Abstract
Pathways that regulate mitochondrial biogenesis are potential therapeutic targets for the amelioration of endothelial dysfunction and vascular disease. Resveratrol was shown to impact mitochondrial function in skeletal muscle and the liver, but its role in mitochondrial biogenesis in endothelial cells remains poorly defined. The present study determined whether resveratrol induces mitochondrial biogenesis in cultured human coronary arterial endothelial cells (CAECs). In CAECs resveratrol increased mitochondrial mass and mitochondrial DNA content, upregulated protein expression of electron transport chain constituents, and induced mitochondrial biogenesis factors (proliferator-activated receptor-coactivator-1α, nuclear respiratory factor-1, mitochondrial transcription factor A). Sirtuin 1 (SIRT1) was induced, and endothelial nitric oxide (NO) synthase (eNOS) was upregulated in a SIRT1-dependent manner. Knockdown of SIRT1 (small interfering RNA) or inhibition of NO synthesis prevented resveratrol-induced mitochondrial biogenesis. In aortas of type 2 diabetic (db/db) mice impaired mitochondrial biogenesis was normalized by chronic resveratrol treatment, showing the in vivo relevance of our findings. Resveratrol increases mitochondrial content in endothelial cells via activating SIRT1. We propose that SIRT1, via a pathway that involves the upregulation of eNOS, induces mitochondrial biogenesis. Resveratrol induced mitochondrial biogenesis in the aortas of type 2 diabetic mice, suggesting the potential for new treatment approaches targeting endothelial mitochondria in metabolic diseases.
Keywords: vasoprotection, histone deacetylase, endothelial dysfunction, diabetes, obesity
endothelial mitochondria have a crucial role in vascular pathophysiology (1, 12, 25, 34). Mitochondria are highly dynamic organelles, and their biogenesis is likely to be involved in the regulation of endothelial cell metabolism, redox regulation, and signal transduction. Impairment of mitochondrial biogenesis is frequently observed in diabetes and the metabolic syndrome (28) and is thus likely to contribute to cellular energetic imbalance, oxidative stress, and endothelial dysfunction in these pathological conditions (16). Previous studies show that dysregulation of mitochondrial biogenesis represents an early manifestation of endothelial dysfunction, shifting cell metabolism toward metabolic hypoxia in animals with impaired nitric oxide (NO) bioavailability (1). Since increased mitochondrial production of reactive oxygen species (ROS) due to impaired mitochondrial biogenesis also appears to be a key event in the development of aging-induced vascular pathologies (6, 41, 42), identification of mechanisms that promote mitochondrial biogenesis in the endothelial cells may contribute to the development of improved pharmacological approaches to promote vascular health in both patients with diabetes (20) and the elderly.
In mice treatment with resveratrol, (3,5,4'-trihydroxystilbene), a diet-derived polyphenol, improved mitochondrial function and biogenesis in the skeletal muscle (21) and the liver (3). Studies in diabetic mice demonstrated that resveratrol treatment improves endothelial function and attenuates vascular inflammation in diabetes mellitus (32, 35, 37, 38, 46) and extends longevity (3, 32). Similar protective effects of resveratrol treatment were observed in aged mice (32, 42). As noted above, both diabetes and aging are characterized by impaired mitochondrial biogenesis, yet the effects of resveratrol on mitochondria in the endothelial cells remain incompletely understood.
The present study was conducted to determine whether resveratrol induces mitochondrial biogenesis in endothelial cells. The effects of resveratrol treatment on mitochondrial mass and the induction of factors regulating mitochondrial biogenesis were assessed in primary human coronary arterial endothelial cells (HCAECs). We focused on the mechanistic role of sirtuin 1 (SIRT1) activation and endothelial NO synthase (eNOS) induction in the effects of resveratrol. The relevance of the effects of resveratrol in vivo was tested on vascular mitochondrial content in type 2 diabetic (db/db) mice.
METHODS
Cell cultures and SIRT1 knockdown.
Primary HCAECs (purchased from Cell Applications) in culture were treated with resveratrol (5-[(1E)-2-(4-hydroxyphenyl)ethenyl]-1,3-benzenediol; 1–10 μmol/l; for 24–48 h) as described (8, 39). Resveratrol (purity ≥98%) was purchased from Cayman Chemical Company (Ann Arbor, MI). To disrupt SIRT1 signaling, downregulation of SIRT1 was achieved by RNA interference using proprietary small interfering RNA (siRNA) sequences (Superarray) and the Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as our laboratory has previously reported (5). Specific gene silencing was verified with quantitative (Q)RT-PCR and Western blotting (at the mRNA and protein level, respectively) as described (7). The SIRT1 siRNA used in our studies did not affect the expression of the reference genes β-actin, HPRT, and YWHAZ. Cell density at transfection was 30%. Experiments were performed on day 2 after the transfection, when gene silencing was optimal. Specific gene silencing was verified with Western blotting (5, 7). Nω-nitro-l-arginine methyl ester (l-NAME; 3 × 10−4 M) was used to inhibit NO synthesis.
SIRT1 activity assay.
Nuclear SIRT1 activity was measured in cells treated with resveratrol. In brief, cells were suspended in lysis buffer containing 10 mM Tris·HCl (pH 7.5), 10 mM NaCl, 15 mM MgCl2, 250 mM sucrose, 0.5% Nonidet P-40, and 0.1 mM EGTA and vortexed for 10 s followed by incubation for 15 min on ice. The cells were centrifuged through 4 ml of sucrose cushion [30% sucrose, 10 mM Tris·HCl (pH 7.5), 10 mM NaCl, and 3 mM MgCl2] at 1,300 g for 10 min at 4°C. The nuclear pellet was washed once with cold 10 mM Tris·HCl (pH 7.5) and 10 mM NaCl. The isolated nuclei were suspended in 50 μl of extraction buffer containing 50 mM HEPES KOH (pH 7.5), 420 mM NaCl, 0.5 mM EDTA Na2, 0.1 mM EGTA, and 10% glycerol; sonicated for 30 s; and incubated on ice for 30 min, followed by centrifugation (15,000 rpm for 10 min). The nuclear extract was collected, and the protein concentration was determined by the Bradford method. SIRT1 was immunoprecipitated from the samples using a rabbit polyclonal antibody directed against the COOH terminus of SIRT1 (Abcam no. ab28170). SIRT1 activity in the samples was measured using the Cyclex SIRT1 Deacetylase Fluorimetric Assay Kit according to the manufacturer's protocols (CycLex, Nagano, Japan). In brief, this assay is based on the principle that upon NAD-dependent deacetlyation of the specific substrate by SIRT1 (in the presence of trichostatin A, a potent inhibitor of SIRT1-independent histone deactelylases), the fluorosubstrate peptide is cleaved by a lysyl endopeptidase, separating the quencher from the fluorophore. Specific activity of SIRT1 was assessed by measuring time-dependent changes in fluorescence intensity, normalized to protein concentration. Standard assay controls included the use of a fluorodeacetylated peptide (to control for lysyl endopeptidase activity), no enzyme control, no NAD+ control, and no inhibitor control. We also assessed resveratrol-induced increases in the specific activity of recombinant SIRT1 in the presence and absence of the specific SIRT1 inhibitor sirtinol (10−4 mol/l).
Measurement of mitochondrial mass in coronary arterial endothelial cells using Mitotracker red staining.
Coronary arterial endothelial cells (CAECs) were treated with resveratrol (10 μmol/l for 48 h) in the absence or presence of SIRT1 knockdown (siRNA). Mitochondrial mass in CAECs was determined by selectively loading the mitochondria with the red fluorescent dye Mitotracker red (Invitrogen, Carlsbad, CA). The fluorescent dyes calcein (green) and Hoechst 33258 (blue) were used to stain the cytoplasm and nuclei, respectively. Optical sections of CAECs were captured at ×60 magnification, and the ratio of mitochondrial area densities to cytoplasmic volume was calculated using the Zeiss Axiovision imaging software. Only cells with intact cytoplasmic calcein staining were included in the analysis. In separate experiments the mitochondria were loaded with Mitotracker red and fluorescence signal intensities were assessed by flow cytometry. The impact of pretreatment with l-NAME (3 × 10−4 mol/l) or SIRT1 siRNA on the effects of resveratrol treatment was assessed.
Measurement of mitochondrial DNA content in CAECs.
Total DNA was isolated from CAECs (DirectPCR; Viagen Biotech). Mitochondrial DNA (mtDNA) copy number was determined by QRT-PCR as described (1), using cytochrome oxidase III and β-actin as markers for the copy numbers of mtDNA and genomic DNA, respectively. The impact of pretreatment with l-NAME (3 × 10−4 mol/l) or SIRT1 siRNA on the effects of resveratrol treatment was assessed.
Measurement of protein expression of electron transport chain constituents.
To elucidate the effect of resveratrol (10 μmol/l) on protein expression of electron transport chain constituents, Western blotting was performed as described (41). Primary antibodies directed against complex I, complex II, complex III, complex V (Molecular Probes/Invitrogen), and cytochrome oxidase (COX-IV, no. 4844; Cell Signaling) were used. We also assessed levels of porin, the most abundant protein of the mitochondrial outer membrane. Due to its abundance, porin is often used as a marker for cellular mitochondrial mass (17). Anti-β-actin (no. 6276; Abcam) was used for normalization purposes.
Measurement of mRNA expression of mitochondrial biogenesis factors by QRT-PCR.
We have used a QRT-PCR technique to determine the effect of resveratrol (10 μmol/l, for 24 h) on mRNA expression of mitochondrial biogenesis factors nuclear respiratory factor-1 (Nrf-1), mitochondrial transcription factor A (Tfam), and peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1 (PGC-1α) in CAECs using the Strategen MX3000 as reported (5, 9–11). Total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (10, 11). Efficiency of the PCR reaction was determined using dilution series of a standard sample. Quantification was performed using the efficiency-adjusted ΔΔCT method. The housekeeping gene HPRT was used for internal normalization. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel. The impact of SIRT1 siRNA treatment on the effects of resveratrol treatment was also determined.
In separate experiments aortic segments of eNOS−/− and wild-type control mice (Jackson Laboratories, Bar Harbor, ME) (22) were maintained in organoid culture under sterile conditions in F12 medium (Gibco) containing antibiotics (100 UI/l penicillin, 100 mg/l streptomycin, and 10 μg/l fungizone) and supplemented with 5% FCS (Boehringer-Mannheim) as previously described (39) in the presence or absence of resveratrol (10−5 mol/l, for 24 h). After the culture period expression of mitochondrial biogenesis factors was assessed by QRT-PCR.
Measurement of eNOS induction by resveratrol.
CAECs were treated with increasing concentrations of resveratrol (for 24 h), and eNOS expression was assessed at the mRNA and protein level with QRT-PCR and Western blotting, respectively. The impact of SIRT1 knockdown on the effects of resveratrol treatment was also determined.
Analysis of mitochondrial biogenesis in aortas of db/db mice.
Animal-use protocols were approved by the Institutional Animal Care and Use Committees of the New York Medical College (Valhalla, NY) and the University of Missouri (Columbia, MO). Heterozygote control m Leprdb mice (control) and homozygote type 2 diabetic Leprdb mice (db/db) were purchased from Jackson Laboratories. At the age of 10 wk, control and db/db mice were treated with resveratrol (20 mg·kg−1·day−1; Cayman Chemical) orally for 4 wk as described (32). This dose was shown to be effective to extend the lifespan of type 2 diabetic mice and is vasoprotective without any toxic side effect (32). After the treatment period the animals were euthanized and the aortas were isolated and snap-frozen in liquid nitrogen. To estimate mitochondrial mass in the aortas, mtDNA copy number was determined by QRT-PCR. We also assessed vascular expression of mitochondrial biogenesis factors (QRT-PCR). It is an advantage of the db/db model that although resveratrol improves hyperglycemic status and insulin sensitivity in high fat-induced obese rodents and type 1 diabetic animal models (32), it does not affect significantly fasting blood glucose levels and plasma insulin levels in db/db mice (47). Thus the direct endothelial effects of resveratrol can be studied in this model of diabetes independent of the secondary vascular alterations due to metabolic improvement.
Data analysis.
Data were normalized to the respective control mean values and are expressed as means ± SE. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Resveratrol induces mitochondrial biogenesis in endothelial cells.
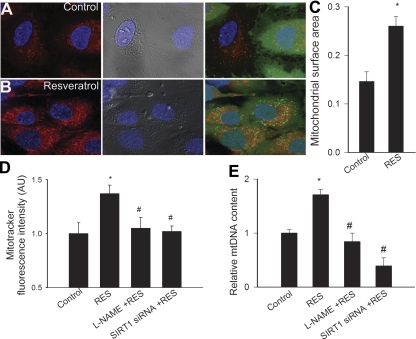
Mitotracker staining showed that mitochondria are localized in the perinuclear region in CAECs (Fig. 1, A and B). The ratio of mitochondrial area densities to cytoplasmic volume in Mitotracker-labeled endothelial cells was significantly increased by resveratrol treatment (Fig. 1, B and C). Analysis of Mitotracker fluorescence intensities in CAECs confirmed that resveratrol treatment increased mitochondrial mass significantly in treated compared with untreated animals (Fig. 1D). Knockdown of SIRT1 (siRNA; Fig. 3C) and pretreatment with l-NAME prevented resveratrol-induced increase in mitochondrial mass (Fig. 1D).
Fig. 1.
When compared with untreated controls (A), resveratrol (Res; B; 10 μM for 48 h) significantly increased the number of mitochondria in cultured coronary arterial endothelial cells (CAECs). Relative mitochondrial mass was estimated by using Mitotracker red staining. The cell body was visualized by differential interference contrast and by green calcein fluorescence (blue: nuclei). Only cells with intact calcium staining were analyzed. Summary data for mitochondrial surface area are shown in C. D: Mitotracker Fluorescent intensities in CAECs were measured to assess mitochondrial biogenesis. Knockdown of sirtuin 1 (SIRT1) [small interfering RNA (siRNA)] or Nω-nitro-l-arginine methyl ester (l-NAME) prevented resveratrol-induced mitochondrial biogenesis. E: resveratrol increased mitochondrial DNA (mtDNA) content in CAECs, which was prevented by l-NAME or SIRT1 siRNA. Data are means ± SE. *P < 0.05; #P < 0.05 vs. resveratrol only. au, Arbitrary units.
Resveratrol induces SIRT1 and eNOS in endothelial cells.
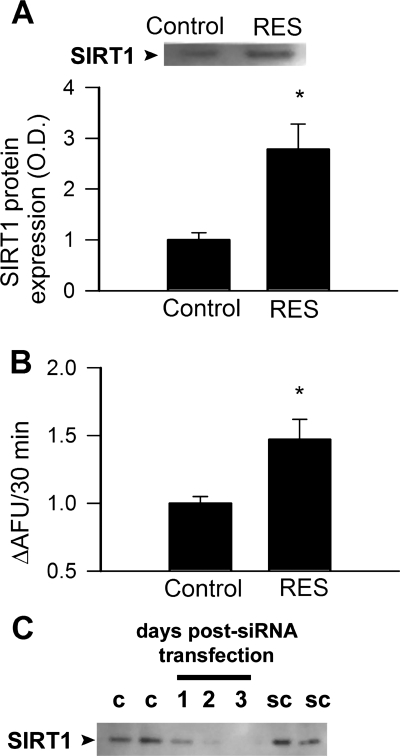
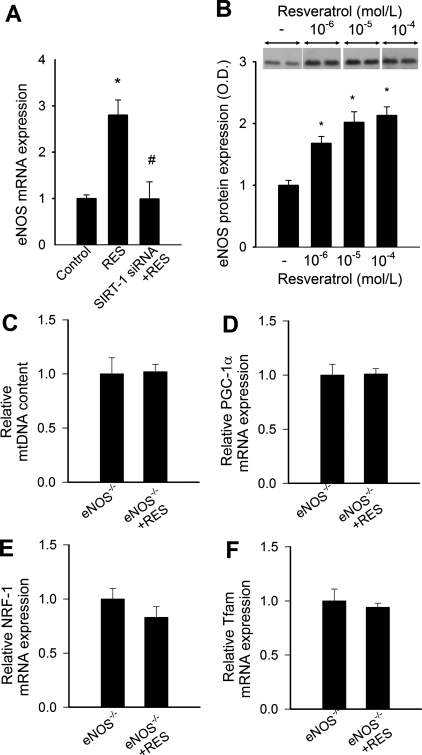
Resveratrol upregulated protein expression of SIRT1 and increased SIRT1 enzymatic activity in endothelial cells (Fig 2). Resveratrol significantly increased mRNA and protein expression of eNOS, which were prevented by knockdown of SIRT1 (Fig. 3, A and B).
Fig. 2.
Treatment of CAECs with resveratrol (Res; 10 μmol/l) upregulated SIRT1 protein expression (A; Western blotting) and activity (B; data from fluorimetric SIRT1 activity assay). C: time course for siRNA knockdown of SIRT1 in CAECs (Western blotting). OD, optical density; AFU, arbitrary fluorescence units; c, control; sc, scrambled siRNA control. *P < 0.05 vs. control.
Fig. 3.
A: effect of resveratrol on mRNA expression of endothelial nitric oxide synthase (eNOS) in CAECs. Knockdown of SIRT1 (siRNA) prevented resveratrol-induced upregulation of eNOS. *P < 0.05 vs. control; #P < 0.05 vs. resveratrol only. B: representative Western blot showing that in vitro resveratrol treatment elicited a concentration-dependent induction of eNOS protein. *P < 0.05. Bar graphs are densitometric data. C–F: effect of resveratrol on mtDNA copy number (C) and mRNA expression of proliferator-activated receptor-coactivator-1α (PGC-1α; D), nuclear respiratory factor-1 (Nrf1; E), and mitochondrial transcription factor A (Tfam; F) in cultured aortic segments of eNOS−/− mice (n = 5; differences are not significant).
Resveratrol upregulates electron transport chain constituents in endothelial cells.
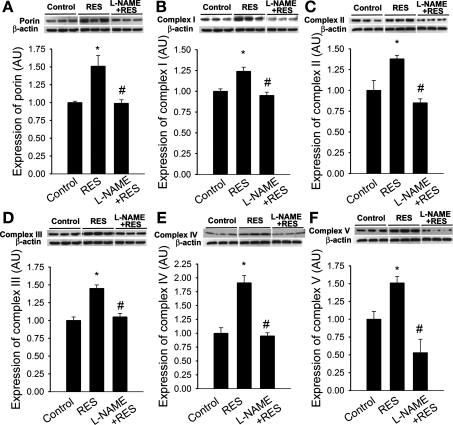
Western blotting revealed that when compared with untreated CAECs, expression of porin was significantly increased in resveratrol-treated cells (Fig. 4A). Levels of porin (which controls the diffusion of small metabolites through the outer membrane) correlate with the cellular mitochondrial volume. Expression of complex I, complex II, complex III, complex IV, and complex V in CAECs (Fig. 4, B–F) was also increased by resveratrol treatment. Pretreatment with l-NAME prevented resveratrol-induced increases in mitochondrial protein expression (Fig. 4).
Fig. 4.
In CAECs resveratrol (Res; 10 μM for 48 h) significantly upregulated protein expression of porin (A) and complexes I (B), II (C), III (D), IV (E), and V (F; Western blotting). l-NAME prevented resveratrol-induced mitochondrial biogenesis. Data are means ± SE. *P < 0.05 vs. untreated; #P < 0.05 vs. resveratrol only.
Resveratrol upregulates mitochondrial biogenesis factors.
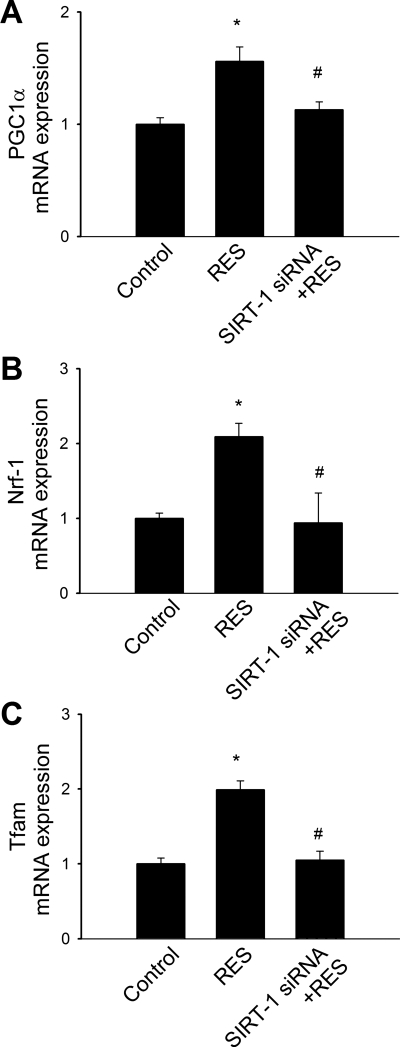
Mitochondrial biogenesis involves the integration of multiple transcriptional pathways controlling both nuclear and mitochondrial gene expression. The PPAR coactivator PGC-1α, Nrf-1, and Tfam are considered key regulators of mitochondrial biogenesis in multiple tissues. QRT-PCR measurements revealed that the expression of the mitochondrial biogenesis factors PGC-1α, Nrf-1, and Tfam in CAECs (Fig. 5, A–C) was significantly increased by resveratrol treatment. Knockdown of SIRT1 prevented resveratrol-induced induction of mitochondrial biogenesis factors in CAECs (Fig. 5). In cultured aortic segments isolated from eNOS−/− mice, resveratrol treatment failed to increase mtDNA content (Fig. 5C) and did not induce mitochondrial biogenesis factors (Fig. 3, D–F).
Fig. 5.
Effect of resveratrol (Res) on mRNA expression of PGC-1α (A), Nrf-1 (B), and Tfam (C) in CAECs. Knockdown of SIRT1 (siRNA) prevented resveratrol-induced upregulation of mitochondrial biogenesis factors. *P < 0.05 vs. control; #P < 0.05 vs. resveratrol only; n = 5 in each group.
Resveratrol treatment induces mitochondrial biogenesis in aortas of type 2 diabetic mice.
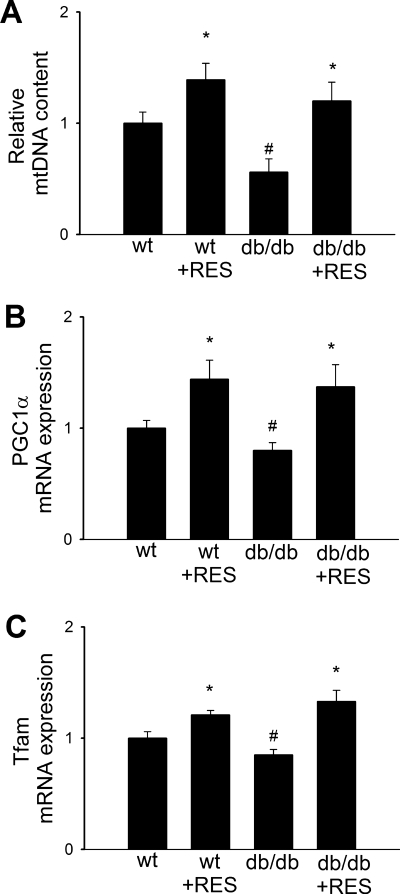
The relevance of our findings to in vivo studies was determined using control and type 2 diabetic db/db mice treated with resveratrol for 4 wk. We found that in aortas of db/db mice relative mtDNA content (Fig. 6A) was significantly decreased and expression of PGC-1α and Nrf-1 was downregulated (Fig. 6, B and C), as compared with vessels of nondiabetic control mice. Resveratrol treatment of db/db mice significantly increased vascular mtDNA content (Fig. 6A) and induced the expression of mitochondrial biogenesis factors (Fig. 6, B and C) thus eliminating the difference between the two groups.
Fig. 6.
In aortas of type 2 diabetic db/db mice, relative mtDNA content (A) was significantly decreased compared with vessels of control mice. In aortas of db/db mice, expression of PGC-1α and Nrf-1 was downregulated (B). Resveratrol (Res) treatment significantly increased vascular mtDNA content (A) and induced the expression of mitochondrial biogenesis factors (B and C) in aortas of db/db mice, eliminating the difference between the 2 groups. Data are means ± SE. *P < 0.05 vs. no resveratrol; #P < 0.05 vs. control mice. wt, Wild-type.
DISCUSSION
Pathways that regulate mitochondrial biogenesis have recently emerged as potential therapeutic targets for the amelioration of endothelial dysfunction and vascular disease observed in metabolic diseases (34). Our studies show that resveratrol induces mitochondrial biogenesis in CAECs. Formation of new mitochondria is associated with activation of SIRT1, upregulation of eNOS, and induction of specific mitochondrial biogenesis factors. We also show that resveratrol treatment induces mitochondrial biogenesis in aortas of type 2 diabetic mice.
Resveratrol is a prototype of a new class of drugs referred to as caloric restriction mimetics (4), which are being developed to reverse organ pathologies associated with aging and metabolic diseases. Resveratrol was shown to exert diverse antiaging effects (3, 18, 19, 32, 44). Previous studies focused on the effects of resveratrol on proinflammatory pathways and antioxidant defense mechanisms in endothelial cells (7, 8, 39) but provided little information on its effects on endothelial mitochondria. Our data support the finding that resveratrol increases mitochondrial content in CAECs (Fig. 1, A–D). Increased mitochondrial biogenesis in resveratrol-treated cells is also indicated by increased cellular mtDNA content (Fig. 1E) and increased protein expression of respiratory chain components (Fig. 4). Multiple mechanisms may explain resveratrol-induced mitochondrial biogenesis and its contribution to vascular health. Impaired mitochondria (e.g., in diabetes and in aging) may diminish ATP production, thereby impairing the synthesis and secretion of endothelium-derived factors that serve as paracrine signals in the vascular wall. Lack of ATP also affects transport functions of the vascular endothelium. Resveratrol-induced mitochondrial biogenesis would correct this impairment. Furthermore, because mitochondrial proliferation reduces the flow of electrons per unit mitochondria, resveratrol-induced mitochondrial biogenesis may reduce mitochondrial ROS production in endothelial cells. Indeed, our recent studies suggest that resveratrol, at physiologically relevant concentrations, attenuates mitochondrial oxidative stress in endothelial cells (7).
To determine whether the increased number of mitochondria in resveratrol-treated endothelial cells is a consequence of the induction of mitochondrial biogenesis factors, we used QRT-PCR to examine the expression of Nrf-1, Tfam, and PGC-1α. Nrf-1 activates the transcription of a number of the nuclear-encoded components of the electron transport chain and also regulates Tfam, which is responsible for the transcription of mtDNA-encoded genes. The regulatory function of Nrf-1 and other mitochondrial biogenesis factors is modulated by PGC-1α. We found that in CAECs, resveratrol induced Nrf-1, Tfam, and PGC-1α (Fig. 5, A–C). It has been established that the altered expression of these factors modulates mitochondrial biogenesis activity (27, 29–31).
The NAD+-dependent protein deacetylase SIRT1 plays a critical role in resveratrol-induced effects in endothelial cells. Accordingly, resveratrol induces SIRT1 in endothelial cells (Fig. 2), thus extending previous observations in other cell types (18, 21). Resveratrol also lowers the Km of SIRT1 for the acetylated substrate and for NAD+ (18). Knockdown of SIRT1 prevented resveratrol-induced mitochondrial biogenesis (Fig. 1, D and E) and the induction of mitochondrial biogenesis factors (Fig. 5). Overexpression of SIRT1 also induces mitochondrial biogenesis in CAECs, mimicking the effects of resveratrol (unpublished observations). These findings are in accord with previous studies, which showed that resveratrol and SIRT1 regulate mitochondrial function and mitochondrial biogenesis in skeletal muscle and the liver (15, 21). SIRT1 likely regulates multiple pathways involved in mitochondrial biogenesis in the endothelial cells, among which NO-dependent pathways appear to play a key role. In addition, SIRT1 may also directly deacetylate PGC-1α, increasing its activity (21, 27).
We demonstrated that inhibition of NO synthesis prevents resveratrol-induced mitochondrial biogenesis in CAECs (Fig. 1, D and E, and Fig. 4), suggesting that NO has an autocrine function in mediating the effects of resveratrol/SIRT1 in endothelial cells. Accordingly, we also found that genetic lack of eNOS prevents resveratrol-induced mitochondrial biogenesis and the upregulation of mitochondrial biogenesis factors in cultured mouse arteries (Fig. 3, C–F). In that regard, it is significant that resveratrol in a SIRT1-dependent manner upregulates eNOS in both cultured arteries and CAECs (Fig 2, A and B), confirming results of previous studies (26, 39, 43). Overexpression of SIRT1 also induces eNOS in cultured rat aortas and CAECs, mimicking the effects of resveratrol (unpublished observations). Although the precise role of eNOS in the SIRT1-induced signaling pathway is unclear, recent evidence supports that NO plays a critical role in initiating and integrating signaling events underlying mitochondrial biogenesis in various tissues. Importantly, inhibition of NO synthesis significantly decreases mitochondrial content in the vasculature (1). Treatment with NO donors also increases mitochondrial mass in various cell types, including brown adipocytes and 3T3-L1, U937, and HeLa cells (29–31). Previous studies also suggest that expression of mitochondrial biogenesis factors may be directly regulated by bioavailability of NO (2, 31).
The association between the pathogenesis of diabetes and its complications and mitochondrial biogenesis has been recently reported (28, 33). Because resveratrol has been reported to exert a possible additional benefit in preventing diabetes complications, we investigated the effect of resveratrol on vascular mitochondrial biogenesis in type 2 diabetes mellitus. In db/db mice, type 2 diabetes diminishes mitochondrial biogenesis in the aorta, as indicated by the decreased mtDNA content (Fig. 6A) and downregulation of mitochondrial biogenesis factors (Fig. 6, B and C). Resveratrol treatment significantly increased vascular mtDNA content (Fig. 6A) and induced the expression of mitochondrial biogenesis factors (Fig. 6, B and C) in both control and db/db mice, eliminating the difference between the two groups. These results extend previous findings that showed chronic resveratrol treatment in type 2 diabetic mice induces mitochondrial biogenesis in the liver and skeletal muscle (3, 21), demonstrating the in vivo relevance of our in vitro findings. Type 2 diabetes mellitus is associated with both macrovascular and microvascular dysfunction, which is characterized by impaired bioavailability of NO in the coronary arteries and other vascular beds (13, 14, 45), which is thought to lead to macrovascular complications of diabetes. We have recently demonstrated that resveratrol induces eNOS and significantly increases NO bioavailability in animal models of type 2 diabetes, including mice fed a high-fat diet (32) and db/db mice (47). Because NO appears to be a key regulator of endothelial mitochondrial content (1), we attribute the resveratrol-induced mitochondrial biogenesis in diabetic mice to the restoration of NO bioavailability. A recent study utilizing a systems biology approach showed that resveratrol can recapitulate many of the molecular events downstream of caloric restriction in vivo (36). In that context it is significant that caloric restriction also induces eNOS (40) and promotes mitochondrial biogenesis (23, 24). In addition, resveratrol may also improve the endocrine regulation of cellular metabolism (3, 21) that contributes to its vasoprotective effects. Several clinical trials are currently underway for various therapeutic indications of resveratrol treatment in humans (4), which confirm that use of resveratrol in humans is safe and does not result in any significant side effects.
In conclusion, resveratrol, at physiologically relevant concentrations (4), increases mitochondrial content in endothelial cells via activating SIRT1. We propose that SIRT1, via a pathway that involves upregulation of eNOS, induces mitochondrial biogenesis factors (including Nrf1, Tfam, and PGC-1α), promoting mitochondrial biogenesis. The finding that resveratrol treatment induces mitochondrial biogenesis in the aortas of type 2 diabetic mice suggests the potential for caloric restriction mimetics to target endothelial mitochondria in metabolic diseases.
GRANTS
This work was supported by grants from the American Diabetes Association (to Z. Ungvari); the American Heart Association (Grant 110350047A to C. Zhang); the American Federation for Aging Research (to A. Csiszar); National Institutes of Health (NIH) Grants HL-077256 and HL-43023 (to Z. Ungvari), CA-111842 (to J. Pinto), and RO1-HL077566 and RO1-HL085119 (to C. Zhang); the Hungarian Science Fund (OTKA 68758 to G. Losonczy); and the Intramural Research Program of NIH (to P. Pacher and R. de Cabo).
REFERENCES
- 1.Addabbo F, Ratliff B, Park HC, Kuo M, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol 174: 34–43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25: 3900–3911, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev 5: 493–506, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 168: 629–638, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses 67: 904–908, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111: 2364–2372, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J 17: 1183–1185, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100: 1128–1141, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115: 245–254, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gao X, Zhang H, Schmidt AM, Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 295: H491–H498, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 99: 924–932, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hanson BJ, Carrozzo R, Piemonte F, Tessa A, Robinson BH, Capaldi RA. Cytochrome c oxidase-deficient patients have distinct subunit assembly profiles. J Biol Chem 276: 16296–16301, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J 26: 3169–3179, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127, 2006. [PubMed] [Google Scholar]
- 21.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol 37: 671–680, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103: 1768–1773, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol 43: 813–819, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res 100: 460–473, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Clementi E, Carruba MO, Moncada S. Defective mitochondrial biogenesis: a hallmark of the high cardiovascular risk in the metabolic syndrome? Circ Res 100: 795–806, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101: 16507–16512, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56: 1751–1760, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF Jr. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation 118: 1347–1357, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 76: 69–75, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Smith JJ, Kenney RD, Gagne DJ, Frushour BP, Ladd W, Galonek HL, Israelian K, Song J, Razvadauskaite G, Lynch AV, Carney DP, Johnson RJ, Lavu S, Iffland A, Elliott PJ, Lambert PD, Elliston KO, Jirousek MR, Milne JC, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol 3: 31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 290: E1339–E1346, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med 43: 720–729, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-α and NF-κB via IKK-β pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol (April 10, 2009). doi: 10.1152/ajpheart.01199.2008. [DOI] [PMC free article] [PubMed]
- 46.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF-α and vascular oxidative stress. Atheroscler, Thromb, Vasc Biol. In press. [DOI] [PMC free article] [PubMed]