Abstract
Cardiac surgery with global myocardial ischemia-reperfusion (I/R) induces a myocardial inflammatory response that impairs cardiac recovery. Chemokines contribute to the overall myocardial inflammatory response through inducing leukocyte infiltration. Although Toll-like receptor 4 (TLR4) has an important role in postischemic myocardial injury, the relative roles of myocardial tissue and leukocyte TLR4 in leukocyte infiltration, as well as the role of TLR4 in myocardial chemokine expression, are unclear. Our recent study, in an isolated mouse heart model of global I/R, found that the 70-kDa heat shock cognate protein (HSC70) is released from cardiac cells and mediates the expression of cardiodepressant cytokines via a TLR4-dependent mechanism. In the present study, we tested the hypotheses that myocardial tissue TLR4 has a major role in mediating neutrophil infiltration and that myocardial TLR4 and extracellular HSC70 contribute to the mechanisms underlying cardiac chemokine response to global I/R. We subjected hearts isolated from TLR4-defective and TLR4-competent mice to global I/R and examined myocardial neutrophil infiltration and expression of keratinocyte-derived chemokine (KC) and monocyte chemoattractant protein-1 (MCP-1). TLR4-defective hearts exhibited reduced neutrophil infiltration regardless of the phenotypes of neutrophils perfused during reperfusion and expressed lower levels of KC and MCP-1. HSC70-specific antibody reduced myocardial expression of KC and MCP-1 after I/R. Furthermore, perfusion of HSC70 increased KC and MCP-1 expression in TLR4-competent hearts but not in TLR4-defective hearts, and HSC70 also induced the chemokine response in macrophages in a TLR4-dependent fashion. A recombinant HSC70 fragment lacking the substrate-binding domain was insufficient to induce chemokine expression in hearts and cells. This study demonstrates that myocardial tissue TLR4, rather than neutrophil TLR4, is the determinant of myocardial neutrophil infiltration after global I/R. TLR4 mediates myocardial chemokine expression, and the mechanisms involve extracellular HSC70. These results imply the HSC70-TLR4 interaction as a novel mechanism underlying the myocardial chemokine response to global I/R.
Keywords: reperfusion, receptor, keratinocyte-derived chemokine, Toll-like receptor 4, heat shock cognate protein 70, monocyte chemoattractant protein-1
cardiac surgery with cardiopulmonary bypass induces a myocardial inflammatory response characterized by increased expression of proinflammatory cytokines and chemokines (2, 20, 32, 33). This response contributes to myocardial injury associated with the obligatory global myocardial ischemia-reperfusion (I/R) (12) and is proposed to be a therapeutic target for myocardial protection (5, 25). Previous studies demonstrate that chemokines, IL-8, and monocyte chemoattractant protein-1 (MCP-1) in particular increase both in the circulation (6, 11, 19, 26, 31) and in myocardial tissue (16) after cardiac surgery. Since chemokines facilitate the infiltration and activation of neutrophils, lymphocytes, and monocytes, they play an important role in propagation of the postoperative myocardial inflammatory response and the development of the systemic inflammatory response syndrome (25). Regulation of myocardial chemokine expression appears to be critical for suppression of the myocardial inflammatory response associated with cardiac surgery with global myocardial I/R. However, the mechanisms responsible for upregulation of chemokine expression after global I/R have not been fully elucidated.
A number of studies have implicated Toll-like receptor 4 (TLR4) signaling in myocardial injury after I/R (9, 21, 24). Furthermore, this innate immune receptor appears to have a role in the myocardial inflammatory response to I/R (8, 14). In a mouse model of cardiac transplantation, which involves hypothermic global myocardial I/R, myocardial neutrophil infiltration is reduced when both graft and host are TLR4 defective (17). Furthermore, attenuated myocardial neutrophil infiltration is observed after regional I/R in mice lacking MyD88, a signaling adaptor for multiple TLRs (13). These studies indicate that TLR4 contributes to the mechanisms of myocardial neutrophil infiltration caused by global and regional I/R. However, it remains unknown whether myocardial tissue TLR4, neutrophil TLR4, or both are involved in neutrophil infiltration to the myocardium after myocardial I/R. In mouse models of regional myocardial I/R, myocardial expression of mRNA encoding chemokines macrophage inflammatory protein-1α (MIP-1α), MIP-2, and MCP-1 was significantly lower in TLR4-defective mice or mice treated with a TLR4 antagonist (9, 28). Although TLR4 appears to have an important role in the upregulation of myocardial chemokine expression following I/R, the relative roles of myocardial TLR4 and circulating cell TLR4 in the myocardial chemokine response are unclear. Furthermore, it is unclear how I/R activates myocardial TLR4 to express chemokines. Identification of TLR4 ligands and elucidation of the mechanisms of ligand-TLR4 interaction may lead to the development of novel approaches for prevention of myocardial inflammation and injury associated with cardiac surgery with global I/R.
We recently demonstrated in a murine model of global myocardial I/R that the 70-kDa heat shock cognate protein 70 (HSC70) is released into the myocardial extracellular space and coronary effluent and that extracellular HSC70 induces myocardial expression of proinflammatory cytokines TNF-α, IL-1β, and IL-6 via a TLR4-dependent mechanism (36). It is likely that myocardial TLR4 and extracellular HSC70 contribute to the mechanisms underlying cardiac chemokine response to global I/R. The current study was undertaken to determine 1) the relative roles of myocardial TLR4 and neutrophil TLR4 in neutrophil infiltration to the myocardium after global I/R, 2) whether myocardial TLR4 mediates myocardial expression of keratinocyte-derived chemokine (KC, a human IL-8 analog) and MCP-1 (CCL2) induced by global I/R, 3) whether HSC70 is involved in the myocardial chemokine response to global I/R, 4) whether the purified HSC70 induces chemokines expression in the myocardium and isolated macrophages through TLR4, and 5) whether the HSC70 substrate-binding domain (SBD) is required for its effect on the myocardium and cells.
MATERIALS AND METHODS
Animals.
Male C3H/HeJ (TLR4 defective, expressing dysfunctional TLR4 caused by mutation) and C3H/HeN (TLR4 competent) mice, with body weight of 23–28 g, were used in this study. All experiments were approved by the Animal Care and Research Committee of the University of Colorado Denver, and this investigation conforms to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996).
Chemicals and reagents.
Recombinant bovine HSC70 (rHSC70), recombinant HSC70 fragment (44 kDa, without the SBD), rat monoclonal anti-HSC70, and rabbit polyclonal anti-HSC70 were purchased from Assay Designs (Ann Arbor, MI). The recombinant HSC70 was a low-endotoxin preparation, with endotoxin <5.0 pg/μg protein (<0.05 EU/μg protein) as measured by Limulus assay. In a preliminary study, we used immunoblotting to confirm that both HSC70 antibodies had no cross-reactivity with recombinant heat shock protein 70. Other reagents were purchased from Sigma Chemical (St. Louis, MO).
Isolation and culture of neutrophils.
Neutrophils were isolated from bone marrow by negative selection as described previously (29). Mouse femur and tibia were flushed with Roswell Park Memorial Institute growth medium (RPMI) 1640, tissue debris was removed by filtration through a glass-wool column, and cells were collected by centrifugation. Cell pellets were resuspended in RPMI 1640 with 2% FCS and incubated for 15 min at 4°C with an antibody mixture (StemCell Technologies) specific for cell surface markers of T cells, B cells, erythrocytes, monocytes, and macrophages (F4/80, CD4, CD45R, CD5, and TER119). Cells were then incubated with 100 μl of antibiotin tetrameric antibody complexes for 15 min at 4°C and subsequently incubated with 60 μl of colloidal magnetic dextran iron particles for 15 min at 4°C. The entire cell suspension was placed into a column surrounded by a magnet. T cells, B cells, erythrocytes, and monocytes were captured in the column, but neutrophils were allowed to pass through. Of the cells collected, neutrophils accounted for >97%, as determined by Wright's-stained cytospin preparations, and mononuclear cells accounted for <0.3%. Viability, as determined by Trypan blue exclusion, was consistently >98%. Neutrophils were cultured in RPMI 1640 with 0.1% FCS for 2 to 4 h. We have characterized the neutrophil isolates by examining their baseline activity and response to TLR4 ligand for cytokine release. The isolates release low levels of TNF-α (mean value, 32 pg/ml) and IL-1β (mean value, 23 pg/ml) without stimulation, indicating a low level of activation by isolation and culture. However, they release significantly higher levels of cytokines (mean values, 460 and 241 pg/ml, respectively, for TNF-α and IL-1β) in response to TLR4 stimulation by LPS, indicating that their TLR4 response is intact.
Isolation and culture of macrophages.
Peritoneal macrophages were collected by lavage from C3H/HeN and C3H/HeJ mice. Animals were anesthetized (pentobarbital sodium, 50 mg/kg iv), and the peritoneal cavity was flushed with cold (4°C) serum-free D-MEM/F-12 medium. Medium was recovered, and cells were collected by centrifugation at 1,100 rpm for 10 min at 4°C (IEC Centra MP4R; International Equipment Needham). Cells were plated and incubated for 2 h in a CO2 incubator. Nonadherent cells were removed by three washes with D-MEM/F-12 medium. During treatment with HSC70, serum concentration in the medium was reduced to 2%.
Isolated heart perfusion.
Isolated hearts were perfused by the isovolumetric Langendorff technique as described previously (3, 7). Mice were anesthetized (pentobarbital sodium, 50 mg/kg ip) and heparinized (sodium heparin, 300 units ip). Their hearts were rapidly excised into oxygenated ice-cold perfusion buffer and retrograde perfused in nonrecirculating mode at a constant pressure of 70 mmHg with Krebs-Henseleit solution (pH 7.4). An ultrathin latex balloon was inserted into the left ventricle, and balloon volume was adjusted to achieve left ventricular end-diastolic pressure of 10–15 mmHg during the initial equilibration. Pacing wires were fixed to the right atrium, and all hearts were paced at 400 beats/min. Hearts were equilibrated for 20 min and then subjected to 20 min normothermic global ischemia followed by 60 min reperfusion. During ischemia, hearts were placed in a normal saline-filled organ bath chamber without pacing, and the temperature of perfusion buffer in the chamber was maintained at 37°C. For anti-HSC70 experiments, hearts were perfused with rabbit polyclonal anti-HSC70 or control IgG, 0.5 μg/ml in coronary circulation, for 10 min before ischemia and 30 min immediately after initiation of reperfusion. For HSC70 perfusion experiments, hearts were perfused with HSC70 or HSC70 fragment, 0.5 μg/ml in coronary circulation, for 30 min followed by a 60-min washout.
Neutrophils were infused into isolated hearts during reperfusion through a side-arm using an infusion pump. Cells were suspended in perfusion buffer and delivered to the aortic root at a concentration of 200,000 cells/ml.
Quantitative RT-PCR.
Total RNA was extracted from tissue homogenate by the RNeasy Mini Kit (Qiagen, Valencia, CA). Purified RNA was used for RT-PCR with the GeneAmp Gold RNA PCR Kit (Applied Biosystems, Foster City, CA). Custom primers for mouse GAPDH, MCP-1, and KC were designed according to GenBank data (Invitrogen, Carlsbad, CA). The Rotor-Gene 3000 Real-Time DNA Detection System (Corbett Research, Sydney, Australia) was used for all reactions.
Chemokine assay.
Aliquots of tissue homogenate and cell culture supernatant were used to assess chemokine peptide levels using ELISA kits (R&D Systems, Minneapolis, MN). Recombinant peptides were used to construct standard curves. Absorbance of standards and samples was determined spectrophotometrically at 450 nm using a microplate reader (Bio-Rad). Results were plotted against the linear portion of the standard curve.
HSC70 immunoassay.
The method has been previously described (1), and we modified the method for analysis of HSC70 in coronary effluent. A rat monoclonal antibody to murine HSC70 (cross-reaction with bovine and human HSC70) was utilized for the immunoassay. Samples were loaded into wells of an immunoassay plate. The volume was brought to 100 μl with PBS. The plate was covered and incubated overnight at 4°C. Following five washes with 200 μl PBS, remaining unbound sites in the wells were blocked by adding 300 μl blocking buffer (PBS containing 1% BSA and 4% sucrose) and incubating at 37°C for 1 h. The plate was washed five times with PBS, and 100 μl primary antibody (1:500 dilution with PBS containing 1% BSA) was added. The plate was incubated at 37°C for 1.5 h followed by five washes with 200 μl PBS containing 0.05% Tween 20. The amount of bound primary antibody was determined by the addition of 100 μl horseradish peroxidase conjugated goat-anti-rat IgG (1:1,000 dilution with PBS containing 1% BSA) and incubation for 1 h at 37°C. The plate was once again washed five times with 200 μl PBS containing 0.05% Tween 20. The activity of the peroxidase was measured by the initial rate of conversion of 2,2′-azino-bis-di(3-ethylbenzothiazoline) sulphonic acid (ABTS) to a chromogenic product in the presence of hydrogen peroxide. The substrate buffer contained 1.0 mg/ml ABTS and 0.015% hydrogen peroxide in citrate-phosphate buffer [0.05 M citric acid and 0.05 M Na2HPO4 (pH 4.0)]. The rate was measured immediately in a 96-well plate kinetic plate reader using a 405-nm filter. Recombinant HSC70 was used to generate a standard curve, and HSC70 concentrations in samples were calculated against the standard curve.
Immunofluorescent staining of neutrophils.
Immunofluorescent staining was performed as described previously (36). Myocardial cryosections (5 μm thick) were treated with a mixture of 30% methanol and 70% acetone and fixed in 4% paraformaldehyde. Sections were incubated with blocking solution (10% BSA in PBS) for 45 min. Sections were then incubated with a mixture of rat monoclonal antibody against mouse neutrophil marker protein (Clone 7/4; ABD Serotec, Oxford, UK) and rabbit polyclonal antibody against mouse macrophage marker protein CD68 (Santa Cruz Biotechnology, Santa Cruz, CA), 5 μg/ml each in antibody buffer, for 90 min. Sections were then incubated for 45 min with a mixture of Cy3-labeled goat anti-rat IgG (labeling neutrophils red; 1:250 dilution with antibody buffer) and Alexa488-conjugated goat anti-rabbit IgG (labeling macrophages green; 1:400 dilution with antibody buffer). Sections were counterstained with Alexa Fluor 350-labeled wheat germ agglutinin (for outlining myocardial cells). To assess the staining specificity, adjacent sections were incubated with a mixture of nonimmune rat IgG and nonimmune rabbit IgG (5 μg/ml each in antibody buffer) in replacement of the primary antibodies and then processed in identical conditions. Microscopic observation and photography were performed with a Leica DMRXA digital microscope. Images were analyzed using SlideBook 2.6 software (I. I. I., Denver, CO) to obtain quantitative estimates of area. Numbers of neutrophils were counted in three random fields by a blinded observer.
Statistical analysis.
Data are presented as means ± SE. Statistical analyses were performed using ANOVA with a post hoc Bonferroni/Dunn test. Statistical significance was accepted within a 95% confidence limit.
RESULTS
Myocardial TLR4 has a major role in neutrophil infiltration in the heart following global myocardial I/R.
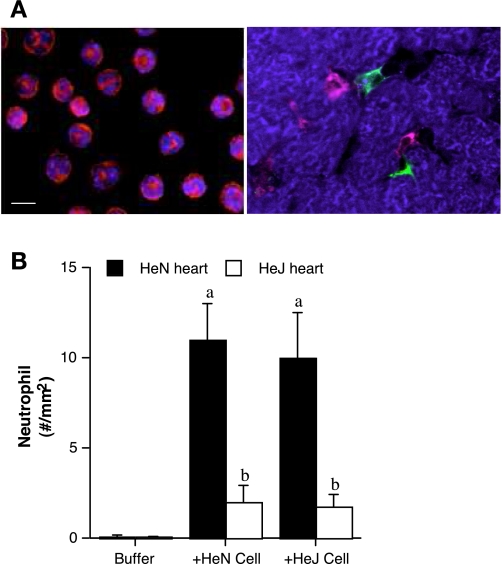
To determine the relative roles of myocardial TLR4 and neutrophil TLR4 in neutrophil infiltration to the myocardium following global I/R, we perfused bone marrow-derived neutrophils to the buffer-perfused heart during reperfusion. As shown in Fig. 1A, neutrophils appeared mature after in vitro incubation and were capable of migrating into myocardial interstitial spaces (as identified with the presence of resident macrophages). Interestingly, comparable neutrophil infiltration was observed in TLR4-competent hearts perfused with TLR4-competent or TLR4-defective cells (Fig. 1B). However, TLR4-defective hearts, which express dysfunctional TLR4, exhibited significantly less neutrophil infiltration regardless of the neutrophil phenotype (Fig. 1B). The results indicate that myocardial TLR4, but not neutrophil TLR4, has a pivotal role in postischemic neutrophil infiltration in the myocardium.
Fig. 1.
Neutrophil infiltration to the myocardium is dependent on myocardial Toll-like receptor 4 (TLR4) rather than neutrophil TLR4. A: neutrophils derived from bone marrow (left) were stained red with rat anti-mouse neutrophil marker protein, and their nuclei were stained blue with bis-benzimide. Cells appeared mature after in vitro incubation. Right: neutrophils (red) infiltrated into the space between myocytes (stained blue with wheat germ agglutinin) and were found in close proximity to resident macrophages (stained green with polyclonal anti-mouse CD68) and myocytes after perfusing ischemic heart with buffer containing neutrophils. Bar = 10 μm. B: hearts isolated from C3H/HeN (TLR4 competent) and C3H/HeJ (TLR4 defective) mice were subjected to global ischemia-reperfusion (I/R; 20 min ischemia/60 min reperfusion). After ischemia, hearts were infused with TLR4-competent or TLR4-defective neutrophils. Immunostaining demonstrates that TLR4-competent hearts exhibit greater neutrophil infiltration than TLR4-defective hearts. Perfusion of TLR4-defective neutrophils did not influence cell infiltration in TLR4-competent hearts. Data are expressed as means ± SE; n = 5 in each group. aP < 0.05 vs. buffer-perfused heart; bP < 0.05 vs. HeN heart.
Myocardial TLR4 mediates chemokine expression in the heart following global myocardial I/R.
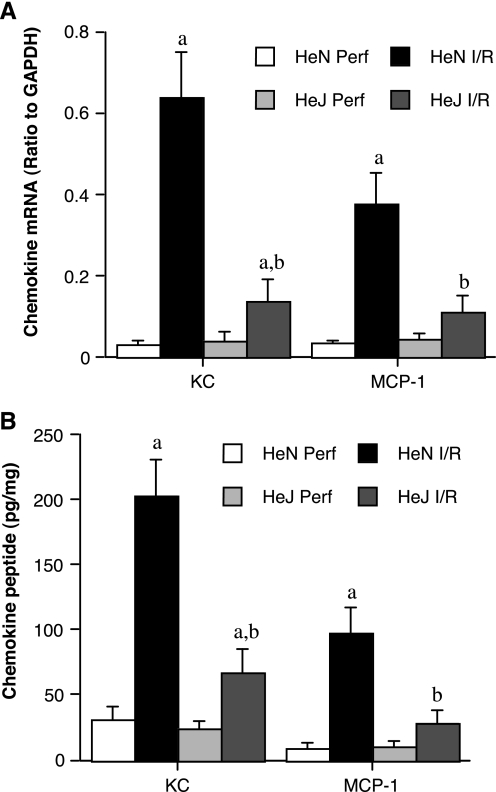
To examine whether reduced neutrophil infiltration in the TLR4-defective heart correlates with reduced myocardial expression of IL-8 and MCP-1, two chemokines critical for neutrophil infiltration, we examined their mRNA and protein levels after global ischemia followed by reperfusion with buffer. As shown in Fig. 2, A and B, mRNA and protein levels of KC and MCP-1 increased markedly in TLR4-competent hearts, and these changes were significantly attenuated in TLR4-defective hearts that express dysfunctional TLR4. The results suggest that myocardial TLR4 is also important for the chemokine response to global I/R.
Fig. 2.
Myocardial TLR4 mediates chemokine response to global I/R. Hearts isolated from C3H/HeN (TLR4 competent) and C3H/HeJ (TLR4 defective) mice were subjected to global I/R (20 min ischemia/60 min reperfusion). Control hearts (Perf) were perfused without subjecting to I/R. mRNA (A) and peptide (B) levels of keratinocyte-derived chemokine (KC) and monocyte chemoattractant protein-1 (MCP-1) after I/R are significantly lower in TLR4-defective hearts than in TLR4-competent hearts. Data are expressed as means ± SE; n = 5 in each group. aP < 0.05 vs. phenotype perfusion control; bP < 0.05 vs. HeN I/R.
Myocardial chemokine expression following global I/R involves HSC70.
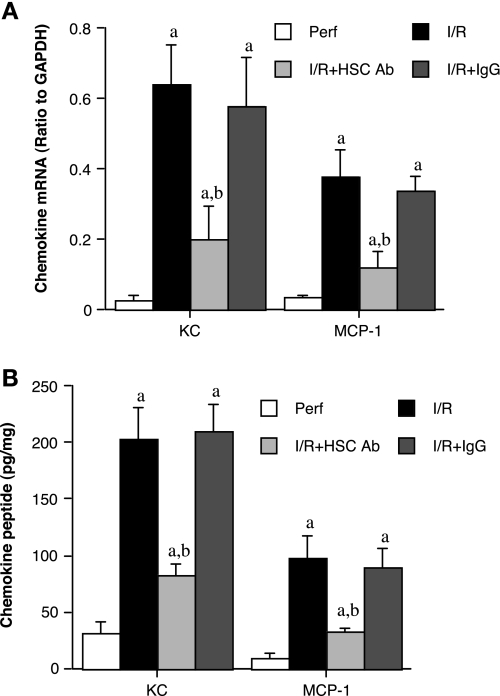
We have found that the HSC70 is released from the myocardium following global myocardial I/R (36). In this study, we applied an immunoassay to analyze HSC70 levels in the coronary effluent. Although a trace amount of HSC70 was found in the coronary effluent of before ischemia (2.1 ± 0.2 ng/ml), HSC70 levels in coronary effluent increased markedly following ischemia (90 ± 19 ng/ml at 0–5 min of reperfusion and 11 ± 2.1 ng/ml at 55–60 min of reperfusion, both P < 0.05 vs. perfusion controls). Comparable levels of HSC70 were found in coronary effluent of TLR4-competent and TLR4-defective hearts. To determine whether HSC70 has a role in postischemic myocardial expression of KC and MCP-1, we perfused hearts isolated from TLR4-competent mice with either a polyclonal antibody specifically against HSC70 or nonimmune IgG (0.5 μg/ml in perfusion buffer) for 10 min before ischemia and throughout reperfusion. As shown in Fig. 3A, both KC and MCP-1 mRNA levels were lower at the end of reperfusion in the anti-HSC70 group than in the untreated I/R group or the nonimmune IgG group. In addition, KC and MCP-1 peptide levels were 60% and 67% lower (both P < 0.05), respectively, in hearts treated with anti-HSC70 than in the untreated I/R group (Fig. 3B). These results demonstrate that endogenous HSC70 released from the myocardium contributes to the mechanisms of myocardial chemokine expression following global myocardial I/R.
Fig. 3.
Extracellular heat shock cognate protein 70 (HSC70) plays a critical role in the myocardial chemokine response following global I/R. A and B: hearts isolated from C3H/HeN (TLR4 competent) mice were subjected to global I/R (20 min ischemia/60 min reperfusion) with or without treatment with polyclonal anti-HSC70 (HSC Ab; 0.5 μg/ml) or control IgG (IgG; 0.5 μg/ml) for 10 min before ischemia and for 30 min after initiation of reperfusion. Control hearts (Perf) were perfused with perfusion buffer without subjecting to I/R. Anti-HSC70 reduced myocardial levels of KC and MCP-1 mRNA and peptides, whereas control IgG had minimal effects. Data are expressed as means ± SE; n = 6 in each group. aP < 0.05 vs. phenotype perfusion control; bP < 0.05 vs. phenotype I/R control.
The HSC70-induced myocardial chemokine response requires TLR4 and the SBD of HSC70.
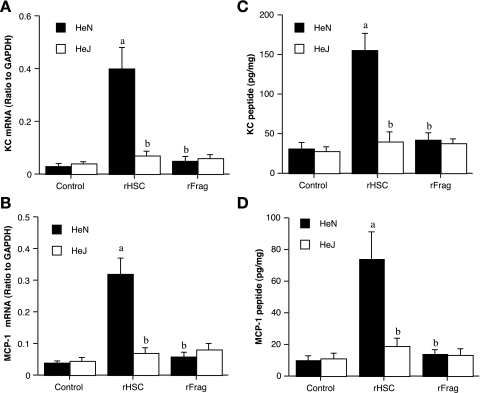
To examine the effect of extracellular HSC70 on chemokine expression, we perfused hearts isolated from TLR4-competent and TLR4-defective mice with rHSC70 (0.5 μg/ml) for 30 min followed by 60-min washout. Treatment with rHSC70 increased KC and MCP-1 mRNA levels by 13- and eightfold, respectively, in TLR4-competent hearts (Fig. 4, A and B). Similarly, KC and MCP-1 peptide levels increased by five- and sevenfold, respectively, in TLR4-competent hearts (Fig. 4, C and D). In contrast, both mRNA and peptide levels of KC and MCP-1 increased slightly, but insignificantly, following rHSC70 treatment in hearts with defective TLR4 (Fig. 4, A–D). Therefore, HSC70 applied extracellularly can induce myocardial chemokine expression. In addition, the HSC70-induced expression of KC and MCP-1 requires functional TLR4.
Fig. 4.
Induction of myocardial chemokine response by HSC70 is TLR4 dependent and requires the peptide-binding domain. Hearts isolated from C3H/HeN (TLR4 competent) and C3H/HeJ (TLR4 defective) mice were perfused with either recombinant full-length HSC70 (rHSC; 0.5 μg/ml) or recombinant HSC70 fragment (rFrag; without the substrate-binding domain; 0.5 μg/ml) for 30 min followed by 60 min washout. Control hearts were perfused with perfusion buffer only. A and B: TLR4-competent hearts expressed KC and MCP-1 mRNA in response to HSC70 treatment, but myocardial KC and MCP-1 mRNA levels were reduced in TLR4-defective hearts. C and D: KC and MCP-1 peptide levels increased in TLR4-competent hearts treated with HSC70, but this effect of HSC70 was reduced in TLR4-defective hearts. The HSC70 fragment without the substrate-binding domain failed to upregulate mRNA or peptide expression of KC and MCP-1 (A–D). Data are expressed as means ± SE; n = 6 in each group. aP < 0.05 vs. phenotype control; bP < 0.05 vs. HeN treated with HSC70 (rHSC).
HSC70 has two functional domains: the nucleotide-binding domain (NBD) and the SBD. The SBD is critical for HSC70 to interact with proteins (10, 23, 34). To determine whether the SBD is required for HSC70 to induce TLR4-dependent chemokine expression, we contrasted the effects of a 44-kDa recombinant HSC70 fragment without the SBD and full-length rHSC70 on chemokine expression in TLR4-competent and TLR4-defective hearts. Treatment of either TLR4-competent or TLR4-defective hearts with the rHSC70 fragment (0.5 μg/ml) failed to induce chemokine expression. Neither KC nor MCP-1 mRNA levels in TLR4-competent hearts were greater than those of perfusion controls (Fig. 4, A and B). KC and MCP-1 peptide levels in TLR4-competent hearts treated with the rHSC70 fragment were slightly greater than controls, but the differences were insignificant (Fig. 4, C and D). In comparison with those in hearts treated with full-length rHSC70, mRNA and peptide levels of those chemokines were significantly lower in hearts treated with the rHSC70 fragment without SBD. These results show that the rHSC70 fragment without the SBD is insufficient to induce a myocardial chemokine response. Taken together, our results demonstrate that both functional TLR4 and the SBD of HSC70 are required for HSC70 to induce a myocardial chemokine response.
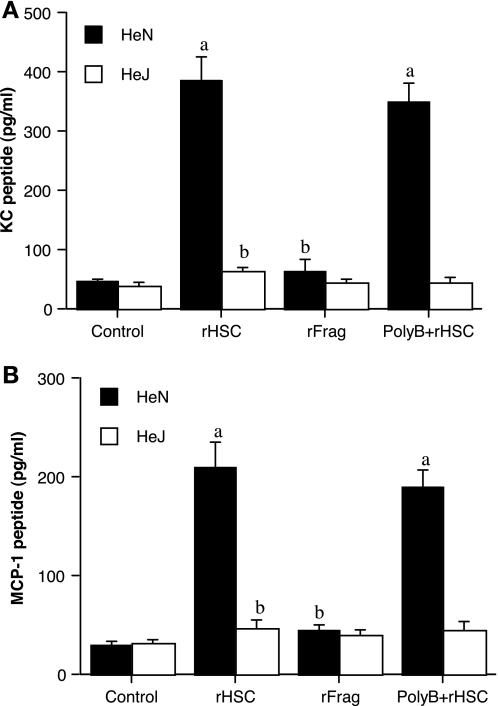
To confirm our findings in the heart, we performed experiments on macrophages since macrophages have a higher density of TLR4 on cell surfaces and they are the main source of tissue chemokine expression. Due to an extremely low yield of isolating cardiac macrophages, we used peritoneal macrophages to examine the effect of HSC70 on chemokine expression. We treated peritoneal macrophages isolated from TLR4-competent and TLR4-defective mice with full-length rHSC70, rHSC70 fragment without the SBD and examined KC and MCP-1 levels in the culture media 4 h after incubation. Correlating with our results on hearts, full-length rHSC70 induced a chemokine response in cells in a TLR4-dependent fashion, but the rHSC70 fragment had a minimal effect (Fig. 5, A and B). The results demonstrate that the chemokine response in macrophages induced by HSC70 also requires functional TLR4 and the SBD of HSC70. To address the issue of endotoxin contamination, although in a trace amount, in the recombinant protein preparations, we added the endotoxin antagonist polymyxin B (5.0 μg/ml) to cell culture media and found that it had a minimal influence on rHSC70 induction of macrophage production of KC and MCP-1 (Fig. 5, A and B), indicating that the HSC70 protein per se was responsible for the observed effects.
Fig. 5.
HSC70 induces macrophage expression of KC and MCP-1 through a TLR4-dependent mechanism. A: peritoneal macrophages from C3H/HeN (TLR4 competent) mice were incubated with recombinant HSC70 (rHSC; 0.5 μg/ml) or recombinant HSC70 fragment (rFrag; without the substrate-binding domain; 0.5 μg/ml) for 4 h or left untreated (control). Recombinant HSC70 induced the release of KC (A) and MCP-1 (B) in TLR4-competent cells but not in TLR4-defective cells. The recombinant HSC70 fragment had no effect on the release of KC or MCP-1. Note that recombinant HSC70 with and without polymyxin B (PolyB) had a comparable effect on cells. Data are presented as means ± SE; n = 4. aP < 0.05 vs. phenotype control; bP < 0.05 vs. HeN treated with HSC70 (rHSC).
DISCUSSION
Global myocardial I/R induces an inflammatory response that contributes to postischemic myocardial injury. Although the inflammatory response is a tempting target for prevention of postischemic myocardial injury in cardiac surgery with global I/R, the underlying mechanisms of the response are incompletely understood. We have previously found that hearts from TLR4 mutant mice and TLR4 knockout mice exhibit reduced myocardial NF-κB activation and proinflammatory cytokine (TNF-α, IL-1β, and IL-6 ) expression, indicating that this innate immune receptor has a major role in the myocardial inflammatory response induced by I/R (7, 36). We also found that HSC70 is released from cells of mouse heart and mediates myocardial NF-κB activation and cytokine expression via a TLR4-dependent mechanism. Thus it appears extracellular HSC70 has a role in TLR4-mediated myocardial inflammatory response to I/R. Studies on patients undergoing cardiopulmonary bypass have demonstrated that circulating levels of IL-8 and MCP-1 increase in early reperfusion (11, 18, 26, 31), and the myocardium is an important source of these chemokines in circulation. It is well known that chemokine expression and neutrophil infiltration are key components of myocardial inflammation. However, the role of TLR4 in these events, especially in the setting of global myocardial I/R, remains unclear.
In the present study, we tested the hypotheses that myocardial TLR4 mediates neutrophil infiltration and that myocardial TLR4 and extracellular HSC70 contribute to the mechanisms underlying cardiac chemokine response to global I/R. We found that TLR4-defective hearts exhibited reduced neutrophil infiltration regardless of the phenotypes of neutrophils perfused. In correlation to the reduced neutrophil infiltration, TLR4-defective hearts expressed significantly lower levels of KC and MCP-1. Similarly, treatment of TLR4-competent hearts with a polyclonal antibody against HSC70 reduced myocardial expression of KC and MCP-1 after I/R. Furthermore, perfusion of recombinant HSC70 induced myocardial expression of KC and MCP-1 in TLR4-competent hearts but not in TLR4-defective hearts, and a 44-kDa recombinant fragment of HSC70 that does not have the peptide-binding domain failed to induce the chemokine response in TLR4-competent hearts. Together, the results demonstrate that myocardial TLR4, rather than neutrophil TLR4, is the determinant of myocardial neutrophil infiltration after global I/R. Furthermore, myocardial TLR4 mediates the upregulation of chemokine expression induced by global I/R, and the mechanisms of TLR4-mediated upregulation involve extracellular HSC70.
TLR4 is important for host defense against gram-negative bacterial infection and is expressed by all cells examined, including cardiac cells (15) and neutrophils (4). In vivo studies demonstrate a critical role of TLR4 in myocardial neutrophil infiltration after regional I/R (24) or heart transplant (17). Although neutrophil TLR4 has been found to mediate cellular expression of TNF-α and IL-1β (4), the relative role of neutrophil TLR4 and myocardial TLR4 in I/R-induced neutrophil infiltration to the myocardium has not been determined. We perfused bone marrow-derived neutrophils to the buffer-perfused heart during reperfusion to determine the relative roles of myocardial TLR4 and neutrophil TLR4. Although neutrophils were barely found in nonischemic hearts, perfusion of cells after ischemia increased myocardial neutrophil numbers in TLR4-competent hearts. However, significantly fewer neutrophils were found in TLR4-defective hearts after perfusion of either TLR4-competent cells or TLR4-defective cells. Apparently, functional TLR4 in the myocardium is required for neutrophil infiltration. Interestingly, comparable infiltration was observed in TLR4-competent hearts perfused with TLR4-competent cells and TLR4-defective cells. Thus neutrophil TLR4 is not required for its infiltration to postischemic myocardium. It should be noted that myocardial neutrophil infiltration is not robust in this model of isolated global I/R. One explanation is that the reperfusion protocol is short, and myocardial injury is not severe. However, cells may have lower activities in perfusion buffer than in blood. Nevertheless, the buffer perfusion model provides the simplicity for determination of the roles of myocardial and circulating factors, and the results of this study demonstrate that neutrophils migration into ischemic myocardium in ex vivo perfused hearts requires functional TLR4 in the myocardium. It is likely that myocardial TLR4 has a critical role in myocardial expression of chemoattractant proteins that mediate neutrophil adhesion and migration.
In this study, we found that myocardial expression of KC and MCP-1 at both mRNA and peptide levels increased after reperfusion. TLR4-defective hearts expressed significantly less KC and MCP-1 at both mRNA and peptide levels, although KC and MCP-1 levels remained higher in these hearts compared with baseline levels. Thus myocardial TLR4 has a major role in regulation of the expression of KC and MCP-1. Previous studies implicated complements and proinflammatory cytokines in the chemokine response to global myocardial I/R (25). In this regard, we observed that TNF-α induces the expression of IL-8 and MCP-1 in human lung microvascular cells (30). Since TNF-α expression is rapid in the myocardium after global ischemia (27), and is under the control by myocardial TLR4 (7), TNF-α may serve as an intermediate in TLR4-mediated myocardial expression of chemokines, including IL-8 and MCP-1. Further studies are needed to determine whether TNF-α links TLR4 signaling to chemokine expression. However, TLR4 mediates myocardial NF-κB activation induced by global I/R (7, 36), and both KC and MCP-1 genes are controlled by NF-κB. Thus it is most likely that TLR4 regulates I/R-induced KC and MCP-1 expression through NF-κB. An important question is how I/R induces TLR4 signaling.
In a recent study we demonstrated that global ischemia causes mouse heart to release HSC70 (36). In the present study, we found increased levels of HSC70 in coronary effluent immediately after ischemia. We applied a polyclonal antibody specific to HSC70 to determine the role of extracellular HSC70 in the mechanisms of postischemic chemokine expression. We found that the chemokine response is mediated, at least in part, by extracellular HSC70 since treatment with the antibody reduced the expression of KC and MCP-1 at both mRNA and peptide levels after I/R. To further determine the role of extracellular HSC70 in the induction of myocardial KC and MCP-1, we perfused hearts and treated macrophages with recombinant HSC70. Treatment with HSC70 increased myocardial levels of KC and MCP-1 in TLR4-competent hearts, but it had a minimal effect on TL4-defective hearts. Similarly, extracellular HSC70 induced macrophage release KC and MCP-1 in a TLR4-dependent manner. Together, these results demonstrate that extracellular HSC70 is both necessary in and adequate to induce the TLR4-mediated chemokine expression. HSC70 is constitutively expressed in the myocardium, and its release does not require de novo protein synthesis. Therefore, the HSC70-TLR4 signaling mechanism that we have identified in this study is consistent with the rapid increase in chemokine levels following cardiopulmonary bypass. We have previously observed that recombinant HSC70 interacts with cell surface TLR4 when incubated with macrophages in vitro (36). It remains unknown from the present study whether extracellular HSC70 interacts with cardiomyocytes and other cardiac cells. Further study is needed to address this important question.
HSC70 is a cytoplasmic protein that functions primarily as a molecular chaperone (35). It has two main functional domains: an ATPase or NBD and a SBD (10, 23, 34). In our earlier study we found that the NBD domain alone is inadequate to induce cytokine production in mouse hearts (36). In this study we found that the HSC70 fragment without the SBD failed to induce a chemokine response in hearts ex vivo and also in macrophages in vitro. Therefore, HSC70-induced myocardial chemokine expression requires both TLR4 and the SBD of HSC70. It is most likely that the SBD of HSC70 interacts with TLR4 to induce a chemokine response. This mechanism is also consistent with the current paradigm of TLR4 signaling for endogenous stimuli (22). Since endotoxin activates TLR4, it is possible that endotoxin contributes to TLR4 activation by any recombinant protein preparation containing a trace amount of endotoxin. To address the issue of endotoxin contamination, we used the endotoxin antagonist polymyxin B (5.0 μg/ml) in macrophages, which are sensitive to low levels of endotoxin. In addition, we compared the effect of recombinant HSC70 with that of a recombinant HSC70 fragment, which also contains a trace amount of endotoxin. The results from macrophage experiments show that the endotoxin antagonist had no effect on chemokine release induced by HSC70 and that recombinant HSC70 and recombinant HSC70 fragment are distinct in the induction of chemokine release. From these results we can conclude that the effects of HSC70 that we observed on chemokine expression are due to the actions of HSC70 protein and not to endotoxin contamination.
In summary, this study demonstrates that myocardial tissue TLR4, rather than neutrophil TLR4, is the determinant of myocardial neutrophil infiltration after global I/R. TLR4 mediates myocardial chemokine expression, and the mechanisms involve extracellular HSC70. Since global myocardial I/R induces an inflammatory response that contributes to postischemic myocardial injury, myocardial TLR4 and extracellular HSC70 might be tempting targets for prevention of myocardial injury in cardiac surgery with global I/R.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grant HL-079051. This study was presented at the American Heart Association Scientific Sessions 2008.
REFERENCES
- 1.Anderson RL, Wang CY, vanKersen I, Lee KJ, Welch WJ, Lavagnini P, Hahn GM. An immunoassay for heat shock protein 73/72: use of the assay to correlate HSP73/72 levels in mammalian cells with heat response. Int J Hyperthermia 9: 539–552, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Gaudino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg 25: 304–311, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Ao L, Song Y, Fullerton DA, Dinarello CA, Meng X. The interaction between myocardial depressant factors in endotoxemic cardiac dysfunction: role of TNF-alpha in TLR4-mediated ICAM-1 expression. Cytokine 38: 124–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol 172: 2522–2529, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Abraham R, Weinbroum AA, Dekel B, Paret G. Chemokines and the inflammatory response following cardiopulmonary bypass—a new target for therapeutic intervention? A review. Paediatr Anaesth 13: 655–661, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Abraham R, Weinbroum AA, Lotan D, Dagan O, Schreriber-Scheffer R, Mishali D, Harel R, Vishne T, Barzilay Z, Paret G. Interleukin-8 secretion following cardiopulmonary bypass in children as a marker of early postoperative morbidity. Paediatr Anaesth 12: 156–161, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cha J, Wang Z, Ao L, Zou N, Dinarello CA, Banerjee A, Fullerton DA, Meng X. Cytokines link Toll-like receptor 4 signaling to cardiac dysfunction after global myocardial ischemia. Ann Thorac Surg 85: 1678–1685, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao W Toll-like receptor signaling: a critical modulator of cell survival and ischemic injury in the heart. Am J Physiol Heart Circ Physiol 296: H1–H12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chou CC, Forouhar F, Yeh YH, Shr HL, Wang C, Hsiao CD. Crystal structure of the C-terminal 10-kDa subdomain of Hsc70. J Biol Chem 278: 30311–30316, 2003. [DOI] [PubMed] [Google Scholar]
- 11.de Mendonca-Filho HT, Pereira KC, Fontes M, Vieira DA, de Mendonca ML, Campos LA, Castro-Faria-Neto HC. Circulating inflammatory mediators and organ dysfunction after cardiovascular surgery with cardiopulmonary bypass: a prospective observational study. Crit Care 10: R46, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhardt F, Mehlhorn U, Larose K, DeVivie ER, Dhein S. Structural myocardial changes after coronary artery surgery. Eur J Clin Invest 30: 938–946, 2000. [PubMed] [Google Scholar]
- 13.Feng Y, Zhao H, Xu X, Buys ES, Raher MJ, Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U, Chao W. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol 295: H1311–H1318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frantz S, Ertl G, Bauersachs J. Toll-like receptor signaling in the ischemic heart. Front Biosci 13: 5772–5779, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 271–280, 1999. [DOI] [PMC free article] [PubMed]
- 16.Gabrielsen A, Lawler PR, Yongzhong W, Steinbruchel D, Blagoja D, Paulsson-Berne G, Kastrup J, Hansson GK. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J Mol Cell Cardiol 42: 870–883, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, Tobita K, Zuckerbraun BS, McCurry KR, Murase N, Billiar TR. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation 84: 1279–1287, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Karth GD, Buberl A, Nikfardjam M, Meyer B, Wollenek G, Grimm M, Lassnigg A, Brannath W, Hiesmayr M, Heinz G. Role of amiodarone on the systemic inflammatory response induced by cardiac surgery: proinflammatory actions. Can J Anaesth 54: 262–268, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Liakopoulos OJ, Schmitto JD, Kazmaier S, Brauer A, Quintel M, Schoendube FA, Dorge H. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg 84: 110–119, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Meldrum DR, Meng X, Dinarello CA, Ayala A, Cain BS, Shames BD, Ao L, Banerjee A, Harken AH. Human myocardial tissue TNFalpha expression following acute global ischemia in vivo. J Mol Cell Cardiol 30: 1683–1689, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Meng X, Ao L, Song Y, Raeburn CD, Fullerton DA, Harken AH. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-α receptor. Am J Physiol Regul Integr Comp Physiol 288: R600–R606, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock 26: 430–437, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Morshauser RC, Hu W, Wang H, Pang Y, Flynn GC, Zuiderweg ER. High-resolution solution structure of the 18 kDa substrate-binding domain of the mammalian chaperone protein Hsc70. J Mol Biol 289: 1387–1403, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Raja SG, Dreyfus GD. Modulation of systemic inflammatory response after cardiac surgery. Asian Cardiovasc Thorac Ann 13: 382–395, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Risnes I, Ueland T, Lundblad R, Mollnes TE, Baksaas ST, Aukrust P, Svennevig JL. Changes in the cytokine network and complement parameters during open heart surgery. Interact Cardiovasc Thorac Surg 2: 19–24, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Shames BD, Barton H H, Reznikov LL, Cairns CB, Banerjee A, Harken AH, Meng X. Ischemia alone is sufficient to induce TNF-α mRNA and peptide in the myocardium. Shock 17: 114–119, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation 114: I270–I274, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Mitra S, Arcaroli J, Kuhn K, Abraham E. Modulation of bone marrow-derived neutrophil signaling by H2O2: disparate effects on kinases, NF-κB, and cytokine expression. Am J Physiol Cell Physiol 286: C683–C692, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Su X, Ao L, Zou N, Song Y, Yang X, Cai GY, Fullerton DA, Meng X. Post-transcriptional regulation of TNF-induced expression of ICAM-1 and IL-8 in human lung microvascular endothelial cells: an obligatory role for the p38 MAPK-MK2 pathway dissociated with HSP27. Biochim Biophys Acta 1783: 1623–1631, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomic V, Russwurm S, Moller E, Claus RA, Blaess M, Brunkhorst F, Bruegel M, Bode K, Bloos F, Wippermann J, Wahlers T, Deigner HP, Thiery J, Reinhart K, Bauer M. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation 112: 2912–2920, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Valen G, Paulsson G, Vaage J. Induction of inflammatory mediators during reperfusion of the human heart. Ann Thorac Surg 71: 226–232, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Wan S, DeSmet JM, Barvais L, Golstein M, Vincent JL, LeClerc JL. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg 112: 806–811, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Wang TF, Chang JH, Wang C. Identification of the peptide binding domain of Hsc70. J Biol Chem 268: 26049–26051, 1993. [PubMed] [Google Scholar]
- 35.Young JC, Barral JM, Hartl FU. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci 28: 541–547, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Zou N, Ao L, Cleveland JC Jr, Yang X, Su X, Cai GY, Banerjee A, Fullerton DA, Meng X. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol 294: H2805–H2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]