Abstract
The ventricles of small mammals express mostly α-myosin heavy chain (α-MHC), a fast isoform, whereas the ventricles of large mammals, including humans, express ∼10% α-MHC on a predominately β-MHC (slow isoform) background. In failing human ventricles, the amount of α-MHC is dramatically reduced, leading to the hypothesis that even small amounts of α-MHC on a predominately β-MHC background confer significantly higher rates of force development in healthy ventricles. To test this hypothesis, it is necessary to determine the fundamental rate constants of cross-bridge attachment (fapp) and detachment (gapp) for myosins composed of 100% α-MHC or β-MHC, which can then be used to calculate twitch time courses for muscles expressing variable ratios of MHC isoforms. In the present study, rat skinned trabeculae expressing either 100% α-MHC or 100% β-MHC were used to measure ATPase activity, isometric force, and the rate constant of force redevelopment (ktr) in solutions of varying Ca2+ concentrations. The rate of ATP utilization was ∼2.5-fold higher in preparations expressing 100% α-MHC compared with those expressing only β-MHC, whereas ktr was 2-fold faster in the α-MHC myocardium. From these variables, we calculated fapp to be approximately threefold higher for α-MHC than β-MHC and gapp to be twofold higher in α-MHC. Mathematical modeling of isometric twitches predicted that small increases in α-MHC significantly increased the rate of force development. These results suggest that low-level expression of α-MHC has significant effects on contraction kinetics.
Keywords: α-myosin heavy chain, rate constants of cross-bridge attachment and detachment, rate of rise of force
the myosin molecule, a hexamer composed of two heavy chains (∼220 kDa) and four light chains (16–25 kDa), is the principal component of the thick filament and is responsible for the generation of force in mammalian striated muscle. The rod-shaped COOH-terminal domain of the myosin molecule (light meromyosin) forms the backbone of the thick filament, whereas the globular NH2-terminus (subfragment 1) contains the actin-binding and catalytic domains (13). Thus, S1 is an important determinant of contractile speed and power developed in the mammalian myocardium.
Two isoforms of myosin heavy chain (MHC), α and β, have been identified in cardiac muscle (23). These MHC isoforms share 93% amino acid sequence homology (32), and yet they confer distinct functional properties to the myocardium: α-MHC has been shown to have markedly higher ATPase activity, faster shortening velocity (Vmax), and faster rates of force development (42), whereas β-MHC exhibits a lower tension cost and is thus more efficient (1, 24, 40).
Expression levels of the ventricular MHC isoforms are species dependent and are both developmentally and hormonally regulated (8, 30, 42). The predominant fetal isoform in all mammalian ventricular myocardium is β-MHC, wheras in adults there is a considerable variation in α-MHC content that scales inversely with size: in rodents, the predominant adult isoform is α-MHC (30), whereas the myocardium of larger adult mammals, including humans, expresses predominately β-MHC (29). Several other factors are known to affect the relative proportions of α-MHC and β-MHC in the myocardium. Elevation of thyroid hormone increases the expression of α-MHC, whereas hypothyroidism, aging, and pressure overload all increase the proportion of β-MHC (46). In addition, hypothyroidism completely abolishes α-MHC expression in the rodent ventricular myocardium, whereas hyperthyroidism increases α-MHC expression to virtually 100% of the total MHC content (46). Moreover, MHC content in mammalian myocardium varies regionally, as characterized by the increased expression of α-MHC in the epicardium compared with the endocardium (7).
Animal models of heart failure have often focused on myosin isoform composition as a possible cause of depressed myocardial performance due to the reduced expression of α-MHC. The higher levels of β-MHC in heart failure confer greater contractile efficiency due to reduced tension cost, but this advantage comes at the expense of a slower rate of rise of force and reduced power. It was long believed that the myosin expressed in adult human ventricles was 100% β-MHC (18), and thus it seemed unlikely that alterations in MHC isoform content had any bearing on depressed contractile kinetics in heart failure. However, recent work has shown that normal adult human ventricles express nearly 10% (average of 7% and up to 13% of total myosin content) α-MHC on a background of ∼90% β-MHC, and this distribution switches to 100% β-MHC in heart failure (34). In addition, α-MHC mRNA decreases from 25–35% of total MHC mRNA in normal human hearts to 2% during end-stage ventricular failure (31, 35).
Because of the faster turnover kinetics of α-MHC, we propose that the small amounts of α-MHC on a predominately β-MHC background normally accelerate the rate of myocardial force development and that reduced α-MHC expression (as in heart failure) depresses the kinetics of force development. Consistent with this idea, increased β-MHC expression due to thyroid deficiency significantly slows contractile kinetics. Euthyroid rats expressing ∼80% α-MHC had markedly greater unloaded shortening velocities and rates of force development compared with myocardium from thyroid-deficient rats expressing 100% β-MHC (12), and shortening velocities under load were also faster in euthyroid compared with hypothyroid rats (20). While single molecule experiments have indicated that there is no difference in force per cross-bridge for α-MHC and β-MHC isoforms, the duty cycle (fraction of the cross-bridge cycle spent in force-generating states) of β-MHC is approximately twice that for α-MHC (37). Another study (21) varied MHC isoform expression by thyroid manipulation and showed that the expression of ∼10% α-MHC on a predominately β-MHC background increased shortening velocities at intermediate loads and thus increased power compared with preparations expressing 0% α-MHC.
We tested the idea that low-level expression of α-MHC on a predominately β-MHC background significantly accelerates force development in myocardium. We first measured isometric force, ATP utilization, and the rate constant of force redevelopment (ktr) in rats expressing either 100% α-MHC or 100% β-MHC and then used those values to derive the rate constants of cross-bridge attachment (fapp) and detachment (gapp) for both MHC isoforms. These rate constants were used to mathematically model an isometric twitch to predict the functional consequences of small changes in the level of expression of α-MHC.
MATERIALS AND METHODS
Experimental Animals
Control and thyroidectomized female Sprague-Dawley rats (Harlan Laboratories, Madison, WI) were housed in groups of 2–3 rats/cage in a light- and temperature-controlled (22–23°C) room. Rats (6–9 mo of age) were divided into two separate groups, and each group was fed a specific diet for a period of 5 wk (27). All thyroidectomized rats (n = 17) were fed a diet of 0.15% propylthiouracil (PTU; Sigma, 0.15 g PTU/100 g rat meal), which has been shown to eliminate any residual plasma triiodothyronine and thyroxine after thyroidectomy (19). Control rats (n = 17) were fed a diet of 0.15% thyroid extract (Sigma, 0.15 g thyroid extract/100 g rat meal). An additional group of 2.5- to 3-mo-old female Sprague-Dawley rats (n = 8) was thyroidectomized but not treated with PTU to generate myocardium with low-level expression of α-MHC, wheras a final group of young (2.5–3 mo old) euthyroid Sprague-Dawley rats (n = 7) was used to obtain myocardium expressing ∼80% α-MHC. All rats had access to food and water ad libitum. No animal showed any signs of distress throughout the feeding protocol, and therefore none of the rats were excluded from the study. Animals were anesthetized by the deep inhalation of isoflurane (15% isoflurane in mineral oil). All animal use was conducted under the strict guidelines and approval of the University of Wisconsin Animal Care and Use Committee.
Experimental Preparations
Skinned trabeculae were used to measure isometric force and ATPase activity. Animals were handled with care to minimize stress levels and limit alterations in contractile protein phosphorylation status. The heart was rapidly removed from the anesthetized animal and placed in a dissecting dish filled with Ringer solution containing (in mM) 120 NaCl, 5 KCl, 1.2 MgSO4, 1 CaCl2, 19 NaHCO3, 1.2 Na2HPO4, and 10 glucose. The solution was bubbled with 95% O2-5% CO2. Long unbranched trabeculae (1–3 mm × 75–250 μm) were located in the right ventricle and then equilibrated for 10 min in fresh Ringer solution containing 15 mM 2,3-butanedione monoxime before dissection to minimize mechanical damage. Trabeculae were then cut free, tied to wooden dowels with 10-0 suture, and bathed in relaxing solution (4°C) containing 1% Triton X-100. After being skinned for ∼24 h to remove membranes, trabeculae were washed for 60 min in cold relaxing solution and stored at −20°C in 50% glycerol and relaxing solution for up to 5 days before use.
Measurements of ktr were performed in separate preparations in a setup having a high-speed force transducer. This was necessary because of the low-frequency response of the transducer in the ATPase setup, which necessarily has a stylus of large mass. To prepare skinned myocardial preparations for measurements of ktr, frozen ventricles that had been rapidly frozen in liquid nitrogen were homogenized in relaxing solution for ∼2 s using a Polytron, which yielded multicellular preparations of 100–250 × 600–900 μm. The homogenate was centrifuged at 120 g for 1 min, and the resulting pellet was washed with fresh relaxing solution and resuspended in relaxing solution containing 1% Triton X-100. After a 30-min permeabilization period, skinned preparations were washed with fresh relaxing solution and then dispersed in relaxing solution in a glass petri dish. The dish was kept on ice at all times except during the selection of preparations for mechanical experiments.
Solutions
The compositions of solutions used for mechanical experiments were calculated using the computer program of Fabiato (9) and the stability constants (corrected to pH 7 and 20°C) listed by Godt and Lindley (17). The relaxing solution (pH 7.0) used for bathing skinned trabeculae contained (in mM) 100 KCl, 20 imidazole, 7 MgCl2, 2 EGTA, and 4 ATP. In addition, the pCa 9.0 solution contained 5.86 ATP, 6.53 MgCl2, 20 EGTA, 48.23μM CaCl2, 100 N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), the pCa 4.5 solution contained 5.89 ATP, 6.01 MgCl2, 20 EGTA, 19.85 CaCl2, and 100 BES, and the preactivating solution contained 0.5 EGTA. In additon, pCa 9.0, pCa 4.5, and preactivating solutions contained 5 NaN3, 0.01 leupeptin, 0.01 P1-P5-di(adenosine-5′)pentaphosphate, and 10 phospho(enol)pyruvic acid. The ionic strength of all solutions was adjusted to 200 mM with potassium propionate. Solutions containing a range of free Ca2+ concentrations ([Ca2+]free; i.e., pCa 5.8–5.4) were prepared by mixing the appropriate volumes of pCa 9.0 and pCa 4.5 solutions. Pyruvate kinase (0.91 mg/ml) and lactate dehydrogenase (0.31 mg/ml) were added to all pCa and preactivating solutions before mechanical experiments. Solutions used for the measurements of ktr were as previously described (43).
Experimental Protocols
ATPase experiments.
While immersed in relaxing solution, a trabecula was attached to the arms of a high-speed length controller (model 312B, Aurora Scientific) and a force transducer (model 403A, Aurora Scientific) by fixing each end to a stainless steel trough with a 0.5-mm length of 4-0 monofilament secured in place with loops of 10-0 monofilament. This procedure was optimized to virtually eliminate compliance at the ends and leave 750–1,000 μm of trabecula exposed to the solution, which allowed accurate measurement of sarcomere length via the diffraction pattern obtained with a He-Ne laser, as described below.
Three variables were measured to calculate fapp and gapp (3): ATPase activity per preparation volume [proportional to (f × g)/(f + g)], tension cost (proportional to g/unified force), which is defined as the rate of ATP turnover divided by isometric force, and ktr (ktr = f + g) in response to a brief period of unloaded isotonic shortening followed by rapid restretch. Measurements of preparation dimensions were done during the experiment to allow calculations of sample volume, and cross-sectional area was determined assuming an elliptical geometry.
Isometric force and ATPase activity were recorded in solutions of varying Ca2+ concentrations (pCa 5.8–4.5) at 20°C at a mean sarcomere length of 2.1 μm. The active force at each pCa was measured as the difference between total steady-state force and passive force determined by applying a 20% slack step to the preparation in a solution of pCa 9.0. Active force was normalized to the cross-sectional area of the preparation. A He-Ne laser (wavelength 632.8 nm) was directed toward the mounted preparation, and the resulting diffraction pattern was projected onto a screen. Measurements of the distance between the zero- and first-order lines were then used to calculate sarcomere length, a method that is accurate to within 0.1 μm. In addition, careful monitoring of sarcomere length throughout each experiment quickly revealed compliance in the attachments, and, in such cases, the preparation was discarded.
ATP consumption was measured using a NADH-coupled enzyme system (16, 44). ADP production during ATP hydrolysis was measured as the change in NADH concentration:
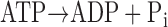 |
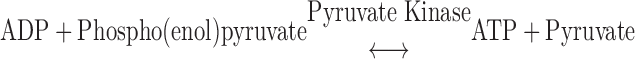 |
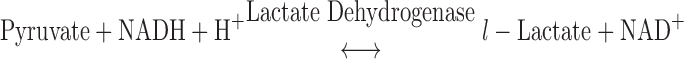 |
NADH absorbs UV light at a wavelength of 339 nm, whereas NAD+ does not; thus, ATP consumption can be measured as the change in absorbance at 339 nm (16, 44). The experimental chamber consisted of a 15-μl well enclosed on two sides by quartz windows. Near-UV light was directed to the skinned myocardial preparation in the experimental chamber, and the transmitted beam was split and directed to two photodiodes: one to record the transmitted light at 339 nm (for the determination of NADH concentration) and the other to correct the signal for photobleaching and to determine nonspecific absorbance at a reference wavelength other than 339 nm. NADH (70 mM) was injected into the measuring chamber before the incubation of the trabecula while calibration of the ATPase signal was performed with 25-nl injections of 10 mM ADP into the measuring chamber. During experiments, the solution in the measuring chamber was continually stirred via motor-driven displacement of a latex membrane on the bottom of the chamber. Recording of the two signals was necessary to minimize noise that may arise from irregularities due to continuous mixing. The rate of NADH consumption was taken as the ATPase activity and determined by linear regression analysis of the measured absorption trace.
Ca2+ sensitivity and kinetics of force redevelopment.
The active force at each pCa was measured as the difference between total steady-state force and passive force determined by applying a 20% slack step to the preparation in a solution of pCa 9.0. Force-pCa relationships were derived by expressing submaximal force (P) at each pCa (range: 6.1–5.5) as a fraction of maximum force (Po) determined at pCa 4.5 (P/Po). The force required for half-maximal activation (pCa50) was determined by fitting the force-pCa data with the following Hill equation: P/Po = [Ca2+]nH/(knH + [Ca2+]nH), where nH is the Hill coefficient and k is the Ca2+ concentration required for pCa50.
ktr was estimated in rat skinned multicellular preparations expressing 100% α-MHC or 100% β-MHC using a modified experimental protocol originally described by Brenner and Eisenberg (5). After a 1-min incubation in preactivating solution, each preparation was transferred to solutions ranging from maximal [Ca2+]free (pCa 4.5) to submaximal [Ca2+]free (pCa 6.1–5.5). Once steady-state force had developed, the preparation was rapidly slackened (<2 ms) by 20% of its original length, resulting in a reduction in force to near zero. After a brief period (10 ms) of unloaded isotonic shortening, the preparation was rapidly restretched to its original length and force redeveloped to the original steady-state level. The apparent ktr was estimated by linear transformation of the half-time of force redevelopment (t1/2), i.e., ktr = 0.693/t1/2, as previously described (43).
Isolation of single myocytes with enzymatic digestion.
In addition to measurements in trabeculae, single ventricular myocytes were used to measure isometric force, as previously described (45). The thyroid status of female Sprague-Dawley rats was manipulated to generate hypothyroid and hyperthyroid rats expressing 100% β-MHC or 100% α-MHC, respectively. Rats were anesthetized as described above. The heart was rapidly excised, and the aorta was cannulated for retrograde perfusion on a modified Langendorff apparatus. The heart was first perfused for 2–5 min with Ringer solution (pH 7.0) containing (in mM) 118 NaCl, 4.8 KCl, 4.2 NaH2PO4, 25 HEPES, 11 glucose, 1.2 MgCl2, and 1 CaCl2 followed by perfusion with Ca2+-free Ringer solution. After ∼2 min, the heart was perfused with Ca2+-free Ringer solution containing 2.5 mg/ml collagenase (type II, Worthington) and 3.0 mg/ml hyaluronidase (Sigma) for 10 min followed by the addition of 250 μl of 0.1 M CaCl2 and perfusion for an additional 15 min. The heart was then minced into small fragments and incubated in the same solution for 20 min followed by filtration through Teflon mesh. Cells were washed three times in Ringer solution containing 0.2 mM Ca2+ and resuspended in 0.3% Triton X-100 relaxing solution for 10 min. After being skinned, cells were washed with relaxing solution, centrifuged for 1 min at low rpm, resuspended in fresh relaxing solution, and then stored on ice until use. All solutions were maintained at 37°C and bubbled with 100% O2 during the perfusion process.
Single myocyte experiments.
Single rod-shaped myocytes were fixed between thin metal wires with silicone adhesive (Dow Corning) to the stage of a Zeiss inverted microscope. One wire was attached to a force transducer (model 403A, Cambridge Technologies), and the other wire was attached to a length controller (model 6800 HP, Cambridge Technologies). Each element could be maneuvered independently via micromanipulators (Narishige). Isometric force was measured at maximal Ca2+ concentration at 20°C and at a mean sarcomere length of 2.1 μm. The active force was measured as the difference between total steady-state force and passive force determined by applying a 20% slack step to the preparation in a solution of pCa 9.0. Active force was normalized to the cross-sectional area of the preparation. Force was sampled (16-bit resolution, DAP5216a, Microstar Laboratories, Bellevue, WA) at rates of 2 kHz using SLControl software developed in our laboratory (6) and saved to data files for analysis.
Quantification of MHC expression.
After each mechanical experiment, the trabecula was cut free at the points of attachment and placed in 10 μl SDS sample buffer (62.5 mM Tris, 75 mM DTT, 25% glycerol, 25 mM SDS, and 0.01% bromophenol blue) and stored at −80°C until the subsequent analysis of MHC isoform content by SDS-PAGE. α- and β-MHC isoforms were separated using 6% acrylamide gels, which were stained using a modified silver stain technique as previously described (15, 47). This method reproducibly detects 2 ng protein/band and has a linearity of stain between 2 and 70 ng protein/band. The proportions of ventricular MHC isoforms were then determined by densitometric analysis of silver-stained gels using an imaging densitometer and LaserPix software (Bio-Rad Laboratories). The proportions of α-MHC and β-MHC were estimated by expressing the area under the peak for each isoform as a fraction of the total area for the two isoforms. To assure the linearity of staining and accuracy of measurements, all samples were serially diluted and run in parallel lanes next to MHC standards from myocardium expressing 100% α-MHC or 100% β-MHC.
Determination of the level of phosphorylation of myofibrillar proteins.
Myofibrillar proteins from control, hyperthyroid, and hypothyroid rat myocardium were separated by SDS-PAGE using 12.5% Tris·HCl Criterion gels (Bio-Rad Laboratories). To determine the level of protein phosphorylation, different volumes (3, 4, 6, and 8 μl) from control, hyperthyroid, and hypothyroid skinned myocardium were loaded on the same gel. Phosphoproteins were detected using a Pro-Q Diamond staining protocol (Molecular Probes) followed by staining with SYPRO ruby (Molecular Probes) for the determination of total protein content. Briefly, gels were fixed in 50% methanol-10% glacial acetic acid for 1.5 h with solution changes every 30 min, washed with double-distilled H2O six times for 5 min each, stained with Pro-Q Diamond stain for 1.5 h, and then destained with Pro-Q Diamond destaining solution (Molecular Probes) overnight. For the determination of myofibrillar protein content, gels were stained with SYPRO ruby (Molecular Probes) for 3 h and destained with SYPRO ruby destain solution (10% methanol-7% glacial acetic acid) for 2 h with solution changes every 30 min. Immediately after each staining protocol, gels were imaged using a UVP BioImaging System (UVP, Upland, CA) and analyzed using LabWorks 4.5 (UVP). The area of protein and phosphoprotein bands was multiplied by mean optical density and plotted versus volume loaded, and a first-order linear regression was fitted to the data points to determine the slope of the relationship between optical density and volume loaded.
Data analysis.
All data are reported as means ± SE. To test for the significance of effects of altered MHC content on ATP utilization, steady-state force, and ktr at different levels of Ca2+ activation, one-way ANOVA with a Tukey post hoc test for significance (P < 0.05) was used.
RESULTS
Alterations in MHC Expression Due to Manipulation of Thyroid Status
SDS-PAGE analysis of all myocardial preparations used for mechanical measurements revealed that the thyroidectomy of rats and dietary supplementation with PTU completely abolished the expression of α-MHC (Fig. 1) and thus converted the rat myocardium to 100% β-MHC (99.8 ± 0.3%, n = 10). In contrast, euthyroid rats that received dietary supplementation with thyroid extract expressed nearly 100% α-MHC (97.8 ± 0.5%, n = 12). Previous studies (12, 42) using manipulation of rat thyroid status have shown large changes in the expression of ventricular MHC isoforms without altering the expression of myosin light chains (MLC1V or MLC2V) or thin filament regulatory proteins. In addition, analysis of phosphoprotein gels using Pro-Q Diamond and SYPRO ruby staining (Fig. 2) indicated that the basal levels of phosphorylation of cardiac myosin-binding protein C, cardiac troponin T, cardiac troponin I, and myosin regulatory light chain were similar in the control, hyperthyroid, and hypothyroid rat myocardium. Thus, the observed differences in kinetics in hyperthyroidism and hypothyroidism can be attributed solely to the distribution of MHC isoforms.
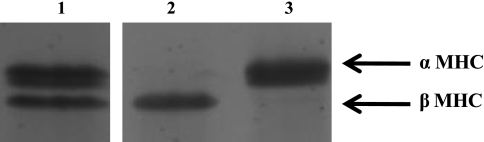
Fig. 1.
Myosin heavy chain (MHC) composition of the rat skinned myocardium. MHC isoform expression was determined with 6% SDS-PAGE. The relative proportions of α-MHC and β-MHC were determined by densitometric analysis (LabWorks software) of silver-stained gels. This representative gel shows that hypothyroid rats typically expressed 100% (99.8 ± 0.2, n = 10) β-MHC (lane 2) and hyperthyroid rats expressed nearly 100% (97.8 ± 0.5, n = 12) α-MHC (lane 3). Lane 1 shows the MHC content of control euthyroid rat myocardium (65% α-MHC and 35% β-MHC).
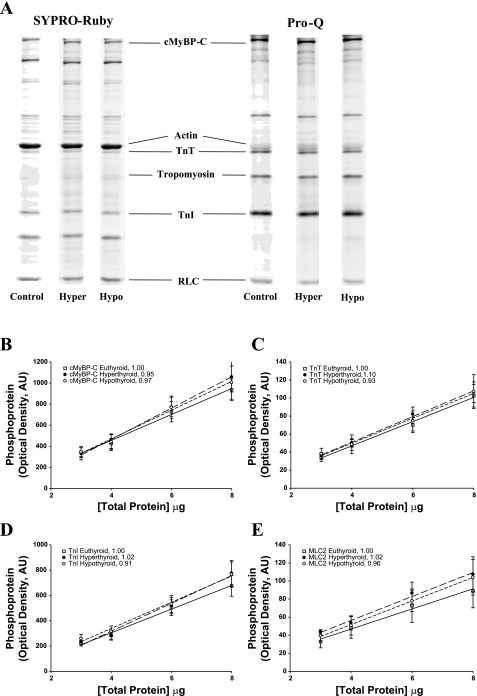
Fig. 2.
A: determination of protein phosphorylation in the rat myocardium. The level of myofibrillar protein phosphorylation was determined in control, hyperthyroid, and hypothyroid rat myocardium (n = 4 from each group) using SYPRO ruby staining for total protein content (left) and Pro-Q Diamond staining for the determination of protein phosphorylation, as shown in this representative SDS-PAGE gel at a single loading concentration (6 μl; right). Volumes of 3, 4, 6, and 8 μl were loaded in successive lanes for each sample preparation. cMyBP-C, cardiac myosin-binding protein-C; TnT, troponin T; TnI, troponin I; RLC, myosin regulatory light chain. B–E: regression analysis of myocardial protein phosphorylation in rat myocardium. Levels of protein phosphorylation in euthyroid, hyperthyroid, and hypothyroid rat myocardium were compared in Pro-Q Diamond-stained SDS-PAGE gels for the following proteins: cMyBP-C (B), TnT (C), TnI (D), and RLC [myosin light chain 2 (MLC2); E]. Regression lines for optical band intensity versus protein load were determined for both Pro-Q Diamond- and SYPRO ruby (not shown)-stained gels, and the ratio of regression line slopes was calculated. The ratio was normalized to euthyroid rat myocardium for each protein (see insets). AU, arbitrary units.
Steady-State Force Development in the Rat Myocardium With Altered MHC Composition
Table 1 shows the steady-state mechanical properties of rat skinned trabeculae expressing either 100% α-MHC or 100% β-MHC. Altered MHC composition had no effect on the Ca2+ sensitivity of force, and the steepness of the force-pCa relationship (nH) did not vary between the preparations, indicating that MHC composition does not influence the apparent cooperativity of tension development. Our findings did reveal that maximum Ca2+-activated force (pCa 4.5) was reduced in preparations expressing 100% β-MHC (Table 1), which differs from previous reports (12, 20, 41, 43) showing that maximum force did not change with the expression of MHC isoforms. However, measurements of maximal force per cross-sectional area in enzymatically digested skinned single myocytes from hypothyroid and hyperthyroid rat myocardium revealed no differences (6.5 ± 1.0 and 7.4 ± 0.7 kN/m2, respectively). These results suggest that the depressed force in the trabeculae of hypothyroid rats may be due to changes in the expression of extracellular elements such as collagen, which has been shown to be increased in rats treated with PTU (49). Alterations of force due to increased collagen would not have been evident in homogenized preparations obtained via mechanical disruption, as in previous reports (12, 20, 41, 43).
Table 1.
Effect of MHC composition on steady-state mechanical properties of rat myocardium
| n | Ca2+-Activated Force in Trabeculae, mN/mm2 | nH | pCa50 | |
|---|---|---|---|---|
| 100% α-MHC | 12 | 41.4±2.3 | 4.06±0.32 | 5.78±0.01 |
| 60% α-MHC | 10 | 41.8±1.6 | 4.31±0.35 | 5.78±0.02 |
| 0% α-MHC | 10 | 31.3±1.6* | 4.13±0.36 | 5.79±0.01 |
Data are means ± SE; n, number of cardiac preparations. MHC, myosin heavy chain; Ca2+-activated force, difference between maximal force generated at pCa 4.5 and rest force generated at pCa 9.0; nH, Hill coefficient for the total force-pCa relationship; pCa50, pCa required for half-maximal force generation.
Significantly different from hyperthyroid (100% α-MHC) myocardium (P < 0.05).
It should be noted that there were no differences in passive tension between euthyroid (1.46 ± 0.11 mN/mm2), hyperthyroid (1.65 ± 0.16 mN/mm2), and hypothyroid (1.34 ± 0.1 mN/mm2) rat myocardium. Passive tension would most likely be influenced by the distribution and expression of titin isoforms (N2B and N2BA), and while it is unlikely that alterations in titin exist in the hyperthyroid and hypothyroid myocardium used in the present study, titin would not play a role in passive tension until sarcomere lengths greater than those used here (>2.2 μm).
Effect of MHC Content on the Kinetics of Force Redevelopment
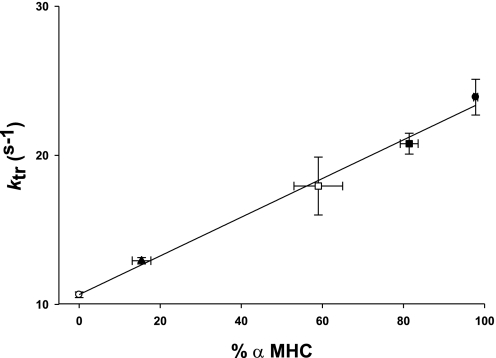
A modified release-restretch protocol (5) was used to determine ktr in steadily Ca2+-activated skinned myocardium expressing 100% α-MHC or 100% β-MHC. ktr, which is equal to fapp + gapp, provides information about the kinetics of cross-bridge transitions between non-force-generating and force-generating states. Consistent with other results (12, 41), expression of α-MHC significantly increased maximal ktr compared with preparations expressing 100% β-MHC (Table 1). In addition, we measured ktr in myocardium expressing variable levels of α-MHC (15%, 59%, and 81%) and found that ktr increased linearly with the expression of α-MHC (Fig. 3). This finding is in agreement with other studies (11, 41) investigating the rate of tension development as a function of MHC isoform expression and suggests that the presence of slower cycling β-MHC cross-bridges is unlikely to impose significant drag on ktr. ktr values in the groups expressing intermediate levels of α-MHC (15%, 59%, and 81%) were 12.93 ± 0.21, 17.94 ± 1.4, and 20.78 ± 0.7 s−1, respectively, and the relationship between ktr and the expression of α-MHC shown in Fig. 3 was well fit by linear regression (r2 = 0.994).
Fig. 3.
Effect of the percent α-MHC expression on the maximal rate constant of force redevelopment (ktr) after a rapid slack-restretch maneuver. ktr was measured in four groups of rat myocardium with variable expression of α-MHC: 0% α-MHC, hypothyroid [thyroidectomized with propylthiouracil (PTU) treatment; ○]; 15.4% α-MHC, hypothyroid (thyroidectomized without PTU treatment; ▴); 59% α-MHC, euthyroid (□); 81.4% α-MHC (▪); and 97.8% α-MHC, hyperthyroid (thyroid hormone treatment; •). The groups expressing 15.4% and 81.4% α-MHC were obtained from young (2.5–3 mo old) Spraque-Dawley rats. Data are means ± SE.
Effects of MHC content on the Rate of ATP Utilization
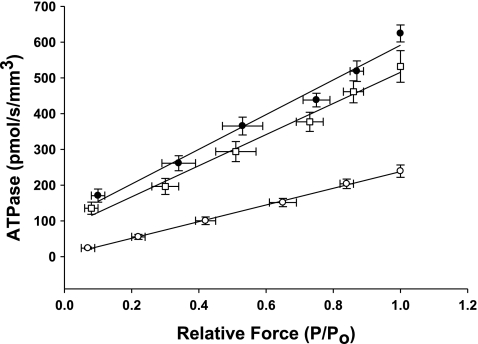
The effect of MHC content on ATPase activity was measured using an ATP-enzyme-coupled assay as described above and is shown in Table 2. Linear regression of the absorbance signal of NADH indicated that the preparations expressing 100% α-MHC exhibited rates of ATP utilization that were ∼2.5-fold higher than preparations expressing 100% β-MHC (Fig. 4). This finding is consistent with the ktr data from preparations of α-MHC or β-MHC, a correlation that presumably manifests in greater rates of cross-bridge cycling in myocardium expressing 100% α-MHC.
Table 2.
Effect of MHC composition on parameters of cross-bridge cycling
| n | ktr, s−1 | ATPase, pmol·s−1·mm−3 | Tension Cost, pmol·s−1·mm−1·mN−1 | fapp | gapp | |
|---|---|---|---|---|---|---|
| 100% α-MHC | 12 | 23.9±1.2 | 624.29±23.7 | 15.09±0.75 | 6.84 | 17.06 |
| 60% α-MHC | 10 | 17.9±1.4* | 531.91±44.2 | 13.25±1.15 | NA | NA |
| 0% α-MHC | 10 | 11.4±0.7* | 247.92±13.9* | 7.92±0.95* | 2.47 | 8.93 |
Data are means ± SE; n, number of cardiac preparations. ktr, rate constant of force redevelopment in response to a rapid slack-restretch maneuver; ATPase, rate of ATP hydrolysis; tension cost, ratio of ATPase to isometric force; fapp, derived rate constant of cross-bridge attachment; gapp, derived rate constant of cross-bridge detachment. Derivations of rate constants were performed only in myocardium expressing either 100% or 0% α-MHC. NA, not applicable.
Significantly different from hyperthyroid (100% α-MHC) myocardium (P < 0.05).
Fig. 4.
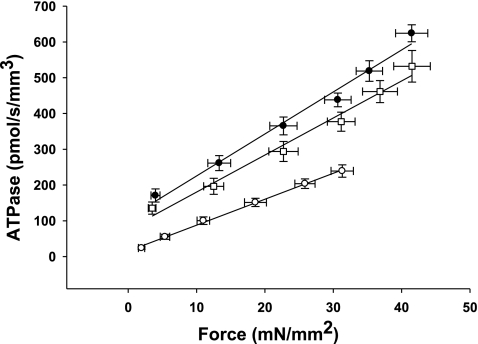
Effect of MHC composition on the rate of ATP utilization in the rat skinned myocardium. The rate of ATP utilization was measured in the skinned myocardium from euthyroid (∼60% α-MHC, n = 10; □), hyperthyroid (100% α-MHC, n = 12; •), and hypothyroid (0% β-MHC, n = 10; ○) rats and plotted against relative force (P/Po). Forces recorded at submaximal free Ca2+ (P) were expressed relative to the maximal force measured at pCa 4.5 (Po). Data are means ± SE.
By measuring both isometric force and ATPase activity in each preparation, we were able to calculate tension cost, which is defined as theATPase rate divided by isometric force and is directly proportional to gapp (3). Tension cost was approximately twofold higher in preparations expressing 100% α-MHC compared with those expressing 100% β-MHC (Fig. 5). This finding is consistent with a previous study (41) and indicates that β-MHC is a more economical isoform, i.e., less ATP is hydrolyzed to maintain a given isometric force.
Fig. 5.
Effect of MHC composition on tension cost in rat skinned myocardium. The rate of ATP utilization was measured in the skinned myocardium from euthyroid (∼60% α-MHC, n = 10; □), hyperthyroid (100% α-MHC, n = 12; •), and hypothyroid (0% α-MHC, n = 10; ○) rats and plotted against Ca2+-activated isometric force. The slope of the line is equal to tension cost. Force and ATPase were measured simultaneously in solutions of varying free Ca2+ concentrations (pCa 4.5 and 5.4–5.8). Data are means ± SE.
Calculation of fapp and gapp and Modeling of an Isometric Twitch
To model the effects of MHC content on twitch kinetics, fapp and gapp were calculated for each isoform assuming the two-state cross-bridge model originally described by Brenner (4). This model assumes that myosin cross-bridges are either attached to the thin filament and generating force or detached and not generating force. We calculated the rate constants of cross-bridge cycling using data obtained from measurements of isometric force [proportional to f/(f + g)], ktr (ktr = f + g), and ATPase activity [proportional to (f × g)/(f + g)]. Mechanical measurements yielded ATPase activity per volume of 624 ± 24 pmol·s−1·mm−3 for α-MHC and 248 ± 14 pmol·s−1·mm−3 for β-MHC, a tension cost of 15.1 ± 0.8 pmol·s−1·mm−1·mN−1 for α-MHC and 7.9 ± 1.0 pmol·s−1·mm−1·mN−1 for β-MHC, and ktr values of 23.9 ± 1.2 s−1 for α-MHC and 11.4 ± 0.7 s−1 for β-MHC. Together, these values can be used to solve a system of four equations with four unknowns:
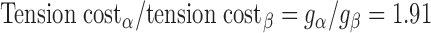 |
(1) |
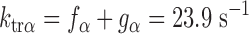 |
(2) |
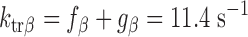 |
(3) |
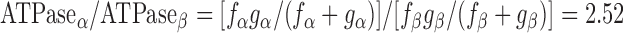 |
(4) |
The following rate constants were obtained: f = 6.84 s−1 and g = 17.06 s−1 α-MHC and f = 2.47 s−1 and g = 8.93 s−1 for β-MHC.
These rate constants were then used to model the myocardial twitch in response to a standardized Ca2+ transient, calculated as the difference between two exponential functions:
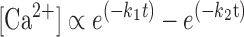 |
where k1 and k2 represent variables that can be altered to vary the duration of the Ca2+ transient. Simulations included force responses in the presence of 0%, 10%, 20%, or 30% α-MHC, all of which were calculated using the same Ca2+ transient. The mathematical model is in the form of one ordinary differential equation and one algebraic equation, which are solved by the standard fourth-order Runge-Kutta method.
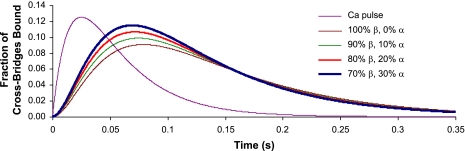
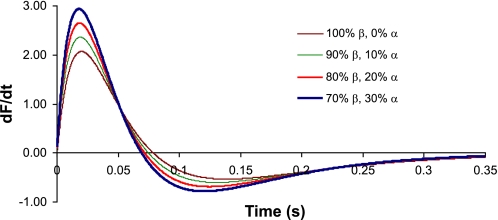
Calculations based on these rate constants predict that the kinetics of the isometric twitch are significantly accelerated as low-level expression of α-MHC is progressively increased on a mostly β-MHC background (Fig. 6). Our model predicts that the maximum rate of rise of force (dF/dtmax) for 10% α-MHC/90% β-MHC is ∼18% faster than for 100% β-MHC (Fig. 7), whereas increases in α-MHC from 0% to 20% and 0% to 30% result in increases of 37% and 54%, respectively, in dF/dtmax. Moreover, the greatest reductions in the time required to reach peak force were observed for α-MHC expression in the range of 0–10% (Table 3).
Fig. 6.
Model predictions of twitch time courses in myocardium with variable ratios of α-MHC and β-MHC. The purple line indicates the Ca2+ pulse (in AU) that was used in each of the simulations. The vertical axis indicates the fraction of cross-bridges bound; the horizontal axis is time (in s). Traces represent 0%, 10%, 20%, and 30% α-MHC of the total MHC content.
Fig. 7.
Model predictions for changes in the rate of force development (dF/dt) and relaxation in myocardium with variable expression of α-MHC. dF/dt is shown on the vertical axis; the horizontal axis is time (in s). Traces represent 0%, 10%, 20%, and 30% α-MHC of the total MHC content.
Table 3.
Effect of low-level expression of α-MHC on the time required to reach peak force
| Time Required to Reach Peak Force, ms | Percent Decrease in Time to Peak Force From 0% α-MHC | Fold Decrease in Time to Peak Force From 0% α-MHC | |
|---|---|---|---|
| 0% α-MHC | 76.8 | 1 | |
| 10% α-MHC | 72.8 | 5.2 | 0.95 |
| 20% α-MHC | 70.0 | 8.9 | 0.91 |
| 30% α-MHC | 67.6 | 12 | 0.88 |
Data were obtained from model predictions of twitch time courses in myocardium with variable ratios of α-MHC and β-MHC. Peak force was determined as the maximal force elicited by a Ca2+ transient. The fold decrease in time to peak force from 0% α-MHC was computed as the ratio of the time to peak force from 10%, 20%, and 30% α-MHC to 0% α-MHC. The time to peak force decreased with each incremental addition of α-MHC, with a concomitant increase in the rate of rise of force. The greatest increase in rate was observed when 10% α-MHC was compared with 0% α-MHC. When each incremental addition of α-MHC was compared with the iteration before it (e.g., 20% α-MHC compared with 10% α-MHC), the increase in the rate of rise of force became progressively smaller.
DISCUSSION
In the present study, we modeled an isometric twitch in the rat myocardium as a function of variable, low-level expression of α-MHC on a predominately β-MHC background using derived values for fapp and gapp for each of these isoforms. To accurately predict the rate constants of cross-bridge cycling, we generated myocardium expressing purely (100%) α-MHC or β-MHC by manipulating thyroid status. This method reliably alters the distribution of MHC isoforms without changing the expression levels of other myofibrillar proteins, thereby ensuring that the contractile differences simulated by the model are based solely on alterations in the distribution of MHC isoforms. Our model predicts significant increases in the ability of the myocardium to generate twitch force (in response to a standardized Ca2+ transient) as the level of α-MHC increases and agrees conceptually with previous work (22) showing that β-MHC behaves as a negative inotrope in rat cardiac myocytes, possibly due to a decreased number of activated cross-bridges in response to a Ca2+ transient. Here, small amounts of α-MHC (10% α-MHC/90% β-MHC) accelerated dF/dtmax by ∼18% compared with myocardium expressing 100% β-MHC. This increase is due to accelerated rates of both cross-bridge attachment (fapp) and detachment (gapp) and, from a functional standpoint, would be expected to increase ejection during systole. In addition, the rate of relaxation, which is based in part on gapp, increased to an even greater extent in preparations expressing α-MHC, which would aid ventricular filling. On the other hand, in conditions such as heart failure, reduced expression of α-MHC and correspondingly slower rates of cross-bridge cycling would increase the economy of contraction by increasing the cross-bridge force-time integral (38) and prolonging the time a cross-bridge remains in the force-generating state, i.e., a longer duty cycle (37). While there is thus an improvement in the energetic efficiency of the pump, the loss of α-MHC would be detrimental to myocardial performance since the rates of force development and relaxation would be slowed, twitch force would be reduced, and work capacity would be diminished (20, 21).
Changes in the Rate of ATP Utilization and Rate Constants of Cross-Bridge Cycling With MHC Expression
As mentioned above, animal models of heart failure and cardiac hypertrophy show a redistribution of myosin isoforms characterized by increased expression of β-MHC and a corresponding reduction in α-MHC, a phenomenon that is especially evident in small mammals such as rodents, which normally express α-MHC as the predominant isoform in adult ventricles (46). In addition, adult human ventricles express ∼10% α-MHC; yet, in conditions of heart failure, there is a significant reduction in α-MHC at both the transcriptional and protein levels (31, 34, 35). Thus, it is possible that the slower kinetics and decreased contractile force characteristic of heart failure involve the redistribution of myosin isoforms to nearly 100% expression of β-MHC and virtually no α-MHC. Our measurements of ATPase activity are consistent with this idea and indicate that increased content of β-MHC in failing hearts would at least account for the reduced twitch kinetics because it is a slower motor protein.
To predict the functional effects of alterations in MHC in the present study, it was necessary to determine fapp and gapp in myocardial preparations expressing 100% α-MHC or 100% β-MHC. Our estimates of these rate constants indicate that myosin composed of α-MHC exhibits a fapp value that was approximately threefold faster than for β-MHC myocardium, whereas the the gapp value was nearly twofold greater in α-MHC myocardium. In a functional context, rapid cross-bridge turnover due to higher levels of α-MHC would increase dF/dtmax in response to a Ca2+ transient and enhance power generation during systole, particularly when the heart is operating at or near maximal conditions. This idea is consistent with previous work (28) showing that power output varies linearly with α-MHC in both single myocytes and isolated working heart preparations from the rat. Moreover, an accelerated rate of cross-bridge detachment at the end of systole would enhance force relaxation and increase ventricular filling, thus increasing the volume of blood ejected with each beat. The influence of the myosin isoform on gapp values calculated in the present study is in agreement with other studies. Using the photolabile caged Ca2+ chelator diazo-2, Fitzsimons and colleagues (12) showed that tension relaxation, which is determined in part by gapp, was slower in hypothyroid rat myocardium expressing 100% β-MHC than in euthyroid rats expressing predominately α-MHC. It should be noted that a reduction in α-MHC during heart failure is not the sole cause of impaired relaxation. A previous study (2) has shown that Ca2+ transients are blunted and prolonged in human heart failure. In conjunction with the loss of α-MHC in heart failure, a reduction in the amplitude of the Ca2+ transient would further suppress the rate of force development during systole, whereas an increase in the duration of the Ca2+ transient would prolong the twitch, thereby slowing the rate of ventricular relaxation and impairing diastolic filling.
Effect of MHC Isoform Expression on the Ca2+ Sensitivity of Force and ktr in Rat Myocardium
Consistent with previous studies (12, 41, 43), we found no alterations in the slope of the force-pCa relationship or in the Ca2+ sensitivity of force in the rat skinned myocardium, indicating that the changes in rate constants did not alter the steady-state numbers of cross-bridges bound to actin at each submaximal Ca2+ concentration. Other studies, however, have reported a decrease (33) or an increase (14) in the Ca2+ sensitivity of force in hypothyroid rats, and, in both cases, the difference in pCa50 was much larger than that observed here. While the mechanisms underlying these shifts are not known, similar levels of myofibrillar protein content and phosphorylation combined with the lack of change in Ca2+ sensitivity of force observed in the present study indicate that hypothyroidism did not alter the isoform expression or phosphorylation states of thin filament regulatory proteins (i.e., troponin T and troponin I).
Although we found no differences in passive tension among euthyroid, hyperthyroid, and hypothyroid rat myocardium, our data did show a significant decrease in the maximum Ca2+-activated isometric force in hypothyroid rats (β-MHC), which differs from the findings of others (12, 20, 41, 43). Recent work by Wu et al. (49) has demonstrated an upregulation of type I and type III collagen expression in PTU-treated rats. While we do not completely understand the basis for the reduced force in the present study, it is possible that the preparations selected for this study (trabeculae) exhibit different structural characteristics than the mechanically disrupted preparations used in other studies, a possibility that is reinforced by the fact that our measurements of maximal Ca2+-activated force in enzymatically digested skinned single myocytes indicated no change in force per cross-sectional area. If the extracellular collagen matrix remained intact in trabeculae, this would reduce the fraction of cross-sectional area occupied by contractile protein and account for a decrease in force normalized to cross-sectional area. Moreover, while it is possible that the observed changes in force could be attributed to changes in the cytoskeleton, this is unlikely since a previous study (25) has shown that rats treated with PTU do not display alterations in the density of microtubules and yet still exhibit contractile dysfunction.
It is also unlikely that alterations in the distribution of titin isoforms existed in the hyperthyroid and hypothyroid myocardium used here. The study by Wu et al. (49) showed that the expression of the more compliant cardiac titin isoform (N2BA) increased with PTU treatment in rats but did not appear to change significantly for the length of treatment used here (5 wk); the N2BA-to-N2B ratio was ∼0.3 compared with 0.2 in untreated rats. Moreover, alterations in titin isoforms would most likely affect passive tension; we observed no such changes here.
In addition to measurements of ATP utilization and isometric force, a critical component of our estimation of cross-bridge rate constants was the determination of ktr. ktr is taken to be the sum of fapp and gapp as myosin cross-bridges transition between force-generating and non-force-generating states (5). Therefore, any change in the magnitude of ktr is due to a change in one or both of the rate constants. Consistent with recent studies (11, 12, 41, 43), we found that ktr was significantly slower in preparations expressing 100% β-MHC across all levels of activation compared with preparations expressing 100% α-MHC. In addition, Fig. 3 shows a strong linear correlation between ktr and the expression of α-MHC, indicating that 1) low-level expression of α-MHC significantly accelerates contractile kinetics over myocardium with no detectable α-MHC; and 2) under steady-state conditions where the number of cycling cross-bridges is constant, there appears to be no undue drag imposed on the faster cycling α-MHC cross-bridges by the presence of β-MHC, either in the form of V2 or V3, or in cases where α-MHC is incorporated in discrete regions of the thick filament (48).
Interestingly, low-level expression of α-MHC increased the rate of force development to a similar extent in both our mathematical simulations and measurements of ktr. As mentioned above, the derived dF/dtmax was ∼18% greater in myocardium expressing 10% α-MHC compared with 0% α-MHC, whereas we found that ktr was ∼13% greater in the group of hypothyroid rats expressing ∼15% α-MHC compared with those expressing 0% α-MHC. It is important to note that the measurements of ktr were performed during conditions of constant Ca2+ concentration rather than the limited-duration Ca2+ transient used for mathematical modeling. While ktr would thus underestimate the accelerating effect of a small population of α-MHC cross-bridges due to the presence of a larger number of slower cycling β-MHC cross-bridges than would bind during a Ca2+ transient, qualitatively the effect would be similar in that dF/dtmax would be accelerated.
Implications of Altered MHC Expression on the Rate of Rise of Pressure
Our model predictions of the kinetics of an isometric twitch reveal that myocardium with greater expression of α-MHC has a greater peak twitch force in response to a Ca2+ transient, as a direct consequence of a larger number of attached cross-bridges. In addition, the time to reach peak force progressively decreases (with a concomitant increase in dF/dtmax) as the levels of α-MHC increase (Table 3). Moreover, the maximum decrease in the time to reach peak force, and thus the greatest increase in rate, is observed when 10% α-MHC is compared with 0% α-MHC. This observation indicates that a small population of fast cycling cross-bridges disproportionately accelerates dF/dtmax in response to a limited-duration Ca2+ transient. It is important to note that an increase in α-MHC would have only proportionate effects on steady-state measurements of cross-bridge cycling (i.e., ATPase activity) due to the fact that both α-MHC and β-MHC reach steady-state distributions between attached and detached states.
At the whole organ level, our modeling data indicate that low-level expression of α-MHC on a predominately β-MHC background would accelerate dP/dtmax, thus facilitating both systolic ejection and diastolic filling. Conversely, a reduction in α-MHC content would lead to a decrease in dP/dtmax and a functional deficit, which, to some degree, would be compensated by an increase in sympathetic tone and reexpression of atrial essential MLC-1 in the ventricles of the human failing myocardium (10, 26, 39). While these observations are suggestive of an important role of α-MHC expression in the human myocardium, such an extension of the present results to humans is premature since the ATP turnover rates for both α-MHC and β-MHC are slower in humans and not known for certain. However, recent evidence has suggested that the rate of ATP utilization by β-MHC is nearly fivefold slower in the human myocardium than in the rodent (36). In light of this observation, our model would predict that expression of α-MHC on the order of 10% would dramatically increase the rate of force development and thus enhance dP/dtmax.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-61635 (to R. L. Moss).
REFERENCES
- 1.Alpert NR, Mulieri LA. Heat, mechanics, and myosin ATPase in normal and hypertrophied heart muscle. Fed Proc 41: 192–198, 1982. [PubMed] [Google Scholar]
- 2.Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85: 1046–1055, 1992. [DOI] [PubMed] [Google Scholar]
- 3.Brenner B Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner B The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single skinned rabbit psoas fibers to characterize cross-bridge kinetics in muscle for correlation with the actomyosin-ATPase in solution. Basic Res Cardiol 81, Suppl 1: 1–15, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci USA 83: 3542–3546, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell KS, Moss RL. SLControl: PC-based data acquisition and analysis for muscle mechanics. Am J Physiol Heart Circ Physiol 285: H2857–H2864, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Carnes CA, Geisbuhler TP, Reiser PJ. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats. J Appl Physiol 97: 446–453, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Everett AW, Sinha AM, Umeda PK, Jakovcic S, Rabinowitz M, Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry 23: 1596–1599, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Fabiato A Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol 157: 378–417, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Fewell JG, Hewett TE, Sanbe A, Klevitsky R, Hayes E, Warshaw D, Maughan D, Robbins J. Functional significance of cardiac myosin essential light chain isoform switching in transgenic mice. J Clin Invest 101: 2630–2639, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzsimons DP, Patel JR, Moss RL. Aging-dependent depression in the kinetics of force development in rat skinned myocardium. Am J Physiol Heart Circ Physiol 276: H1511–H1519, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol 513: 171–183, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem 68: 687–728, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Gibson LM, Wendt IR, Stephenson DG. Contractile activation properties of ventricular myocardium from hypothyroid, euthyroid and juvenile rats. Pflügers Arch 422: 16–23, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem 129: 277–287, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Glyn H, Sleep J. Dependence of adenosine triphosphatase activity of rabbit psoas muscle fibres and myofibrils on substrate concentration. J Physiol 365: 259–276, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279–297, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorza L, Mercadier JJ, Schwartz K, Thornell LE, Sartore S, Schiaffino S. Myosin types in the human heart. An immunofluorescence study of normal and hypertrophied atrial and ventricular myocardium. Circ Res 54: 694–702, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Haddad F, Masatsugu M, Bodell PW, Qin A, McCue SA, Baldwin KM. Role of thyroid hormone and insulin in control of cardiac isomyosin expression. J Mol Cell Cardiol 29: 559–569, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol 281: H1217–H1222, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res 90: 1150–1152, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Herron TJ, Vandenboom R, Fomicheva E, Mundada L, Edwards T, Metzger JM. Calcium-independent negative inotropy by beta-myosin heavy chain gene transfer in cardiac myocytes. Circ Res 100: 1182–1190, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hoh JF, McGrath PA, Hale PT. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol 10: 1053–1076, 1978. [DOI] [PubMed] [Google Scholar]
- 24.Holubarsch C, Litten RZ, Mulieri LA, Alpert NR. Energetic changes of myocardium as an adaptation to chronic hemodynamic overload and thyroid gland activity. Basic Res Cardiol 80: 582–593, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi Y, Takahashi M, Isomatsu Y, Qiao F, Iijima Y, Shiraishi H, Simsic JM, Baicu CF, Robbins J, Zile MR, Cooper GT. Role of microtubules versus myosin heavy chain isoforms in contractile dysfunction of hypertrophied murine cardiocytes. Am J Physiol Heart Circ Physiol 285: H1270–H1285, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Khalina YN, Bartsch H, Petzhold D, Haase H, Podlubnaya ZA, Shpagina MD, Morano I. Reconstitution of ventricular myosin with atrial light chains 1 improves its functional properties. Acta Biochim Pol 52: 443–448, 2005. [PubMed] [Google Scholar]
- 27.Kiss E, Brittsan AG, Edes I, Grupp IL, Grupp G, Kranias EG. Thyroid hormone-induced alterations in phospholamban-deficient mouse hearts. Circ Res 83: 608–613, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Korte FS, Herron TJ, Rovetto MJ, McDonald KS. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol 289: H801–H812, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lompre AM, Mercadier JJ, Schwartz K. Changes in gene expression during cardiac growth. Int Rev Cytol 124: 137–186, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259: 6437–6446, 1984. [PubMed] [Google Scholar]
- 31.Lowes BD, Minobe W, Abraham WT, Rizeq MN, Bohlmeyer TJ, Quaife RA, Roden RL, Dutcher DL, Robertson AD, Voelkel NF, Badesch DB, Groves BM, Gilbert EM, Bristow MR. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest 100: 2315–2324, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNally EM, Kraft R, Bravo-Zehnder M, Taylor DA, Leinwand LA. Full-length rat alpha and beta cardiac myosin heavy chain sequences. Comparisons suggest a molecular basis for functional differences. J Mol Biol 210: 665–671, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Metzger JM, Wahr PA, Michele DE, Albayya F, Westfall MV. Effects of myosin heavy chain isoform switching on Ca2+-activated tension development in single adult cardiac myocytes. Circ Res 84: 1310–1317, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86: 386–390, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest 100: 2362–2370, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narolska NA, van Loon RB, Boontje NM, Zaremba R, Penas SE, Russell J, Spiegelenberg SR, Huybregts MA, Visser FC, de Jong JW, van der Velden J, Stienen GJ. Myocardial contraction is 5-fold more economical in ventricular than in atrial human tissue. Cardiovasc Res 65: 221–229, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Palmiter KA, Tyska MJ, Dupuis DE, Alpert NR, Warshaw DM. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J Physiol 519: 669–678, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez NG, Hashimoto K, McCune S, Altschuld RA, Marban E. Origin of contractile dysfunction in heart failure: calcium cycling versus myofilaments. Circulation 99: 1077–1083, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Ritter O, Luther HP, Haase H, Baltas LG, Baumann G, Schulte HD, Morano I. Expression of atrial myosin light chains but not alpha-myosin heavy chains is correlated in vivo with increased ventricular function in patients with hypertrophic obstructive cardiomyopathy. J Mol Med 77: 677–685, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Rossmanith GH, Hamilton AM, Hoh JF. Influence of myosin isoforms on tension cost and crossbridge kinetics in skinned rat cardiac muscle. Clin Exp Pharmacol Physiol 22: 423–429, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Rundell VL, Manaves V, Martin AF, de Tombe PP. Impact of β-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am J Physiol Heart Circ Physiol 288: H896–H903, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Stelzer JE, Brickson SL, Locher MR, Moss RL. Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J Physiol 579: 161–173, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stienen GJ, Roosemalen MC, Wilson MG, Elzinga G. Depression of force by phosphate in skinned skeletal muscle fibers of the frog. Am J Physiol Cell Physiol 259: C349–C357, 1990. [DOI] [PubMed] [Google Scholar]
- 45.Strang KT, Sweitzer NK, Greaser ML, Moss RL. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res 74: 542–549, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Swynghedauw B Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev 66: 710–771, 1986. [DOI] [PubMed] [Google Scholar]
- 47.Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 320: 149–151, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Wenderoth MP, Eisenberg BR. Incorporation of nascent myosin heavy chains into thick filaments of cardiac myocytes in thyroid-treated rabbits. J Cell Biol 105: 2771–2780, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. J Mol Cell Cardiol 42: 186–195, 2007. [DOI] [PubMed] [Google Scholar]