Abstract
To determine whether impaired endothelium-dependent dilation (EDD) in older adults is associated with changes in the expression of major vasoconstrictor or vasodilator proteins in the vascular endothelium, endothelial cells (EC) were obtained from the brachial artery and peripheral veins of 56 healthy men, aged 18–78 yr. Brachial artery EC endothelin-1 (ET-1) [0.99 ± 0.10 vs. 0.57 ± 0.10 ET-1/human umbilical vein EC (HUVEC) intensity, P = 0.01] and serine 1177 phosphorylated endothelial nitric oxide synthase (PeNOS) (0.77 ± 0.09 vs. 0.44 ± 0.07 PeNOS/HUVEC intensity, P < 0.05) (quantitative immunofluorescence) were greater, and EDD (peak forearm blood flow to intrabrachial acetylcholine) was lower (10.2 ± 0.9 vs. 14.7 ± 1.7 ml·100 ml−1·min−1, P < 0.05) in older (n = 18, 62 ± 1 yr) vs. young (n = 15, 21 ± 1 yr) healthy men. EDD was inversely related to expression of ET-1 (r = −0.39, P < 0.05). Brachial artery EC eNOS expression did not differ significantly with age, but tended to be greater in the older men (young: 0.23 ± 0.03 vs. older: 0.33 ± 0.07 eNOS/HUVEC intensity, P = 0.08). In the sample with venous EC collections, EDD (brachial artery flow-mediated dilation) was lower (3.50 ± 0.44 vs. 7.68 ± 0.43%, P < 0.001), EC ET-1 and PeNOS were greater (P < 0.05), and EC eNOS was not different in older (n = 23, 62 ± 1 yr) vs. young (n = 27, 22 ± 1 yr) men. EDD was inversely related to venous EC ET-1 (r = −0.37, P < 0.05). ET-1 receptor A inhibition with BQ-123 restored 60% of the age-related impairment in carotid artery dilation to acetylcholine in B6D2F1 mice (5–7 mo, n = 8; 30 mo, n = 11; P < 0.05). ET-1 expression is increased in vascular EC of healthy older men and is related to reduced EDD, whereas ET-1 receptor A signaling tonically suppresses EDD in old mice. Neither eNOS nor PeNOS is reduced with aging. Changes in ET-1 expression and bioactivity, but not eNOS, contribute to vascular endothelial dysfunction with aging.
Keywords: endothelium-dependent dilation, brachial artery flow-mediated dilation
vascular endothelial function is impaired in healthy sedentary middle-aged and older adult humans, as indicated by reduced endothelium-dependent dilation (4, 36). However, our understanding of the molecular events associated with age-related reductions in endothelium-dependent dilation in humans is limited. Changes in the expression of key proteins implicated in endothelium-mediated vasoconstriction and vasodilation could be involved, but no information is available.
Endothelin-1 (ET-1) is the most potent vasoconstrictor protein synthesized in and released by vascular endothelial cells (44). Recent findings indicate that ET-1 signaling is increased in healthy older sedentary adult humans and contributes to tonic vasoconstriction in peripheral arteries (37, 38, 41). In culture, ET-1 synthesis is greater in aortic endothelial cells obtained from older compared with young adult donors with various pathologies (39). However, it is unknown if ET-1 protein expression is increased in the vascular endothelium of healthy older humans and, if so, is related to impaired endothelium-dependent dilation.
Endothelial nitric oxide synthase (eNOS) is the enzyme expressed in endothelial cells that produces nitric oxide (NO), the most important vasodilatory molecule synthesized by the endothelium (20). Reduced production and/or bioavailability of NO is a primary mechanism mediating impaired endothelium-dependent dilation in healthy older humans (35). However, it is unknown if the expression of eNOS and/or its activated serine 1177 phosphorylated isoform (PeNOS) is reduced in endothelial cells from older adults, and if such reductions are related to age-associated decreases in endothelium-dependent dilation. Based on results from a study in rodents (40), it is possible that eNOS activity actually is greater in older adults, possibly as a compensatory attempt to increase NO bioavailability (40).
To gain insight into these issues, we measured ET-1, eNOS, and PeNOS protein expression in endothelial cells obtained from the brachial artery or peripheral veins of groups of healthy young and older men. Endothelium-dependent dilation was assessed by measuring either the increase in forearm blood flow (FBF) to an intra-arterial infusion of acetylcholine (brachial artery studies) or brachial artery flow-mediated dilation (FMD) (peripheral vein studies). Because the results of these investigations in humans indicated a potential role for increased ET-1 expression with aging, we then studied the contribution of ET-1 bioactivity using pharmacological inhibition of ET-1 A (ETA) receptors in young and old B6D2F1 mice. Endothelium-independent dilation, a control for vascular smooth muscle responsiveness to NO, was assessed in all protocols.
METHODS
Experiments in Young and Older Human Subjects
Subjects.
Data were obtained on 56 healthy men: 29 young (aged 18–30 yr) and 27 older (55–78 yr), previously phenotyped for endothelium-dependent dilation. All subjects had resting blood pressure < 140/90 mmHg, body mass index < 30 kg/m2, and were free of cardiovascular diseases (CVD), diabetes, and other clinical disorders, as assessed by medical history, physical examination, and blood chemistries. Subjects >40 yr of age were further screened for CVD using electrocardiogram and blood pressure responses to incremental treadmill exercise performed to volitional exhaustion. Subjects were nonsmokers, not taking medications or dietary supplements (including antioxidants), and not regularly exercising. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder and conform with the principles outlined in the Declaration of Helsinki for use of human subjects and tissue. The nature, benefits, and risks of the study were explained to the volunteers, and their written, informed consent was obtained before participation.
Study procedures.
All measurements were performed at the University of Colorado at Boulder General Clinical Research Center after an overnight fast and a 24-h abstention from alcohol and physical activity.
Subject characteristics.
Body mass, body mass index, resting arterial blood pressure and heart rate, habitual physical activity, fasting blood chemistries, and plasma concentrations of ET-1, oxidized low-density lipoprotein (LDL), and total antioxidant status were measured as previously described (13, 29).
Endothelium-dependent and -independent dilation.
For experiments involving brachial artery catheter placements (young: n = 15, 21 ± 1 yr; older: n = 18, 62 ± 1 yr), endothelium-dependent dilation and endothelium-independent dilation were determined as the peak FBF (measured by venous occlusion plethysmography) responses to an incremental intrabrachial artery infusion of acetylcholine at 1.0, 2.0, 4.0, and 8.0 μg·dl forearm tissue−1·min−1, and sodium nitroprusside at 0.5, 1, and 2.0 μg·dl forearm tissue−1·min−1, respectively, as described previously (9, 12, 30). For experiments involving only peripheral venous catheter placements (young: n = 27, 22 ± 1 yr; older: n = 23, 62 ± 1 yr), ultrasonography was used to assess endothelium-dependent dilation via measurement of brachial artery FMD and endothelium-independent dilation via measurement of brachial artery dilation in response to sublingual nitroglycerin, as previously described by our laboratory (13, 16–18, 21).
Endothelial cell protein expression.
The procedures used for collection of endothelial cells and measurement of protein expression were described originally by Feng et al. (19) and Colombo et al. (7) and more recently by our laboratory (11, 13, 16, 21, 32). Briefly, J-wires were advanced into a brachial artery and/or an antecubital vein ∼4 cm beyond the tip of the catheter and withdrawn, and cells were recovered by washing and centrifugation. Collected cells were fixed with 3.7% formaldehyde and plated on slides. After blocking nonspecific binding sites with 5% donkey serum (Jackson Immunoresearch), cells were incubated with monoclonal antibodies for one of the following: ET-1 (Affinity BioReagents), eNOS (Transduction Laboratories) or serine 1177 PeNOS (Calbiochem). Nitrotyrosine (Abcam), a cellular marker of oxidative stress (3), was assessed, and its relation to ET-1, eNOS, and serine 1177 PeNOS was determined in a subset of subjects. Cells were next incubated with CY3-conjugated secondary antibodies (Research Diagnostics).
Slides were systematically scanned to identify endothelial cells (positive staining of von Willebrand factor), and nuclear integrity was confirmed using 4′,6′-diamidino-2-phenylindole hydrochloride staining. Once endothelial cells with intact nuclei were identified, images were captured and then analyzed using Metamorph Software (Universal Imaging, Downingtown, PA) to quantify the intensity of CY3 staining (i.e., average pixel intensity). Values are reported as ratios of endothelial cell protein expression/human umbilical vein endothelial cell (HUVEC). Reporting ratios minimize the possible confound of differences in intensity of staining among different staining sessions. A single technician analyzed each batch of slides. Technicians were blinded to subject identity during the staining and analysis procedures.
Experiments in Young and Old B6D2F1 Mice
Animals.
Eight young (5–7 mo) and 11 older (30 mo) male B6D2F1 mice were obtained from the National Institute on Aging rodent colony. All mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12-h light-dark cycle and fed standard rodent chow ad libitum. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996) and were approved by the University of Colorado Animal Care and Use Committee.
Endothelium-dependent and -independent dilation.
Measurements were made in isolated vessels studied ex vivo using a modification of methods described previously (14, 15, 24). Mice were euthanized by exsanguination via cardiac puncture while under isoflurane anesthesia. Carotid arteries were excised and placed in myograph chambers (DMT) containing EDTA buffered physiological saline solution, cannulated onto glass micropipettes, and secured with nylon (11–0) suture. Once cannulated, the carotid arteries were warmed to 37°C, pressurized to 50-mmHg intraluminal pressure, and allowed to equilibrate for 1 h. All arteries then were submaximally preconstricted with phenylepherine (2 μM), because the arteries do not exhibit spontaneous tone. Increases in luminal diameter in response to increasing concentrations of the endothelium-dependent dilator acetylcholine (1 × 10−9 to 1 × 10−4 M) and endothelium-independent dilator sodium nitroprusside (1 × 10−10 to 1 × 10−4 M) were determined.
To assess the influence of ET-1 bioactivity, dose responses to acetylcholine were repeated in the presence of the ETA antagonist BQ-123, (10−6 M, 60-min incubation), which inhibits ETA-mediated vasoconstriction (15, 22, 26). Only an ETA receptor antagonist was used because ETA receptors are responsible for most of the vasoconstriction induced by ET-1 within its physiological range (15).
Data Analysis
Experiments in human subjects.
As in our previous studies (13, 16–18, 21), because there were no differences between groups in peak shear rate (young: 458 ± 16 s−1, older 440 ± 20 s−1), normalizing FMD for the hyperemic stimulus in young (n = 11) and older (n = 12) subjects did not alter the results (still P < 0.05). Accordingly, FMD is expressed as millimeters and percent change from baseline diameter, consistent with recently published guidelines (10). Statistical analyses were performed with SPSS (version 16.0). Group differences were determined by T-tests for independent sample comparisons, and FBF responses to incremental doses of acetylcholine and sodium nitroprusside were analyzed by repeated-measures ANOVA. Pearson correlation analysis was used to determine relations of interest. Statistical significance for all analyses was set at P < 0.05.
Experiments in mice.
For animal and vessel characteristics (maximum dilation and IC50), group differences were determined by one-way ANOVA or T-test where appropriate. For all dose responses, group differences were determined by repeated-measures ANOVA. Data are presented as means ± SE. Significance was set at P < 0.05.
RESULTS
Experiments in Young and Older Human Subjects
Subject characteristics.
Characteristics of the young and older subjects are shown in Table 1. Body mass, body mass index, resting blood pressure, fasting plasma glucose, total cholesterol, high-density lipoprotein, and low-density lipoprotein cholesterol, and triglyceride concentrations were higher in the older men (all P < 0.05), but all values were within clinically normal ranges. Habitual physical activity was not different in the groups. Plasma ET-1 and oxidized LDL concentrations were greater in the older men (P < 0.05), whereas total antioxidant status was not different.
Table 1.
Subject characteristics total
| Young | Older | |
|---|---|---|
| n | 29 | 27 |
| Age, yr | 22±1 | 61±1* |
| Mass, kg | 80±1 | 83±1* |
| Body mass index, kg/m2 | 25±1 | 26±1* |
| Physical activity, MET h/wk | 60±8 | 75±10 |
| Systolic blood pressure, mmHg | 117±2 | 124±2* |
| Diastolic blood pressure, mmHg | 63±2 | 78±2* |
| Fasting glucose, mg/100 ml | 87±1 | 97±1* |
| Total cholesterol, mg/100 ml | 158±4 | 199±3* |
| HDL cholesterol, mg/100 ml | 45±1 | 50±2* |
| LDL cholesterol, mg/100 ml | 93±3 | 123±3* |
| Triglycerides, mg/100 ml | 105±6 | 122±16* |
| Endothelin-1, pg/ml | 5.57±0.21 | 6.55±0.33* |
| Oxidized LDL, IU/l | 41±3 | 60±3* |
| Total antioxidant status, mmol/l | 1.30±0.04 | 1.29±0.04 |
Values are means ± SE; n, no. of subjects. MET, metabolic equivalent; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 vs. young.
Endothelium-dependent and -independent dilation.
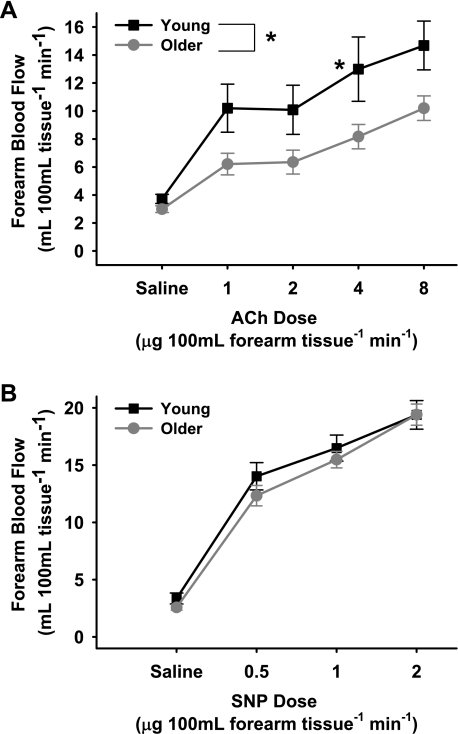
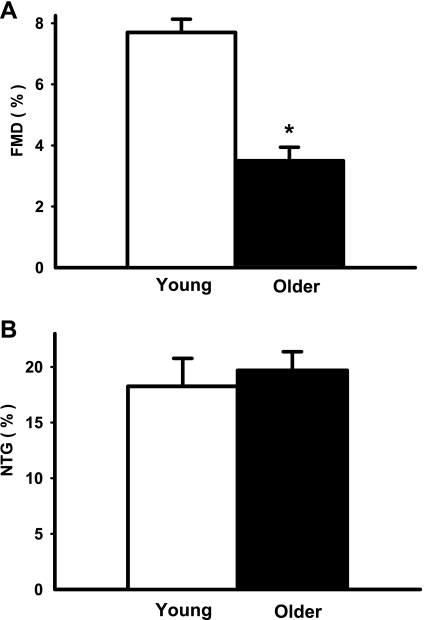
In subjects with brachial artery catheters, FBF in response to acetylcholine was lower (P < 0.01) in the older compared with the young men (Fig. 1A) (peak FBF: 10.2 ± 0.9 vs. 14.7 ± 1.7 ml·100 ml−1·min−1). In contrast, the FBF response to sodium nitroprusside was similar in the groups (Fig. 1B) (peak FBF: 19.4 ± 0.9 vs. 19.4 ± 1.2 ml·100 ml−1·min−1, P = 0.75). In subjects with peripheral vein catheters only, brachial artery FMD was lower (P < 0.001) in the older (0.15 ± 0.02 mm, 3.50 ± 0.44%) compared with the young (0.30 ± 0.01 mm, 7.68 ± 0.43%) men (Fig. 2A). In contrast, brachial artery dilation in response to sublingual nitroglycerin was similar in the groups (older: 0.86 ± 0.07 mm, 19.67 ± 1.77% vs. young: 0.80 ± 0.09 mm, 18.22 ± 2.52%, P > 0.55) (Fig. 2B).
Fig. 1.
Endothelium-dependent dilation (forearm blood flow to intrabrachial artery infusion of ACh; 1.0, 2.0, 4.0, and 8.0 μg/100 ml forearm tissue; A) and endothelium-independent dilation (forearm blood flow to intrabrachial artery infusion of SNP; 0.5, 1.0, and 2.0 μg/100 ml forearm tissue; B) in young and older men. Values are means ± SE. *P < 0.01 vs. young.
Fig. 2.
Endothelium-dependent dilation [brachial artery flow-mediated dilation (FMD); percent change; A] and endothelium-independent dilation [brachial artery dilation with sublingual nitroglycerin (NTG); percent change; B] in young and older men. Values are means ± SE. *P < 0.001 vs. young.
ET-1 protein expression.
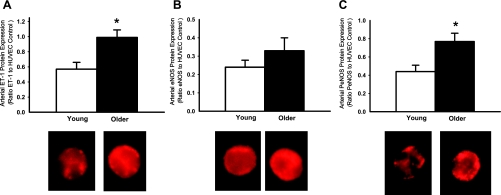
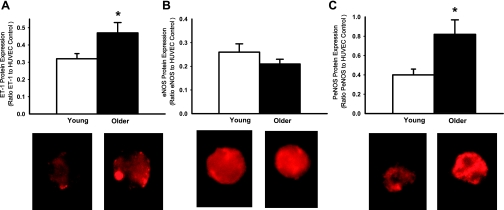
In subjects with brachial artery samples, expression of ET-1 was higher in endothelial cells of older compared with young men (0.99 ± 0.10 vs. 0.57 ± 0.10 ET-1/HUVEC intensity, P = 0.01, Fig. 3A). In the overall sample, peak FBF to acetylcholine was inversely related to ET-1 expression in endothelial cells (r = −0.39, P < 0.05). Similarly, in subjects with peripheral vein samples only, expression of ET-1 was higher in endothelial cells obtained from older compared with young men (0.47 ± 0.05 vs. 0.32 ± 0.04 ET-1/HUVEC intensity, P < 0.05) (Fig. 4A), and brachial artery FMD was inversely related to ET-1 expression (r = −0.37, P < 0.05). The age group differences in ET-1 expression were not related to age-associated elevations in any risk factor, plasma ET-1, or plasma oxidized LDL. ET-1 expression was positively related to nitrotyrosine abundance in endothelial cells obtained from both brachial artery (r = 0.65, P < 0.001) and peripheral vein (r = 0.63, P < 0.005) sampling.
Fig. 3.
Endothelin-1 (ET-1) (A), eNOS (B), and serine 1177 PeNOS protein expression (C) in endothelial cells obtained from brachial arteries in young and older men. Values are means ± SE. *P < 0.05 vs. young.
Fig. 4.
ET-1 (A), endothelial nitric oxide synthase (eNOS; B), and serine 1177 phosphorylated eNOS (PeNOS) protein expression (C) in endothelial cells obtained from antecubital veins in young and older men. Values are means ± SE. HUVEC, human umbilical vein endothelial cell. *P < 0.05 vs. young.
eNOS and PeNOS protein expression.
In subjects with brachial artery samples, expression of eNOS in endothelial cells was not significantly different with age, but tended to be greater in the older men (0.33 ± 0.07 vs. 0.23 ± 0.03 eNOS/HUVEC intensity, P = 0.08) (Fig. 3B). In subjects with peripheral vein samples only, expression of eNOS was not different with age (young: 0.26 ± 0.04 vs. older: 0.21 ± 0.02 eNOS/HUVEC intensity, P = 0.21) (Fig. 4B).
In contrast to eNOS, expression of PeNOS in endothelial cells was consistently greater in the older compared with the young men, both in groups with brachial artery samples (0.77 ± 0.09 vs. 0.44 ± 0.07 PeNOS/HUVEC intensity, P < 0.05, Fig. 3C) and in subjects with peripheral vein samples only (0.82 ± 0.15 vs. 0.40 ± 0.06 PeNOS/HUVEC intensity, P < 0.05) (Fig. 4C).
Neither eNOS nor PeNOS was related to endothelium-dependent dilation in either sample. PeNOS was positively related to body mass index in subjects with brachial artery samples (r = 0.40, P < 0.05) and, as observed previously (32), in subjects with peripheral vein samples (r = 0.49, P < 0.05). Neither eNOS nor PeNOS was related to any other age-associated difference in subject characteristics (Table 1). eNOS and PeNOS generally were not related to nitrotyrosine, although PeNOS was related to nitrotyrosine in peripheral vein samples (r = 0.58, P < 0.05).
Experiments in Young and Old B6D2F1 Mice
Animal and artery characteristics.
The old mice weighed more than the young mice (35 ± 1 vs. 32 ± 1 g, P < 0.05), but there was no difference in soleus muscle weights (old: 0.016 ± 0.002 vs. young: 0.014 ± 0.002 g, P > 0.05). Carotid artery maximal diameter was similar in the two groups (older: 399 ± 10 vs. young: 391 ± 7 μm, P > 0.05), as was the vascular tone after preconstriction (older: 18 ± 1 vs. young: 20 ± 2% tone from maximum diameter, P > 0.05).
Carotid artery endothelium-dependent and -independent dilation.
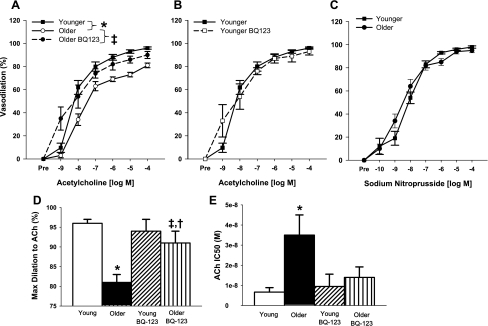
Acetylcholine-mediated dilation was attenuated in carotid arteries of old compared with young control mice (P < 0.05, Fig. 5A). Inhibition of the ET-1 ETA receptor with BQ-123 improved acetylcholine-mediated dilation in old mice (P < 0.05, Fig. 5A), although it did not restore peak vasodilation to levels observed in young control mice (older: 81 ± 2% vs. older BQ-123: 90 ± 3% vs. young: 96 ± 1% of maximal vasodilation, P < 0.05, Fig. 5D). BQ-123 did, however, increase sensitivity to acetylcholine in the older mice (P < 0.05) to that of young mice (Fig. 5E). In contrast to old mice, BQ-123 had no effect on responses to acetylcholine in young mice (P > 0.05, Fig. 5, B and D). There were no differences in carotid artery dilation in response to sodium nitroprusside in the young and old mice (Fig. 5C, P > 0.05).
Fig. 5.
Endothelial-dependent dilation (acetylcholine 1 × 10−9 to 1 × 10−4 M) in untreated and BQ-123-treated carotid arteries from old mice (A) (dose-response curve of young mice is provided as a reference), and untreated and BQ-123-treated carotid arteries from young mice (B). C: endothelial-independent dilation (sodium nitroprusside, 1 × 10−10 to 1 × 10−4 M) of carotid arteries isolated from young and old mice. D: maximal dilation to acetylcholine in untreated and BQ-123-treated carotid arteries from young and old mice. E: sensitivity (IC50) to acetylcholine-mediated dilation in untreated and BQ-123-treated carotid arteries from young and old mice. Values are means ± SE. *P < 0.05 vs. young untreated mice. ‡P < 0.05 vs. untreated old mice. †P < 0.05 vs. young untreated mice.
DISCUSSION
The novel findings of the present studies are as follows. First, expression of ET-1, an endothelium-derived vasoconstrictor protein, is greater in endothelial cells from older compared with young, healthy men. Second, age-associated reductions in endothelium-dependent dilation are inversely related to these increases in endothelial cell ET-1 protein expression. Third, ETA signaling tonically suppresses endothelium-dependent dilation in old, but not in young, mice. Fourth, neither eNOS, the enzyme responsible for production of the potent vasodilator molecule NO, nor PeNOS, the activated isoform of eNOS, is reduced in endothelial cells from older compared with young men. Indeed, PeNOS expression is greater in endothelial cells from older men. Collectively, these results provide experimental evidence that increases in ET-1 expression and bioactivity contribute to vascular endothelial dysfunction with aging, but that reductions in eNOS and eNOS activation are not obviously involved.
ET-1 Protein Expression and Modulation of Endothelium-Dependent Dilation with Aging
Our findings on ET-1 extend current knowledge in at least two ways. First, recent findings in humans (37, 38, 41) indicate that increased signaling through ETA contributes to peripheral vasoconstriction in healthy older sedentary humans, consistent with earlier findings in rats (15). However, the mechanisms involved remain undetermined (37, 41). The present results provide experimental evidence that bioavailability of ET-1 is greater in endothelial cells of older adults and may contribute to increased ET-1 signaling with aging. This is consistent with previous findings of greater ET-1 synthesis in cultured endothelial cells obtained from older compared with young donors with various pathologies (39). Second, our findings of a relation between vascular reactivity and ET-1 expression in humans and ETA receptor blockade in mice provide compelling support for the idea that increased ET-1 signaling may contribute to reductions in endothelium-dependent dilation with aging. The most direct evidence was that ETA inhibition with BQ-123 restored 60% of the age-related impairment in carotid artery dilation to acetylcholine in B6D2F1 mice and completely restored sensitivity to acetylcholine. This is consistent with the idea that increased ETA receptor activity tonically suppresses endothelium-dependent dilation with aging.
Recently, it was suggested that increased ET-1-mediated vasoconstriction with aging in humans could be linked to oxidative stress (41). In agreement with this idea, in the present study, ET-1 expression was positively related to abundance of nitrotyrosine, a cellular marker of oxidative stress (3, 31), in both arterial and venous endothelial cells obtained from healthy young and older men. However, although our results are consistent with the possibility, the present data do not provide direct evidence that ET-1 suppression of endothelium-dependent dilation with aging is mediated by vascular oxidative stress.
In the present study, plasma ET-1 concentrations were greater in the older men. Previous observations by our laboratory and others indicate that in healthy adults, plasma ET-1 either increases modestly or does not change with aging (2, 25, 27, 28, 38). Of interest, there was no relation between plasma concentrations and endothelial cell ET-1 in the present investigation. This may be due to the fact that plasma concentrations are the net result of production and release into and clearance from the plasma compartment. In addition, the majority of ET-1 produced by endothelial cells is released abluminally and, because of its negative charge, diffuses toward vascular smooth muscle cells in the media (42).
eNOS and Activated eNOS with Aging
eNOS protein expression has been reported to be greater (5, 34, 40), not different (34, 43), and lower (8, 43) in arteries obtained from older compared with young rodents, whereas eNOS mRNA expression appears to be lower in arteries from older animals (1, 6, 8). The only previous data in endothelial cells per se reported sevenfold higher eNOS expression in endothelium extracted from aortas of older compared with young rats (40). The present findings are the first data on endothelial cell eNOS protein expression reported in young and older humans. eNOS did not differ significantly with age in endothelial cells obtained from either brachial artery or antecubital vein sampling, although there was a strong trend for greater eNOS in arterial cells from older men. Moreover, we found no relation between eNOS protein in endothelial cells and brachial artery FMD. Together, these observations indicate that the age-associated impairment of EDD in humans is not associated with reductions in eNOS protein expression.
Recent findings in rats indicate that PeNOS and the PeNOS-to-total eNOS ratio are lower in aortas of older compared with young animals (33). In contrast, in the present study, PeNOS expression was markedly greater in endothelial cells from older men, suggesting activation of the enzyme. The greater endothelial cell PeNOS in the older men could reflect an unsuccessful attempt to stimulate NO production in the face of increased superoxide scavenging and reduced bioavailability of NO (35, 40). If eNOS is uncoupled, however, as appears to be the case with aging (23), the greater activation of eNOS could actually contribute to further development of oxidative stress in the older men by producing greater amounts of superoxide anion (45, 48). That the older men demonstrated higher plasma concentrations of oxidized LDL, a marker of systemic oxidative stress (Table 1), and endothelial cell abundance of nitrotyrosine (0.53 ± 0.08 vs. 0.27 ± 0.03 nitrotyrosine/HUVEC intensity, n = 13 older, n = 11 young, P < 0.05), as reported previously (13), is consistent with this possibility. This concept also is in agreement with previous findings that NO production is substantially lower in aortic endothelial cells from older compared with young rats, despite marked increases in eNOS protein and activity (40). We did not, however, find consistent relations between eNOS or PeNOS and nitrotyrosine in endothelial cells obtained from arterial and venous sampling in the present study.
Limitations
We recognize at least three additional limitations of our study discussed previously (13, 32). First, because of the limited number of endothelial cells available from our sampling procedure, we were not able to measure mRNA gene expression. Second, because we studied only healthy men, our results may not reflect differences with aging in healthy females or adults with clinical disease or CVD risk factors. Third, we focused our analysis on the key vasoconstrictor and vasodilatory proteins of the vascular endothelium, ET-1 and isoforms of eNOS, because of recent studies in humans indicating a role for ET-1 in age-associated vasoconstriction (37, 38, 41) and previous work in rodents examining eNOS in endothelial dysfunction with aging (40). However, we acknowledge that the expression of other vasoactive proteins may differ in young and older adults and contribute to impaired endothelium-dependent dilation.
Summary and Conclusions
The results of the present study provide new insight into the molecular events involved in age-related vascular endothelial dysfunction. We show that ET-1 protein expression is increased in vascular endothelial cells of healthy sedentary older men and is related to a reduction in endothelium-dependent dilation. Consistent with this, inhibition of ET-1 signaling selectively improves endothelium-dependent dilation in old mice and restores vasodilatory responsiveness to acetylcholine to levels observed in young mice. Our data also indicate that neither eNOS protein nor its activation is reduced in vascular endothelial cells of older men, and that eNOS activation actually is increased with age. Together, these findings provide novel translational evidence that ET-1 expression and bioactivity, but not eNOS, contribute to vascular endothelial dysfunction with aging.
GRANTS
This study was supported by National Institutes of Health Grants AG006537, AG029337, AG013038, AG022241, AG000279, HL007851, and RR00051.
Acknowledgments
We thank Rhea Chiang, Cassandra Roeca, and Brooke Lawson for technical assistance.
REFERENCES
- 1.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension 30: 817–824, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Battistelli S, Gori S, Borgogni T, Manasse G. [Variation in the plasma endothelin levels in relation to age]. Minerva Cardioangiol 44: 111–114, 1996. [PubMed] [Google Scholar]
- 3.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83: 279–286, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Challah M, Nadaud S, Philippe M, Battle T, Soubrier F, Corman B, Michel JB. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol Heart Circ Physiol 273: H1941–H1948, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol 92: 1331–1338, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 9.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol 51: 1959–1964, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7: 805–812, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol 105: 1359–1363, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol 579: 115–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res 66: 393–401, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology 212: 655–664, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol 31: 5–14, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Ihara M, Ishikawa K, Fukuroda T, Saeki T, Funabashi K, Fukami T, Suda H, Yano M. In vitro biological profile of a highly potent novel endothelin (ET) antagonist BQ-123 selective for the ETA receptor. J Cardiovasc Pharmacol 20, Suppl 12: S11–S14, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol 293: H1344–H1350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci 64: 9–20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda S, Tanabe T, Miyauchi T, Otsuki T, Sugawara J, Iemitsu M, Kuno S, Ajisaka R, Yamaguchi I, Matsuda M. Aerobic exercise training reduces plasma endothelin-1 concentration in older women. J Appl Physiol 95: 336–341, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell D, Bihari A, Sandig M, Tyml K. Endothelin-a receptor in rat skeletal muscle microvasculature. Microvasc Res 64: 179–185, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Miyauchi T, Yanagisawa M, Iida K, Ajisaka R, Suzuki N, Fujino M, Goto K, Masaki T, Sugishita Y. Age- and sex-related variation of plasma endothelin-1 concentration in normal and hypertensive subjects. Am Heart J 123: 1092–1093, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol 102: 890–895, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension 45: 1107–1112, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem 275: 32460–32466, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 115: 627–637, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 101: 1751–1759, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Thijssen DH, Hopman MT, Levine BD. Endothelin and aged blood vessels: one more reason to get off the couch? Hypertension 50: 292–293, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Tokunaga O Immunohistologic localization in aorta and biosynthesis by cultured human aortic endothelial cells. Lab Invest 67: 210–217, 1992. [PubMed] [Google Scholar]
- 40.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50: 403–409, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem 267: 16066–16068, 1992. [PubMed] [Google Scholar]
- 43.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93: 1685–1690, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. [DOI] [PubMed] [Google Scholar]