Abstract
Nitric oxide (NO) inhibits transient receptor potential channel 3 (TRPC3) channels via a PKG-dependent mechanism. We sought to determine 1) whether NO inhibition of TRPC3 occurs in freshly isolated smooth muscle cells (SMC); and 2) whether NO inhibition of TRPC3 channels contributes to NO-mediated vasorelaxation. We tested these hypotheses in freshly isolated rat carotid artery (CA) SMC using patch clamp and in intact CA by vessel myograph. We demonstrated TRPC3 expression in whole CA (mRNA and protein) that was localized to the smooth muscle layers. TRPC1 protein was also expressed and coimmunoprecipitated with TRPC3. Whole cell patch clamp demonstrated nonselective cation channel currents that were activated by UTP (60 μM) and completely inhibited by a TRPC channel inhibitor, La3+ (100 μM). The UTP-stimulated current (IUTP) was also inhibited by intracellular application of anti-TRPC3 or anti-TRPC1 antibody, but not by anti-TRPC6 or anti-TRPC4 control antibodies. We next evaluated the NO signaling pathway on IUTP. Exogenous NO [(Z)-1-{N-methyl-N-[6(N-methylammoniohexyl)amino]}diazen-1-ium-1,2-diolate (MAHMA NONOate)] or a cell-permeable cGMP analog (8-bromo-cGMP) significantly inhibited IUTP. Preapplication of a PKG inhibitor (KT5823) reversed the inhibition of MAHMA NONOate or 8-bromo-cGMP, demonstrating the critical role of PKG in NO inhibition of TRPC1/TRPC3. Intact CA segments were contracted with UTP (100 μM) in the presence or absence of La3+ (100 μM) and then evaluated for relaxation to an NO donor, sodium nitroprusside (1 nM to 1 μM). Relaxation to sodium nitroprusside was significantly reduced in the La3+ treatment group. We conclude that freshly isolated SMC express TRPC1/TRPC3 channels and that these channels are inhibited by NO/cGMP/PKG. Furthermore, NO contributes to vasorelaxation by inhibition of La3+-sensitive channels consistent with TRPC1/TRPC3.
Keywords: transient receptor potential channel, protein kinase G, transient receptor potential 3, transient receptor potential 1
the canonical transient receptor potential channels (TRPCs) are nonselective cation channels that have been demonstrated to play a variety of roles in regulation of vascular tone (23). TRPC3 is one of the less well-characterized members of the TRPC family (TRPC1–7) within the vasculature. Nevertheless, TRPC3 has recently been shown to be involved in receptor-mediated constriction within the smooth muscle cells (SMCs) of cerebral arteries (18). Activation of the TRPC3 channel permits Ca2+ and Na+ entry into the cell; however, the major mechanism of Ca2+ entry in SMC is thought to be secondary to Na+ entry and smooth muscle depolarization (18). Depolarization from Na+ entry leads to activation of L-type Ca2+ channels with subsequent Ca2+ entry and smooth muscle contraction.
Nitric oxide (NO) is well known as a vasodilator. However, the mechanism by which NO promotes smooth muscle relaxation is still incompletely resolved. At this time, it is believed that NO leads to smooth muscle relaxation by multiple pathways, including activation of potassium channels (6), activation of the sarco(endo)plasmic reticulum Ca2+-ATPase (8), inhibition of Rho kinase (7, 20), and stimulation of myosin light chain phosphatase (16). Recent TRPC3 studies indicate that these channels are regulated by NO as well (13), raising the question of whether inhibition of TRPCs may be yet another pathway by which NO regulates smooth muscle relaxation.
Initial studies by Kwan et al. (13) demonstrated that TRPC3 overexpressed in human embryonic kidney (HEK)-293 cells can be inhibited by NO through PKG-dependent phosphorylation of the protein at positions T11 and S263. Since structurally related TRPC6 and TRPC7 share several of these PKG phosphorylation consensus sites, these channels have been proposed to share the same regulatory potential of the NO signaling pathway (13). Indeed, a more recent examination of TRPC6 has shown that PKG-dependent phosphorylation does occur in overexpressed TRPC6 channels, as well as in a rat aorta SMC line, A7r5 cells (22).
While the evidence for negative regulation of TRPCs by the NO signaling pathway in vascular smooth muscle is mounting, it is still incomplete. Even with the recent reporting of NO/cGMP/PKG negative regulation of TRPC6 in A7r5 cells (22), data regarding similar regulation of TRPC3 or TRPC7 in SMC are lacking. Furthermore, the existing data regarding this potentially novel aspect of NO-mediated vasorelaxation is entirely derived from studies performed with a SMC line (22). This is an especially relevant point, since it is known that expression of proteins involved in Ca2+ handling (including TRPC3 and TRPC6) changes significantly in SMC culture (3). Thus studies are needed in freshly isolated vascular SMCs (VSMCs) to demonstrate that this mechanism is not unique to SMC culture or cell lines. Additionally, the proposed model of NO inhibition of TRPC3 needs to be examined in intact arteries to determine whether this mechanism comprises a meaningful component of NO-mediated vasorelaxation.
The present study was originally designed to test the following hypotheses: 1) NO/cGMP/PKG-mediated inhibition of TRPC3 channels occurs in native SMCs; and 2) NO-mediated vasorelaxation is partially dependent on inhibition of TRPCs in intact carotid artery (CA). However, in the course of these studies, we obtained novel data suggesting that TRPC3 might act within a TRPC1/TRPC3 heteromer. We subsequently modified hypothesis 1 to incorporate this new data as follows: NO/cGMP/PKG-mediated inhibition of TRPC1/TRPC3 channels occurs in native SMCs. These hypotheses were tested in the rat CA at the cellular level (whole cell patch clamp) and at the whole artery level (isolated vessel myograph). These studies show for the first time that VSMC exhibit TRPC1/TRPC3 channel currents that are inhibited by the NO signaling pathway. Furthermore, these studies provide preliminary evidence for a NO/TRPC1/TRPC3 feedback system that is functionally involved in NO-mediated vasorelaxation of intact arteries.
MATERIALS AND METHODS
Experiments were carried out in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the Animal Protocol Review Committee at Baylor College of Medicine. Male Long-Evans rats (230–330g) from Charles River Laboratories were anesthetized with isoflurane.
RT-PCR analysis.
Total RNA from CA was extracted using the RNeasy MicroKit (Qiagen) per manufacturer's instructions. The mRNA was converted to cDNA using SuperScript III (Invitrogen), according to manufacturer instructions. RNase H was added and incubated for 20 min at 37°C to degrade any remaining RNA. Control samples (RT−) were prepared by substitution of dH2O for reverse transcriptase.
PCR amplification was performed using Platinum Taq DNA polymerase (Invitrogen) according to the recommended protocol. Amplification parameters were as follows: 97°C activation (2 min) followed by 94°C dissociation (15 s), 56°C anneal (45 s), and 72°C elongation (45 s) for 33 cycles. Primer pairs were as follows: TRPC3 forward: 5′-ctggccaacatagagaaggagt-3′, TRPC3 reverse: 5′-caccgattccagatctccat-3 (5)′; TRPC6 forward: 5′-gacagaaatcagctggcaca-3′, TRPC6 reverse: 5′-tccccttcgttcacttcatc-3′; TRPC7 set 1 forward: 5′-catggtgtttgtggccttca-3′, TRPC7 set 1 reverse: 5′-cgtctgcgtcttcctcgatt-3′; TRPC7 set 2 forward: 5′-tgctcaacatgctgatagcc-3′, TRPC7 set 2 reverse: 5′-aaagggagcagggagagttc-3′. Negative controls were performed with RT− samples to confirm the absence of genomic DNA amplification. PCR products were analyzed by electrophoresis and digitally imaged for analysis (Kodak). The predicted size of the PCR amplification products are 141, 176, 137, and 293 for TRPC3, TRPC6, and TRPC7 primer sets 1 and 2, respectively.
Immunohistochemistry.
The CA was removed and immediately frozen in 2-methylbutane. Frozen segments were mounted in a cryostat (−20°C) and cut into 10-μm sections. The sections were fixed with 4% paraformaldehyde for 10 min and then washed with phosphate-buffered saline (PBS) (3 × 5 min). Sections were then permeabilized and blocked in PBS containing goat serum (10%), BSA (0.5%), and Tween 20 (0.1%) for 30 min at RT. Incubation with rabbit anti-TRPC3 antibody (Sigma) or PBS alone was performed overnight at 4°C at 1:300 dilution in the blocking solution above. Sections were next washed with PBS (3 × 5 min) and incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG secondary (Jackson ImmunoResearch, West Grove, PA). The secondary detection antibody was incubated covered at room temperature (60 min) at 1:1,000 dilution. The sections were washed with PBS (5 × 10 min) and stained with 4,6-diamidino-2-phenylindole (1:10,000 dilution) before coverslips were applied. Imaging was performed using an Olympus IX81 motorized fluorescence microscope with Retiga 2000RV camera and Slidebook 4.2 software. Sections were acquired at 0.3-μm intervals using a ×60 oil objective (numerical aperture 1.40).
Western blot analysis.
Two-millimeter sections of CA were harvested, cleaned of connective tissue and blood, and frozen in liquid nitrogen. The segments were pulverized with a pestle, homogenized in RIPA buffer (Sigma) containing 50 mM Tris·HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate with protease inhibitor cocktail (Roche, Indianapolis, IN). After centrifugation (15 min at 15,000 g), a portion of the supernatant was used for total protein by DC Protein Assay (Bio-Rad, Hercules, CA). Equal amounts of sample protein were mixed with LDS sample buffer and sample reducing agent (Invitrogen, Carlsbad, CA), and heated at 70°C for 10 min. Two to eight micrograms of protein were loaded onto each lane of the gel (10% NuPAGE Bis-Tris gel). The gel was run at room temperature at 130 V for 1.5 h and then transferred to a nitrocellulose membrane by iBlot Dry Blotting System (Invitrogen), according to the manufacturer's instructions. The membrane was blocked in PBS supplemented with 5% nonfat dry milk and 0.2% Tween 20 (block solution) for 2 h, followed by incubation in a rabbit polyclonal anti-TRPC3 antibody (1:800) or anti-TRPC6 antibody (1:400) at 4°C overnight (both from Sigma). The membrane was washed in PBS with 0.2% Tween 20 (PBS-T, 4 × 10 min) and then incubated in a goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:10,000 diluted in block solution, Thermo Fisher, Rockford, IL) for 1 h. The membrane was washed with PBS-T (4 × 10 min) and then layered with Pierce SuperSignal West Femto Maximum Sensitivity Substrate (4 min), followed by exposure to film.
Coimmunoprecipitation.
Total CA protein was extracted as above, except with a buffer containing 50 mM Tris·HCl (pH 8.0), 1% Nonidet P-40, and protease inhibitor cocktail. Coimmunoprecipitation was performed using Dynabeads protein A (Invitrogen) with anti-TRPC3 (3 μg in 200 μl volume) as the immunoprecipitating antibody. Washes were performed with the same buffer used for protein extraction. The eluted protein was run out on a gel, transferred to nitrocellulose membrane, and probed with anti-TRPC1 (1:400, Sigma) at 4°C overnight. The secondary antibody and detection was performed as described above.
Isolation of rat CA SMC.
Single rat CA SMC were enzymatically isolated using a modification of a previously described protocol (9). Both CA were harvested, cleaned of connective tissue, and placed in cold (4°C) digestion buffer, containing 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 10 mM glucose, 10 mM HEPES, with pH adjusted to 7.4 with NaOH. After being washed once in digestion buffer, the CA were cut into ∼2-mm-long segments. Each segment was cut longitudinally into two pieces. Two segments were incubated in digestion buffer with 5 mg/ml BSA for 10 min and then digested with 3.5 mg/ml papain (Sigma, P4762), 5 mg/ml dithiothreitol, and 5 mg/ml BSA in digestion buffer for 30 min at 37°C. Afterwards, the tissue was washed with digestion buffer twice and further digested with 5 mg/ml collagenase H (Sigma, C8051), 5 mg/ml hyaluronidase (Sigma, H3506), and 5 mg/ml BSA in digestion buffer containing 50 μM CaCl2 for 10 min at 37°C. The tissue was washed several times with digestion buffer and triturated with a fire-polished Pasteur pipette. The cells were placed on ice and used within 8 h.
Patch-clamp electrophysiology.
The whole-cell patch-clamp configuration was used for measurements of whole-cell currents in freshly isolated SMC using a MultiClamp 700B amplifier (Axon Instruments) and pCLAMP 10 software (Axon Instruments, Union City, CA), as reported previously (4). Patch electrodes were pulled from borosilicate glass (1.65 outer diameter, 1.28 inner diameter; Warner Instruments) and polished to a pipette resistance of ∼2 MΩ. In some instances, currents were normalized to the cell membrane capacitance and presented as current densities (pA/pF).The pipette buffer contained (in mM) 156 CsCl, 1 MgCl2, 0.91 CaCl2, 2 EGTA, 7.85 CsOH, and 10 HEPES; pH was adjusted to 7.2 with CsOH. The calculated free Ca2+ concentration was 131 nM. The bath buffer contained (mM) 156 NaCl, 1.8 CaCl2, 10 glucose, and 10 HEPES; pH was adjusted to 7.4 with NaOH. The isolated cells were placed in a chamber on the stage of an inverted microscope and continually superfused with bath buffer. Whole-cell currents were recorded in the voltage-clamp mode over the voltage range of −110 to +80 mV (sweep rate of 0.131 mV/ms; −50 mV holding potential). Whole cell ionic currents were measured in the absence or presence of 60 μM UTP. Lanthanum chloride (100 μM) was added 5 min after UTP stimulation. In some cases, 10 μM gadolinium chloride was added instead of lanthanum chloride. Data were digitized and filtered at 1 kHz. All recordings were performed at room temperature (20–22°C).
Anti-TRPC3 antibody targeted to an intracellular region of the TRPC3 protein near the COOH-terminus, anti-TRPC1 antibody targeted to an intracellular epitope, as well as anti-TRPC4 and anti-TRPC6 antibodies. These antibodies were thus added to the pipette solution to allow access to the antigenic region of the channels. Since the anti-TRPC antibodies contain sodium azide (NaN3) as preservative, we also investigated the effect of 0.00025% NaN3 (concentration of NaN3 when anti-TRPC antibodies are diluted at 1:200) in pipette solution. This concentration of NaN3 did not alter the evoked UTP currents. UTP stimulation was started 10 min after obtaining whole cell access. 1-Oleoyl-2-acetyl-sn-glycerol (OAG) and KT 5823 (BIOMOL, Plymouth Meeting, PA) were dissolved in DMSO. (Z)-1-{N-methyl-N-[6(N-methylammoniohexyl)amino]}diazen-1-ium-1,2-diolate (MAHMA NONOate) (Cayman, Ann Arbor, MI) was dissolved in 0.01 M NaOH and added in both bath and pipette solution. The presence of 0.01 mM NaOH (concentration as 10 μM MAHMA NONOate) in pipette solution does not alter the basal current or UTP-stimulated current (IUTP) (data not shown). 8-Bromo-cGMP (8-BrcGMP) (Tocris, Elliville, MO) and other compounds were dissolved in distilled water.
Isometric tension recordings.
Isolated CAs from Long Evans rats were placed in ice-cold physiological solution containing the following (in mM): 137 NaCl, 5.6 KCl, 1 MgCl2, 10 glucose, 2.5 CaCl2, and 10 HEPES (pH 7.4). Each artery was subsequently cut into four ring segments that were 2.5 mm in length. The rings were then mounted in an eight-channel artery myograph (ChuelTech, Houston, TX) containing the same physiological solution at 37°C. This bicarbonate-free buffer was used in these experiments due to precipitation of LaCl3 in bicarbonate-containing solutions (unpublished observation).
Each ring was stretched to a resting tension of 1.5 g based on length-tension studies. Rings were then subjected to four 40 mM KCl exposures, followed by one 60 mM KCl exposure before subsequent study. In one study, concentration-response curves were performed to UTP (1 × 10−6 to 3 × 10−4 M) in the presence and absence of LaCl3 (100 μM). In a second study, rings were constricted with UTP (100 μM) in the presence or absence of LaCl3 (100 μM). This concentration of UTP was used because the level of tension did not differ between groups. Following preconstriction with UTP, concentration responses were generated with sodium nitroprusside (SNP, 1 × 10−9 to 1 × 10−6 M) in log steps. In the summary plot, relaxations were normalized according to the formula (TSNP − TUTP)/(TUTP − Tbase) * 100, where Tbase is the resting tension, TUTP is the tension in response to 100 μM UTP, and TSNP is the tension in response to each concentration of SNP. Data was digitized and analyzed with Powerlab/8SP with Chart version 4.24.
Statistical analysis.
Data are presented as means ± SE. Comparisons between treatment groups containing multiple points were made by two-way repeated measures-ANOVA; comparison between individual points was evaluated by the Holm-Sidak method. Comparisons between treatment groups containing single points were made by one-way ANOVA (SigmaPlot 11).
RESULTS
Demonstration of TRPC3/6/7 mRNA and protein in CA.
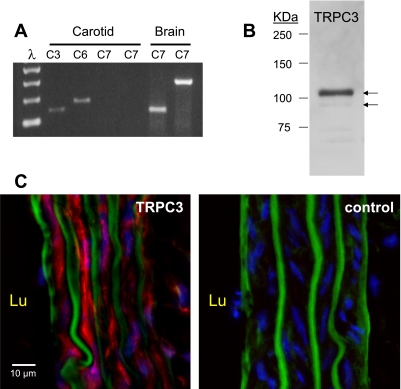
We first examined CA for expression of TRPC3/6/7 mRNA by RT-PCR. Single products of expected size were obtained for TRPC3 and TRPC6; however, no product was obtained for TRPC7 with either of two sets of PCR primers (Fig. 1A). The TRPC7 primers were validated using cDNA obtained from whole brain.
Fig. 1.
A: evaluation of transient receptor potential channel (TRPC) 3/6/7 mRNA expression in rat carotid artery (CA) by RT-PCR. PCR amplicons of predicted size were detected for TRPC3 and TRPC6, but not for TRPC7. Brain cDNA was used as a positive control for the TRPC7 primers. A 100-bp ladder is shown to the left. B: evaluation of TRPC3 protein expression in rat CA by Western blot. The arrows indicate the doublet corresponding to the glycosylated and nonglycosylated forms of the TRPC3 protein. C: immunofluorescence analysis of TRPC3 expression in CA frozen sections. TRPC3 expression (red) is present throughout the smooth muscle layers, but is not seen in the endothelial cells. The green signal reflects the elastic laminae recorded as the autofluorescence in the green channel. Cell nuclei appear in the blue channel. Lu, luminal (endothelial) side.
We next determined whether TRPC3 protein was expressed in CA by Western analysis. A doublet corresponding to the glycosylated and unglycosylated forms of the protein was obtained at the expected mass of TRPC3 (Fig. 1B) (10). Heart protein was used as a positive control and yielded a lower mass band of ∼84 kDa (not shown), consistent with a known splice variant expressed in heart (17). TRPC6 protein was not detected in CA, although it was detected in cerebral arteries and in brain (Supplemental Fig. 1). (The online version of this article contains supplemental data.) TRPC7 was not examined for protein due to the lack of mRNA.
We performed immunofluorescence with CA frozen sections to determine which cells within the CA expressed TRPC3 (Fig. 1C). An autofluorescence recording in the green channel demonstrates the multiple elastic layers within the CA. The innermost elastic layer (luminal side, Lu) reflects the boundary between the endothelial and smooth muscle layers. The 4,6-diamidino-2-phenylindole-stained nuclei are visible in the blue channel. TRPC3 immunofluorescence (red channel) was present throughout the smooth muscle layers, but was not detected in the endothelium.
Demonstration of TRPC1/TRPC3 protein interaction.
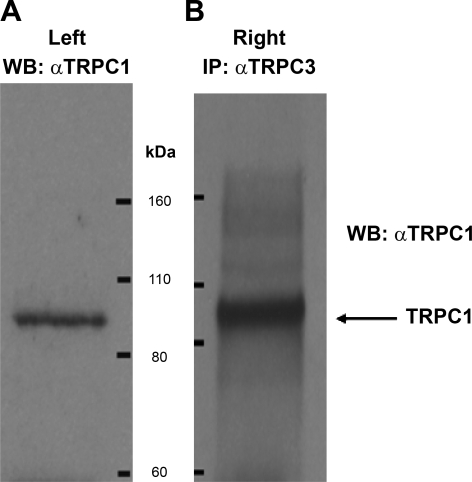
During the course of these studies, data emerged suggesting a role for TRPC1 or a TRPC1-containing heteromer. As TRPC1 and TRPC3 have demonstrated the capacity to form a heteromeric channel in expression systems and native embryonic brain tissue (15, 21), we investigated this possibility in CA. TRPC1 protein was detected by Western as a single band at ∼88 kDa (Fig. 2A). Next, coimmunoprecipitation was performed using anti-TRPC3 as the precipitating antibody, and the Western blot was probed with anti-TRPC1. A prominent single band was detected corresponding to the expected mass of TRPC1 (Fig. 2B). A total of three separate co-IP experiments were performed with protein extracts from freshly isolated CA. We similarly found a protein interaction of TRPC1/TRPC3 in cultured CA SMCs by coimmunoprecipitation (Supplemental Fig. 2).
Fig. 2.
A: evaluation of TRPC1 protein expression in rat CA by Western blot (WB). The blot shows a band at the expected mass of TRPC1. B: coimmunoprecipitation of TRPC1 and TRPC3 from rat CA. Protein was immunoprecipitated with anti-TRPC3 and the membrane probed with anti-TRPC1.
Demonstration of TRPC1/TRPC3 currents in isolated VSMCs.
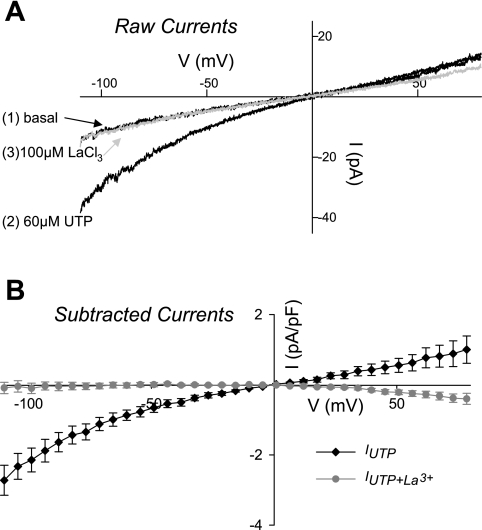
We utilized whole cell patch clamp to determine whether TRPC1/TRPC3 channel currents were present in freshly isolated CA SMC. Initial studies were performed using OAG (100 μM) to stimulate TRPC currents. OAG, a cell-permeable analalog of diacylglycerol, is a known direct activator of the TRPC family. OAG produced increased inward and outward currents, as would be expected (Supplemental Fig. 3). We next evaluated the potential of UTP to stimulate TRPC currents. This agonist has recently been demonstrated to stimulate TRPC3 channel currents in rat cerebral artery SMCs (18). Figure 3A shows raw current-voltage recordings at rest, after UTP (60 μM), and following addition of a nonselective TRPC1 and TRPC3 channel blocker, 100 μM La3+. The IUTP was primarily inward and reversed near 0 mV in these conditions. A summary of the subtracted currents (UTP − basal and UTP + La3+ − basal) normalized for capacitance are shown in Fig. 3B. The IUTP was abolished by the application of La3+.
Fig. 3.
A: representative current-voltage (I-V) plots in freshly isolated rat CA smooth muscle cells (SMCs). The currents reflect the baseline current (1), the current following application of 60 μM UTP (2), and then the cumulative application of UTP and 100 μM La3+ (3). B: summary of the UTP-stimulated current (IUTP) (subtracted currents; UTP-basal) before (diamonds) and after application of La3+ (circles). Currents are normalized to cell capacitance. Administration of La3+ abolished the IUTP.
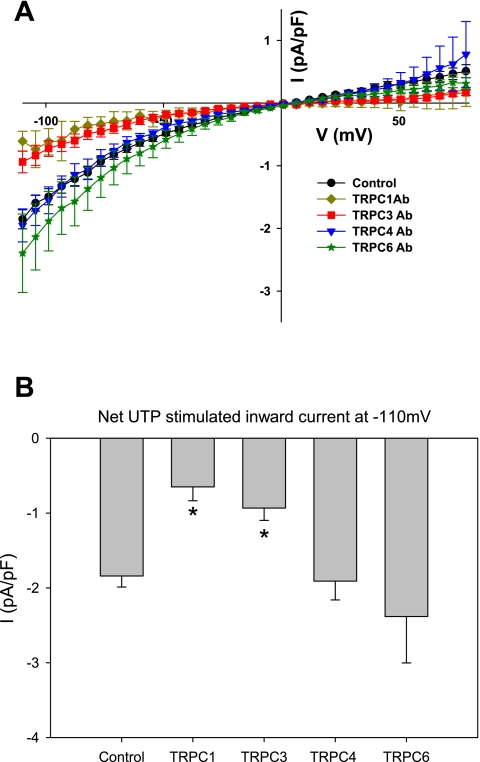
To confirm that UTP was indeed stimulating TRPC1/TRPC3 currents in CA SMCs, we applied TRPC1 or TRPC3 channel antibody to the inside of the cell via the patch pipette solution (Fig. 4A). These antibodies recognize an intracellular epitope and thus were expected to interfere with normal channel function, as previously demonstrated (1, 2, 11, 12, 19). Application of TRPC1 channel antibody (3 μg/ml) or TRPC3 channel antibody (3 μg/ml) produced a significant decrease in IUTP. In contrast, application of other TRPC antibodies (TRPC4 and TRPC6) did not decrease IUTP, demonstrating the functional role for TRPC1 and TRPC3, but not TRPC6. TRPC4 antibody was used as a negative control, as there was no expectation that TRPC4 channels were involved in the smooth muscle response to UTP. As an additional control, application of sodium azide (a preservative present in antibody solutions) also did not decrease IUTP. The summary IUTP are presented as subtracted currents normalized to cell capacitance, as in Fig. 3B. The IUTP at −110 mV is summarized for the different antibodies in Fig. 4B. The peak inward IUTP with either TRPC1 or TRPC3 antibody is significantly reduced compared with control or to TRPC4 and TRPC6 antibodies. When taken with the coimmunoprecipitation results, these data suggest that IUTP is carried by a TRPC1/TRPC3 heteromer in rat CA SMC.
Fig. 4.
A: summary I-V plots showing IUTP normalized to cell capacitance (current density). Control recordings were performed with standard patch pipette solution (circles) as well as with sodium azide as a vehicle control (not shown). Antibody (Ab) for TRPC1 (diamonds), TRPC3 (squares), TRPC4 (inverted triangles), and TRPC6 (stars) was included in the pipette solution to selectively inhibit the respective TRPCs. Note that there is significant reduction of current with intracellular application of TRPC1 or TRPC3 Ab. B: summary of IUTP at −110 mV for the different TRPC Abs in the pipette solution. Treatment with the TRPC1 or TRPC3 Ab, but not TRPC4 or TRPC6 Abs, resulted in significantly reduced inward current. *P < 0.05 vs. control.
Demonstration of TRPC1/TRPC3 current inhibition by NO/PKG.
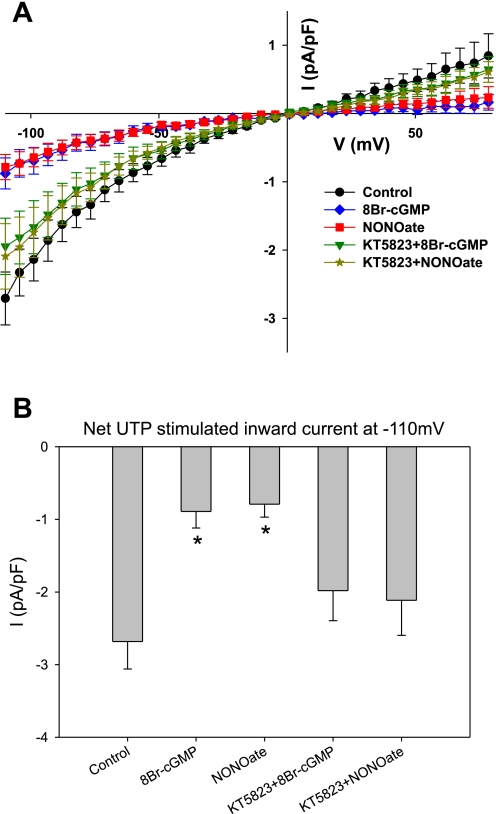
From the above studies, we demonstrated that the IUTP in CA SMCs was largely dependent on TRPC1/TRPC3 channels. We next sought to determine whether the TRPC1/TRPC3 channel current was inhibited by NO or products of the NO-signaling pathway (Fig. 5A). Application of a NO donor, MAHMA NONOate (10 μM), results in a significant inhibition of IUTP. This concentration of MAHMA NONOate produces a near, but not complete, dilation of cerebral arteries and arterioles, suggesting that this concentration of NO donor is sufficient but not excessive to promote dilation of an artery (28). Similar reduction of IUTP was obtained with the cell-permeable cGMP analog, 8-BrcGMP (100 μM). To evaluate the role of PKG in the mechanism, we used a PKG inhibitor (KT5823; 1 μM) to reverse the effect of MAHMA NONOate or 8-BrcGMP. Thus, if NO and cGMP act through PKG activation, inhibition of PKG should reverse the inhibitory effect of MAHMA NONOate and 8-BrcGMP. Figure 5A further demonstrates that KT5823 does indeed inhibit the effect of MAHMA NONOate and 8-BrcGMP to block IUTP. Notice that 8-BrcGMP and MAHMA NONOate significantly reduce IUTP, whereas IUTP is restored to near control levels when the PKG inhibitor is present. Thus the TRPC1/TRPC3 current is inhibited by NO and cGMP. Furthermore, these regulatory effects are mediated by PKG. The peak inward IUTP at −110 mV are summarized in Fig. 5B. Peak inward currents are significantly reduced compared with control, as well as the KT5823 + MAHMA NONOate or KT5823 + 8-BrcGMP groups.
Fig. 5.
A: summary I-V plots showing IUTP normalized to cell capacitance (current density). Recordings were performed with UTP alone (circles) or following pretreatment with 8-bromo-cGMP (8-BrcGMP; diamonds), (Z)-1-{N-methyl-N-[6(N-methylammoniohexyl)amino]}diazen-1-ium-1,2-diolate (MAHMA NONOate; squares), KT5823 + 8-BrcGMP (inverted triangles), or KT5823 + MAHMA NONOate (stars). The IUTP was inhibited by the nitric oxide (NO) donor, as well as the cGMP analog. Note that the inhibition of the NO donor or cGMP analog was reversed in the presence of a PKG inhibitor. B: summary of UTP-stimulated current at −110 mV evaluating regulation by the NO signaling pathway. *P < 0.05 vs. control.
Role of TRPC-like currents in NO-mediated vasorelaxation.
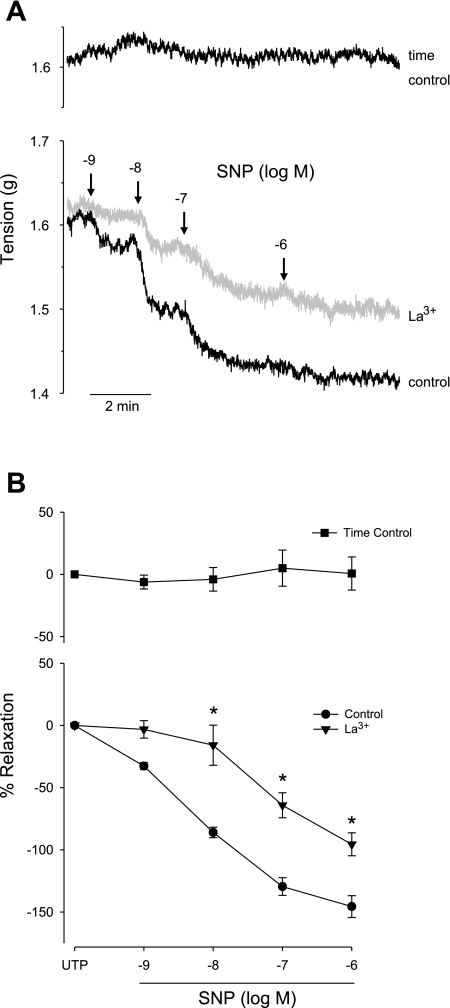
We used CA segments in an isometric tension ring bath to evaluate the role of TRPC1/TRPC3 channels in NO-mediated vasorelaxation. NG-nitro-l-arginine methyl ester (50 μM) was included to inhibit endogenous production of NO. UTP (100 μM) was used to preconstrict arteries in the absence or presence of La3+ (100 μM). This was the highest concentration of UTP that still produced comparable tension in all groups. Relaxations were then elicited by the delivery of SNP (10−9 to 10−6M). Figure 6A shows a representative experiment, including a control (no La3+), 100 μM La3+, and a time control (no SNP added) to evaluate the stability of the UTP constriction. The summarized data (Fig. 6B) demonstrate a significant inhibition of the NO-mediated relaxation by 100 μM La3+.
Fig. 6.
A: representative artery myograph traces from isolated CA segments. CA segments were preconstricted with 100 μM UTP before addition of sodium nitroprusside (SNP) in the absence (control) or presence of 100 μM La3+. The arrows indicate the points at which SNP was delivered. A time control was performed, in which SNP was not administered, to demonstrate the stability of the UTP response over the time period of the SNP study. B: summary of the SNP-mediated relaxation responses for control (circles) and for 100 μM La3+ (triangles). A time control was performed in which no SNP was administered (squares). The La3+ treatment group was significantly different from the controls. *P < 0.05 vs. control and time control groups.
DISCUSSION
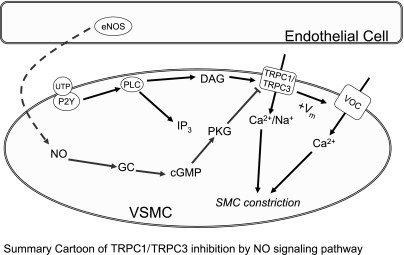
Our goal in this study was to test the following two hypotheses regarding the regulation of vascular tone by NO: 1) NO/cGMP/PKG-mediated inhibition of TRPC1/TRPC3 channels occurs in native SMC; and 2) NO-mediated vasorelaxation is partially dependent on inhibition of TRPCs in intact CA. As a result of these studies, we conclude that CA smooth muscle expresses a TRPC1/TRPC3 heteromer, which can be activated in response to UTP stimulation. The TRPC1/TRPC3 heteromer is significantly inhibited by addition of exogenous NO or a cell-permeable analog of cGMP. Furthermore, this inhibition requires the specific activation of PKG. Last, we present evidence that this mechanism of TRPC1/TRPC3 channel inhibition contributes to the mechanism of NO-mediated vasorelaxation in the intact artery. These findings are summarized in Fig. 7 and are discussed further below.
Fig. 7.
Cartoon depicting the proposed mechanism of NO regulation of vascular tone via TRPC1/TRPC3 heteromeric channels. In the proposed mechanism, UTP stimulation results in activation of PLC with subsequent production of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). DAG [and possibly IP3 (24)] stimulates TRPC1/TRPC3, which promotes influx of Ca2+ and Na+. The influx of Na+ results in the depolarization of the SMC and increases the opening of voltage-operated Ca2+ channels (VOC). This opening of the VOCs is the main source of Ca2+ influx that promotes SMC constriction. NO diffuses from the endothelium and stimulates guanylate cyclase (GC) to produce cGMP within the SMC. The resulting cGMP activates PKG, which then phosphorylates and inactivates the TRPC1/TRPC3 channels, thus inhibiting the depolarizing stimulus. eNOS, endothelial NO synthase; VSMC, vascular SMC; Vm, membrane potential.
Expression of the NO-sensitive TRPCs in native CA smooth muscle.
Of the TRPCs, TRPC3 and TRPC6 have been demonstrated to be inhibited by NO in heterologous expression systems, as well as a SMC line A7r5 cells (13, 14, 22). The critical PKG phosphorylation sites identified for TRPC3 and TRPC6 are conserved in hTRPC7, thus suggesting that TRPC7 may similarly be regulated by NO (13, 22). However, a comprehensive study evaluating such potential regulation of TRPC7 has yet to be reported. For the purposes of this study, however, we considered all of the TRPC3/6/7 channels as potentially NO sensitive.
In the present study, we evaluated native CA smooth muscle for the expression of TRPC3/6/7. We demonstrated the presence of TRPC3 and TRPC6 mRNA in whole CA. However, expression of TRPC7 was not detected with either of two validated sets of PCR primers. TRPC3 protein was readily detected by Western analysis and localized to the SMC by immunofluorescence. Interestingly, we did not detect TRPC3 expression in the endothelial cells in this artery, even though it has been shown to be expressed in the endothelium of some other vascular beds (22a, 26) and in cultured endothelial cells (17a, 20a). TRPC6 protein was not detectable in CA by Western analysis, although it was detectable at low levels in whole cerebral artery and brain protein extracts (Supplemental Fig. 1). While these findings demonstrated a significant presence of TRPC3 in carotid smooth muscle, we could not exclude some low level of expression of TRPC6 as well. Therefore, while studies continued predominantly with TRPC3, we continued to consider a possible role for TRPC6 in our experimental design (see below).
The functional expression of TRPC3 channels in carotid SMCs was evaluated by patch clamp. We used UTP to elicit TRPC currents, given that this agonist is reported to produce mainly TRPC3 channel activation in cerebral artery SMC (18). UTP (60 μM) produced reproducible currents that reversed at ∼0 mV and shared similar rectification compared with other reported TRPC3 recordings (10, 18). Since there is a lack of selective pharmacological tools with which to study TRPC3 and TRPC6 function and RNA silencing proved ineffective in the CA smooth muscle, we evaluated the role of these channels in IUTP by inhibition with selective TRPC antibodies delivered via the patch pipette solution. This type of strategy has been used successfully by others to inhibit TRPC1, TRPC3, TRPC5, and TRPC6 channels (1, 2, 11, 12, 19, 25). Surprisingly, we found significant inhibition of IUTP by intracellular incubation with anti-TRPC1 or anti-TRPC3 antibodies. Given the unexpected finding suggesting a role for TRPC1 in the IUTP, we performed coimmunoprecipitation studies to evaluate the possible existence of TRPC1/TRPC3 heteromeric channel. These studies suggested a prominent protein interaction between TRPC1 and TRPC3, consistent with the existence of TRPC1/TRPC3 heteromers in native SMCs. Although a TRPC1/TRPC3 heteromer has not previously been demonstrated in SMC, a TRPC1/TRPC3 heteromer has been reported in embryonic rat brain (21), as well as by coexpression studies (15). Lintschinger et al. (15) further demonstrated that the TRPC1/TRPC3 heteromer channel takes on characteristics that are unique from the respective homomeric channels. In particular, the TRPC1/TRPC3 channel adopts a more inwardly rectifying current compared with the homomeric TRPC3 channel, and the basal activity is increased by the removal of extracellular calcium (15). Our studies clearly demonstrate an inwardly rectifying current (Fig. 2B), as well as potentiation of basal current following the removal of extracellular bath calcium (not shown). Although Reading et al. (18) concluded that UTP stimulated a TRPC3 channel in cerebral SMC, their data could still be explained by a TRPC1/TRPC3 heteromer. Reading et al. (18) knocked down TRPC3 by RNA silencing and demonstrated loss of IUTP by patch clamp. However, it seems highly plausible that knockdown of one of the components of a TRPC1/TRPC3 heteromer might also have profound effects on the functionality of the channel and produce the same functional knockdown. Thus, when evaluated as a whole, our data are consistent with TRPC3 acting within a TRPC1/TRPC3 heteromeric channel in native SMC.
Inhibition of TRPC1/TRPC3 by the NO signaling pathway in native SMCs.
Inhibition of TRPCs by the NO signaling pathway was first shown with TRPC3 heterologously expressed in HEK-293 cells (13), and more recently with TRPC6 in A7r5 cells (22). The present study extends these findings to TRPC1/TRPC3 channels in freshly isolated SMCs, thus demonstrating that this NO/TRPC feedback system is not unique to cell culture or overexpression systems. We have demonstrated that endogenous TRPC1/TRPC3 channels responsible for IUTP could be significantly inhibited by exogenous NO or a cell-permeable analog of cGMP in native SMCs. Furthermore, we demonstrated the obligatory role of PKG in the signaling pathway by reversing the inhibitory effect of NO and cGMP by PKG inhibition. While NO has also been reported to modulate TRPCs through cysteine S-nitrosylation (27), the nearly complete reversal of NO inhibition by inhibiting PKG suggests that the action of NO in this system is predominantly through activation of cGMP/PKG.
PKG phosphorylation of TRPC3 is clearly supported by studies evaluating homomeric TRPC3 channels. In a TRPC1/TRPC3 heteromeric channel, PKG regulation could be imparted via the regulatory sites of TRPC3 alone or in combination through yet-to-be-described sites on TRPC1. In support of the latter possibility, an evaluation of predicted PKG phosphorylation sites with rat TRPC1 returns two possible sites (KinasePhos). Future studies will be needed to determine whether either of these predicted phosphorylation sites contributes to TRPC1 channel regulation.
Inhibition of NO-mediated vasorelaxation by TRPCs.
As stated earlier, a functional role of NO-dependent inhibition of TRPCs in vasorelaxation has not previously been reported. We used vessel myography to evaluate the role of TRPCs in NO-mediated vasorelaxation in intact CA. The arteries were preconstricted with UTP in the presence or absence of La3+, a broadly acting TRPC blocker. Based on our patch clamp data, we expected activation of TRPC1/TRPC3 channels by UTP. When an exogenous NO donor was delivered, significantly less relaxation occurred in the La3+-treated arteries compared with controls. These data are consistent with the involvement of a La3+-sensitive channel that is stimulated by UTP and inhibited by NO. As La3+ can act to inhibit multiple pathways of Ca2+ entry, further studies will be required to definitively prove the specific role of TRPC1/TRPC3. Nevertheless, the isolated smooth muscle data and the intact artery myograph data are consistent with NO inhibition of a Ca2+ entry pathway leading to UTP-mediated constriction. The data thus far are consistent with TRPC1/TRPC3 as the molecular target for NO-mediated inhibition of Ca2+ entry.
Physiological significance.
We have presented evidence for a feedback system involving NO and TRPC1/TRPC3 channels in the control of vascular tone (see Fig. 7). This system involves receptor-mediated activation of TRPC1/TRPC3 channels, which, in turn, contributes to SMC depolarization and voltage-operated Ca2+ channel opening. The resulting Ca2+ influx leads to smooth muscle contraction and increased vascular tone. NO contributes to vasorelaxation through multiple previously described mechanisms, and we now add inhibition of TRPC1/TRPC3 channels to that list.
GRANTS
This work was supported by American Heart Association Grant 0665100Y and National Heart, Lung, and Blood Institute Grant R01 HL088435 to S. P. Marrelli.
Supplementary Material
REFERENCES
- 1.Albert AP, Pucovsky V, Prestwich SA, Large WA. TRPC3 properties of a native constitutively active Ca2+-permeable cation channel in rabbit ear artery myocytes. J Physiol 571: 361–369, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez J, Coulombe A, Cazorla O, Ugur M, Rauzier JM, Magyar J, Mathieu EL, Boulay G, Souto R, Bideaux P, Salazar G, Rassendren F, Lacampagne A, Fauconnier J, Vassort G. ATP/UTP activate cation-permeable channels with TRPC3/7 properties in rat cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H21–H28, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295: C779–C790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan RM, You J, Phillips SC, Andresen JJ, Lloyd EE, Rogers PA, Dryer SE, Marrelli SP. Evidence for two-pore domain potassium channels in rat cerebral arteries. Am J Physiol Heart Circ Physiol 291: H770–H780, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem 281: 33487–33496, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Carrier GO, Fuchs LC, Winecoff AP, Giulumian AD, White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol Heart Circ Physiol 273: H76–H84, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Chitaley K, Webb RC. Nitric oxide induces dilation of rat aorta via inhibition of rho-kinase signaling. Hypertension 39: 438–442, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res 84: 210–219, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich A, Mederos YS, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25: 6980–6989, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich A, Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem 278: 47842–47852, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium 45: 38–54, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Fauconnier J, Lanner JT, Sultan A, Zhang SJ, Katz A, Bruton JD, Westerblad H. Insulin potentiates TRPC3-mediated cation currents in normal but not in insulin-resistant mouse cardiomyocytes. Cardiovasc Res 73: 376–385, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci USA 101: 2625–2630, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwan HY, Huang Y, Yao X. Protein kinase C can inhibit TRPC3 channels indirectly via stimulating protein kinase G. J Cell Physiol 207: 315–321, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem 275: 27799–27805, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101: 712–722, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Ohki G, Miyoshi T, Murata M, Ishibashi K, Imai M, Suzuki M. A calcium-activated cation current by an alternatively spliced form of Trp3 in the heart. J Biol Chem 275: 39055–39060, 2000. [DOI] [PubMed] [Google Scholar]
- 17a.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem 281: 13588–13595, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol 288: H2055–H2061, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577: 479–495, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauzeau V, Rolli-Derkinderen M, Marionneau C, Loirand G, Pacaud P. RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem 278: 9472–9480, 2003. [DOI] [PubMed] [Google Scholar]
- 20a.Smedlund K, Vazquez G. Involvement of native TRPC3 proteins in ATP-dependent expression of VCAM-1 and monocyte adherence in coronary artery endothelial cells. Arterioscler Thromb Vasc Biol 28: 2049–2055, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278: 39014–39019, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, Mori MX, Honda A, Inoue R. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol 586: 4209–4223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Thilo F, Loddenkemper C, Berg E, Zidek W, Tepel M. Increased TRPC3 expression in vascular endothelium of patients with malignant hypertension. Mod Pathol 22: 426–430, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther 118: 337–351, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res 102: 1118–1126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol 291: H2653–H2659, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, Michel V, Yew DT, Yao X. Expression of TRPC homologs in endothelial cells and smooth muscle layers of human arteries. Histochem Cell Biol 122: 553–561, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2: 596–607, 2006. [DOI] [PubMed] [Google Scholar]
- 28.You J, Johnson TD, Marrelli SP, Bryan RM Jr. Functional heterogeneity of endothelial P2 purinoceptors in the cerebrovascular tree of the rat. Am J Physiol Heart Circ Physiol 277: H893–H900, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.