Abstract
The exercise pressor reflex is evoked by both mechanical and metabolic stimuli arising in contracting skeletal muscle. Recently, the blockade of acid-sensing ion channels (ASICs) with amiloride and A-316567 attenuated the reflex. Moreover, amiloride had no effect on the mechanoreceptor component of the reflex, prompting us to determine whether ASICs contributed to the metaboreceptor component of the exercise pressor reflex. The metaboreceptor component can be assessed by measuring mean arterial pressure during postcontraction circulatory occlusion when only the metaboreceptors are stimulated. We examined the effects of amiloride (0.5 μg/kg), A-317567 (10 mM, 0.5 ml), and saline (0.5 ml) on the pressor response to and after static contraction while the circulation was occluded in 30 decerebrated cats. Amiloride (n = 11) and A-317567 (n = 7), injected into the arterial supply of the triceps surae muscles, attenuated the pressor responses both to contraction while the circulation was occluded and to postcontraction circulatory occlusion (all, P < 0.05). Saline (n = 11), however, had no effect on the pressor responses to contraction while the circulation was occluded or to postcontraction circulatory occlusion (both, P > 0.79). Our findings led us to conclude that ASICs contribute to the metaboreceptor component of the exercise pressor reflex.
Keywords: lactic acid, group III and IV afferents, skeletal muscle, amiloride, A-317567
static exercise is well known to increase mean arterial pressure and sympathetic neural activity to maintain an adequate perfusion of metabolically active muscles. The pressor and sympathetic responses to static exercise are believed to be caused in part by the exercise pressor reflex (3, 23, 25, 39). The afferent arm of the reflex consists of group III and IV afferents (23), the endings of which are stimulated by both mechanical and metabolic stimuli arising in contracting muscles (16, 17). For the most part, group III afferents are thought to be responsive to mechanical stimuli, whereas group IV afferents are thought to be responsive to metabolic stimuli arising in the contracting muscles (15).
Lactic acid is believed to be one of the substances that evoke the metabolic component of the exercise pressor reflex. For example, lactic acid concentrations in the muscle interstitium are increased by contraction (22). In addition, lactic acid injected into the arterial supply of skeletal muscle reflexly increased arterial pressure, heart rate, and ventilation (32), as well as increased the discharge of group III and IV muscle afferents (31). Furthermore, blunting lactic acid production by either dichloroacetate or glycogen depletion decreased the exercise pressor reflex (7, 35).
If lactic acid is involved in evoking the exercise pressor reflex, then the blockade of its receptors, namely, acid sensing ion channels (ASICs), should attenuate the pressor response to exercise. In fact, the injection of amiloride, an ASIC antagonist, decreased the pressor response to exercise (10). Amiloride, however, is not specific to ASICs because it also blocks epithelial sodium channels (ENaCs), channels that are mechano- and metabosensitive and thought to play a role in cardiovascular regulation (5). Moreover, the concentration of amiloride needed to block ENaCs is 10-fold lower than that needed to block ASICs (18). When an ASIC-specific antagonist A-317567 became available, we used it to block ASICs during a static contraction. We found that A-317567, which does not appear to block ENaCs, reduced the pressor response to static contraction of freely perfused muscles (12). Our finding suggested that lactic acid working through ASICs, and not ENaCs, may play an important role in evoking the exercise pressor reflex.
The question then became, Do ASICs evoke the exercise pressor reflex through activation of mechanoreceptors, metaboreceptors, or a combination of both? To answer this question, the reflex effects of mechanoreceptors and metaboreceptors need to be separated. Therefore, we first investigated the role played by ASICs on mechanoreceptors by analyzing renal sympathetic nerve activation at the first 5 s of static contraction (24). The renal sympathetic nerve responses to contraction, during this time period, were not different before and after amiloride injection, a finding that suggested that the mechanoreceptor component of the exercise pressor reflex was not mediated by ASICs (24). Thus the present study is our effort to determine whether ASICs on thin fiber muscle afferents play a role in evoking the metabolic component of the exercise pressor reflex. The reflex effect evoked by metaboreceptors was isolated from those evoked by mechanoreceptors by measuring arterial pressure and renal sympathetic nerve responses during postcontraction circulatory occlusion, a maneuver that trapped metabolites in the vasculature of the hindlimb muscles (1).
We used amiloride and A-317567 to determine the effect of ASIC blockade on the pressor responses to both static contraction during circulatory occlusion and to postcontraction circulatory occlusion in decerebrated cats. We tested the hypothesis that ASICs play a role in evoking the metabolic component of the exercise pressor reflex.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center.
Surgical Preparation
Adult cats (n = 30; 3.3 ± 0.4 kg; and range, 2.6–5.0 kg) of either sex (8 males, and 22 females) were anesthetized with a mixture of 5% isoflurane-95% oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids as well as for the measurement of arterial blood pressure. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham) to monitor blood pressure. Heart rate was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within normal range by either adjusting the ventilation or the intravenous administration of sodium bicarbonate (8.5%). A temperature probe was passed through the mouth to the stomach. The temperature was continuously monitored and maintained at 37–38°C by a water-perfused heating pad and a heat lamp.
In 10 cats, the left external iliac artery and common iliac vein were isolated, and snares were placed around these vessels, which, when tightened, trapped the injectate in the circulation of the leg. The left triceps surae muscles, left popliteal artery, and left tibial nerve were isolated. In 20 cats, the left common iliac vein and abdominal aorta were isolated, and snares were placed around these vessels to trap the drugs in the circulation of the leg. In addition, the sacral artery that perfuses the tail was ligated. A catheter with its tip pointing toward the heart was passed into the right femoral artery. When the snare placed around the abdominal aorta was tightened, the fluid injected from the right femoral artery flowed into the left external iliac artery. This was checked in every cat by injecting saline into the catheter in the right femoral artery and seeing blood exit the left external iliac artery, leaving it clear. The volume of saline needed to clear the left femoral artery, which was usually 0.15 to 0.2 ml, was used to flush the drugs used in this experiment. In these 20 cats, a laminectomy was also performed to expose the lower lumbar and sacral portions of the spinal cord.
Following the placement in a Kopf stereotaxic frame, each cat was decerebrated at the midcollicular level under isoflurane anesthesia. Dexamethasone (4 mg) was injected intravenously just before the decerebration procedure to minimize brain edema. The left common carotid artery was tied off to reduce bleeding. All neural tissue rostral to the midcollicular section was removed, and the cranial vault was filled with agar.
A renal nerve bundle was carefully isolated from the renal plexus and surrounding connective tissue near the renal artery and vein. The nerve was cut and its central end was draped over a pair of silver wire electrodes (bare diameter, 76 μm) insulated with Teflon (A-M Systems). The nerve-electrode complex was then covered with a mixture of silicone gel (Kwik Sil, World Precision Instruments). The electrode wire for recording renal sympathetic nerve activity (RSNA) was placed outside of the incision site, and the abdominal wound was closed. The electrode was attached in series with a high-impedance probe (model HIP 511, Grass) and then amplified (model P511, Grass). RSNA was displayed on a storage oscilloscope (Hewlett-Packard) and made audible. The amplifier was filtered between 100 Hz and 3 kHz. The cat was then placed in a Kopf spinal unit.
In the 20 cats in which a laminectomy was performed, we used the skin on the back to form a pool that was filled with warm (37°C) mineral oil. The dura of the cord was cut and reflected, allowing a visual identification of the spinal roots. The left L6, L7, and S1 ventral roots were identified and cut. The peripheral cut ends were draped over a stimulating electrode. In all cats, the left calcaneal bone was cut, and its tendon was attached to a force transducer (model FT-10C, Grass) for measurement of the tension developed during static contraction of the left triceps surae muscles. The knee joint was secured to a post. Once the surgeries were completed, the anesthesia was withdrawn from the decerebrated cat and the lungs were ventilated with room air.
Experimental Protocols
Amiloride.
We assessed the effect of amiloride (0.5 μg/ kg; Sigma-Aldrich, St. Louis, MO) on the reflex pressor, cardioaccelerator, and renal sympathetic nerve responses to four stimuli: 1) static contraction of the left triceps surae muscles while their circulation was occluded, 2) circulatory occlusion immediately after static contraction of the left triceps surae muscles, 3) static contraction of the freely perfused left triceps surae muscles, and 4) injection of lactic acid (24 mM; 0.5–1.0 ml) into the left popliteal artery or left femoral artery. Static contraction is a combined mechanical and metabolic stimulus, whereas the circulatory occlusion immediately after static contraction is purely a metabolic stimulus. Contraction and postcontraction circulatory occlusion were each applied for 60 s. The triceps surae muscles were contracted statically by electrically stimulating the left tibial nerve (40 Hz, 25 μs, 2 times motor unit threshold) or L6, L7, and S1 ventral roots (40 Hz, 100 μs, 2 times motor unit threshold). Baseline tension was set at 0.5–0.7 kg. Arterial blood pressure, heart rate, and RSNA were recorded for 60 s before, during, and after static contractions. Injections of lactic acid were accomplished by gently inserting a 30-gauge needle into the popliteal artery or injecting into the contralateral femoral artery catheter. Lactic acid was injected over ∼10 s into the vasculature of the triceps surae muscles. Before injecting lactic acid into the popliteal or femoral artery, we paralyzed the cat by injecting intravenously rocuronium bromide (0.5–0.7 mg/kg). The maneuvers were repeated 20–30 min after the injection of amiloride (0.5 μg/ kg) into the left popliteal artery or left femoral artery. Immediately before injecting amiloride, we tightened the snare placed around the left common iliac artery and vein or abdominal aorta and left common iliac vein. Amiloride was then injected into the left popliteal or contralateral femoral artery, and the snares were maintained for 10 min, after which they were released and the hindlimb was freely perfused. At this point, the left triceps surae muscles were contracted, followed by an injection of lactic acid. Amiloride exhibits peak attenuation of the exercise pressor response by 30 min and is no longer effective 60 min after injection at this dose (0.5 μg/ kg) (10).
A-317567 compound protocol.
We ran the protocol described above for the amiloride protocol for static contraction while the circulation was occluded and postcontraction circulatory occlusion before and after injection of A-317567 (10 mM; 0.5 ml; A-317567 was prepared by Dr. J. Rainer, University of Utah). However, based on our prior study (12), the blockade of ASICs by A-317567 loses its efficacy starting 20 min after its injection. Time constraints prevented us from contracting a freely perfused muscle or injecting lactic acid. Immediately before injecting A-317567, we tightened the snare placed around the abdominal aorta and left common iliac vein. A-317567 was injected into the contralateral femoral artery, and the snares were maintained for 3 min, after which they were released and the hindlimb was freely perfused. Static contraction while the circulation was occluded was repeated at 10 min after the injection of A-317567 (10 mM; 0.5 ml) into the left femoral artery.
Control protocol.
We ran the exact protocol described above for the A-317567 compound protocol, but instead of injecting A-317567, we injected saline (0.9% sodium chloride; 0.5 ml). We used saline, the vehicle used for dissolving amiloride and A-317567, to ensure that the pressor, cardioaccelerator, and RSNA responses to ischemic contraction were repeatable in our preparation and that the attenuation we documented in the two previous protocols was due to the injection of amiloride or A-317567.
Data Analysis
Tibial nerve and ventral root stimulation produced similar results; thus the data were pooled. Mean arterial blood pressure, heart rate, and RSNA values were expressed as means ± SE. Baseline mean arterial blood pressure and heart rate were measured immediately before a maneuver, and peak mean arterial blood pressure and heart rate were measured during injection of lactic acid as well as during the 60 s of static contraction or postcontraction circulatory occlusion. RSNA was rectified and integrated (Spike 2), and 60-s values were used to compare the differences between baseline and the response to each maneuver. The integrated voltage found after hexamethonium injection (see results) was subtracted from the integrated RSNA values. The tension-time index was calculated by integrating the area between the tension trace and the baseline level (Spike 2) (in kg·s) (29). Statistical comparisons were performed with either a one- or two-way repeated-measures ANOVA. If significant main effects were found with an ANOVA, post hoc tests were performed with the Tukey test between individual means. The criterion for statistical significance was P < 0.05.
RESULTS
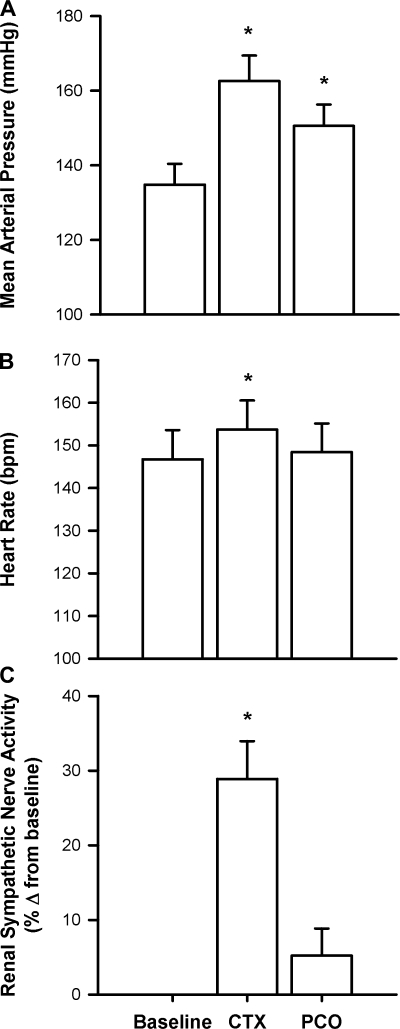
We pooled the control circulatory occluded contraction data from the 30 cats to determine the average mean arterial pressure, heart rate, and renal sympathetic nerve responses to the circulatory occluded contraction and the postcontraction circulatory occlusion. We found that, on average, mean arterial pressure was increased during circulatory occluded contraction and remained above baseline in the postcontraction circulatory occlusion (P < 0.05; Fig. 1). Heart rate and RSNA were increased during circulatory occluded contraction (P < 0.05) but were no longer elevated above baseline during postcontraction circulatory occlusion (both, P > 0.28; Fig. 1). To isolate metaboreceptors from mechanoreceptors, we examined variables that had significant increases over baseline values during the postcontraction circulatory occlusion. Consequently, we focused on analyzing the pressor response to postcontraction circulatory occlusion before and after amiloride, A-317567, or saline injections to test our hypothesis.
Fig. 1.
The peak pressor (n = 30 cats; A) and cardioaccelerator (n = 30 cats; B), and renal sympathetic nerve (n = 28 cats; C) responses to both circulatory occluded contraction (CTX) and postcontraction circulatory occlusion (PCO). Bars represent means ± SE. *P < 0.05, significant difference between baseline and peak.
Amiloride
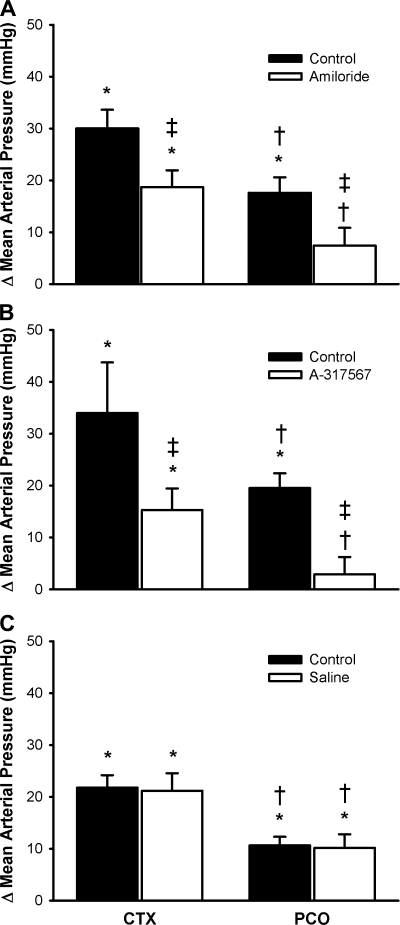
After a latent period of 30 min, amiloride attenuated the pressor response to a static contraction with the circulation occluded (n = 12; P < 0.05; Fig. 2). Before amiloride, the peak pressor response during postcontraction circulatory occlusion was lower than the peak pressor occurring during static contraction with the circulation occluded, but it was still elevated above baseline (P < 0.05). After amiloride, the mean arterial pressure was not increased in response to postcontraction circulatory occlusion (P = 0.18). The tension-time index before amiloride injection for the static contraction with the circulation occluded was not different from that at 30 min after injection (P = 0.40; and Table 1).
Fig. 2.
Effects of amiloride (n = 12 cats; A), A-317567 (n = 7 cats; B), and saline (n = 11 cats; C) on the pressor responses to contraction while the circulation was occluded (CTX) and to postcontraction circulation occlusion (PCO). Bars represent means ± SE. *P < 0.05, significant difference between baseline and peak; †P < 0.05, significant difference between CTX and PCO; ‡P < 0.05, significant difference between control and amiloride.
Table 1.
TTIs and peak tension for static contraction while the circulation was occluded before and after amiloride, A-317567, and saline injections
| n | Before Drug TTI, kg·s | Before Drug Peak Tension, kg | After Drug TTI, kg·s | After Drug Peak Tension, kg | |
|---|---|---|---|---|---|
| Amiloride | 12 | 110±13 | 3.48±0.32 | 102±10 | 3.64±0.30 |
| A-317567 | 7 | 139±30 | 4.62±0.31 | 124±30 | 4.36±0.34 |
| Saline | 11 | 119±10 | 4.14±0.27 | 111±10 | 4.47±0.30 |
Values are means ± SE; n, number of cats. TTI, tension-time index.
A-317567
After a latent period of 10 min, A-317567 reduced the pressor response to contraction with the circulation occluded and the pressor response to postcontraction circulatory occlusion (n = 7; P < 0.05; Fig. 2). Before A-317567, the peak pressor response during the postcontraction circulatory occlusion was lower than the peak pressor occurring during static contraction with the circulation occluded, but it was still elevated above baseline (P < 0.05). After A-317567, the mean arterial pressure was not increased in response to postcontraction circulatory occlusion (P = 0.70). The tension-time index before A-317567 injection for the static contraction with the circulation occluded was not different from that at 10 min after injection (P = 0.50; and Table 1).
Saline
After a latent period of 10 min, saline had no effect on the pressor response to contraction with the circulation occluded or the pressor response to postcontraction circulatory occlusion (n = 11; P > 0.79; Fig. 2). The mean arterial pressure was reduced from the contraction with the circulation occluded to the postcontraction circulatory occlusion (P < 0.05) but remained above baseline (P < 0.05). The tension-time index before saline injection for the static contraction with the circulation occluded was not different from that at 10 min after injection (P = 0.50; and Table 1).
Control Experiments
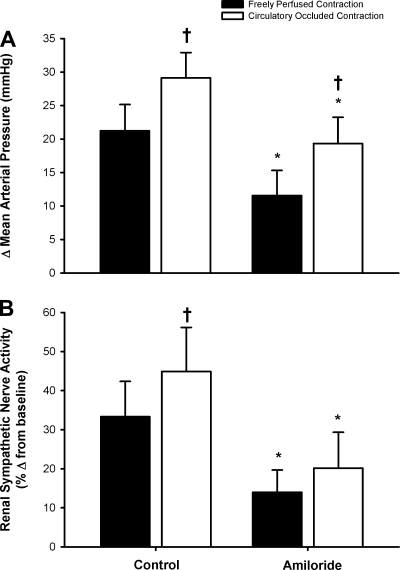
In nine cats, we compared the pressor and renal sympathetic nerve responses to a freely perfused contraction with those to a circulatory occluded contraction. We found that the pressor and renal sympathetic nerve responses to contraction were greater when the circulation was occluded than those to contraction when the muscles were freely perfused (both, P < 0.05; Fig. 3). We also found that in each of the 9 cats tested, amiloride attenuated the pressor response to the freely perfused contraction (before, 25 ± 5; and after, 15 ± 5 mmHg; P < 0.05). The tension-time index before amiloride injection for the freely perfused static contraction was not different from that at 30 min after injection (before, 114 ± 13 vs. after, 111 ± 10 kg·s; P = 0.65).
Fig. 3.
Effects of amiloride on pressor (A) and renal sympathetic nerve (B) responses to static contraction while the muscles were freely perfused and while the circulation was occluded both before and after amiloride. Bars represent means ± SE. *P < 0.05, significant difference between control and amiloride; †P < 0.05, significant difference between freely perfused and circulatory occluded contraction. Data are means ± SE; n = 9 cats.
In 10 cats in which we contracted the triceps surae muscles, we compared the pressor and renal sympathetic nerve responses to lactic acid, injected into the popliteal artery, before amiloride with those to lactic acid, ∼30 min after amiloride was injected into the popliteal artery. We found that in each of the 10 cats tested, amiloride attenuated the pressor (before, 36 ± 4; and after, 19 ± 3 mmHg) and renal sympathetic nerve (before, 317 ± 94; and after, 83 ± 22% change from baseline) responses (both P < 0.05).
In addition, an intravenous injection of hexamethonium bromide (20 mg/kg) abolished the renal sympathetic discharge in each of the 30 cats tested.
DISCUSSION
The purpose of our experiments was to determine whether ASICs on thin fiber muscle afferents played a role in evoking the metabolic component of the exercise pressor reflex. We blocked ASICs on these afferents by injecting two structurally different ASIC antagonists, amiloride and A-317567, into the arterial circulation of the muscles being contracted. The efficacy of the blockade with amiloride was confirmed by showing that the pressor and renal sympathetic nerve responses to popliteal arterial injection of lactic acid were attenuated by about half. In addition, we ensured that the attenuation of the pressor response to contraction was due to the blockade of ASICs and not to a deterioration of our preparation by finding that the pressor response was matched before and after saline injection. We found that the pressor response to postcontraction circulatory occlusion, when only metaboreceptors were stimulated, was attenuated with both ASIC antagonists. This finding suggests that ASICs play a role in evoking the metabolic component of the exercise pressor reflex.
RSNA increased in response to static contraction while the circulation was occluded. During postcontraction circulatory occlusion, however, the activity returned to baseline levels, even though mean arterial pressure remained elevated. In a small subset of cats (8 of 30), renal nerve activity was elevated (i.e., >10% increase above baseline) in the postcontraction period. The lack of an overall renal sympathetic nerve response to postcontraction circulatory occlusion could mean that, in the majority of cats, the renal sympathetic outflow is more under the control of mechanoreceptors than metaboreceptors. In support of this notion, Momen and colleagues (27) determined that mechanoreceptors played a primary role in the activation of renal vasoconstriction during static exercise in humans. Alternatively, the lack of a renal sympathetic nerve response to postcontraction circulatory occlusion could be due to baroreflex control of RSNA. Thus the baroreflex could be shutting off RSNA when muscle contraction stops, even though metaboreceptors in skeletal muscle are still being stimulated, albeit at a lower level than that during contraction. Thus other vascular beds that are under more modest baroreflex control will have increased sympathetic activation, thus contributing to the pressor response noted during postcontraction circulatory occlusion.
The present study provides further support that ASICs, but not ENaCs, contribute to the exercise pressor reflex. The findings from the freely perfused protocol (i.e., the pressor and RSNA responses were attenuated with amiloride) are consistent with our previous study investigating the role of ASICs on the stimulation of mechanoreceptors during exercise (24). Unfortunately, a limitation in that study was that we did not know whether the attenuation of the pressor and renal sympathetic nerve responses were from ASICs and/or ENaCs. We would like to suggest that amiloride blocked ASICs in that protocol because A-317567 also attenuated the pressor and renal sympathetic nerve responses to ischemic contraction. We are fairly confident in this suggestion since amiloride and A-317567 are structurally different and A-317567 is a specific antagonist for ASICs (6).
ASICs activate metaboreceptors but not mechanoreceptors during static contraction (24); we cannot say whether ASICs activate group IV and/or group III afferents. The majority of group III afferents are mechanoreceptors, whereas the majority, if not all group IV afferents, are metaboreceptors (11, 15). However, close arterial injections of lactic acid, in concentrations consistent with those generated by muscle contraction, stimulated both group III and IV afferents (8, 31, 34, 38). In addition, the reduction of lactic acid production with dichloroacetate reduced the discharge rate of group III afferents (34). Electrophysiological investigations will determine whether ASICs activate group IV and/or group III afferents during exercise.
A strong role for lactic acid working through ASICs has also been found in the sensation of pain, especially that caused by ischemia. Of the four ASIC subtypes, ASIC3 seems to be the most likely channel responding to muscle contraction. Specifically, ASIC3 was found to be expressed almost exclusively in dorsal root ganglion neurons (20, 40, 41) and in very high levels on neurons sensitive to metabolites (2, 26, 37). In addition, ASIC3 knockout mice did not respond to noxious stimuli, whereas their wild-type counterparts did (30). Moreover, ASIC3 channels open when the pH drops from 7.4 to 7.0 (37) and can sustain current at a pH of 7.0 (42); this pH is consistent with that found during ischemic exercise (4, 33).
Lactic acid is not the only metabolite that has been suggested to evoke the metabolic component of the exercise pressor reflex. Individual blockade of ATP receptors or prostaglandin production can each reduce the exercise pressor reflex by half (9, 19, 36). One wonders how all of these substances can be responsible for the reflex when adding the individual magnitudes of the reduction in the reflex far exceeds 100%. An answer to this question comes from two lines of evidence suggesting that the combination of metabolites is the key to activation of group III and IV afferents during muscular exercise. The first line of evidence offered by McCleskey and colleagues found that lactate and ATP greatly potentiated the effect of an acidic pH (∼7.0–6.8) on the activation of ASIC3. When ASIC3 was activated by a pH of 7.0, the current was 80% greater in the presence of physiological levels of lactate than when lactate was not present (13), an effect that was attributed to the chelation of calcium by lactate (14). In addition, the levels of ATP that are consistent with those released during ischemic contraction also increase current through ASIC3. Lactate has a relatively quick effect on ASICs, whereas ATP needs 15–60 s after its application for a peak effect on ASICs; moreover, current through the channel remains high for minutes even after ATP has been removed (14, 28). The second line of evidence found that combinations of protons, lactate, and ATP were needed to activate cultured dorsal root ganglion cells (21). When the cells were exposed to just one of the metabolites, only a small stimulatory effect on cells was measured, whereas when the cells were exposed to a combination of the three metabolites, the effect far exceeded the summation of each one individually (21). These investigations provide evidence that not just one metabolite or receptor can be the sole contributor to the reflex but that combinations of metabolites act synergistically on two or more receptors for the full expression of the reflex.
In conclusion, we found that the pressor response to postcontraction circulatory occlusion, when only metaboreceptors were stimulated, was attenuated with both ASIC antagonists. This finding suggests that ASICs play a role in evoking the metabolic component of the exercise pressor reflex. Nevertheless, as discussed, more than one receptor is likely responsible of the full expression of the exercise pressor reflex.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-051503.
Acknowledgments
We thank Jon Rainer (University of Utah) for supplying A-317567 and Jennifer Probst for technical assistance.
REFERENCES
- 1.Alam M, Smirk FH. Observation in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ Res 84: 921–928, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornett JA, Herr MD, Gray KS, Smith MB, Yang QX, Sinoway LI. Ischemic exercise and the muscle metaboreflex. J Appl Physiol 89: 1432–1436, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117: 88–96, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Ettinger S, Gray K, Whisler S, Sinoway L. Dichloroacetate reduces sympathetic nerve responses to static exercise. Am J Physiol Heart Circ Physiol 261: H1653–H1658, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Graham R, Jammes Y, Delpierre S, Grimaud C, Roussos Ch. The effects of ischemia, lactic acid and hypertonic sodium chloride on phrenic afferent discharge during spontaneous diaphragmatic contraction. Neurosci Lett 67: 257–262, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol 94: 1437–1445, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol 581: 1271–2323, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol 587: 873–882, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1720–H1725, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, chapt. 10, p. 381–447.
- 16.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Kindig AE, Hayes SG, Kaufman MP. Blockade of purinergic 2 receptors attenuates the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 293: H2995–H3000, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Krishtal OA, Marchenko SM, Pidoplichko VI. Receptor for ATP in the membrane of mammalian sensory neurones. Neurosci Lett 35: 41–45, 1983. [DOI] [PubMed] [Google Scholar]
- 21.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X and TRPV1. J Neurophysiol 100: 1184–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean DA, LaNoue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitial metabolite responses in the cat. J Appl Physiol 85: 1583–1592, 1998. [DOI] [PubMed] [Google Scholar]
- 23.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCord JL, Hayes SG, Kaufman MP. Acid sensing ion and epithelial sodium channels do not contribute to the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 295: H1017–H1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: the role of the muscle mechanoreflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res 38: 1561–1569, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Gonzalez JF Factors determining the blood pressure responses to isometric exercise. Circ Res 48: I-76–I-86, 1981. [PubMed] [Google Scholar]
- 30.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Rotto DM, Stebbins CL, Kaufman MP. Reflex cardiovascular and ventilatory responses to increasing H+ activity in cat hindlimb muscle. J Appl Physiol 67: 256–263, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol 66: 429–436, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol 69: 1053–1059, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Sinoway LI, Wroblewski KJ, Prophet SA, Ettinger SM, Gray KS, Whisler SK, Miller G, Moore RL. Glycogen depletion-induced lactate reductions attenuate reflex responses in exercising humans. Am J Physiol Heart Circ Physiol 263: H1499–H1505, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res 59: 645–654, 1988. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA 98: 711–716, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thimm F, Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscles. Pflügers Arch 410: 143–152, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res 65: 468–476, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Waldmann R, Champigny G, Lingueglia E, De W Jr, Heurteaux C, Lazdunski M. H+-gated cation channels. Ann NY Acad Sci 868: 67–76, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99: 501–509, 2006. [DOI] [PubMed] [Google Scholar]