Abstract
We performed the present study to determine whether hibernating myocardium is chronically protected from ischemia. Myocardial tissue was rapidly excised from hibernating left anterior descending coronary regions (systolic wall thickening = 2.8 ± 0.2 vs. 5.4 ± 0.3 mm in remote myocardium), and high-energy phosphates were quantified by HPLC during simulated ischemia in vitro (37°C). At baseline, ATP (20.1 ± 1.0 vs. 26.7 ± 2.1 μmol/g dry wt, P < 0.05), ADP (8.1 ± 0.4 vs. 10.3 ± 0.8 μmol/g, P < 0.05), and total adenine nucleotides (31.2 ± 1.3 vs. 40.1 ± 2.9 μmol/g, P < 0.05) were depressed compared with normal myocardium, whereas total creatine, creatine phosphate, and ATP-to-ADP ratios were unchanged. During simulated ischemia, there was a marked attenuation of ATP depletion (5.6 ± 0.9 vs. 13.7 ± 1.7 μmol/g at 20 min in control, P < 0.05) and mitochondrial respiration [145 ± 13 vs. 187 ± 11 ng atoms O2·mg protein−1·min−1 in control (state 3), P < 0.05], whereas lactate accumulation was unaffected. These in vitro changes were accompanied by protection of the hibernating heart from acute stunning during demand-induced ischemia. Thus, despite contractile dysfunction at rest, hibernating myocardium is ischemia tolerant, with reduced mitochondrial respiration and slowing of ATP depletion during simulated ischemia, which may maintain myocyte viability.
Keywords: energy metabolism, mitochondrial respiration, stunned myocardium
although reversible myocardial dysynergy can arise from a number of specific pathophysiological states, chronic hibernating myocardium is unique, in that it is characterized by regional contractile dysfunction and reduced resting flow in the absence of infarction (4, 18). In chronic hibernating myocardium, regional O2 consumption is reduced at rest, as well as during increases in myocardial O2 demand, yet evidence of acute myocardial ischemia is absent (11), which contrasts with findings in short-term hibernating myocardium (45, 46). This appears to be partially related to a downregulation in many of the enzymes involved in oxidative metabolism and electron transport (39). Thus this regional dissociation between O2 consumption and external workload protects the heart during submaximal increases in demand and attenuates apoptosis-induced myocyte loss in the chronic state.
Collectively, these findings raise the possibility that hibernating myocardium may be chronically protected against ischemia. In support of this notion, Ausma et al. (1) demonstrated increased tolerance to ischemia in myocytes from hibernating myocardium. Similarly, Milei et al. (34) demonstrated a reduction in ischemia-reperfusion injury during cardiopulmonary bypass in hibernating myocardium compared with segments with normal systolic function. Unfortunately, these previous studies were indirect, since they relied on quantification of histopathological changes indicative of irreversible injury. Nevertheless, they support the notion that, despite the presence of contractile dysfunction at rest, human hibernating myocardium may be chronically protected from irreversible injury following acute ischemia.
The present study was designed to determine whether chronic hibernating myocardium in pigs with a chronic left anterior descending coronary artery (LAD) occlusion is protected against acute ischemic injury. Since collateral-dependent myocardium precludes use of an infarct model to assess protection against irreversible injury, we studied the depletion of high-energy phosphates (HEP) in tissue subjected to anoxia in vitro to simulate myocardial ischemia (22). Previous studies demonstrated that ATP depletion is slowed after acute preconditioning, providing a potential surrogate that is independent of in vivo alterations in contractile function or collateral flow (22, 37). In additional studies, we assessed ex vivo mitochondrial respiration and determined whether hibernating myocardium is protected from stunning during demand-induced ischemia in vivo. Our results demonstrate that mitochondrial respiration is depressed in chronic hibernating myocardium. This reduces ATP depletion during simulated ischemia and protects the dysfunctional heart from acute stunning during increases in myocardial O2 consumption when coronary flow reserve is chronically impaired. Both effects are consistent with intrinsic protection from irreversible injury.
METHODS
Experimental procedures and protocols conformed to institutional guidelines for the care and use of animals in research and were approved by the University at Buffalo Institutional Animal Care and Use Committee.
Coronary artery instrumentation.
Juvenile farm-bred pigs (8–10 kg body wt) were fasted, premedicated with a Telazol [tiletamine (50 mg/ml) + zolazepam (50 mg/ml)]-ketamine (100 mg/ml) mixture (0.037 ml/kg im), and given prophylactic antibiotics [cefazolin (0.5 mg iv) and gentamicin (40 mg iv)]. Pigs were intubated and anesthetized with isoflurane (1–2%). A left thoracotomy was performed in the fourth intercostal space. The proximal LAD was dissected free and instrumented with a fixed-diameter Delrin stenosis (1.5 mm ID) (6, 11, 12). The chest incision was closed in layers, the intercostal nerves were infiltrated with 2% lidocaine for analgesia, and the pneumothorax was evacuated. A single postoperative dose of antibiotics was repeated, and analgesics (butorphanol at 0.025 mg/kg) were given postoperatively and repeated as required to alleviate pain. A total of 52 pigs with hibernating myocardium and 29 normal pigs were used in the studies.
Experimental protocol.
At 3 mo after instrumentation with a chronic LAD stenosis, physiological studies were performed in the closed-chest anesthetized state. The animals were sedated with Telazol-xylazine (0.022 ml/kg im) and mechanically ventilated with O2. Anesthesia was maintained with a propofol infusion (5–10 mg·kg−1·h−1 iv) (52). A 6F introducer was placed into the brachial artery, and the side port was used for arterial pressure measurement. A 5F end-hole micromanometer (Millar, Houston, TX) was inserted into the left ventricle (LV) apex for pressure measurement and microsphere administration. Animals were heparinized (100 U/kg iv), and hemodynamics were allowed to equilibrate for 30 min.
Regional myocardial perfusion was quantified with fluorescent microspheres, as previously described (12, 26, 52). Briefly, 3 × 106 fluorescently labeled microspheres (15 μm diameter; Triton) were injected into the LV after initiation of a 90-s reference arterial withdrawal sample (6 ml/min). The injections were given at rest and during adenosine vasodilation (0.9 mg·kg−1·min−1 iv), with coinfusion of phenylephrine (10.0 ± 1.3 μg·kg−1·min−1) to maintain arterial pressure (12, 52). At the same time, hemodynamics were measured. Finally, coronary angiography was performed to assess severity of stenosis and extent of collateralization.
Using a 2.5-MHz phased-array transducer (GE Vivid 7, GE Healthcare), we assessed regional and global LV function with transthoracic echocardiography at the midventricular level through a right parasternal approach. Thickness of the anteroseptal and posterior wall was measured using anatomic M-mode echocardiography. End diastole was defined as the onset of the QRS complex, and end systole was the point of minimum chamber diameter, as recommended by the American Society of Echocardiography (43). Regional function was quantified using myocardial systolic wall thickening (end-systolic thickness − end-diastolic thickness). Fractional shortening, an assessment of global function, was defined as follows: 100 × (LV end-diastolic dimension − LV end-systolic dimension)/LV end-diastolic dimension.
Terminal study and myocardial sampling for flow and ATP.
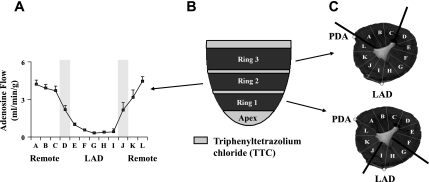
The animals were allowed to recover, and the terminal study was performed ≥3 days after the physiological studies. Pigs were intubated and anesthetized using propofol (20–75 μg·kg−1·min iv)-ketamine (30–125 μg·kg−1·min−1 iv) (52). After hemodynamics equilibrated for 30 min, the heart was exposed, and Tru-cut needle biopsies were obtained from the center of LAD and normally perfused remote regions (2–3 biopsies per region, ∼20–30 mg each). The biopsies were flash frozen (within 5 s) in liquid nitrogen for baseline HEP measurements. Subsequently, hearts were arrested with KCl, excised, and rapidly sectioned into multiple concentric rings (Fig. 1). For ATP depletion studies and isolation of mitochondrion, a large wedge of LAD-perfused tissue was sampled from the most apical ring (ring 1), and the posterobasal ring (ring 3) was used for remote normally perfused tissue. The midventricular ring (ring 2) and remaining samples from rings 1 and 3 were used for quantification of myocardial perfusion (6, 52). LAD and remote region perfusion measurements reflected the weighted average of all samples within a perfusion territory after review of the vasodilated perfusion map and exclusion of border zone samples (Fig. 1) (12). Three intervening rings were stained with 2,3,5-triphenyltetrazolium chloride for assessment of tissue necrosis (6), which was quantified as a percentage of LV mass.
Fig. 1.
Myocardial sampling. A: circumferential distribution of segmental perfusion during adenosine vasodilation from a representative experiment. Average flows are the weighted mean of samples selected using the vasodilated flow map to exclude border zone samples from results (gray bars). LAD, left anterior descending coronary artery; A–L, segments. B: sectioning of the heart for tissue sampling. Three rings were used; 4 thin concentric rings between them (gray rings) were taken for 2,3,5-triphenyltetrazolium chloride staining to exclude myocardial infarction. C: tissue samples from central regions within hibernating LAD and remote myocardium for assessment of myocardial ATP depletion in vitro. Remote, normally perfused myocardium included wedges A, B, and C from basal ring 3. LAD-perfused myocardium included wedges G, H, and I of apical ring 1. PDA, posterior descending artery.
HEP measurements and tissue lactate assays.
In the first series of experiments (n = 22 hibernating and 8 normal control), simulated global ischemia (zero flow) was produced in vitro as previously described (23). Myocardial samples were rapidly excised and placed into Zip-lock plastic bags and incubated in a 37°C water bath. Subendocardial and subepicardial samples from LAD and remote myocardium were removed at 2, 5, 10, 20, 40, 60, and 80 min and immediately frozen in liquid nitrogen. ATP, ADP, AMP, creatine (Cr), and Cr phosphate (CP) were quantified (μmol/g dry wt) by HPLC using a Dynamax SD-200 system (Rainin Instrument). The HEP compounds were eluted from a Vydac 218TP54 column (4.6 × 250 mm; The Nest Group) at room temperature at a flow rate of 1 ml/min. We used an isocratic ion-pair reverse-phase assay (32, 48), which allows for single-run determination of both Cr compounds and adenine nucleotides with the spectrophotometer set at 206 nm (32, 48). Since CP is rapidly depleted during simulated ischemia, it was only assessed in the initial baseline sample obtained by biopsy. For tissue lactate assays, frozen tissue was manually ground with a mortar and pestle under liquid nitrogen. The resulting powder was dissolved in cold 18% perchloric acid for 20 min. The solution was centrifuged at 11,500 rpm for 10 min at 4°C after neutralization with 10 M KOH. The supernatant was collected, and tissue lactate was measured with a lactate assay kit (Biovision Research, Mountain View, CA) (14).
Calculation of myocardial energetic indexes.
Total adenine nucleotides (TAN) were quantified as the sum of ATP, ADP, and AMP. Myocardial free ADP levels were calculated from the Cr kinase equilibrium expression (27): [ADP] = ([ATP] × [free Cr])/([CP] × [H+] × Keq), where the equilibrium constant (Keq) = 1.66 × 109, cytosolic pH = 7.1, and [ADP], [ATP], [free Cr], [CP], and [H+] are concentrations of ADP, ATP, free Cr, CP, and H+. Free Cr was calculated by subtraction of the CP values from total Cr measured in the biopsy tissue. McFalls et al. (32) showed that the ratio of CP to ATP, measured by in vivo 31P-NMR spectroscopy of the LAD region of beating hearts in hibernating and control pigs, was 2.15 and 2.11, respectively. Thus we used these in vivo CP-to-ATP ratios to calculate myocardial free ADP for LAD tissues of hibernating and control biopsies. Free ADP was used to calculate the ATP-to-ADP ratio.
Mitochondrial respiration in vitro.
In a second series of experiments (n = 21 hibernating and 11 sham), mitochondria were isolated for assessment of respiration in vitro, as previously described (40). An enriched mitochondrial fraction was prepared from 300- to 800-mg pieces of fresh subendocardial tissue. Samples were isolated immediately after excision of the heart using the Mitochondrial Isolation Kit (Sigma, St. Louis, MO). Briefly, tissue was minced (1- to 2-mm pieces) with a McIlwain tissue chopper and treated with 0.25 mg/ml trypsin in an extraction buffer of 10 mM HEPES (pH 7.5), 2 M mannitol, 70 mM sucrose, and 1 mM EGTA. After the reaction was quenched with albumin, samples were homogenized in extraction buffer for 20 s and then spun, first at low speed spin (700 g) for 8 min to remove cellular debris and, then, at a higher speed (11,000 g) for 12 min to pellet the mitochondria. Material settling on top of the brown mitochondrial pellet was carefully removed, along with the supernatant, by pipetting. The mitochondrial pellet was resuspended in incubation medium (80 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 1 mM EGTA, and 1 mg/ml delipidated bovine serum albumin), and protein content was determined with the DC Protein Assay (Bio-Rad).
We assessed in vitro mitochondrial O2 consumption at 31°C using a Clark electrode O2-measuring system (Instech Laboratories, Plymouth Meeting, PA), as previously described (19). State 2 (basal) mitochondrial respiration (400-μg samples) was analyzed in the presence of 10 mM pyruvate, and state 3 respiration was assessed with repeated additions of ADP (2 μmol). The average slope (ΔPo2/time) was determined from digitized data (Notocord) by linear regression. Reported values reflect the average of two sequential measurements. The respiratory control ratio (RCR) was calculated as the ratio of state 3 to state 2 respiration.
In vivo protection from myocardial stunning following transient demand-induced ischemia.
In a third series of experiments, we used a transient epinephrine infusion to simulate the effects of increased demand on regional myocardial function in hibernating animals (n = 9), sham animals (n = 7), and pigs chronically instrumented with a flow probe and hydraulic occluder to produce an acute critical stenosis (n = 3), as we previously described (54). In animals subjected to an acute critical stenosis, LAD coronary flow was measured using an LAD flow probe (Transonics, Ithaca, NY) and maintained at a level similar to the resting value by adjustment of the hydraulic occluder as required. After a baseline echocardiogram, a single-stage submaximal infusion of epinephrine was administered (0.35 ± 0.01 μg·kg−1·min−1) to increase heart rate by ∼40 beats/min (2). This condition was maintained for ∼15 min to allow hemodynamics to equilibrate, then echocardiograms were repeated. Functional recovery and acute stunning were reassessed 15 min after cessation of epinephrine stimulation when hemodynamics had returned to baseline.
Data analysis.
Values are means ± SE. Regional differences in physiological parameters, as well as differences following adenosine vasodilation, were assessed with paired t-tests. Baseline HEP levels from biopsies, lactate levels, function, and mitochondrial respiration were assessed with a two-way ANOVA. ATP depletion during ischemia was analyzed with a three-way ANOVA to account for group, region, and layer (subendocardium vs. subepicardium) with repeated measures (ATP levels over time). The Holm-Sidak test was used for post hoc comparisons. P ≤ 0.05 was considered statistically significant.
RESULTS
Regional flow and function in pigs with hibernating myocardium.
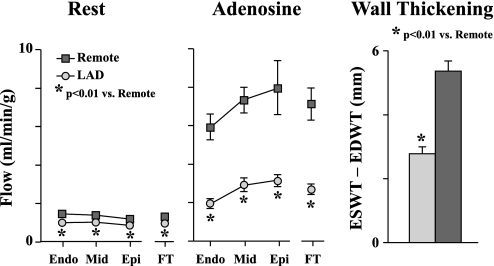
Studies were conducted 3 mo after instrumentation, at which time hibernating myocardium was present. To eliminate potentially confounding effects of patchy necrosis on the metabolic measurements, we excluded four pigs with necrosis involving >0.5% of LV mass. In animals with trivial necrosis (n = 4, 0.3 ± 0.1% of LV mass), ATP values were similar to those where necrosis was completely absent. Thus a total of 18 animals were used to study ATP depletion in vitro. Hemodynamic parameters of animals with hibernating myocardium during physiological studies in animals used for ATP depletion studies are summarized in Table 1. Regional LAD function was reduced with systolic LAD wall thickening averaging 2.8 ± 0.2 mm vs. 5.4 ± 0.3 mm in remote regions (P < 0.01; Fig. 2). Global LV function was normal (30 ± 1% fractional shortening). Resting LAD flow was reduced in subendocardial (0.95 ± 0.08 vs. 1.40 ± 0.10 ml·min−1·g−1 in remote, P < 0.01) and full-thickness samples (0.92 ± 0.08 vs. 1.26 ± 0.08 ml·min−1·g−1 in remote, P < 0.01), consistent with hibernating myocardium (Fig. 2). Similarly, vasodilated LAD flow was markedly reduced in subendocardial (1.96 ± 0.25 vs. 5.93 ± 0.67 ml·min−1·g−1 in remote, P < 0.01) and full-thickness myocardium (2.71 ± 0.28 vs. 7.13 ± 0.83 ml·min−1·g−1 in remote, P < 0.01; Fig. 2).
Table 1.
Closed-chest hemodynamics data in animals with hibernating myocardium
| Heart Rate, beats/min | Mean Aortic Pressure, mmHg | LV Systolic Pressure, mmHg | LVEDP, mmHg | LV dP/dtmax, mmHg/s | LV dP/dtmin, mmHg/s | |
|---|---|---|---|---|---|---|
| Rest | 113±3 | 93±3 | 126±4 | 22±2 | 2,290±78 | −2,518±83 |
| Adenosine | 107±3 | 95±4 | 135±4 | 28±3* | 2,394±170 | −2,100±246 |
Values are means ± SE (n = 18). LVEDP, left ventricular (LV) end-diastolic pressure; dP/dtmax and dP/dtmin, rate of LV pressure rise and fall, respectively.
P < 0.05 vs. rest.
Fig. 2.
Flow and function in hibernating myocardium (n = 18). Left: resting perfusion was significantly reduced in each myocardial layer of LAD-perfused myocardium (LAD) compared with remote normally perfused (remote) region. Middle: regional differences in perfusion were more pronounced during adenosine vasodilation. FT, full thickness. Right: regional wall thickening [end-systolic wall thickness (ESWT) − end-diastolic wall thickness (EDWT)] was significantly reduced in hibernating LAD regions compared with remote regions. Endo, subendocardial; Mid, midmyocardial; Epi, subepicardial.
Baseline HEP levels.
HEP levels obtained from transmural needle biopsies in the beating heart are summarized in Table 2. Compared with normal myocardium (control), ATP and ADP levels were reduced in the hibernating LAD region, while AMP was normal. As a result, TAN were significantly reduced (Table 2). In contrast, total Cr, CP, myocardial free ADP, and ATP-to-ADP ratio were normal in the hibernating heart (Table 2), indicating a stable balanced energetic state. The reductions in ATP, ADP, and TAN levels were global, with similar changes observed in the remote, normally perfused myocardium (Table 2).
Table 2.
Baseline high-energy phosphate levels from needle biopsy
| n | ATP | ADP | AMP | TAN | Creatine | CP | Free ADP | ATP/ADP | |
|---|---|---|---|---|---|---|---|---|---|
| Hibernating | 18 | ||||||||
| LAD | 20.1±1.0* | 8.1±0.4* | 3.0±0.2 | 31.2±1.3* | 80.2±5.3 | 18.9±1.0 | 0.22±0.02 | 101±8 | |
| Remote | 20.9±0.5* | 7.9±0.3* | 2.7±0.2 | 31.4±0.8* | 72.3±2.8 | 17.9±0.8 | |||
| Control | 8 | ||||||||
| LAD | 26.7±2.1 | 10.3±0.8 | 3.1±0.3 | 40.1±2.9 | 88.2±7.3 | 22.1±1.9 | 0.24±0.02 | 113±6 | |
| Remote | 24.5±1.4 | 9.3±0.7 | 3.0±0.3 | 36.8±2.1 | 79.8±4.7 | 19.2±1.2 |
Values are means ± SE, expressed in μmol/g dry wt. LAD, left anterior descending coronary artery; TAN, total adenine nucleotides; CP, creatine phosphate.
P < 0.05 vs. control.
ATP depletion during simulated ischemia.
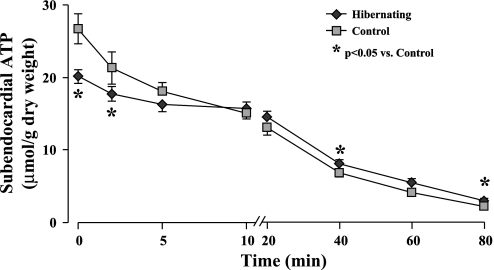
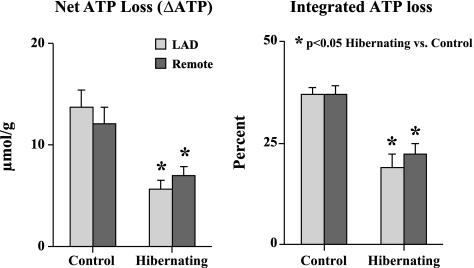
Figure 3 summarizes LAD subendocardial ATP depletion during simulated ischemia in hibernating and control myocardium. The ATP levels for all other regions are provided in Table 3. Along with lower initial ATP values in hibernating myocardium, the rate of ATP depletion was significantly slowed. As a result, ATP values in hibernating subendocardial samples exceeded those in control myocardium by 20–40 min of ischemia. Net ATP loss during the first 20 min of ischemia (from baseline to 20 min; Fig. 4) was also markedly reduced in hibernating myocardium compared with controls (5.6 ± 0.9 vs. 13.7 ± 1.7 μmol/g dry wt, P < 0.05; Fig. 4, left). A similar conclusion was reached using integrated ATP loss from baseline levels (19.0 ± 3.4% vs. 37.1 ± 1.6% in normal, P < 0.05; Fig. 4, right). Similar to baseline reductions in ATP, the fall in ATP following ischemia was slowed in remote normally perfused regions from hibernating hearts, with no significant regional differences between LAD and remote regions in either hibernating or control hearts (Table 3, Fig. 4). There were also no significant transmural variations (subendocardial vs. subepidcardial) with respect to group, region, or time point (Table 3; P = 0.19 by ANOVA).
Fig. 3.
Subendocardial ATP depletion in hibernating and control myocardium. Basal ATP levels (time 0, full-thickness biopsy) were significantly lower in LAD region of animals with hibernating myocardium than in normal controls. During simulated ischemia in vitro, rate of subendocardial ATP depletion was slower in hibernating myocardium, such that ATP levels became higher than in normal control myocardium at 40 and 80 min. There were no differences between LAD and remote regions in either group, and directionally similar results were seen in corresponding subepicardial samples (Table 3).
Table 3.
ATP at baseline and during simulated ischemia
| n | Baseline |
Ischemia |
||||||
|---|---|---|---|---|---|---|---|---|
| 20 min | 40 min | 80 min | ||||||
| Subendocardium | ||||||||
| Hibernating | 18 | |||||||
| LAD | 20.1±1.0* | 14.5±0.8 | 8.1±0.5* | 3.0±0.2* | ||||
| Remote | 20.9±0.5* | 13.9±0.7 | 7.8±0.4* | 2.6±0.2 | ||||
| Control | 8 | |||||||
| LAD | 26.7±2.1 | 13.0±1.0 | 6.8±0.6 | 2.2±0.3 | ||||
| Remote | 24.5±1.4 | 12.4±1.2 | 6.6±0.5 | 2.1±0.2 | ||||
| Subepicardium | ||||||||
| Hibernating | 18 | |||||||
| LAD | 14.2±0.6* | 7.2±0.5 | 2.5±0.2 | |||||
| Remote | 12.8±0.7 | 7.3±0.5 | 2.4±0.2 | |||||
| Control | 8 | |||||||
| LAD | 12.6±0.9 | 6.9±0.5 | 1.9±0.3 | |||||
| Remote | 11.0±1.0 | 6.1±0.5 | 2.0±0.2 | |||||
Values are means ± SE, expressed in μmol/g dry wt.
P < 0.05 vs. corresponding control.
Fig. 4.
Absolute and integrated loss of subendocardial ATP during the first 20 min of simulated ischemia in vitro. Rate of ATP depletion was markedly reduced in hibernating compared with normal control hearts. This was evident in absolute ATP loss from baseline (5.6 ± 0.9 vs. 13.7 ± 1.7 μmol/g dry weight, P < 0.05; left), as well as integrated ATP loss from baseline (19.0 ± 3.4 vs. 37.1 ± 1.6%, P < 0.05; right). Attenuation in rate of ATP depletion was similar in dysfunctional LAD and normally perfused remote location in pigs with hibernating myocardium.
Tissue lactate during simulated ischemia.
Table 4 summarizes subendocardial lactate accumulation during simulated ischemia in hibernating and control myocardium. Lactate in LAD and remote regions increased during the first 40 min of simulated ischemia, consistent with persistent glycolysis. The rate of lactate accumulation slowed after 40 min of simulated ischemia, indicating a gradual cessation of glycolysis. Interestingly, throughout the period of simulated ischemia (2, 5, 20, 40, and 80 min), there were no significant differences between hibernating and normal myocardium in LAD or remote regions of the same heart. This suggests that anaerobic glycolysis was not altered in hibernating myocardium.
Table 4.
Lactate levels in subendocardium during simulated ischemia
| n |
Ischemia |
|||||
|---|---|---|---|---|---|---|
| 2 min | 5 min | 20 min | 40 min | 80 min | ||
| Hibernating | 17 | |||||
| LAD | 19.1±4.0 | 41.0±4.6 | 88.1±9.6 | 128.3±7.9 | 131.2±12.5 | |
| Remote | 21.5±6.6 | 40.9±4.6 | 89.0±6.7 | 121.9±12.5 | 117.3±12.9 | |
| Control | 8 | |||||
| LAD | 23.8±3.6 | 41.4±3.3 | 92.6±4.1 | 133.5±6.1 | 136.3±18.9 | |
| Remote | 21.5±4.0 | 40.8±3.3 | 89.2±6.2 | 137.1±4.2 | 144.2±11.6 | |
Values are means ± SE, expressed in μmol/g dry wt.
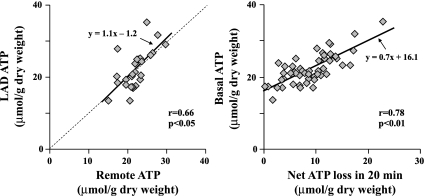
Determinants of ATP depletion.
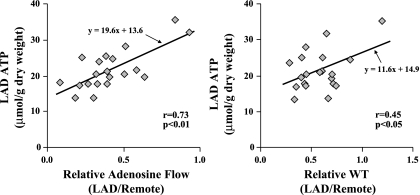
In individual animals, ATP levels in hibernating LAD regions were closely correlated with measurements in remote normally perfused regions (r = 0.66, P < 0.05; Fig. 5, left). There was also a very close correlation between the net ATP loss (ΔATP) and the basal ATP values (r = 0.78, P < 0.01; Fig. 5, right). To test the possibility that this was related to the severity of regional ischemia or degree of LAD dysfunction, we assessed the relation among tissue ATP, relative coronary flow reserve, and wall thickening in the occluded region (Fig. 6). Basal ATP levels in hibernating myocardium were correlated with relative adenosine flow (LAD/remote, r = 0.73, P < 0.01; Fig. 6, left) and relative wall thickening (r = 0.45, P < 0.05; Fig. 6, right). There was no correlation between ATP levels and absolute resting flow.
Fig. 5.
Determinants of basal ATP levels. There was a close correlation between basal ATP levels in LAD and normally perfused remote regions (r = 0.66, P < 0.05; left). Basal ATP levels were also directly correlated with net ATP loss in the initial 20 min of ischemia (r = 0.78, P < 0.01; right).
Fig. 6.
Interrelation among basal ATP, vasodilated flow, and resting function in hibernating myocardium. Significant correlations between basal ATP level in the hibernating LAD region and both relative adenosine flow (LAD/remote adenosine flow; r = 0.73, P < 0.01; left) and relative wall thickening (LAD/remote WT; r = 0.45, P < 0.05; right) support the notion that reductions in baseline ATP are related to the propensity of the heart to develop regional ischemia.
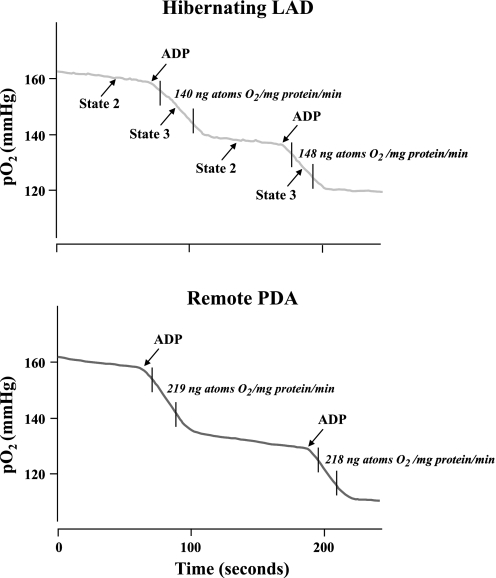
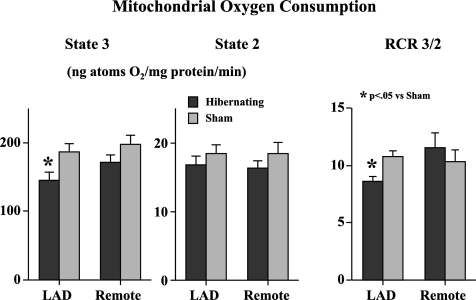
Subendocardial mitochondrial respiration in vitro.
Figure 7 shows dynamic traces of mitochondrial respiration in freshly isolated subendocardial mitochondria in the presence of pyruvate. The results of experiments in pigs with hibernating myocardium and sham controls are summarized in Fig. 8. State 2 respiration in hibernating LAD regions averaged 16.8 ± 1.3 vs. 18.4 ± 1.3 ng atoms O2·mg protein−1·min−1 in sham myocardium and was similar to that in remote regions of hibernating myocardium. In contrast, state 3 respiration was reduced in hibernating LAD regions compared with normal myocardium (145 ± 13 vs. 187 ± 11 ng atoms O2·mg protein−1·min−1, P < 0.05). Remote regions were intermediate between normal and hibernating myocardium but significantly greater than paired samples from hibernating LAD regions (171 ± 11.4, P < 0.05 vs. hibernating LAD). The reductions in state 3 respiration led to a significant reduction in the RCR in hibernating myocardium compared with remote myocardium (8.6 ± 0.4 vs. 11.6 ± 0.6, P < 0.05).
Fig. 7.
Representative traces of mitochondrial respiration in hibernating LAD region vs. remote myocardium. Mitochondrial respiration was calculated in the presence of pyruvate as the regression slope of the relation between Po2 and time from digitized recordings. State 2 respiration was similar in hibernating and normal myocardium. State 3 respiration was depressed after addition of ADP.
Fig. 8.
Mitochondrial respiration in subendocardial samples from 21 animals with hibernating myocardium and 11 normal sham animals. There were no differences in state 2 respiration among hibernating LAD regions, remote myocardium, and sham animals. O2 consumption in state 3 respiration was significantly reduced in hibernating LAD region compared with sham LAD region. State 3 respiration in remote regions from hibernating hearts was also lower than in sham animals, but the difference was of borderline significance. As a result of these changes, respiratory control ratio (RCR) was significantly lower in hibernating myocardium.
Regional LAD function after transient metabolic stimulation.
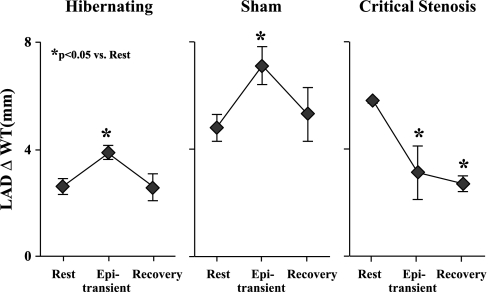
Figure 9 illustrates LAD wall thickening at rest, after 15 min of transient inotropic stimulation with epinephrine, and 15 min after recovery. Despite reduced systolic wall thickening at rest in animals with hibernating myocardium (2.6 ± 0.3 vs. 5.9 ± 0.5 mm in remote myocardium, P < 0.05), regional LAD wall thickening increased in response to inotropic stimulation (3.9 ± 0.3, P < 0.05). During recovery, function returned to the previous baseline level, with no evidence of superimposed acute stunning (2.6 ± 0.5, P = not significant). This was similar to the pattern in sham animals. In contrast, pigs with normal myocardium and an acute critical stenosis developed regional dysfunction during transient epinephrine stimulation (3.1 ± 1.6 mm during epinephrine vs. 5.8 ± 0.1 mm at rest, P < 0.05), and LAD wall thickening remained depressed during recovery (2.7 ± 0.3 mm, P < 0.05 vs. rest), consistent with acute stunning from demand-induced ischemia.
Fig. 9.
Protection of hibernating myocardium from acute demand-induced stunning following transient increases in demand during epinephrine infusion, determined by regional LAD wall thickening (ΔWT). Animals with hibernating myocardium (n = 9) were compared with normal sham animals (n = 7) as well as animals subjected to an acute flow-limiting stenosis (n = 3) on the LAD. Hemodynamic changes were similar among groups. Left: depression of LAD wall thickening in pigs with hibernating myocardium (rest), increase during β-adrenergic stimulation (Epi transient), and return to identical depressed baseline level 15 min into recovery. Middle: temporal increase and return to normal in sham animals. Right: contractile dysfunction during epinephrine stimulation in animals instrumented to place an acute critical stenosis prevented flow from increasing during vasodilation. Regional function remained depressed 15 min into recovery, indicative of acute stunning. These results indicate a preconditioning-like effect of acute myocardial stunning in pigs with chronic hibernating myocardium.
DISCUSSION
Several important new findings related to energetics during acute ischemia in chronically conditioned hibernating myocardium from the present study are as follows. 1) There are modest reductions in baseline ATP levels in hibernating myocardium, while total Cr, CP, and ATP-to-ADP ratio are preserved. 2) Hibernating myocardium exhibits a preconditioning-like effect during simulated ischemia in vitro, with decreased ATP depletion and higher absolute levels of ATP during the later stages of ischemia. The reduction in ATP depletion is not related to a slowing of glycolysis but appears to arise from an intrinsic downregulation in mitochondrial respiration. 3) The alterations in energetics and mitochondrial respiration are functionally relevant in vivo and accompanied by protection from acute stunning following demand-induced ischemia in the hibernating heart. Collectively, these findings support the hypothesis that intrinsic metabolic adaptations characteristic of hibernating myocardium serve to downregulate ATP utilization so as to chronically protect the heart from acute stunning and irreversible injury. This supports the results of histopathological studies in humans suggesting that hibernating myocardium is tolerant of ischemia.
Baseline HEP levels in hibernating myocardium.
Reductions in myocardial ATP levels can be observed in stunned myocardium, hypertrophied myocardium, remodeled myocardium following infarction, and the failing heart (3, 20, 21). All these changes occur in the absence of confounding tissue fibrosis. A number of groups have reported baseline HEP levels in chronically dysfunctional myocardium with disparate findings. This may partially relate to inclusion of a broad array of pathophysiological states associated with reversible dysynergy and fibrosis that are not related to chronic repetitive ischemia (5). Elsasser et al. (10) found severely reduced CP and ATP levels, along with markedly increased lactate levels and tissue acidosis, compared with biopsies from patients with normal LV function. In contrast, Wiggers et al. (56) demonstrated that ATP and CP levels were preserved and similar in dysfunctional and remote regions of patients with hibernating myocardium. They found that contractile dysfunction with preserved energetics was reversible after revascularization. In contrast, the pattern of depressed ATP and CP with tissue acidosis described by Elsasser et al. predicted irreversible dysfunction (56). The disparity between the two studies is difficult to resolve but may reflect differences arising from inclusion of patients with a degenerative vs. an adaptive myocyte phenotype (5). In support of this notion, fibrosis volume fraction was markedly increased in the study of Elsasser et al. (∼30%) when severe ATP depletion was present. In swine with hibernating myocardium and minimal fibrosis, McFalls et al. (30) reported results similar to those of Wiggers et al., with ATP and CP levels that were not different from remote regions. Consonant with these studies, our results confirm the lack of regional reductions in CP and ATP levels in hibernating myocardium. Nevertheless, when comparing results in LAD hibernating regions with remote regions and normal myocardium from control animals, we observed a global reduction of absolute ATP, ADP, and TAN levels. Despite reduced ATP, the total Cr and CP levels were preserved with normal ATP-to-ADP ratios, suggesting a stable energetic adaptation in chronic hibernating myocardium. McFalls et al. (32) also observed a normal energetic state within hibernating myocardium, as supported by normal phosphocreatine-to-ATP ratio detected by 31P spectroscopy in chronic hibernating myocardium. Interestingly, a similar pattern of energetic adaptation is typical of prolonged acute ischemia or short-term myocardial hibernation. For example, Pantely et al. (41) found that continued moderate ischemia in the pig heart caused persistent reduction in ATP, whereas after an initial decrease, CP recovered at 60 min of ischemia. Similarly, Martin et al. (29) demonstrated that the free energy change of ATP hydrolysis returned to normal at 90 min of ischemia in short-term hibernating myocardium. Taken together, a stable reduction in energetic state of hibernating myocardium appears to correlate with the reduced flow and contractile function, despite reduced baseline ATP values. The changes in ATP are similar to those following acute preconditioning with brief ischemia (37) but contrast with failing myocardium, where CP, total Cr, and ATP-to-ADP ratio are abnormal, with an imbalance of energy supply and demand (21, 28). Thus variable reductions in absolute ATP with preservation of stable energetic state are hallmarks of hibernating myocardium.
Preservation of ATP during simulated ischemia in vitro.
Murry et al. (37) demonstrated that ischemic preconditioning attenuates the rate of ATP depletion following prolonged ischemia in vivo. After ischemic preconditioning, ATP levels were higher and the onset of ultrastructural evidence of irreversible injury was delayed. A similar slowing of in vitro ATP depletion was also reported after acute ischemic preconditioning with use of an experimental approach that was identical to the present study (22). Our results in an animal model of chronic repetitive ischemia are similar to those observed in hearts with acute ischemic preconditioning. Although hibernating tissue started at lower initial ATP levels, the slower rate of ATP depletion resulted in higher ATP levels during late ischemia than in normal tissue. Since tissue ATP values are associated with the onset of sarcolemmal disruption and necrosis during infarction, the results support the notion that hibernating myocardium is chronically preconditioned against irreversible injury.
In contrast to a slowing of ATP depletion, we found no effect of hibernating myocardium on lactate accumulation. This contrasts with results of others during ischemic preconditioning, where lactate accumulation was modestly reduced when hearts were preconditioned with brief repetitive ischemia. It is, however, similar to the lack of effect of pharmacological preconditioning on lactate accumulation during simulated ischemia (55). Our results suggest that anaerobic glycolysis in hibernating myocardium is unchanged. The difference with repetitive ischemia likely relates to the fact that glycogen is, if anything, maintained or increased in hibernating myocardium, whereas it is markedly reduced after repetitive ischemia. Thus the attenuated rate of lactate accumulation in acute ischemic preconditioning probably reflects substrate availability.
Mitochondrial respiration in vitro.
The slower rate of ATP depletion we observed was not related to chronically reduced contractile function, since myocardial tissue was studied in an arrested state, where ATP consumption from contractile activity is absent. Moreover, a similar (albeit less pronounced) attenuation of ATP depletion was also found in normally contracting remote myocardium from hibernating hearts. One possibility is that these changes are the result of a reduction in mitochondrial respiration, reflecting an intrinsic adaptation of the heart to repetitive ischemia. In support of this theory, McFalls et al. (33) recently demonstrated a reduction in the RCR in mitochondria isolated from pigs with hibernating myocardium that results from insignificant reductions in state 3 respiration and increases in state 2 respiration. Our results provide further support for a primary mitochondrial adaptation by demonstrating a significant reduction in mitochondrial function in vitro. Similar to the finding of McFalls et al., we found a significant reduction in the RCR in pigs with hibernating myocardium. In contrast, this was predominantly due to a reduction in state 3 respiration, whereas state 2 respiration remained unchanged in the present study, compared with an increase in state 2 respiration, as in the previous study. The exact reasons for the differences are unclear but could reflect variability in the mechanism of contractile dysfunction between studies (chronic stunning vs. hibernating myocardium), the duration of repetitive ischemia to which swine were subjected, or variations in the physiological severity or chronicity of the stenosis.
Metabolic mechanisms involved in chronic adaptations to ischemia have recently been elucidated through proteomic analyses of viable dysfunctional myocardium by Page et al. (39) and Duan et al. (9). In pigs with hibernating myocardium, there was a coordinate downregulation of many mitochondrial enzymes, including entry points to oxidative metabolism and enzymes involved in electron transport. Importantly, there was a downregulation in the mitochondrial ATPase in hibernating myocardium. When contraction ceases under anaerobic conditions, the major utilization of ATP arises from the energy required for the mitochondrial ATPase (24), which reversibly catalyzes the conversion of ATP to ADP in the presence of ischemia. Jennings et al. (24) demonstrated that inhibition of this ATPase with oligomycin greatly slows ATP depletion in simulated ischemia. Therefore, reduced ATPase activity in hibernating myocardium could serve as a potential mechanism of reduced ATP depletion in hibernating heart exposed to ischemia.
Mechanisms related to delayed preconditioning (7) could also be involved in preserved ATP levels during simulated ischemia. It has been demonstrated that inducible nitric oxide (NO) synthase (NOS) is upregulated and total NOS activity is increased in chronic hibernating myocardium (25, 31) and that endogenous NO production from endothelial NOS helps the myocardium adapt to ischemia in short-term hibernation (17). Increased NO production could decrease myocardial O2 uptake by promoting the myocardial utilization of glucose, a mechanism that is independent of contractile activity, although it is not supported by the similarity of in vitro lactate accumulation (51). Alternatively, opening of the mitochondrial ATP-dependent potassium (KATP) channel has been shown to preserve the adenine nucleotide pool during simulated ischemia (8) and could contribute to cardioprotection via an action similar to ischemic preconditioning. An argument against the latter possibility is the finding that energy conservation during a catecholamine challenge in chronic hibernating myocardium was not affected by blockade of KATP with glibenclamide (32). Similarly, Schulz et al. (47) showed that KATP channels are not involved in metabolic adaptation in a swine model of short-term hibernation. Thus the conservation of energy in hibernating myocardium seems to be independent of the opening of the KATP channels.
Global changes in energetics in diseased hearts.
Our study also suggests that the remote normally perfused region in a dysfunctional hibernating heart develops an attenuation of HEP depletion and reduction in mitochondrial O2 consumption somewhat similar to the ischemic LAD region. Surprisingly, the baseline ATP levels in the LAD and remote regions were closely correlated within individual animals. Thus, although many mechanisms responsible for chronic hibernating myocardium are regional, global molecular, metabolic, and morphological remodeling may occur in the chronic setting. In support of remote remodeling in hibernating myocardium, Thomas et al. (54) demonstrated that glycogen content and myolysis increase to a similar extent in remote regions of hibernating hearts and in the ischemic LAD region. Similarly, structural proteins, such as titin and cardiotin, were also found to exhibit global, rather than regional, alterations in swine with hibernating myocardium (53). Global structural changes were also reported in human biopsies by Wiggers et al. (56).
The mechanism that drives global pathological and energetic changes in the hibernating heart is unclear. One possibility is that a circulating factor is released during myocardial ischemia. In this regard, it is interesting that reductions in baseline ATP levels in hibernating and remote regions were closely related to relative reductions in vasodilated LAD flow, which is a major predictor of demand-induced ischemia. The fact that global myocardial preconditioning has been elicited following remote ischemia of other organs supports a more central role for a circulating factor released by ischemia in animals as well as humans (13, 15). Circulating hormones, such as adiponectin (49) and leptin (36, 50), were reported to activate the AMP-activated protein kinase and the reperfusion injury salvage kinase pathways, both of which have a potential role in ischemic preconditioning (16, 42). Recently, macrophage migration inhibitory factor, a paracrine factor released in ischemic heart, was also reported to activate AMP-activated protein kinase (35).
An alternative explanation would be that myocardial stretch resulting from cyclical elevations in preload in response to acute regional ischemia elicits global remodeling, as has been demonstrated in canine myocardium (38). More recently, Schott et al. (44) showed that a short-term increase in preload in rabbit myocardium caused alteration of a variety of mitochondrial enzymes involved in energy metabolism. The long-term effect of elevated preload in regulating enzymes associated with energy metabolism is unknown, and further studies are needed.
Methodological limitations.
Our values of CP from needle biopsies are slightly lower than those reported in some previous studies (10, 48). The rapid decline in CP after sampling probably reflects small differences in the time required to freeze myocardial needle biopsy samples. This would not affect our primary conclusions, which are based on comparisons between groups with similar sampling strategies, rather than the absolute values.
We did not measure inorganic phosphate and, therefore, did not calculate free energy change of ATP hydrolysis, which recovers toward normal during short-term hibernation following prolonged acute ischemia (29). Nevertheless, chronic hibernating myocardium shows a normal ATP-to-ADP ratio, which supports a preserved, rather than depleted, energetic state.
Previous studies demonstrated a small increase in connective tissue that reduces myocyte volume fraction by ∼2% in chronic hibernating myocardium in swine (12). This can vary among samples from the same heart and was not quantified in the tissue samples, nor was it used to correct LAD tissue metabolites in hibernating swine. The lack of correction would not have affected the rate of ATP depletion and, if anything, would underestimate the preservation of ATP compared with normal tissue over time.
Many anesthetic regimens have been suggested to improve functional recovery after acute ischemia, but the effects of propofol and ketamine on myocardial infarct size are not known. Although they could alter ATP depletion rates, as well as mitochondrial respiration, our experimental design employed the same regimen when animals were briefly anesthetized for tissue harvest. Although this could have affected absolute measurements, the relative differences between hibernating and normal animals would have been unaffected.
Clinical implications.
The results of our study support the notion that, in the adapted state, hibernating myocardium is chronically protected against irreversible injury, as supported by studies examining myocyte cellular structure from biopsies of patients with hibernating myocardium (1, 34). The fact that this protection occurs globally is consistent with an indirect mechanism resulting from the release of a circulating factor following ischemia or myocardial stretch related to transient ischemia-induced increases in preload. Further studies are needed to determine whether similar endogenous protection is present in chronic ischemic heart disease when regional LV dysfunction is absent. Such a finding could explain the difficulty in eliciting preconditioning of humans with coronary artery disease compared with the ubiquity of this response in normal animals. It also supports the notion that hibernating myocardium is ischemia tolerant as an intrinsic adaptation to inadequate perfusion that parallels the downregulation in contractile function.
GRANTS
This work was supported by the Department of Veterans Affairs, National Heart, Lung, and Blood Institute Grants HL-55324 and HL-61610, the Albert and Elizabeth Rekate Fund, and the John R. Oishei Foundation.
Acknowledgments
We thank Anne Coe, Beth Palka, Deanna Gretka, Elaine Granica, and Amy Johnson for technical assistance.
REFERENCES
- 1.Ausma J, Thone F, Dispersyn GD, Flameng W, Vanoverschelde JL, Ramaekers FC, Borgers M. Dedifferentiated cardiomyocytes from chronic hibernating myocardium are ischemia-tolerant. Mol Cell Biochem 186: 159–168, 1998. [PubMed] [Google Scholar]
- 2.Banas MD, Baldwa S, Suzuki G, Canty JM, Fallavollita JA. Determinants of contractile reserve in viable, chronically dysfunctional myocardium. Am J Physiol Heart Circ Physiol 292: H2791–H2797, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R Mechanism of myocardial stunning. Circulation 82: 723–738, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Canty JM, Fallavollita JA. Hibernating myocardium. J Nucl Cardiol 12: 104–119, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Canty JM Jr. Coronary blood flow and myocardial ischemia. In: Braunwald's Heart Disease, edited by Libby P, Bonow RO, Mann DL, and Zipes DP. Philadelphia: Elsevier, 2007, p. 1167–1194.
- 6.Canty JM Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res 94: 1142–1149, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Depre C, Vatner SF. Cardioprotection in stunned and hibernating myocardium. Heart Fail Rev 12: 307–317, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP-sensitive K+ channel protects the ischemic heart. Am J Physiol Heart Circ Physiol 283: H284–H295, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Duan X, Young RF, Straubinger RM, Page BJ, Cao J, Wang H, Yu Y, Canty JM Jr, Qu J. A straightforward and highly efficient precipitation/on-pellet digestion procedure coupled to a long gradient nano-LC separation and Oprbitrap mass spectrometry for label-free expression profiling of the swine heart mitochondrial proteome. J Proteome Res. In press. [DOI] [PMC free article] [PubMed]
- 10.Elsasser A, Muller KD, Skwara W, Bode C, Kubler W, Vogt AM. Severe energy deprivation of human hibernating myocardium as possible common pathomechanism of contractile dysfunction, structural degeneration and cell death. J Am Coll Cardiol 39: 1189–1198, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Fallavollita JA, Malm BJ, Canty JM Jr. Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res 92: 48–55, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Fallavollita JA, Perry BJ, Canty JM Jr. 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium: evidence for transmural variations in chronic hibernating myocardium. Circulation 95: 1900–1909, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Gho BCG, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94: 2193–2200, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials 28: 4628–4634, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370: 575–579, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Heusch G Obesity—a risk factor or a RISK factor for myocardial infarction? Br J Pharmacol 149: 1–3, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heusch G, Post H, Michel MC, Kelm M, Schulz R. Endogenous nitric oxide and myocardial adaptation to ischemia. Circ Res 87: 146–152, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol 288: H984–H999, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hoppel C, DiMarco JP, Tandler B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem 254: 4164–4170, 1979. [PubMed] [Google Scholar]
- 20.Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, Swingen C, Zhang G, Feygin J, Ochiai K, Bransford TL, From AH, Bache RJ, Zhang J. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol 291: H648–H657, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 95: 135–145, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Jennings RB, Murry CE, Reimer KA. Energy metabolism in preconditioned and control myocardium: effect of total ischemia. J Mol Cell Cardiol 23: 1449–1458, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Jennings RB, Reimer KA, Hill ML, Mayer SE. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res 49: 892–900, 1981. [DOI] [PubMed] [Google Scholar]
- 24.Jennings RB, Reimer KA, Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol 23: 1383–1395, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Kalra DK, Zhu X, Ramchandani MK, Lawrie G, Reardon MJ, Lee-Jackson D, Winters WL, Sivasubramanian N, Mann DL, Zoghbi WA. Increased myocardial gene expression of tumor necrosis factor-α and nitric oxide synthase-2: a potential mechanism for depressed myocardial function in hibernating myocardium in humans. Circulation 105: 1537–1540, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Kowallik P, Schulz R, Guth BD, Schade A, Paffhausen W, Gross R, Heusch G. Measurement of regional myocardial blood flow with multiple colored microspheres. Circ Res 83: 974–982, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem 254: 6528–6537, 1979. [PubMed] [Google Scholar]
- 28.Lee J, Hu Q, Nakamura Y, Wang X, Zhang X, Zhu X, Chen W, Yang Q, Zhang J. Open-chest 31P magnetic resonance spectroscopy of mouse heart at 4.7 Tesla. J Magn Reson Imaging 24: 1269–1276, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Martin C, Schulz R, Rose J, Heusch G. Inorganic phosphate content and free energy change of ATP hydrolysis in regional short-term hibernating myocardium. Cardiovasc Res 39: 318–326, 1998. [DOI] [PubMed] [Google Scholar]
- 30.McFalls EO, Baldwin D, Palmer B, Marx D, Jaimes D, Ward HB. Regional glucose uptake within hypoperfused swine myocardium as measured by positron emission tomography. Am J Physiol Heart Circ Physiol 272: H343–H349, 1997. [DOI] [PubMed] [Google Scholar]
- 31.McFalls EO, Hou M, Bache RJ, Best A, Marx D, Sikora J, Ward HB. Activation of p38 MAPK and increased glucose transport in chronic hibernating swine myocardium. Am J Physiol Heart Circ Physiol 287: H1328–H1334, 2004. [DOI] [PubMed] [Google Scholar]
- 32.McFalls EO, Kelly RF, Hu Q, Mansoor A, Lee J, Kuskowski M, Sikora J, Ward HB, Zhang J. The energetic state within hibernating myocardium is normal during dobutamine despite inhibition of ATP-dependent potassium channel opening with glibenclamide. Am J Physiol Heart Circ Physiol 293: H2945–H2951, 2007. [DOI] [PubMed] [Google Scholar]
- 33.McFalls EO, Sluiter W, Schoonderwoerd K, Manintveld OC, Lamers JM, Bezstarosti K, van Beusekom HM, Sikora J, Ward HB, Merkus D, Duncker DJ. Mitochondrial adaptations within chronically ischemic swine myocardium. J Mol Cell Cardiol 41: 980–988, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Milei J, Fraga CG, Grana DR, Ferreira R, Ambrosio G. Ultrastructural evidence of increased tolerance of hibernating myocardium to cardioplegic ischemia-reperfusion injury. J Am Coll Cardiol 43: 2329–2336, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young LH. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature 451: 578–582, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 66: 913–931, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol Heart Circ Physiol 266: H137–H146, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Page B, Young R, Iyer V, Suzuki G, Lis M, Korotchkina K, Patel M, Blumenthal K, Fallavollita J, Canty JM Jr. Persistent regional downregulation in mitochondrial enzymes and upregulation of stress proteins in swine with chronic hibernating myocardium. Circ Res 102: 103–112, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–8739, 1977. [PubMed] [Google Scholar]
- 41.Pantely GA, Malone SA, Rhen WS, Anselone CG, Arai A, Bristow J, Bristow JD. Regeneration of myocardial phosphocreatine in pigs despite continued moderate ischemia. Circ Res 67: 1481–1493, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Russell RR 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Schott P, Asif AR, Graf C, Toischer K, Hasenfuss G, Kogler H. Myocardial adaptation of energy metabolism to elevated preload depends on calcineurin activity: a proteomic approach. Basic Res Cardiol 103: 232–243, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz R, Guth BD, Pieper K, Martin C, Heusch G. Recruitment of an inotropic reserve in moderately ischemic myocardium at the expense of metabolic recovery: a model of short-term hibernation. Circ Res 70: 1282–1295, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Schulz R, Rose J, Martin C, Brodde OE, Heusch G. Development of short-term myocardial hibernation: its limitation by the severity of ischemia and inotropic stimulation. Circulation 88: 684–695, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Schulz R, Rose J, Post H, Heusch G. Regional short-term myocardial hibernation in swine does not involve endogenous adenosine or KATP channels. Am J Physiol Heart Circ Physiol 268: H2294–H2301, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Sellevold OFM, Jynge P, Aarstad K. High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds and adenine nucleotides in myocardial tissue. J Mol Cell Cardiol 18: 517–527, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith CCT, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol 149: 5–13, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suto N, Mikuniya A, Okubo T, Hanada H, Shinozaki N, Okumura K. Nitric oxide modulates cardiac contractility and oxygen consumption without changing contractile efficiency. Am J Physiol Heart Circ Physiol 275: H41–H49, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki G, Lee TC, Fallavollita JA, Canty JM Jr. Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96: 767–775, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Thijssen VL, Borgers M, Lenders MH, Ramaekers FC, Suzuki G, Palka B, Fallavollita JA, Thomas SA, Canty JM Jr. Temporal and spatial variations in structural protein expression during the progression from stunned to hibernating myocardium. Circulation 110: 3313–3321, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Thomas SA, Fallavollita JA, Borgers M, Canty JM Jr. Dissociation of regional adaptations to ischemia and global myolysis in an accelerated swine model of chronic hibernating myocardium. Circ Res 91: 970–977, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Vander Heide RS, Reimer KA, Jennings RB. Adenosine slows ischaemic metabolism in canine myocardium in vitro: relationship to ischaemic preconditioning. Cardiovasc Res 27: 669–673, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Wiggers H, Noreng M, Paulsen PK, Bottcher M, Egeblad H, Nielsen TT, Botker HE. Energy stores and metabolites in chronic reversibly and irreversibly dysfunctional myocardium in humans. J Am Coll Cardiol 37: 100–108, 2001. [DOI] [PubMed] [Google Scholar]