Abstract
Human cytochrome P-450 (CYP)2J2 is abundant in heart and active in biosynthesis of epoxyeicosatrienoic acids (EETs). Recently, we demonstrated that these eicosanoid products protect myocardium from ischemia-reperfusion injury. The present study utilized transgenic (Tr) mice with cardiomyocyte-specific overexpression of human CYP2J2 to investigate protection toward toxicity resulting from acute (0, 5, or 15 mg/kg daily for 3 days, followed by 24-h recovery) or chronic (0, 1.5, or 3.0 mg/kg biweekly for 5 wk, followed by 2-wk recovery) doxorubicin (Dox) administration. Acute treatment resulted in marked elevations of serum lactate dehydrogenase and creatine kinase levels that were significantly greater in wild-type (WT) than CYP2J2 Tr mice. Acute treatment also resulted in less activation of stress response enzymes in CYP2J2 Tr mice (catalase 750% vs. 300% of baseline, caspase-3 235% vs. 165% of baseline in WT vs. CYP2J2 Tr mice). Moreover, CYP2J2 Tr hearts exhibited less Dox-induced cardiomyocytes apoptosis (measured by TUNEL) compared with WT hearts. After chronic treatment, comparable decreases in body weight were observed in WT and CYP2J2 Tr mice. However, cardiac function, assessed by measurement of fractional shortening with M-mode transthoracic echocardiography, was significantly higher in CYP2J2 Tr than WT hearts after chronic Dox treatment (WT 37 ± 2%, CYP2J2 Tr 47 ± 1%). WT mice also had larger increases in β-myosin heavy chain and cardiac ankryin repeat protein compared with CYP2J2 Tr mice. CYP2J2 Tr hearts had a significantly higher rate of Dox metabolism than WT hearts (2.2 ± 0.25 vs. 1.6 ± 0.50 ng·min−1·100 μg protein−1). In vitro data from H9c2 cells demonstrated that EETs attenuated Dox-induced mitochondrial damage. Together, these data suggest that cardiac-specific overexpression of CYP2J2 limited Dox-induced toxicity.
Keywords: cytochrome P-450 2J2, heart, function
cytochrome p-450 (CYP) epoxygenases are predominant enzymes responsible for the epoxidation of endogenous arachidonic acid (AA) to four regioisomeric epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EETs). These epoxy fatty acid products have an important role in cellular signaling and possess numerous biological activities in the cardiovascular system (54). The actions of EETs are terminated by conversion to the corresponding and less biologically active dihydroxyeicosatrienoic acids (DHETs) by epoxide hydrolases (41). The role of EETs in cardiovascular homeostasis and protection against ischemia-reperfusion injury has been demonstrated in both rodent and dog (19, 48). While a protective role in ischemia-reperfusion injury is known, it remains to be investigated whether EETs mediate myocardial protection against other pathological stressors.
Doxorubicin (Dox, Adriamycin) is a quinone-containing anthracycline antibiotic commonly used in treatment of a variety of human neoplastic diseases and solid cancers. Its clinical use is limited by irreversible cardiomyopathy and heart failure (11, 39). Dox-induced cardiotoxicity can be characterized by acute myocardial injury, which occurs immediately after an initial treatment. Alternatively, chronic cardiotoxicity, which can lead to conditions such as congestive heart failure, ventricular dysfunction, and arrhythmia, may occur years to decades after treatment (36, 47, 55). It is well established that the adverse effect of Dox treatment is mediated by mechanisms distinct from its therapeutic mode of action, which involves interacting with DNA by intercalation and thereby inhibiting macromolecular biosynthesis (18). Dox-induced cardiotoxicity results from a series of adverse reactions involving increased oxidative stress, alteration in cellular energetics, and initiation of cell death pathways (39, 57, 59). Numerous reports have demonstrated moderate protection against Dox-induced cardiotoxicity, yet the exact mechanism(s) and optimal therapy remain unknown (3, 6, 7, 9, 31).
Recently, we reported (48, 50) that enhanced postischemic recovery of left ventricular (LV) function and reduced cardiac injury are found in transgenic (Tr) mice that overexpress human CYP2J2 and in mice with targeted deletion of soluble epoxide hydrolase. Whether the overexpression of CYP2J2 can protect against Dox-induced cardiotoxicity has not been investigated. To examine the role of human CYP2J2 isoform in a model of Dox-induced toxicity we utilized Tr mice with cardiac-specific overexpression of CYP2J2. Results of these studies indicate that CYP2J2 overexpression provided significant protection in both an acute and a chronic model of Dox-induced injury.
MATERIALS AND METHODS
Animals.
Mice with cardiomyocyte-specific overexpression of human CYP2J2 and their wild-type (WT) littermate controls were utilized (48). All experiments used male and female mice aged 3–5 mo and weighing 25–35 g and were approved by the National Institute of Environmental Health Sciences/National Institutes of Health and University of Alberta Animal Care and Use Committees.
Treatment protocols.
In the acute protocol, mice were randomly divided into three groups and received 0, 5, or 15 mg/kg Dox by intraperitoneal injections (Fig. 1A). Mice were treated with a single dose each day for 3 days (0, 24, and 48 h) and were killed by CO2 asphyxiation on the fourth day (72 h). Hearts were either isolated and perfused in the Langendorff mode to assess heart function (see below) or collected for histological and biochemical analyses.
Fig. 1.
A: acute doxorubicin (Dox) treatment protocol. B: chronic Dox treatment protocol.
In the chronic protocol, mice were randomly divided into three groups and received 0, 1.5, or 3.0 mg/kg Dox by intraperitoneal injections (Fig. 1B). Dox was administered twice a week for 5 wk for a total of 10 treatments. A 2-wk “washout” period was allowed after the last treatment, at which point cardiac function was assessed by echocardiography. Mice were then killed by CO2 asphyxiation, and cardiac specimens were analyzed. All studies were conducted by investigators who were blinded to treatment group assignments.
Biochemical analyses.
At the end of each protocol, blood was drawn from the inferior vena cava to assess levels of lactate dehydrogenase (LDH) and creatine kinase (CK). Serum was collected within 2 h from clotted blood by centrifugation and analyzed with end point assay kits (Sigma Diagnostics, St. Louis, MO). LDH and CK activities were expressed as units per liter. Subcellular fractions were prepared by differential centrifugation from frozen hearts as described previously (15). Catalase activity was measured with a spectrophotometric assay that monitored H2O2 disappearance at 240 nm and used the molar extinction coefficient 43.6 M−1cm−1 (2, 15). Caspase-3 activity was assessed with a spectrofluorometric assay as previously described (49). Briefly, caspase-3 activity was determined in cytosolic fractions by monitoring the release of 7-amino-4-methylcoumarin (AMC) by proteolytic cleavage of the peptide Ac-DEVD-AMC (20 μM) (Sigma-Aldrich, Oakville, ON, Canada). Fluorescence was monitored at wavelengths of 380 nm (excitation) and 460 nm (emission). Specific activities were determined to be within the linear range of a standard curve established with AMC. Protein quantities were determined with a Bradford protein assay kit (Bio-Rad Laboratories).
Isolated, perfused hearts.
Hearts were perfused in the Langendorff mode as described previously (48, 50). Hearts from CYP2J2 Tr and age-/sex-matched WT littermate control animals were perfused in a retrograde fashion at constant pressure (90 cmH2O) with continuously aerated (95% O2-5% CO2) Krebs-Henseleit buffer at 37°C. Hearts were perfused for 40 min (stabilization) and then subjected to 20 min of global no-flow ischemia, followed by 40 min of reperfusion. Recovery of contractile function was taken as LV developed pressure (LVDP) at the end of reperfusion expressed as a percentage of preischemic LVDP.
Transthoracic echocardiography.
Two-dimensional M-mode echocardiography was performed with a Vevo 770 high-resolution imaging system (VisualSonics) as described previously (48, 50). LV end-diastolic dimension (LVDd, mm) and LV end-systolic dimension (LVDs, mm) were assessed. Fractional shortening (FS) of the LV was expressed as %FS = [(LVDd − LVDs)/LVDd] × 100.
Histology and gene expression.
Histological analyses were performed on hearts from both CYP2J2 Tr and WT mice as previously described (48). Briefly, hearts were removed, dissected, fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned for examination. Sections were used for a terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) procedure for detecting apoptotic cardiomyocytes as previously described (16). The percentage of TUNEL-positive nuclei was determined by counting 10 random fields. All tissues were stored at −80°C until preparation of RNA. Total RNA was isolated with an RNeasy Midi kit (Qiagen, Valencia, CA) and concentrated with a Microcon YM-30 column (Millipore, Billerica, MA). A formaldehyde agarose gel containing ethidium bromide was used to assess the quality of the RNA. Semiquantitative PCR analysis was performed for alterations in gene expression. PCR primers for α-myosin heavy chain (α-MHC) were 5′-GGA AGA GTG AGC GGC GCA TCA AGG-3′ (forward) and 5′-CTG CTG GAG AGG TTA TTC CTC G-3′ (reverse); for β- myosin heavy chain (β-MHC) were 5′-GCC AAC ACC AAC CTG TCC AAG TTC-3′ (forward) and 5′-TGC AAA GGC TCC AGG TCT GAG GGC-3′ (reverse); for cardiac ankyrin repeat protein (CARP) were 5′-TGC GAT GAG TAT AAA CGG ACG-3′ (forward) and 5′-GTG GAT TCA AGC ATA TCT CGG AA-3′ (reverse); and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5′-ACC ACA GTC CAT GCC ATC AC-3′ (forward) and 5′-TCC ACC ACC CTG TTG CTG TA-3′ (reverse). Amplification was as follows: 1) 50°C for 2 min; 2) 95°C for 5 min; 3) 30 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 2 min; 4) 72°C for 10 min. The products were fractionated on a 2% agarose gel and visualized by ethidium bromide staining. The ratio of β-MHC to α-MHC and CARP expression were quantitated by assessing relative cDNA levels of the genes compared with GAPDH expression from the same sample. Immunoblots were prepared with S9 fractions (50 μg protein) isolated from hearts and probed with antibodies to anti-CYP2J2pep1 (1:1,000) and GAPDH (1:1,000) (Santa Cruz Biotechnology, Santa Cruz, CA) (32, 48, 50).
Measurement of Dox metabolism.
The ability of WT and CYP2J2 Tr mice to metabolize Dox was evaluated in an HPLC assay. Briefly, microsomal proteins from WT and CYP2J2 Tr hearts (1 mg protein/ml) were incubated with Dox (500 nM) at 37°C for 60 min in a buffer containing 50 mM potassium phosphate, 1.15% KCl, pH 7, and 1 mM NADPH (17, 34). Control experiments were performed with the selective P-450 epoxygenase inhibitor N-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MS-PPOH) (50 μM; generously provided by Dr. J. Falck, University of Texas, Dallas, TX). The reactions were stopped by the addition of 300 μl of acetonitrile, and Dox was extracted with a chloroform and 2-propanol (1:1 vol/vol) procedure, dried, and redissolved in 120 μl of methanol. Samples were injected into a Waters 712 WISP HPLC equipped with a Schima D RF-10AXL fluorescence detector (17, 34, 38). A C18 10-μm Bondapak column was utilized with a formic acid (0.05%):acetonitrile gradient mobile phase in reverse mode. All products were identified based on coelution with authentic standards. Standard curves prepared with Dox (0–1,000 ng/ml) were used to determine concentration differences and specific activity (excitation 470 nm, emission 560 nm).
Mitochondrial assessment.
H9c2 cells (American Type Culture Collection, Manassas, VA) were cultured and grown in DMEM supplemented with 10% bovine serum albumin and antibiotics such as penicillin and streptomycin at 37°C in an atmosphere of 5% CO2-95% air. Cells were loaded with 150 nM tetramethylrhodamine ethyl ester (TMRE) (Invitrogen) for 30 min and then subjected to time-lapse imaging for 60 min at 37°C and 5% CO2. A Zeiss Axio Observer Z1 inverted epifluorescence microscope was used to take z-stack images every minute with 200-ms exposure time. Cells were observed under a ×40 objective, fluorescence was excited at the 555-nm line, and emission was recorded with a band-pass filter of 575–640 nm. Changes in fluorescence were recorded in cells treated with vehicle (0.5% ethanol in PBS), Dox (10 μM), 11,12-EET (1 or 10 μM) or 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) (1 μM; generously provided by Dr. J. Falck, University of Texas). Mitochondrial morphology changes were visualized over the 60-min exposure period, and individual mitochondria were assessed for alterations to the elongated and filamentous appearance found in control cells. The term “punctate mitochondria” was used to describe both condensed and fragmented mitochondrial morphology as described elsewhere (35). Measurements were taken from four or five individual experiments, and intensities were quantified relative to background levels. Individual mitochondria were quantified in multiple images taken at similar magnifications with AxioVision Software (Carl Zeiss Imaging Solutions). Changes in fluorescence were expressed as percent change relative to baseline levels.
Statistical analysis.
Values are expressed as means ± SE. Statistical significance was determined by the unpaired Student's t-test and one-way ANOVA followed by a Duncan's test to assess multiple group comparisons. Values were considered significant if P < 0.05.
RESULTS
Effects of acute Dox administration.
Serum CK and LDH activities increased in a dose-dependent manner after three consecutive daily administrations of Dox in both WT and CYP2J2 Tr mice (Fig. 2, A and B). However, overexpression of CYP2J2 resulted in lower serum CK and LDH activities after Dox treatment, indicative of reduced myocardial injury. Dox-mediated cardiotoxicity has been shown to involve production of reactive oxygen species (ROS) (1, 8). In turn, increased intracellular ROS activate endogenous antioxidant enzymes, such as catalase. Catalase is an important antioxidant enzyme that catalyzes the decomposition of hydrogen peroxide to water and oxygen. Acute Dox treatment resulted in significant increases in cardiac catalase activities in both WT and CYP2J2 Tr mice, although this increase was less pronounced in the latter group (Fig. 2C).
Fig. 2.
Acute exposure to Dox. A and B: serum creatine kinase (CK; A) and lactate dehydrogenase (LDH; B) levels after 3 consecutive daily administrations of Dox (0, 5, or 15 mg/kg); n = 6–8 per group. *P < 0.05 treated vs. control of the same genotype; ^P < 0.05 vs. wild type (WT). CYP2J2, cytochrome P-450 2J2; Tr, transgenic. C: catalase activity in hearts from mice after 3 consecutive daily administrations of Dox (0 or 15 mg/kg); n = 5 per group. *P < 0.05 treated vs. control of the same genotype; ^P < 0.05 vs. WT. D: postischemic left ventricular (LV) developed pressure (LVDP) recovery at 40 min of reperfusion (R40) expressed as % of baseline LVDP in hearts from mice after 3 consecutive daily administrations of Dox (0 or 15 mg/kg); n = 5 per group. *P < 0.05 vs. control of the same genotype; ^P < 0.05 vs. WT.
Postischemic cardiac performance was decreased in Dox-treated WT mice compared with vehicle-treated mice as measured by LVDP (21.8 ± 5% vs. 12.3 ± 3% for the 0 and 15 mg/kg groups, respectively; P < 0.05) (Fig. 2D). CYP2J2 Tr mice exhibited improved postischemic cardiac function compared with WT mice, consistent with previously published results (48). Importantly, no decrease in LVDP was observed in CYP2J2 Tr hearts after Dox treatment (38 ± 3% vs. 36 ± 11% for the 0 and 15 mg/kg groups, respectively) (Fig. 2D).
Evidence suggests that cellular apoptotic responses may be triggered subsequent to Dox exposure. Therefore, we further assessed cardiac injury by analyzing cytosolic fractions for caspase-3 activity. Compared with mice treated with vehicle, caspase-3 activity was significantly higher in both WT and CYP2J2 Tr mice treated with Dox (15 mg/kg) (Fig. 3A). Importantly, caspase-3 activity was significantly higher in WT hearts than in CYP2J2 Tr hearts after Dox treatment (84 ± 8 vs. 55 ± 9 pmol·min−1·mg protein−1, respectively; P < 0.05) (Fig. 3A). Consistent with these results, significant increases in TUNEL-positive nuclei were observed in WT hearts after acute treatment with Dox, whereas no significant changes were observed in CYP2J2 Tr hearts (Fig. 3B). However, similar to a recent report by Hiraumi et al. (21), we did not observe any significant histological changes with hematoxylin and eosin staining on repeated blinded analyses.
Fig. 3.
Apoptotic response following acute Dox. A: cardiac caspase-3 activity. Cytosolic fractions were prepared from hearts after acute exposure to Dox (0 or 15 mg/kg) and analyzed for caspase-3 activity with Ac-DEVD-AMC as substrate; n = 5. *P < 0.05 Dox vs. control of the same genotype; ^P < 0.05 CYP2J2 Tr vs. respective WT mice. B: terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL)-positive cells. Myocardium apoptosis was assessed by TUNEL assay in heart slices after acute exposure to Dox (0 or 15 mg/kg). Values represent % increase in TUNEL-positive nuclei above control groups from the same genotype; n = 10–15 hearts per group. *P < 0.05 Dox vs. control of the same genotype.
Effects of chronic Dox administration.
Chronic treatment with Dox resulted in significant dose-dependent decreases in body weight in both WT and CYP2J2 Tr mice (Fig. 4, A and B). After Dox treatment was stopped and during the “washout” period, body weight stabilized and began to recover in all Dox-treated animals. Mice were killed at the end of the recovery period, after which heart weights were measured and compared with changes in body weight. As shown in Fig. 4C, heart weight-to-body weight ratios increased in a dose-dependent manner after Dox treatment. However, the magnitude of increase was significantly larger in WT mice than in CYP2J2 Tr mice at the highest Dox dose, suggestive of cardiac hypertrophy.
Fig. 4.
Chronic exposure to Dox. A and B: body weight change in WT (A) and CYP2J2 Tr (B) mice. Animal weights were taken during the chronic Dox protocol; n = 12–15. C: analysis of heart weight-to-body weight ratios (HW:BW) in WT and CYP2J2 Tr mice after chronic Dox treatment (0, 1.5, or 3.0 mg/kg); n = 10–15. *P < 0.05 DOX vs. control of the same genotype; ^P < 0.05 CYP2J2 Tr vs. respective WT. D: semiquantitative PCR analysis of myosin heavy chain (MHC) gene expression. β-MHC:α-MHC expression was quantitated by assessing relative cDNA of both genes from the same individual samples; n = 3. *P < 0.05 Dox vs. control. E: semiquantitative PCR analysis of cardiac ankyrin repeat protein (CARP) gene expression. CARP expression was quantitated by assessing relative cDNA to GAPDH expression from the same individual samples; n = 3. *P < 0.05 Dox vs. control. F: Dox metabolism is increased in CYP2J2 Tr compared with WT hearts and attenuated by the P-450 epoxygenase inhibitor N-methylsulfonyl-6-(propargyloxyphenyl)hexanamide (MS-PPOH, 50 μM). Values are means ± SE; n = 4. *P < 0.05 vs. WT.
In contrast to the acute protocol, serum CK and LDH activities were not different in Dox-treated mice compared with vehicle control animals at the end of the chronic protocol (see Supplemental Data for this article).1 Additionally, no significant difference in caspase-3 activity was observed between Dox-treated and vehicle-treated mice in the chronic study (see Supplemental Data). To determine whether chronic Dox treatment resulted in significant changes to the cardiac structure, we examined expression levels of MHC and CARP in WT and CYP2J2 Tr mice. Analysis for MHC gene expression following chronic Dox treatment revealed an increased β-MHC-to-α-MHC ratio in WT mice but not in CYP2J2 Tr mice (Fig. 4D). CARP is a transcriptional cofactor and structural component of the sarcomere involved in cardiogenesis and muscle injury (46). Studies have demonstrated that Dox treatment can induce CARP in vivo but repress CARP expression in cell culture systems (5, 60). As shown in Fig. 4E, chronic Dox treatment increased CARP expression only in WT mice. Together the data indicate that CYP2J2 Tr mice had less cardiac injury than WT mice after chronic Dox treatment.
To examine whether CYP2J2 can metabolize Dox, we incubated microsomes from CYP2J2 Tr and WT mouse hearts and measured Dox turnover. As shown in Fig. 4F, CYP2J2 Tr mice demonstrated a significantly higher rate of Dox metabolism (2.2 ± 0.25 ng·min−1·100 μg protein−1) compared with WT mice (1.6 ± 0.50 ng·min−1·100 μg protein−1). Importantly, the selective epoxygenase inhibitor MS-PPOH abolished the improved metabolism observed in CYP2J2 Tr mice (Fig. 4F). Thus the enzymatic activity was comparable in the two genotypes after treatment with MS-PPOH. Immunoblot analysis demonstrated that Dox treatment did reduce the expression level of CYP2J2 protein in hearts after chronic administration (Supplemental Data).
Cardiac function after chronic administration of Dox.
To determine whether contractile function was affected by chronic Dox administration, LVDd and LVDs were assessed by transthoracic echocardiography at the end of the recovery period and LVFS was calculated. As shown in Fig. 5, there was no significant difference in these parameters between WT mice and CYP2J2 Tr mice in the vehicle control groups, indicating that CYP2J2 Tr mice had normal chamber dimensions and basal contractile function. Dox treatment caused significant dose-dependent increases in both LVDd and LVDs in WT mice (Fig. 5, A and B). In contrast, there were no significant changes in LVDd or LVDs in CYP2J2 Tr mice after Dox treatment. DOX caused a reduction in %FS in WT and CYP2J2 Tr mice, although the decrease in %FS was significantly less in CYP2J2 Tr mice (Fig. 5C). These data suggest that CYP2J2 Tr hearts had significantly better cardiac function than WT hearts after chronic Dox treatment. There were no significant changes in heart rate between the groups (Fig. 5D).
Fig. 5.
Assessment of cardiac function after chronic Dox treatment. A and B: LV end-diastolic dimension (LVDd, mm; A) and LV end-systolic dimension (LVDs, mm; B) after chronic Dox treatment (0, 1.5, or 3.0 mg/kg); n = 12–17. *P < 0.05 Dox vs. control of the same genotype; ^P < 0.05 CYP2J2 Tr vs. respective WT. C: fractional shortening (FS) after chronic Dox treatment (0, 1.5, or 3.0 mg/kg). FS of the LV is expressed as %FS = (LVDd − LVDs)/LVDd × 100; n = 12–17. *P < 0.05 Dox vs. control of the same genotype; ^P < 0.05 CYP2J2 Tr vs. respective WT. D: heart rate (HR) after chronic DOX treatment (0, 1.5, or 3.0 mg/kg); n = 12–17. bpm, beats/min.
EETs limit mitochondrial damage caused by Dox in H9C2 cells.
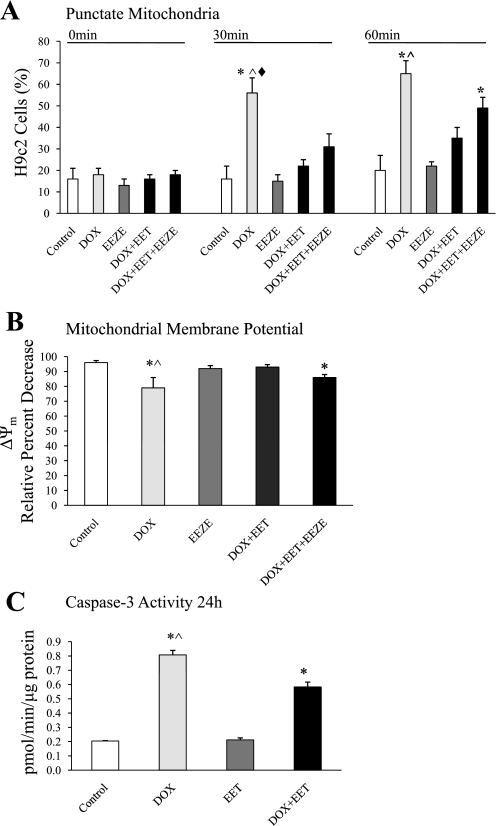
Recent reports indicate that mitochondria undergo dramatic fragmentation and dysfunction in response to Dox-induced toxicity (13, 21, 23, 43). To determine whether EETs can limit Dox injury, we investigated the mitochondrial morphology and membrane potential in H9c2 cells by real-time imaging. Mitochondria, which exhibit elongated and filamentous morphology in healthy control cells, became dramatically shorter and round after Dox exposure in H9c2 cells loaded with TMRE (Fig. 6 and Fig. 7A). Dox exposure resulted in the dissipation of fluorescence from the cells within 30 min (Fig. 7B), indicating changes in mitochondrial membrane potential. Pretreatment of H9c2 cells with 11,12-EET significantly attenuated the fragmentation and conversion of tubular mitochondria to punctate mitochondria (Fig. 6 and Fig. 7A). Blinded quantitative analysis revealed significantly higher relative fluorescence intensity in cells cotreated with 11,12-EET and Dox compared with Dox alone (Fig. 7B). Together these data suggest that EETs attenuated mitochondrial fission and slowed the collapse of the membrane potential. To confirm that the effect was mediated by EETs, we conducted experiments in the presence of 14,15-EEZE. This putative pan-EET receptor antagonist had no effect on mitochondria when administered alone and abrogated the effect of 11,12-EET in H9c2 cells (Figs. 6 and 7). In further analysis of downstream effects of mitochondrial dysfunction, Dox-induced caspase-3 activity was partially attenuated by cotreatment with EETs (Fig. 7C).
Fig. 6.
Assessment of mitochondrial morphology in H9c2 cells. Representative frames from time-lapse series show H9c2 cells treated with vehicle (0.5% EtOH in PBS), 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 1 μM), Dox (10 μM), Dox (10 μM) + 11,12-epoxyeicosatrienoic acid (11,12-EET, 10 μM), or Dox (10 μM) + 11,12-EET (10 μM) + 14,15-EEZE (1 μM) at 0, 30, and 60 min. Mitochondrial morphology, filamentous and tubular shape, of the control cells remains unaltered during this time period. In contrast, Dox-treated cells exhibit significant punctate and fragmented mitochondrial morphology, marked by arrows, which is attenuated in EET-treated cells.
Fig. 7.
Assessment of mitochondrial morphology and membrane potential (ΔΨm) in H9c2 cells. A: mitochondrial morphology. Histograms represent % of punctate mitochondria present in individual cells relative to total mitochondria. Cells were treated with vehicle (0.5% EtOH in PBS), Dox (10 μM), 14,15-EEZE (1 μM), Dox (10 μM) + 11,12-EET (10 μM), or Dox (10 μM) + 11,12-EET (10 μM) + 14,15-EEZE (1 μM). Values represent means ± SE; n = 4 or 5. *P < 0.05 vs. vehicle control; ^P < 0.05 vs. Dox+EET treated; ♦P < 0.05 vs. Dox+EET+EEZE treated. B: ΔΨm. Histograms representing % of tetramethylrhodamine ethyl ester (TMRE) fluorescence lost in H9c2 cells after collapse of ΔΨm. Cells were treated with vehicle (EtOH), Dox (10 μM), 14,15-EEZE (1 μM), Dox (10 μM) + 11,12-EET (10 μM), or Dox (10 μM) + 11,12-EET (10 μM) + 14,15-EEZE (1 μM). Values are mean ± SE % change in relative fluorescence from baseline; n = 4 or 5. *P < 0.05 vs. vehicle control; ^P < 0.05 vs. Dox+EET treated. C: caspase-3 activity in H9c2 cells using Ac-DEVD-AMC as substrate. Cells were treated with vehicle (EtOH), Dox (10 μM), 11,12-EET (10 μM), or Dox (10 μM) + 11,12-EET (10 μM) for 24 h. Values represent means ± SE; n = 4 or 5. *P < 0.05 vs. vehicle control; ^P < 0.05 vs. Dox+EET treated.
DISCUSSION
Several hypotheses have been put forth to explain the cardiotoxicity that limits the therapeutic use of Dox (37, 52, 53), including generation of free radicals in cardiomyocyte mitochondria. There is an increasing amount of literature reporting the functional significance of CYP monooxygenase enzymes in the heart. This is particularly true for CYP2J2, a primarily cardiac P-450 active in the epoxidation of AA to EETs (61). In the present study, we demonstrate that cardiac overexpression of the human CYP2J2 cDNA limits Dox-induced toxicity in mice by maintaining LV function and increased Dox metabolism. Moreover, our in vitro data demonstrate direct protective effects of CYP2J2-derived metabolites, EETs, toward Dox-mediated mitochondrial damage.
Dox-induced injury can be indirectly monitored by the release of CK and LDH into the serum. No differences in baseline CK and LDH were observed between CYP2J2 Tr and WT mice, but acute Dox treatment resulted in significant increases in serum LDH and CK levels suggestive of tissue damage, consistent with other reports (56). Importantly, these levels were significantly lower in mice overexpressing CYP2J2 than in WT mice. Although Dox toxicity occurs more selectively in heart than in other tissues, serum LDH and CK can arise from multiple organs. However, as CYP2J2 was only overexpressed in cardiomyocytes of CYP2J2 Tr mice and circulating EETs levels are similar between WT and CYP2J2 Tr mice (48), the data presented here infer a reduction in cardiac-specific CK and LDH as a result of CYP2J2 overexpression.
Evidence indicates that cardiomyocyte apoptosis plays a significant role in cardiac dysfunction in Dox-induced cardiomyopathy (39, 59). Here we observed that hearts from CYP2J2 Tr mice had reduced activation of caspase-3 and reduced TUNEL-positive cells after acute Dox administration, consistent with reports demonstrating the antiapoptotic effects of CYP2J2-derived EETs in other cell types (12, 26, 63). It is plausible that the increased level of EETs in the hearts of CYP2J2 Tr mice mediated this response or that CYP2J2 was involved in the metabolism of Dox. Although the exact antiapoptotic mechanism(s) of EETs is not known, it appears to involve p42/p44-MAPK and phosphatidylinositol 3-kinase/Akt pathways (12, 26, 63). Manifestation of acute injury results in functional decline in cardiac performance following Dox toxicity. In this regard, cardiac dysfunction was marginally evident during baseline perfusions as evidenced by decline in both inotropy and lusitropy before ischemic insult, consistent with other reports (58). However, the cardioprotective effect of CYP2J2 was prominent after ischemia-reperfusion, when the LVDP of Dox-treated CYP2J2 Tr mice did not differ significantly from that of vehicle-treated mice whereas Dox-treated WT mice had a significant decline in LVDP compared with vehicle-treated mice. These data are strongly suggestive of a protective effect of CYP2J2 in maintaining cardiac function after Dox administration.
To assess the influence of CYP2J2 on late events in Dox-mediated cardiotoxicity, we used a chronic Dox administration protocol. Our results revealed a general toxicity that occurred in both WT and CYP2J2 Tr mice and manifested as a significant decrease in body weight, most likely stemming from severe anorexia, poor oral intake, and dehydration. Interestingly, Dox-induced increases in heart weight-to-body weight ratios, β-MHC-to-α-MHC ratios, and CARP expression were greater in WT mice than in CYP2J2 Tr mice. While the functional role of CARP is not fully understood, evidence suggests its involvement in mechanical or stress responses, where it contributes to tissue repair (46). CARP appears to have a role in structural organization of sarcomeres as well as in the transcriptional machinery of cardiomyocytes, striated muscles, and vasculature (51). Increased expression of CARP has been observed in several cardiovascular injuries, such as LV dilated cardiomyopathy and pressure-overload hypertrophy (4, 46). Interestingly, opposing data are available regarding Dox-mediated regulation of CARP expression, with increased expression reported in vivo (60) and repression reported in vitro; these differences have been attributed to differences in timing and models utilized (46). Data presented here indicate that cardiac overexpression of CYP2J2 resulted in maintenance of control levels of CARP expression after chronic Dox administration, although the significance of this finding for the maintenance of overall cardiac function is unclear. Consistent with adverse effects, there was a decrease in cardiac function in WT mice that was either not apparent (LVDd and LVDs) or not as severe (LVFS) in CYP2J2 Tr mice. As such changes have been well documented in various models of DOX-induced heart failure (28, 29, 42, 44), the results found here are indicative of a significant protection against Dox-induced cardiac dysfunction by CYP2J2 overexpression.
Evidence demonstrates that CYP enzymes are expressed in the heart, where they may participate in the metabolism of therapeutic agents, environmental toxicants, and endogenous compounds (10, 14, 48). Currently limited information is available regarding the regulation and role CYP enzymes play in the pathophysiology of heart diseases and cardiac drug metabolism. Our present results demonstrate that CYP2J2 Tr mice can limit the Dox-induced injury. Recent studies documenting that P-450-derived eicosanoids can affect cardiomyocyte function in vitro (25, 27, 32, 33, 40, 45, 62) and protect against ischemia-reoxygenation injury (19, 20, 48, 50) have led to the hypothesis that these metabolites may have important endogenous functions in the heart. However, the reduced injury observed in our CYP2J2 Tr mice may be partially attributed to the increased Dox metabolism compared with WT mice. Interesting data from H9c2 cell culture experiments show that Dox can induce CYP enzymes, notably CYP2J2 isoforms (65). The increased turnover of Dox in CYP2J2 Tr mice suggests a potential mechanism for the reduced toxicity observed in these animals. Considering that CYP enzymes can produce bioactive metabolites and metabolize foreign compounds, many important questions remain regarding the role of cardiac CYP.
Cellular excitation-contraction and mitochondrial energetics are tightly regulated in cardiac cells to meet the high energetic flux during cardiac work. Importantly, cellular stress conditions can result in distinct morphological changes that reduce mitochondrial dynamics influencing the energetic state of the cell (24). We recently demonstrated that a marked disorganization of cardiomyocyte ultrastructure following ischemia-reperfusion was significantly reduced in CYP2J2 Tr mice; moreover, EETs can minimize adverse effects of stress on mitochondrial function (30). Mitochondria are dynamic organelles that migrate through the cell and undergo continuous fusion or fission processes to maintain proper function and meet cellular demands (22). Significant decreases in fusion or increases in fission resulting from disease or toxicity can lead to punctate, fragmented mitochondria, which are thought to play a critical role in cellular dysfunction and death (22, 35, 64). Dox-induced toxicity can result in mitochondrial swelling and ultrastructural changes and alter function. Recently, Hiraumi et al. (21) demonstrated that Dox-induced mitochondrial damage can begin to occur at an early phase in cardiac injury before apoptotic changes. In the present study using an in vitro model, we demonstrate that Dox-increased mitochondrial fragmentation and membrane depolarization occur within 1 h before caspase-3 activation at 24 h in H9c2 cells. Consistent with our previous data, our present study demonstrates that EETs minimize the adverse effects of Dox on mitochondrial function. While these results are limited to our in vitro model, the implication that EETs can attenuate formation of punctate mitochondria is of particular significance. Indeed, the fact EETs can inhibit apoptotic events and maintain mitochondrial function highlights an interesting dichotomy, where elevated EETs may provide benefit to reduction in cellular injury but can also be detrimental for cancer therapy. Further studies are needed to investigate how EETs might influence mitochondrial fission and fusion and, moreover, how this might affect in vivo cardiac function and cardioprotection and what implications there are for oncogenesis.
In summary, the results obtained here illustrate that cardiac CYP2J2 overexpression limits the progression of cardiac injury and preserves cardiac function in mice after treatment with Dox under two different administration protocols. Indeed, as assessed by various biochemical and functional end points, a more advanced progression toward development of cardiac injury was observed in WT mice compared with CYP2J2 Tr mice after treatment with Dox. These studies are the first to document a protective effect of CYP2J2 in Dox-induced cardiotoxicity and may have implications for treatment of cardiac injury.
GRANTS
This work was supported by a Canadian Institutes of Health Research Grant (MOP79465, J. M. Seubert) and by the Intramural Research Program of the National Institute of Environmental Health Sciences (Z01-ES-025034). J. M. Seubert is the recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Adachi T, Nagae T, Ito Y, Hirano K, Sugiura M. Relation between cardiotoxic effect of adriamycin and superoxide anion radical. J Pharmacobiodyn 6: 114–123, 1983. [DOI] [PubMed] [Google Scholar]
- 2.Aebi H Catalase in vitro. Methods Enzymol 105: 121–126, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed HH, Mannaa F, Elmegeed GA, Doss SH. Cardioprotective activity of melatonin and its novel synthesized derivatives on doxorubicin-induced cardiotoxicity. Bioorg Med Chem 13: 1847–1857, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, Nagai R. Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension 36: 48–53, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Aihara Y, Kurabayashi M, Tanaka T, Takeda SI, Tomaru K, Sekiguchi KI, Ohyama Y, Nagai R. Doxorubicin represses CARP gene transcription through the generation of oxidative stress in neonatal rat cardiac myocytes: possible role of serine/threonine kinase-dependent pathways. J Mol Cell Cardiol 32: 1401–1414, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Arafa HM, Abd-Ellah MF, Hafez HF. Abatement by naringenin of doxorubicin-induced cardiac toxicity in rats. J Egypt Natl Canc Inst 17: 291–300, 2005. [PubMed] [Google Scholar]
- 7.Chularojmontri L, Wattanapitayakul SK, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Srichairat S. Antioxidative and cardioprotective effects of Phyllanthus urinaria L. on doxorubicin-induced cardiotoxicity. Biol Pharm Bull 28: 1165–1171, 2005. [DOI] [PubMed] [Google Scholar]
- 8.D'Alessandro N, Nicotra C, Crescimanno M, Rausa L. Effects of doxorubicin on mouse heart catalase. Drugs Exp Clin Res 13: 601–606, 1987. [PubMed] [Google Scholar]
- 9.Daosukho C, Chen Y, Noel T, Sompol P, Nithipongvanitch R, Velez JM, Oberley TD, St Clair DK. Phenylbutyrate, a histone deacetylase inhibitor, protects against Adriamycin-induced cardiac injury. Free Radic Biol Med 42: 1818–1825, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delozier TC, Kissling GE, Coulter SJ, Dai D, Foley JF, Bradbury JA, Murphy E, Steenbergen C, Zeldin DC, Goldstein JA. Detection of human CYP2C8, CYP2C9, and CYP2J2 in cardiovascular tissues. Drug Metab Dispos 35: 682–688, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng S, Kruger A, Kleschyov AL, Kalinowski L, Daiber A, Wojnowski L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic Biol Med 42: 466–473, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol 294: H724–H735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diotte NM, Xiong Y, Gao J, Chua BH, Ho YS. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta 1793: 427–438, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Elbekai RH, El-Kadi AO. Cytochrome P450 enzymes: central players in cardiovascular health and disease. Pharmacol Ther 112: 564–587, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Ellerby LM, Bredesen DE. Measurement of cellular oxidation, reactive oxygen species, and antioxidant enzymes during apoptosis. Methods Enzymol 322: 413–421, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Falk R, Hacham M, Nyska A, Foley JF, Domb AJ, Polacheck I. Induction of interleukin-1beta, tumour necrosis factor-alpha and apoptosis in mouse organs by amphotericin B is neutralized by conjugation with arabinogalactan. J Antimicrob Chemother 55: 713–720, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Fang C, Gu J, Xie F, Behr M, Yang W, Abel ED, Ding X. Deletion of the NADPH-cytochrome P450 reductase gene in cardiomyocytes does not protect mice against doxorubicin-mediated acute cardiac toxicity. Drug Metab Dispos 36: 1722–1728, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewirtz DA A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57: 727–741, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res 68: 18–25, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol 294: H2838–H2844, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiraumi Y, Iwai-Kanai E, Baba S, Yui Y, Kamitsuji Y, Mizushima Y, Matsubara H, Watanabe M, Watanabe K, Toyokuni S, Nakahata T, Adachi S. Granulocyte colony-stimulating factor protects cardiac mitochondria in the early phase of cardiac injury. Am J Physiol Heart Circ Physiol 296: H823–H832, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Hom J, Sheu SS. Morphological dynamics of mitochondria—a special emphasis on cardiac muscle cells. J Mol Cell Cardiol 46: 811–820, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huigsloot M, Tijdens IB, Mulder GJ, van de Water B. Differential regulation of doxorubicin-induced mitochondrial dysfunction and apoptosis by Bcl-2 in mammary adenocarcinoma (MTLn3) cells. J Biol Chem 277: 35869–35879, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Jendrach M, Mai S, Pohl S, Voth M, Bereiter-Hahn J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion 8: 293–304, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L. A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem 272: 22800–22808, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 65: 4707–4715, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Kanai H, Tanaka T, Aihara Y, Takeda S, Kawabata M, Miyazono K, Nagai R, Kurabayashi M. Transforming growth factor-beta/Smads signaling induces transcription of the cell type-restricted ankyrin repeat protein CARP gene through CAGA motif in vascular smooth muscle cells. Circ Res 88: 30–36, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem 271: 12610–12616, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 100: 1501–1506, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol 46: 867–875, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Kim DS, Kim HR, Woo ER, Kwon DY, Kim MS, Chae SW, Chae HJ. Protective effect of calceolarioside on adriamycin-induced cardiomyocyte toxicity. Eur J Pharmacol 541: 24–32, 2006. [DOI] [PubMed] [Google Scholar]
- 32.King LM, Ma J, Srettabunjong S, Graves J, Bradbury JA, Li L, Spiecker M, Liao JK, Mohrenweiser H, Zeldin DC. Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol Pharmacol 61: 840–852, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Lee HC, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J Physiol 519: 153–168, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licata S, Saponiero A, Mordente A, Minotti G. Doxorubicin metabolism and toxicity in human myocardium: role of cytoplasmic deglycosidation and carbonyl reduction. Chem Res Toxicol 13: 414–420, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ (March 20, 2009); doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed]
- 36.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med 324: 808–815, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Lou H, Kaur K, Sharma AK, Singal PK. Adriamycin-induced oxidative stress, activation of MAP kinases and apoptosis in isolated cardiomyocytes. Pathophysiology 13: 103–109, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Minotti G, Licata S, Saponiero A, Menna P, Calafiore AM, Di Giammarco G, Liberi G, Animati F, Cipollone A, Manzini S, Maggi CA. Anthracycline metabolism and toxicity in human myocardium: comparisons between doxorubicin, epirubicin, and a novel disaccharide analogue with a reduced level of formation and [4Fe-4S] reactivity of its secondary alcohol metabolite. Chem Res Toxicol 13: 1336–1341, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56: 185–229, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Moffat MP, Ward CA, Bend JR, Mock T, Farhangkhoee P, Karmazyn M. Effects of epoxyeicosatrienoic acids on isolated hearts and ventricular myocytes. Am J Physiol Heart Circ Physiol 264: H1154–H1160, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45: 311–333, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 50: 528–536, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhyay P, Rajesh M, Bátkai S, Yoshihiro K, Haskó G, Liaudet L, Szabó C, Pacher P. Role of superoxide, nitric oxide and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296: H1466–H1483, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther 300: 862–867, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Rastaldo R, Paolocci N, Chiribiri A, Penna C, Gattullo D, Pagliaro P. Cytochrome P-450 metabolite of arachidonic acid mediates bradykinin-induced negative inotropic effect. Am J Physiol Heart Circ Physiol 280: H2823–H2832, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Samaras SE, Shi Y, Davidson JM. CARP: fishing for novel mechanisms of neovascularization. J Investig Dermatol Symp Proc 11: 124–131, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz RG, McKenzie WB, Alexander J, Sager P, D'Souza A, Manatunga A, Schwartz PE, Berger HJ, Setaro J, Surkin L. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med 82: 1109–1118, 1987. [DOI] [PubMed] [Google Scholar]
- 48.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res 95: 506–514, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Seubert JM, Darmon AJ, El-Kadi AO, D'Souza SJ, Bend JR. Apoptosis in murine hepatoma hepa 1c1c7 wild-type, C12, and C4 cells mediated by bilirubin. Mol Pharmacol 62: 257–264, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res 99: 442–450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Reitmaier B, Regenbogen J, Slowey RM, Opalenik SR, Wolf E, Goppelt A, Davidson JM. CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue. Am J Pathol 166: 303–312, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900–905, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Singal PK, Li T, Kumar D, Danelisen I, Iliskovic N. Adriamycin-induced heart failure: mechanism and modulation. Mol Cell Biochem 207: 77–86, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43: 55–90, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266: 1672–1677, 1991. [PubMed] [Google Scholar]
- 56.Tesoriere L, Ciaccio M, Valenza M, Bongiorno A, Maresi E, Albiero R, Livrea MA. Effect of vitamin A administration on resistance of rat heart against doxorubicin-induced cardiotoxicity and lethality. J Pharmacol Exp Ther 269: 430–436, 1994. [PubMed] [Google Scholar]
- 57.Tokarska-Schlattner M, Wallimann T, Schlattner U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. C R Biol 329: 657–668, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Tokarska-Schlattner M, Zaugg M, da Silva R, Lucchinetti E, Schaub MC, Wallimann T, Schlattner U. Acute toxicity of doxorubicin on isolated perfused heart: response of kinases regulating energy supply. Am J Physiol Heart Circ Physiol 289: H37–H47, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 41: 389–405, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Torrado M, Lopez E, Centeno A, Castro-Beiras A, Mikhailov AT. Left-right asymmetric ventricular expression of CARP in the piglet heart: regional response to experimental heart failure. Eur J Heart Fail 6: 161–172, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem 271: 3460–3468, 1996. [DOI] [PubMed] [Google Scholar]
- 62.Xiao YF, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J Physiol 508: 777–792, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, Lu ZY, Wang DW, Zeldin DC. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol 293: H142–H151, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79: 341–351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zordoky BN, El-Kadi AO. Induction of several cytochrome P450 genes by doxorubicin in H9c2 cells. Vascul Pharmacol 49: 166–172, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.