GT, Kurjiaka DT. Alteration of Cx43:Cx40 expression ratio in A7r5 cells. Am J Physiol Cell Physiol 280: C500–C508, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Burt JM, Steele TD. Selective effect of PDGF on connexin43 versus connexin40 comprised gap junction channels gap junction channels. Cell Commun Adhes 10: 287–291, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am J Physiol Cell Physiol 281: C1559–C1567, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cottrell GT, Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta 1711: 126–141, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cottrell GT, Wu Y, Burt JM. Functional characteristics of heteromeric Cx40-Cx43 gap junction channel formation. Cell Commun Adhes 8: 193–197, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am J Physiol Cell Physiol 282: C1469–C1482, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Delmar M, Coombs W, Sorgen P, Duffy HS, Taffet SM. Structural bases for the chemical regulation of Connexin43 channels. Cardiovasc Res 62: 268–275, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Delorme B, Dahl E, Jarry-Guichard T, Briand JP, Willecke K, Gros D, Theveniau-Ruissy M. Expression pattern of connexin gene products at the early developmental stages of the mouse cardiovascular system. Circ Res 81: 423–437, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chicken Connexin45.6 gap junctional channels. J Physiol 576: 787–799, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunham B, Liu S, Taffet S, Trabka-Janik E, Delmar M, Petryshyn R, Zheng S, Perzova R, Vallano ML. Immunolocalization and expression of functional and nonfunctional cell-to-cell channels from wild-type and mutant rat heart connexin43 cDNA. Circ Res 70: 1233–1243, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Eckert R Gap-junctional single channel permeability for fluorescent tracers in mammalian cell cultures. Biophys J 91: 565–579, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res 98: 1498–1505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans WH, Martin PE. Gap junctions: structure and function (Review). Mol Membr Biol 19: 121–136, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1: 457–459, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta 1662: 96–101, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Haefliger JA, Castillo E, Waeber G, Bergonzelli GE, Aubert JF, Sutter E, Nicod P, Waeber B, Meda P. Hypertension increases connexin43 in a tissue-specific manner. Circulation 95: 1007–1014, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Harris AL Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94: 120–143, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyman NS, Burt JM. Hindered diffusion through an aqueous pore describes invariant dye selectivity of Cx43 junctions. Biophys J 94: 840–854, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homma N, Alvarado JL, Coombs W, Stergiopoulos K, Taffet SM, Lau AF, Delmar M. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ Res 83: 27–32, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Kanagaratnam P, Rothery S, Patel P, Severs NJ, Peters NS. Relative expression of immunolocalized connexins 40 and 43 correlates with human atrial conduction properties. J Am Coll Cardiol 39: 116–123, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kwak BR, Jongsma HJ. Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Mol Cell Biochem 157: 93–99, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36: 1171–1186, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampe PD, Tenbroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149: 1503–1512, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leaf DE, Feig JE, Vasquez C, Riva PL, Yu C, Lader JM, Kontogeorgis A, Baron EL, Peters NS, Fisher EA, Gutstein DE, Morley GE. Connexin40 imparts conduction heterogeneity to atrial tissue. Circ Res 103: 1001–1008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leithe E, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem 279: 50089–50096, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lin LC, Wu CC, Lin MS, Lin SF, Liu YB. Reducing the cyclic variations of ultrasonic integrated backscatters and myocardial electrical synchronism by reversibly blocking intercellular communications with heptanol. Ultrasound Med Biol 35: 209–218, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Ma M, Dahl G. Cosegregation of permeability and single-channel conductance in chimeric connexins. Biophys J 90: 151–163, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res 90: 450–457, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Moreno AP, Fishman GI, Spray DC. Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J 62: 51–53, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivedal E, Leithe E. Connexin43 synthesis, phosphorylation, and degradation in regulation of transient inhibition of gap junction intercellular communication by the phorbol ester TPA in rat liver epithelial cells. Exp Cell Res 302: 143–152, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Shin JL, Solan JL, Lampe PD. The regulatory role of the C-terminal domain of connexin43. Cell Commun Adhes 8: 271–275, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Solan JL, Fry MD, Tenbroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci 116: 2203–2211, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J 79: 3036–3051, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Veen TA, Van Rijen HV, Jongsma HJ. Physiology of cardiovascular gap junctions. Adv Cardiol 42: 18–40, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Verheule S, van Batenburg CA, Coenjaerts FE, Kirchhoff S, Willecke K, Jongsma HJ. Cardiac conduction abnormalities in mice lacking the gap junction protein connexin40. J Cardiovasc Electrophysiol 10: 1380–1389, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Verheule S, VanKempen MJA, Welscher PHJA, Kwak BR, Jongsma HJ. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ Res 80: 673–681, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Vozzi C, Ullrich S, Charollais A, Philippe J, Orci L, Meda P. Adequate connexin-mediated coupling is required for proper insulin production. J Cell Biol 131: 1561–1572, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J 87: 958–973, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilke CR, Chang P. Correlation of diffusion coefficients in dilute solutions. AIChE J 1: 264–270, 1955. [Google Scholar]
- 46.Yeh TH, Lee DY, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia. In press. [DOI] [PMC free article] [PubMed]