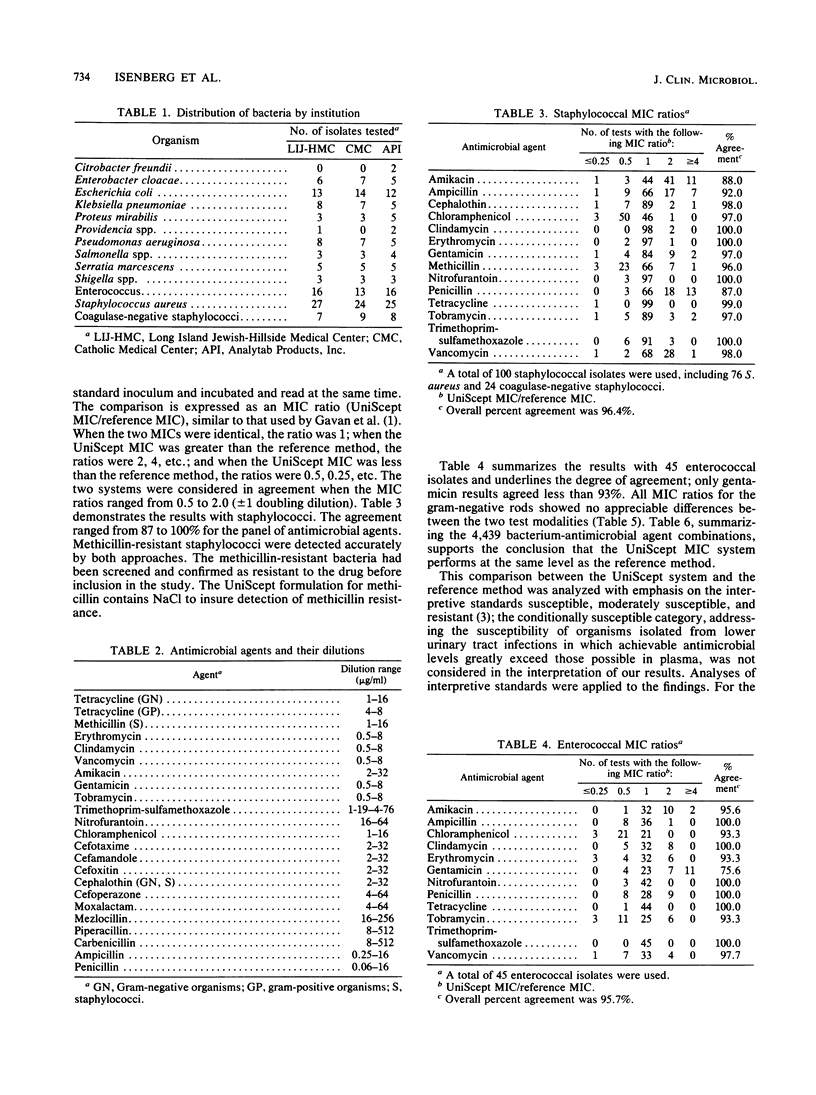
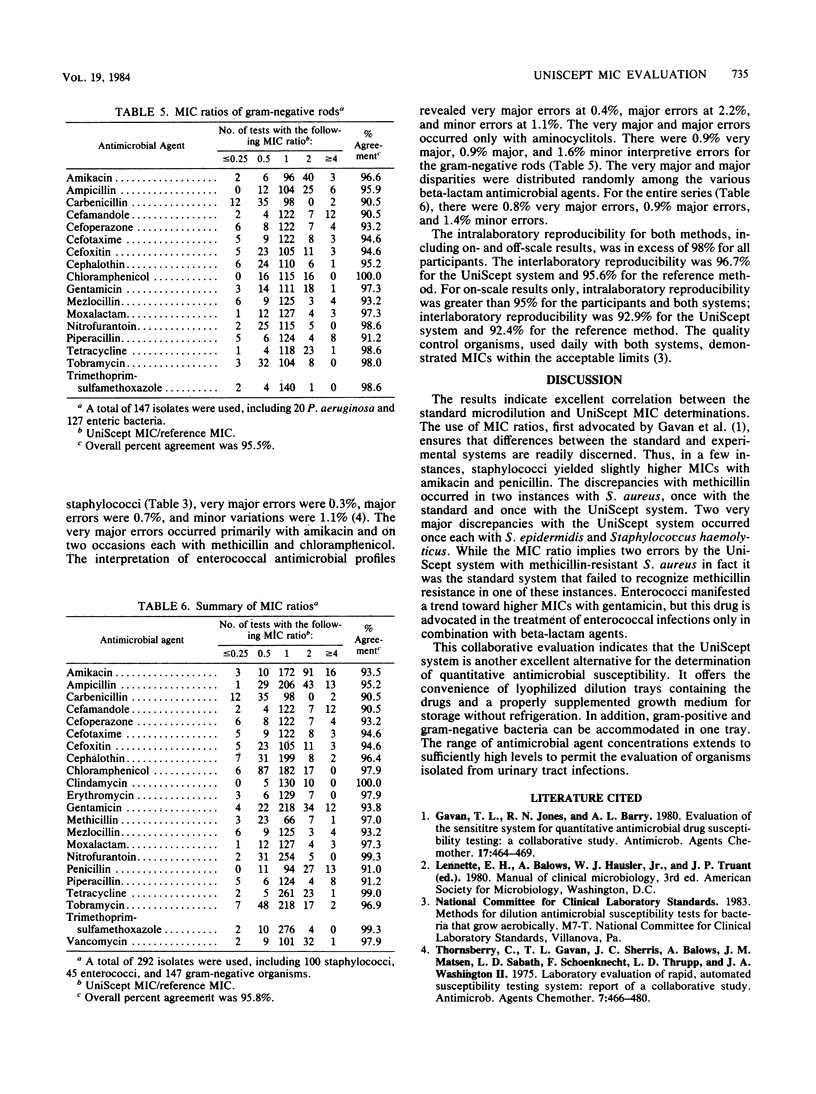
Abstract
UniScept (Analytab Products, Plainview, N.Y.) is a commercially prepared microdilution antimicrobial susceptibility system for the determination, in a single tray, of antimicrobial MICs for gram-negative and gram-positive bacteria and those isolated from urinary tract infections. The system showed excellent correlation with the reference microdilution approach for organisms from clinical specimens and with stock and reference cultures. Intra- and interlaboratory reproducibility was high.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gavan T. L., Jones R. N., Barry A. L. Evaluation of the Sensititre system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1980 Mar;17(3):464–469. doi: 10.1128/aac.17.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]


