Abstract
We have previously shown that increased nitric oxide (NO) production in sepsis impairs arteriolar-conducted vasoconstriction cGMP independently and that the gap junction protein connexin (Cx) 37 is required for this conducted response. In the present study, we hypothesized that NO impairs interendothelial electrical coupling in sepsis by targeting Cx37. We examined the effect of exogenous NO on coupling in monolayers of cultured microvascular endothelial cells derived from the hindlimb skeletal muscle of wild-type (WT), Cx37 null, Cx40 null, and Cx43G60S (nonfunctional mutant) mice. To assess coupling, we measured the spread of electrical current injected in the monolayer and calculated the monolayer intercellular resistance (inverse measure of coupling). The NO donor 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (DETA) rapidly and reversibly reduced coupling in cells from WT mice, cGMP independently. NO scavenger HbO2 did not affect baseline coupling, but it eliminated DETA-induced reduction in coupling. Reduced coupling in response to DETA was also seen in cells from Cx40 null and Cx43G60S mice, but not in cells from Cx37 null mice. DETA did not alter the expression of Cx37, Cx40, and Cx43 in WT cells analyzed by immunoblotting and immunofluorescence. Furthermore, neither the peroxynitrite scavenger 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III), superoxide scavenger Mn(III)tetrakis(4-benzoic acid)porphyrin chloride, nor preloading of WT cells with the antioxidant ascorbate affected this reduction. We conclude that NO-induced reduction of electrical coupling between microvascular endothelial cells depends on Cx37 and propose that NO in sepsis impairs arteriolar-conducted vasoconstriction by targeting Cx37 within the arteriolar wall.
Keywords: cell-to-cell communication, nitric oxide
a locally induced arteriolar constriction or dilation can rapidly spread along a 2- to 3-mm length of the arteriole (16). This conducted diameter response represents a fundamental feature of the arteriole that enables it to sufficiently alter its hemodynamic resistance and to regulate blood flow (47). Arteriolar-conducted response has been shown to involve 1) a local change in membrane potential within the cells of the arteriolar wall and 2) propagation of electrical current between these cells along the vessel length (58). Direct electrophysiological measurements have demonstrated that electrical currents travel through both the endothelial and smooth muscle cell layers (54, 60) but that electrical coupling between endothelial cells mainly underlies arteriolar conduction (9, 10, 60). Intercellular electrical coupling is mediated by gap junctions (19), which have a low electrical resistance (15, 22).
Gap junction channels connect the cytoplasm of two neighboring cells and are formed when two connexons, one in each of the contacting membranes, are joined end-to-end. A connexon is composed of six proteins, which are known as connexins (Cx; see Ref. 39). It has been shown that arteriolar endothelial cells express Cx37, Cx40, and Cx43, whereas the vascular smooth muscle cells express Cx37, Cx40, Cx43, and Cx45 (40). The ability of an arteriole to conduct a vasomotor response could be acutely modulated by altering the cell membrane resistance between the cytosol and extracellular space and/or by changing the permeability of the gap junctions. Conduction could also be modulated long-term by altering the gap junction protein expression.
We have shown that sepsis impairs arteriolar-conducted vasoconstriction (25). Sepsis can be defined as a systemic inflammatory response caused by an infection (8). It leads to circulatory system failure (e.g., hypotension, maldistribution of blood flow) and is associated with multiple organ dysfunction (1, 14, 48). We have also shown that 1) partial restoration of the impaired conducted vasoconstriction occurs after local arteriolar treatment with the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester and 2) exogenous nitric oxide (NO) completely mimics the impairment in control nonseptic tissue (25). Our most recent work in septic mice has demonstrated that the impaired conduction in sepsis is due to an increase in neuronal NOS-derived NO (29).
Based on immunohistochemical examination, Cx37 has been reported to be highly expressed at endothelial cell-to-cell junctions in the mouse cremaster muscle arterioles (27). Recent evidence suggests that NO may reduce vascular cell coupling by targeting Cx37. In communication-deficient HeLa cells transfected with Cx37, exogenous NO caused a cGMP-independent reduction in dye coupling (21). Our laboratory demonstrated that sepsis impairs conducted vasoconstriction cGMP independently, and that genetic deletion of Cx37 inhibits conducted vasoconstriction (29). However, a direct assessment of the effect of NO on vascular cell coupling and the role of Cx37 in this effect has not yet been carried out.
The primary objective of the present study was to determine if NO impairs electrical coupling between mouse microvascular endothelial cells (MMEC). In addition, we investigated which connexin is involved in this impairment by using mice with genetically ablated or reduced Cx37, Cx40, and Cx43. Based on the previous reports suggesting an obligatory role of Cx37 (21, 29), we hypothesized that NO reduces electrical coupling between MMEC by targeting Cx37.
MATERIALS AND METHODS
Reagents.
The S-nitroso-N-acetylpenicillamine (SNAP), 1-H-(1,2,4)oxadiazolo(4,3-a)quinozalin-1 (ODQ), 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (DETA), 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III) (FeTPPS), Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP), and anti-β-actin antibody kit were purchased from Calbiochem (La Jolla, CA). Heparin was purchased from Leo Laboratories (Ajax, ON). FBS, dialyzed FBS, antibiotic-mycotic solution, l-glutamine, and trypsin-EDTA were purchased from GIBCO (Mississauga, ON). DMEM-F-12, HEPES, GS-1 lectin, l-ascorbic acid, 3,8-diamino-5-ethyl-6-phenylphenanthridinium bromide (ethidium bromide), Hoechst 33258, hemoglobin, and hydrogen peroxide were purchased from Sigma Chemical (St. Louis, MO). Endothelial cell growth supplement was purchased from Collaborative Research (Bedford, MA). Magnetic beads and the magnetic particle concentrator were purchased from Dynal (Lake Success, NY). For immunoblotting, mouse monoclonal anti-Cx43 antibody was purchased from Transduction Laboratories (Bio/Can Scientific, Mississauga, ON), and peroxidase-labeled anti-mouse IgG was from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-Cx40 antibody was purchased from Chemicon (Temecula, CA); this antibody does not cross-react with Cx37 or Cx43 (40). Rabbit polyclonal anti-Cx37 antibody was generated and its specificity confirmed in Simon's laboratory (42). Rabbit polyclonal anti-protein kinase G (PKG) antibody was purchased from ABCAM (Cambridge, MA) while rabbit anti-phospho-serine antibody was from Zymed Laboratories (San Francisco, CA). Peroxidase-labeled anti-rabbit IgG antibodies for anti-Cx37, anti-PKG, and anti-phosphoserine antibodies were purchased from Biolynx (Brockville, ON) and Cell Signaling Technology (Beverly, MA). The enhanced chemiluminescence kit was purchased from LUMIGLO, KPL Laboratories (Gaithersburg, MD). SuperSignal West Femto Maximum Sensitivity Substrate was from Pierce Biotechnology (Rockford, IL). For immunofluorescence, rabbit anti-Cx37 and rabbit anti-Cx40 antibodies were obtained from Alpha Diagnostic International (San Antonio, TX). Rabbit anti-Cx43 antibody was from Sigma, and Alexa 488-conjugated secondary antibody was from Invitrogen (Carlsbad, CA).
Mouse strains.
This investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH publication no. 85–23, revised 1996), and all experimental protocols were approved by the Animal Use Subcommittee of the University Council on Animal Care at the University of Western Ontario. We used male wild-type (WT) C57BL/6 mice (from The Jackson Laboratory, Bar Harbor, ME), Cx40 null (Gja5−/−) C57BL/6 mice and Cx37 null (Gja4−/−) C57BL/6 mice (both strains provided by Dr. David Paul, Harvard University, Boston, MA) (43, 44), and Cx43G60S mutant (Gja1Jrt) mice on a mixed C57BL/C3H/HeJ background after two generations of backcrossing with C57BL/6 mice. The Cx43 null mutation is lethal, and homozygous mice do not survive postnatally, although heterozygous mice are normal (35). In contrast, the Gja1Jrt allele encodes Cx43 with a single amino acid substitution (G60S) that renders the protein nonfunctional. Cx43-mediated gap junctional communication is severely reduced due to dominant inhibition of WT Cx43 function (13). Gja1Jrt heterozygous males were obtained from Dr. Janet Rossant of the Centre for Modeling Human Disease at the University of Toronto.
Isolation and culture of MMEC.
Isolation of MMEC was based on a procedure described by us (26). Briefly, the hindlimb muscle of mice was excised, minced, and digested in an enzyme solution. Digest was filtered through a nylon mesh, and cells were collected and washed in DMEM-F-12. Cells were grown to confluence and then subjected to purification by immunoseparation using GS-1 lectin-coated beads. Pure MMEC were then cultured in maintenance medium containing DMEM-F-12, FBS (10%), endothelial growth supplement (100 μl/ml), heparin (5 U/ml), l-glutamine (0.1 μg/ml), and antibiotic-mycotic solution (10 μl/ml) in standard incubator conditions. Before experiments (1 h), the maintenance medium was replaced by a dialyzed serum medium (DSM) consisting of DMEM-F-12, dialyzed FBS (5%), and heparin (5 U/ml), l-glutamine (0.1 μg/ml), and antibiotic-mycotic solution (10 μl/ml). Cells were used between passages 6 and 15. Endothelial phenotype was determined by the presence of von Willebrand factor VIII and GS-1 lectin antigens as detailed by us (55), showing purity near 100%.
Electrophysiology.
To assess coupling, we determined intercellular resistance (i.e., inverse measure of coupling) based on an electrophysiological approach described by us (26). Briefly, cells were grown in a monolayer on a glass cover slip, viewed by a microscope, and then injected with three to four hyperpolarizing pulses (25 nA, 100 ms). The resulting spread of electrical current in the monolayer was assessed by recording deflections from the resting membrane potential (Em) in cells at various distances along the monolayer (5). In practice, we positioned the recording electrode in one cell and then moved the injecting electrode to cells at increasing interelectrode distance. To select these cells, we moved the electrode in random directions from the recording electrode. In WT monolayers, we determined that interelectrode distances of 50, 150, and 250 μm spanned 3.08 ± 0.08, 7.83 ± 0.21, and 12.67 ± 0.19 cells, respectively (n = 12 microscopic fields of view in 3 monolayers, for each particular distance). In the present study, Em deflections ranged 2–30 mV for interelectrode distances in the range of 50–350 μm. Based on the Em deflection recordings, the monolayer thickness of 1.9 μm (26), and a Bessel function model, the intercellular resistance (ri), transmembrane resistivity (Rm), and space constant (λ) were determined as detailed by us (26). Because electrophysiology was done in room air (cell chamber heated to 37°C), cells were covered with normoxic DSM including 25 mM HEPES (i.e., to maintain pH at 7.3). Our previous work in rat microvascular endothelial cells showed variability in baseline intercellular resistance (e.g., reflecting experiment-to-experiment variability). To control for this variability, for each experimental day, we simultaneously prepared the appropriate number of monolayer-covered glass cover slips, which were then randomly assigned to control NO donor treatment groups (DETA or SNAP). Within these groups, cover slips were also randomly assigned to treatment subgroups, including ODQ (10 μM, highly selective, irreversible, heme site inhibitor of soluble guanylyl cyclase), FeTPPS (25 μM, selective peroxynitrite scavenger), MnTBAP (100 μM, superoxide scavenger), antioxidant ascorbate (200 μM), and NO scavenger HbO2 [10 μM, prepared from Hb as described previously (24)]. Concentrations of agents used were based on manufacturer's recommended concentrations, published reports, and on preliminary experiments.
Measurement of nitrate and nitrite.
Nitrite and nitrate (NOx) concentrations were measured to estimate the total NO produced by DETA in the culture medium. The culture medium was filtered through a 10-kDa molecular mass cutoff filter to eliminate proteins. Nitrate was converted to nitrite by nitrate reductase, and total nitrite was measured using a total NOx assay kit (Cayman, Ann Arbor, MI). The samples were mixed with an equal volume of Griess reagent, and the absorbance was measured at 545 nm. The detection limit of NOx level with this assay is ∼2.5 μM.
Western blotting.
Cells were washed and lysed with SDS lysis buffer (containing protease inhibitors). Proteins were resolved on a 10% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Membranes were blocked with 5% nonfat dry milk. For Cx37 and Cx40 immunoblotting, 25 μl/well were loaded, and membranes were incubated with anti-Cx37 or anti-Cx40 antibody (1:5,000) overnight at 4°C. Membranes were then washed and further incubated with peroxidase-labeled anti-rabbit IgG antibody (1:100,000) for 1 h at room temperature. Bands were visualized using West Femto Maximum Sensitivity Substrate and Kodak BIOMAX MS imaging film. For Cx43 immunoblotting, 15 μl/well were loaded, and membranes were incubated with anti-Cx43 antibody (1:1,000) for 1 h at room temperature. Membranes were then washed and further incubated with peroxidase-labeled anti-mouse IgG antibody (1:2,000, 1 h at room temperature). For PKG immunoblotting, 20 μl/well were loaded, membranes were blocked with 3% BSA, and incubated with anti-PKG antibody (1:1,000) overnight at 4°C. For phosphoserine immunoblotting, 20 μl/well were loaded, and membranes were blocked with 3% BSA and incubated with anti-phosphoserine antibody (1:2,000) overnight at 4°C. Membranes were then washed and further incubated with peroxidase-labeled anti-rabbit IgG antibody (1:2,000, 1 h at room temperature). Bands were visualized using an enhanced chemiluminescence kit with Kodak BIOMAX MR imaging film. For protein loading control, all blots were stripped and reprobed for β-actin using β-actin antibody (1:5,000, 1 h at room temperature), washed, and further probed with peroxidase-labeled anti-mouse IgM antibody (1:2,000) and subsequently visualized.
Immunofluorescence and membrane integrity.
For immunofluorescence, cells grown on glass cover slips were fixed with 4% paraformaldehyde at 4°C for 20 min, rinsed with PBS, and prepared for immunostaining. Briefly, the cells were blocked with washing buffer containing 2% BSA (wt/vol) for 1 h, immunolabeled with primary antibody (anti-Cx37 at 1:100, anti-Cx40 at 1:300, or anti-Cx43 at 1:500 dilution) for 1 h at room temperature, washed with PBS, and immunolabeled with Alexa 488-conjugated secondary antibody (1:500 dilution) for 1 h at room temperature in the dark. Cells were washed in PBS, and the nuclei were stained with 0.1% Hoechst 33258 for 10 min followed by washes with PBS and double-distilled H2O. The cover slips were mounted on slides with Airvol (Air Products and Chemicals, Allentown, PA) before storage at 4°C. The cells were imaged using a Zeiss LSM 510 META confocal microscope (Thornwood, NY). Fluorescent signals were captured after excitation with 488 and 730 nm laser lines. Digital images were prepared using Zeiss LSM and Adobe Photoshop 7.0 software.
Membrane integrity was determined using a two-fluorescent-dyes exclusion assay, as described previously (32, 53). Hoechst 33258 stain was used to highlight the cell nuclei, whereas ethidium bromide stained cell nuclei with damaged membranes. Briefly, cells grown on glass cover slips were exposed to control vehicle (DSM) or DETA (500 μM, 3 h) in the presence of ethidium bromide (2.5 mg/ml for 1 h). Cells were then fixed, stained with Hoechst (1:5,000, 10 min, room temperature), and mounted on glass slides. Fluorescence was observed using a Zeiss Axiovert 200M fluorescence microscope. H2O2 (100 mM for 1 h) was used as a positive control for cell damage and effectiveness of the ethidium bromide stain.
Statistics.
Data are presented as means ± SE. MMECs were isolated from at least three different mice, and n indicates the number of monolayers used per treatment group, unless otherwise stated. Data were analyzed by Student's t-test or by ANOVA followed by Dunnett's posttest. We considered P < 0.05 as significant.
RESULTS
Effect of NO on electrical coupling in MMEC.
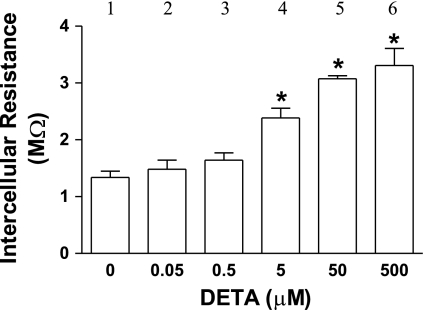
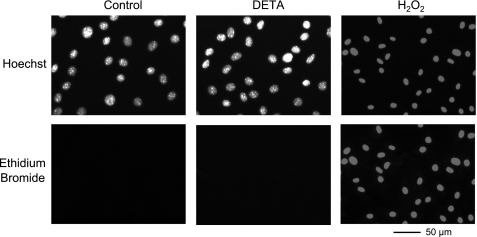
DETA (500 μM, 3 h), an NO donor, did not affect the morphological appearance of the cell monolayer and did not alter the WT cell monolayer's resting Em (control: −15.1 ± 0.4 mV, n = 16; DETA: −14.9 ± 0.3 mV, n = 21) (Fig. 1A). The absolute value of the resting Em is less than that seen in in vivo or ex vivo preparations (20), but it is within the range reported for cultured endothelial cells (6, 30, 51). This low resting Em has been proposed to be due to closing of potassium channels near the potassium equilibrium potential (51). Figure 1A shows a representative example of Em deflections at increasing interelectrode distance in control and DETA-treated (500 μM, 3 h) monolayers. The steeper decay of deflections with distance following DETA (Fig. 1B) indicates an increased intercellular resistance. The 3-h duration of DETA exposure was chosen to model the persistently elevated NO levels associated with sepsis (57). Figure 2 shows that DETA increased intercellular resistance (i.e., reduced coupling) in a concentration-dependent manner. DETA (500 μM) significantly increased ri (control: 1.5 ± 0.1 MΩ, n = 16; DETA: 3.1 ± 0.1 MΩ, n = 21, P < 0.05) and decreased λ (control: 1,068 ± 128 μm, n = 16; DETA: 422 ± 52 μm, n = 21, P < 0.05), but it did not affect the membrane resistivity Rm (control: 14.1 ± 4.9 kΩ·cm2, n = 16; DETA: 9.2 ± 2.0 kΩ·cm2, n = 21, P > 0.05). This indicates that the NO-induced modulation of the spread of electrical current along the monolayer was due to modulation of intercellular resistance, rather than modulation of membrane resistivity. Figure 3 demonstrates that DETA (500 μM, 3 h) did not compromise MMEC membrane integrity. Using the Griess reagent, we found that DETA resulted in NOx levels in the culture medium that were equivalent to the 100 μM plasma NOx levels measured in human septic patients with the Griess reagent (11) (i.e., 104 ± 2, 102 ± 3, and 2.6 ± 0.2 μM for 500 μM DETA 3 h, 500 μM DETA 10 min, and 5 μM DETA 3 h, respectively, n = 3 in each group). For this reason, we used 500 μM DETA in all of the following experiments.
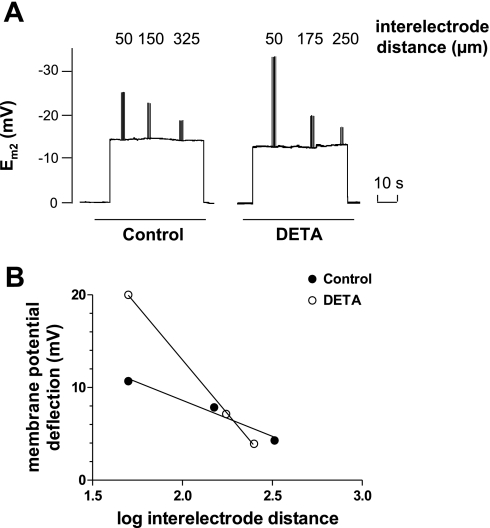
Fig. 1.
Determination of electrical coupling between mouse microvascular endothelial cells (MMEC). A: representative example of electrode recordings from a control monolayer and from a separate monolayer treated with the nitric oxide (NO) donor 2,2′-(hydroxynitrosohydrazino)bis-ethanamine (DETA; 500 μM, 3 h). For the control monolayer, the initial step change in membrane potential (Em2) from 0 to −15 mV indicates insertion of the recording electrode (E2) in the cell. The following superimposed Em2 deflections are caused by current pulse injections in cells impaled by the injecting electrode at interelectrode distances of 50, 150, and 325 μm, respectively. The return of Em2 to 0 indicates the withdrawal of E2 from the cell at the end of the experiment. For DETA-treated monolayer, superimposed Em2 deflections were observed at interelectrode distances of 50, 175, and 250 μm. B: plot of deflections of A against log of interelectrode distance. Based on this plot, monolayer thickness of 1.9 μm, and a Bessel function model (26), the intercellular resistance (ri) was computed and used as an index of cell electrical coupling. Lines represent best fit through the points. The slope of each line represents the intercellular electrical resistance of the monolayer. The computation yielded ri of 1.05 MΩ for the control monolayer and 3.51 MΩ for the DETA-treated monolayer.
Fig. 2.
DETA-induced increase in intercellular resistance is concentration dependent. MMEC monolayers from wild-type mice were exposed to DETA for 3 h. *P < 0.05 compared with control (bar 1); n = 4 experiments/group.
Fig. 3.
Lack of effect of DETA on membrane integrity of MMEC from wild-type mice. MMEC were exposed to 1) control, dialyzed serum for 3 h, 2) 500 μM DETA for 3 h, or 3) 100 mM H2O2 for 1 h (a positive control experiment). Monolayers were then fixed, and nuclear staining was visualized using a fluorescence microscope. Row on top shows representative examples (n = 3) of nuclei of cells identified with Hoechst 33258 stain, whereas the row on bottom shows representative examples (n = 3) of nuclei stained by ethidium bromide (2.5 μg/ml) during the course of the particular treatment.
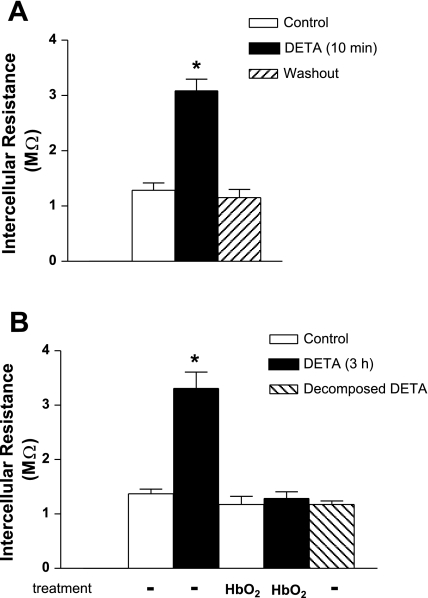
To determine how quickly DETA reduced electrical coupling between WT cells, current injection recordings were taken after 10 min of DETA exposure (Fig. 4A). DETA resulted in the same increase in intercellular resistance as that seen at 3 h (Fig. 4B). Within 10 min of removing DETA, resistance returned to baseline (Fig. 4A). This indicates that NO decreased coupling via a mechanism not involving permanent cell injury and likely not dependent on modulation of gene expression. Decomposed DETA (3 h) had no effect on intercellular resistance (Fig. 4B). Treatment of the control monolayer with the NO scavenger HbO2 (10 μM, 3 h) did not affect intercellular resistance, indicating that baseline endogenous NO produced by MMEC had no effect on this resistance (Fig. 4B). Alternatively, the lack of effect of HbO2 could be due to very low baseline NO produced by MMEC, since undetectable baseline constitutive NOS enzymatic activity was reported for cultured microvascular endothelial cells (56). In contrast to the lack of effect at baseline, HbO2 completely eliminated the DETA-induced increase in resistance (Fig. 4B), indicating that DETA-released NO accounted for this increase.
Fig. 4.
Effect of short- and long-term exposure of DETA on intercellular resistance in wild-type MMEC. A: short-term DETA (500 μM, 10 min) increased intercellular resistance compared with the control group. DETA washout returned intercellular resistance to control level within 10 min. *Significant difference from the control group, P < 0.05; n = 4/group. B: long-term DETA (500 μM, 3 h) increased intercellular resistance compared with the control group, whereas decomposed DETA (i.e., incubated at 4°C for 3 wk) had no effect on resistance following 3 h of exposure. The coincubation with NO scavenger HbO2 (10 μM, 3 h) had no effect on baseline resistance, but it eliminated the DETA-induced increase in resistance. *Significant difference from control, P < 0.05; n = 6 in control and n = 4 in the remaining groups.
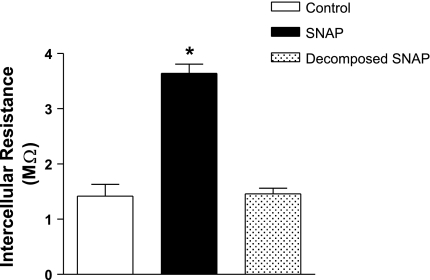
To confirm the results with DETA, we used another NO donor, SNAP. WT cell monolayers exposed to SNAP (5 μM, 10 min) showed a significant increase in resistance compared with control (Fig. 5). Decomposed SNAP had no effect on electrical resistance (Fig. 5).
Fig. 5.
Effect of the NO donor S-nitroso-N-acetylpenicillamine (SNAP) on intercellular resistance in wild-type MMEC. Endothelial cell monolayers were exposed to 5 μM SNAP for 10 min or to decomposed SNAP (incubated at 37°C for 24 h). SNAP increased intercellular resistance compared with the control group. *P < 0.05 compared with control; n = 4 for each group.
Role of NO and peroxynitrite.
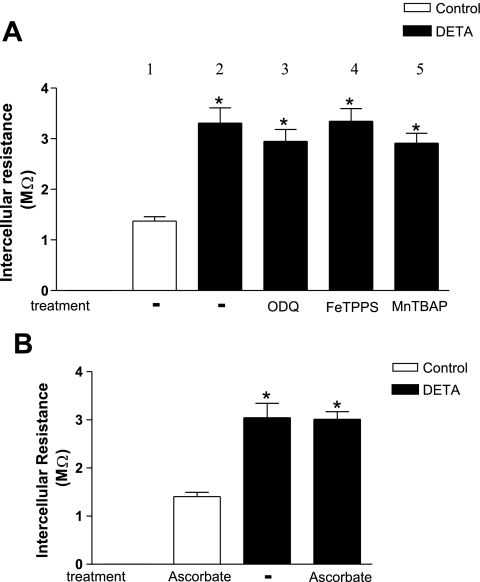
The effect of DETA and SNAP could be due to released NO or, possibly, due to peroxynitrite formed from NO and superoxide. To examine this possibility, cells were coincubated with DETA and peroxynitrite scavenger FeTPPS (25 μM for 3 h), based on published reports showing the effectiveness of this agent in endothelial cells (12, 41, 45). The peroxynitrite scavenger failed to prevent the DETA-induced increase in electrical resistance (Fig. 6A). To confirm this finding, we also coincubated monolayers with the superoxide scavenger MnTBAP (100 μM, 3 h). MnTBAP failed to prevent the DETA-induced increase in electrical resistance (Fig. 6A). The effectiveness of MnTBAP was established in a control experiment (data not shown) where 100 μM MnTBAP inhibited the increase in superoxide production in cultured MMECs following hypoxia/reoxygenation (6). Finally, we preloaded the MMECs with the antioxidant ascorbate (200 μM, 4 h). Ascorbate too failed to prevent the DETA-induced increase in resistance (Fig. 6B). Previous studies performed in our laboratory showed the effectiveness of 200 μM ascorbate as an antioxidant in MMECs (6). Together, these experiments indicated that NO, rather than peroxynitrite, reduced electrical coupling between endothelial cells.
Fig. 6.
Lack of effect of inhibition of cGMP, peroxynitrite, superoxide, and oxidants on DETA-induced increase in intercellular resistance. A: wild-type MMEC coincubated with soluble guanylyl cyclase inhibitor 1-H- (1,2,4)oxadiazolo(4,3-a)quinozalin-1 (ODQ; 10 μM, 3 h), peroxynitrite scavenger 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III) (FeTPPS; 25 μM, 3 h), or superoxide scavenger Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP; 100 μM, 3 h) still showed increased intercellular resistance following exposure to DETA (500 μM, 3 h). *Significant difference from the control group, P < 0.05; n = 8 in group 1, n = 5 in groups 2 and 3, and n = 4 in groups 4 and 5. B: preloading of wild-type cells with the antioxidant ascorbate (200 μM for 4 h) did not affect the increase in intercellular resistance after exposure to DETA (500 μM, 3 h). Control group represents wild-type cells preloaded with ascorbate (200 μM for 4 h) followed by ascorbate (200 μM) in dialyzed serum for 3 h. *Significant difference from the control group, P < 0.05; n = 4 for each group.
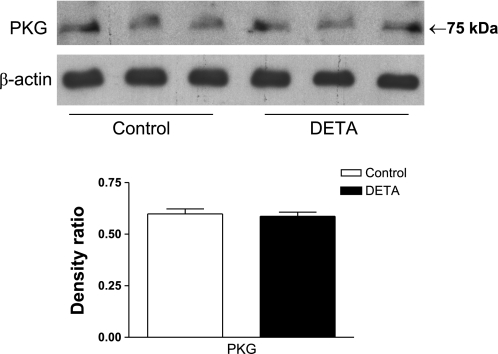
Figure 6A also shows that inhibition of soluble guanylyl cyclase with ODQ (10 μM for 3 h) did not prevent the DETA-induced increase in electrical resistance. This implies that NO reduces electrical coupling in WT cells by a cGMP-independent mechanism. We have previously tested the effectiveness of ODQ in a control experiment where ODQ inhibited the SNAP-mediated dilation in preconstricted arterioles (data not shown). To ensure that the target of cGMP (i.e., PKG) was present in MMEC, we determined PKG protein expression in control and DETA-treated MMEC (500 μM, 3 h). PKG protein was expressed under both conditions, at the same level (Fig. 7).
Fig. 7.
Lack of effect of DETA (500 μM, 3 h) on protein kinase G (PKG) protein expression. Shown are immunoblots of PKG and β-actin proteins, and densitometric ratios of PKG immunoblots to β-actin immunoblots for control and DETA-treated MMEC. DETA had no effect on PKG protein expression; n = 3 in each group.
Effect of DETA on connexin expression.
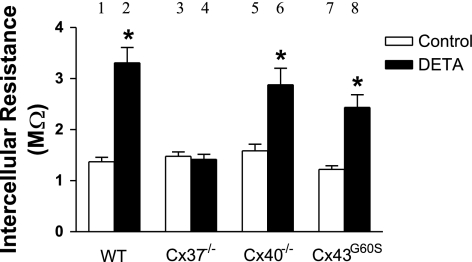
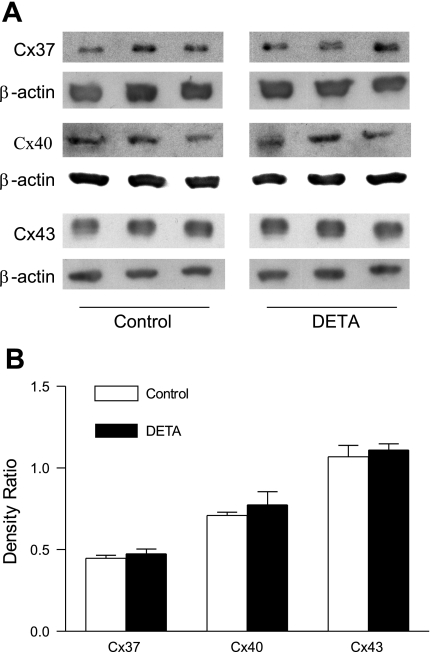
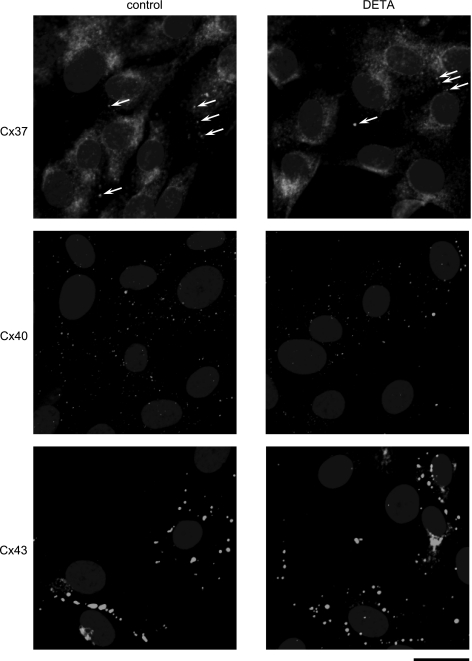
We visually observed that the size of cells from WT, Cx37 null, Cx40 null, and Cx43G60S mice did not differ, indicating that the number of cells spanned by a given interelectrode distance in the monolayer did not differ between the mouse strains. Based on ANOVA, there was no change in baseline intercellular resistance between WT, Cx37 null, Cx40 null, and Cx43G60S cell monolayers (Fig. 8). This indicates that deletion or functional mutation of any of the connexins did not affect the baseline intercellular coupling, and it suggests that the loss of one particular connexin was functionally compensated by another connexin in our MMEC. In Cx40 null and Cx43G60S cells, DETA-induced resistance increase was comparable to that of WT cells (Fig. 8). Interestingly, DETA produced no change in resistance in Cx37 null cells compared with control (Fig. 8), indicating that DETA-induced increase in intercellular resistance was Cx37-dependent. DETA exposure did not result in any changes in protein expression of Cx37, Cx40, and Cx43 in WT cell monolayers analyzed by immunoblotting (Fig. 9, A and B) or any apparent redistribution of the three connexins within WT cells examined by immunofluorescence (Fig. 10). Finally, based on reported serine phosphorylation of Cx37 (23), we examined the possibility that DETA increased Cx37 serine phosphorylation in WT cells. Because we were unable to immunoprecipitate Cx37 with the present anti-Cx37 antibody, we examined instead serine phosphorylation of proteins at 37 kDa in whole cell lysates. Using the same anti-phosphoserine antibody we previously used to reveal altered Cx40 serine phosphorylation in MMEC challenged with lipopolysaccharide (7), DETA did not alter serine phosphorylation at the 37-kDa level in cell lysates (data not shown).
Fig. 8.
Effect of DETA exposure on intercellular resistance in wild-type, connexin (Cx) 37 null, Cx40 null, and Cx43G60S (nonfunctional mutant) MMEC. DETA (500 μM, 3 h) increased resistance in cells derived from wild-type, Cx40 null, and Cx43G60S mice, but not in cells from Cx37 null mice. *Significant difference from the appropriate control group, P < 0.05; n = 16 in group 1 (pooled from all control experiments), n = 21 in group 2 (pooled), and n = 4 in groups 3–8.
Fig. 9.
Lack of effect of DETA on Cx37, Cx40, and Cx43 protein expression in wild-type MMEC. A: Cx37, Cx40, Cx43, and β-actin immunoblots from control and DETA-treated MMEC (500 μM, 3 h). B: densitometric ratios of Cx37, Cx40, and Cx43 to β-actin immunoblots for control and DETA-treated MMEC. DETA had no effect on Cx37, Cx40, or Cx43 protein expression; n = 3 in each group.
Fig. 10.
Lack of effect of DETA on distribution of Cx37, Cx40, and Cx43 within wild-type MMEC. Top: representative examples of control (n = 9) and DETA-treated cells (500 μM, 3 h, n = 6) probed for Cx37. Although most of the immunoreactivity is cytoplasmic, occasional punctate staining (arrows) indicates putative Cx37 gap junctions. Comparable staining was also seen with another anti-Cx37 antibody (i.e., that used for immunoblotting) (data not shown). The superimposed oval uniform gray shapes are cell nuclei identified with Hoechst 33258 stain. Middle: representative examples of control (n = 7) and DETA-treated (n = 7) cells probed for Cx40. Numerous small Cx40 gap junctions are indicated by the punctate staining. The superimposed oval gray shapes are cell nuclei. Bottom: representative examples of control (n = 3) and DETA-treated (n = 3) cells probed for Cx43. Numerous Cx43 gap junctions of various sizes are evident, larger than those containing Cx37 or Cx40, whereas gray ovals indicate cell nuclei. Control experiments where the connexin primary antibodies were omitted showed only gray oval nuclei (data not shown). Bar at bottom right indicates 20-μm scale for all panels.
DISCUSSION
The present study demonstrates for the first time that NO reduces electrical coupling in MMECs in a concentration-dependent manner, that this reduction is rapid and reversible, and that it requires the presence of the gap junction protein Cx37. In contrast, the reduction does not depend on the presence of Cx40 and is not affected by dominant inhibition of Cx43 function. Furthermore, inhibition of soluble guanylyl cyclase does not affect the decrease, indicating that the reduction is cGMP independent. Similarly, a peroxynitrite scavenger, a superoxide scavenger, and the antioxidant ascorbate do not affect the reduction, indicating that NO rather than peroxynitrite mediates the reduction. This lack of effect is consistent with our previous study where hypoxia/reoxygenation reduced electrical coupling in an oxidant- and Cx40-dependent manner (6). Based on that study and the present data, it appears that oxidants modulate signaling that targets Cx40, whereas NO modulates signaling that targets Cx37 in MMEC.
There are several properties of Cx37 that support the idea that Cx37 plays a key function in mediating the spread of electrical signals between endothelial cells. For example, functional Cx37 channels have a much larger unitary conductance (300 pS) (52) than either Cx40 (175 pS) (2, 46) or Cx43 (100 pS) (31) channels. Cx37 is also known to be highly expressed at the border between endothelial cells of an arteriole (27). The endothelium is required for conducted response to occur along an arteriole (10, 16, 60). Given the longitudinal orientation of endothelial cells within a blood vessel and their higher proportion of gap junctions over that of smooth muscle cells (17), endothelial cells are thought to act as a low electrical resistance pathway that allows any change in membrane potential to electrotonically spread from cell to cell (33). This electrical signal is then transmitted to smooth muscle cells via myoendothelial junctions, resulting in a vasomotor response (18).
It has been shown that sepsis-induced increased endogenous NO, and exogenous NO, impairs KCl-induced conducted vasoconstriction (25, 29, 37). Interestingly, exogenous NO does not affect conducted vasodilatation (37). Based on the facts that genetic deletion of Cx37 impairs KCl-induced conducted vasoconstriction (29) and that exogenous NO decreases electrical coupling Cx37 dependently (Fig. 8), we suggest that NO signaling in sepsis impairs this conducted vasoconstriction by targeting Cx37 in arterioles. However, the exact anatomic location of this target is not clear. It has been proposed that conducted vasoconstriction occurs along the arteriolar smooth muscle layer and that NO affects conductivity of gap junctions composed of Cx43 and/or Cx45, as these connexins are expressed in smooth muscle cells (37, 40). However, Cx37 is also found in arteriolar smooth muscle cells (40). Because the KCl-induced conducted vasoconstriction is accompanied by conduced depolarization along the endothelium (54) and because Cx37 protein is expressed in the endothelial layer (27) and myoendothelial junctions (18), the target of NO signaling may be complex and not restricted to a particular cell type or anatomic location. Clearly, because the extent to which our cell culture model mimics the in vivo situation is limited, the present data may offer only a partial insight into the potentially complex mechanism of NO-induced reduction in conducted vasoconstriction.
The inhibitory effect of NO on cellular coupling has been established in several different cell types, but the mechanism of this inhibition varies significantly. In hybrid bass and rabbit retinal cells, NO reduced coupling in a cGMP-dependent mechanism (28, 59). Similarly, NO uncoupled HeLa cells stably transfected with Cx35 in a guanylyl cyclase- and protein kinase A-dependent manner (34). In human primary uterine myocytes, inhibition was achieved through a decrease in Cx43 protein expression (38). Finally, NO inhibited dye coupling in rat astrocytes after reacting with superoxide to form peroxynitrite (4). The present data are consistent with a previously reported study by Kameritsch and coworkers (21) showing that communication-deficient HeLa cells transfected with Cx37 exhibited a cGMP-independent reduction in dye coupling in response to NO. However, in contrast to our results, their group reported that NO had no effect on the electrical coupling between Cx37-transfected HeLa cells (21). This discrepancy could be attributed to differences in the cell models used, since HeLa cells express very low levels of connexins under normal conditions.
Our data show that exogenous NO does not alter Cx37 protein expression nor its distribution within MMEC (Figs. 9 and 10). Because the NO-induced reduction in coupling occurred rapidly (within 10 min), and since it was also rapidly reversible, the mechanism of the NO effect most likely involved fast intracellular events, and it was not brought about by changes in gene expression. One such fast event could be phosphorylation of connexins (50). It has been documented that increased Cx43 phosphorylation results in decreased gap junction permeability (31), whereas increased Cx40 phosphorylation results in increased gap junction permeability (49). However, little is known about the effect of phosphorylation on Cx37 function, since this connexin is difficult to immunoprecipitate with available antibodies (unpublished observation). Using BWEM cells stably transfected with Cx37 construct containing the FLAG moiety, Larson and coworkers (23) detected serine phosphorylated Cx37, manifesting itself as a slower-migrating Cx37 protein band at 38 kDa. In the present study, no such band was detected (Fig. 9A), indicating that NO did not result in serine phosphorylation of Cx37. Consistent with this observation, DETA did not affect the serine phosphorylation banding pattern at 37 kDa in whole cell lysates. Therefore, it is unlikely that NO affected serine phosphorylation of Cx37 in MMEC.
Alternatively, NO could directly affect Cx37 function via nitrosylation, a reversible mechanism that regulates the function of many proteins (3). For example, it has been proposed that increased Cx43 hemichannel permeability in cortical astrocytes exposed to metabolic inhibition is mediated by S-nitrosylation of intracellular Cx43 cysteine residues (36). Because the present anti-Cx37 antibodies did not permit Cx37 precipitation, examination of Cx37 nitrosylation was beyond the scope of the present study. Thus, addressing this particular alternative awaits future investigative effort.
In conclusion, we demonstrate that Cx37 plays a key role in the NO-induced reduction in electrical coupling in MMECs. This reduction is not affected by inhibition of soluble guanylyl cyclase or by scavenging peroxynitrite or superoxide. Based on the reports that 1) increased NO production is responsible for the sepsis-induced decrease in conducted vasoconstriction in the mouse cremaster muscle (29), 2) deletion of Cx37 results in attenuated conduction in control mice (29), and 3) NO decreases electrical coupling in MMECs in a Cx37-dependent manner, our results suggest that Cx37 is a key player in mediating impaired arteriolar-conducted vasoconstriction during sepsis.
GRANTS
This work was supported by Heart and Stroke Foundation of Ontario Grant NA 5941 to K. Tyml, National Heart, Lung, and Blood Institute Grant HL-064232 to A. M. Simon, and and Ontario Graduate Scholarship (salary award to R. L. McKinnon).
Acknowledgments
We thank Drs. Y. Ouellette, J. Dixon, S. Mehta, D. Jones, and D. Lidington for their discussion and technical assistance.
REFERENCES
- 1.Baker CH Vascular endothelium in sepsis and endotoxemia. J Fla Med Assoc 81: 119–122, 1994. [PubMed] [Google Scholar]
- 2.Beblo DA, Wang HZ, Beyer EC, Westphale EM, Veenstra RD. Unique conductance, gating, and selective permeability properties of gap junction channels formed by connexin40. Circ Res 77: 813–822, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology 208: 177–192, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bolanos JP, Medina JM. Induction of nitric oxide synthase inhibits gap junction permeability in cultured rat astrocytes. J Neurochem 66: 2091–2099, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bolon ML, Kidder GM, Simon AM, Tyml K. Lipopolysaccharide reduces electrical coupling in microvascular endothelial cells by targeting connexin40 in a tyrosine-, ERK1/2-, PKA-, and PKC-dependent manner. J Cell Physiol 211: 159–166, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bolon ML, Ouellette Y, Li F, Tyml K. Abrupt reoxygenation following hypoxia reduces electrical coupling between endothelial cells of wild-type but not connexin40 null mice in oxidant- and PKA-dependent manner. FASEB J 19: 1725–1727, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bolon ML, Peng T, Kidder GM, Tyml K. Lipopolysaccharide plus hypoxia and reoxygenation synergistically reduce electrical coupling between microvascular endothelial cells by dephosphorylating connexin40. J Cell Physiol 217: 350–359, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–1655, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol 280: H160–H167, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Feihl F, Waeber B, Liaudet L. Is nitric oxide overproduction the target of choice for the management of septic shock? Pharmacol Ther 91: 179–213, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol 287: C895–C902, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L, Nieman BJ, Peterson K, Qu D, Roscoe W, Shao Q, Tong D, Veitch GI, Voronina I, Vukobradovic I, Wood GA, Zhu Y, Zirngibl RA, Aubin JE, Bai D, Bruneau BG, Grynpas M, Henderson JE, Henkelman RM, McKerlie C, Sled JG, Stanford WL, Laird DW, Kidder GM, Adamson SL, Rossant J. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132: 4375–4386, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol 278: H1480–H1489, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 65: 475–502, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson F, Holstein-Rathlou N. Conducted vasomotor responses in arterioles: characteristics, mechanisms and physiological significance. Acta Physiol Scand 167: 11–21, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res 53: 113–120, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol 291: H2047–H2056, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62: 345–356, 2004. [DOI] [PubMed] [Google Scholar]
- 20.He P, Curry FE. Measurement of membrane potential of endothelial cells in single perfused microvessels. Microvasc Res 50: 183–198, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Kameritsch P, Khandoga N, Nagel W, Hundhausen C, Lidington D, Pohl U. Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J Cell Physiol 203: 233–242, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kumar NM, Gilula NB. The gap junction communication channel. Cell 84: 381–388, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Larson DM, Seul KH, Berthoud VM, Lau AF, Sagar GD, Beyer EC. Functional expression and biochemical characterization of an epitope-tagged connexin37. Mol Cell Biol Res Commun 3: 115–121, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Lepore DA, Stewart AG, Tomasi A, Anderson RL, Hurley JV, Morrison WA. The survival of skeletal muscle myoblasts in vitro is sensitive to a donor of nitric oxide and superoxide, SIN-1, but not to nitric oxide or peroxynitrite alone. Nitric Oxide 3: 273–280, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Lidington D, Ouellette Y, Li F, Tyml K. Conducted vasoconstriction is reduced in a mouse model of sepsis. J Vasc Res 40: 149–158, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Lidington D, Ouellette Y, Tyml K. Endotoxin increases intercellular resistance in microvascular endothelial cells by a tyrosine kinase pathway. J Cell Physiol 185: 117–125, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol 97: 1152–1158, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lu C, McMahon DG. Modulation of hybrid bass retinal gap junctional channel gating by nitric oxide. J Physiol 499: 689–699, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinnon RL, Lidington D, Bolon M, Ouellette Y, Kidder GM, Tyml K. Reduced arteriolar conducted vasoconstriction in septic mouse cremaster muscle is mediated by nNOS-derived NO. Cardiovasc Res 69: 236–244, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Mehrke G, Pohl U, Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol 439: 277–299, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res 74: 1050–1057, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Morris VL, MacDonald IC, Koop S, Schmidt EE, Chambers AF, Groom AC. Early interactions of cancer cells with the microvasculature in mouse liver and muscle during hematogenous metastasis: videomicroscopic analysis. Clin Exp Metastasis 11: 377–390, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Patel LS, Mitchell CK, Dubinsky WP, O'Brien J. Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes 13: 41–54, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science 267: 1831–1834, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodenwaldt B, Pohl U, de WC. Endogenous and exogenous NO attenuates conduction of vasoconstrictions along arterioles in the microcirculation. Am J Physiol Heart Circ Physiol 292: H2341–H2348, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Roh CR, Heo JH, Yang SH, Bae DS. Regulation of connexin 43 by nitric oxide in primary uterine myocytes from term pregnant women. Am J Obstet Gynecol 187: 434–440, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Saez JC, Martinez AD, Branes MC, Gonzalez HE. Regulation of gap junctions by protein phosphorylation. Braz J Med Biol Res 31: 593–600, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res 60: 643–653, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Shelton JL, Wang L, Cepinskas G, Sandig M, Scott JA, North ML, Inculet R, Mehta S. Inducible NO synthase (iNOS) in human neutrophils but not pulmonary microvascular endothelial cells (PMVEC) mediates septic protein leak in vitro. Microvasc Res 74: 23–31, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Simon AM, Chen H, Jackson CL. Cx37 and Cx43 localize to zona pellucida in mouse ovarian follicles. Cell Commun Adhes 13: 61–77, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature 385: 525–529, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol 8: 295–298, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Tan KH, Harrington S, Purcell WM, Hurst RD. Peroxynitrite mediates nitric oxide-induced blood-brain barrier damage. Neurochem Res 29: 579–587, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Traub O, Eckert R, Lichtenberg-Frate H, Elfgang C, Bastide B, Scheidtmann KH, Hulser DF, Willecke K. Immunochemical and electrophysiological characterization of murine connexin40 and -43 in mouse tissues and transfected human cells. Eur J Cell Biol 64: 101–112, 1994. [PubMed] [Google Scholar]
- 47.Tyml K, Wang X, Lidington D, Ouellette Y. Lipopolysaccharide reduces intercellular coupling in vitro and arteriolar conducted response in vivo. Am J Physiol Heart Circ Physiol 281: H1397–H1406, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Tyml K, Yu J, McCormack DG. Capillary and arteriolar responses to local vasodilators are impaired in a rat model of sepsis. J Appl Physiol 84: 837–844, 1998. [DOI] [PubMed] [Google Scholar]
- 49.van Rijen HV, van Veen TA, Hermans MM, Jongsma HJ. Human connexin40 gap junction channels are modulated by cAMP. Cardiovasc Res 45: 941–951, 2000. [DOI] [PubMed] [Google Scholar]
- 50.van Veen TA, van Rijen HV, Jongsma HJ. Physiology of cardiovascular gap junctions. Adv Cardiol 42: 18–40, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Vargas FF, Caviedes PF, Grant DS. Electrophysiological characteristics of cultured human umbilical vein endothelial cells. Microvasc Res 47: 153–165, 1994. [DOI] [PubMed] [Google Scholar]
- 52.Veenstra RD, Wang HZ, Beyer EC, Ramanan SV, Brink PR. Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys J 66: 1915–1928, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vogt CJ, Schmid-Schonbein GW. Microvascular endothelial cell death and rarefaction in the glucocorticoid-induced hypertensive rat. Microcirculation 8: 129–139, 2001. [PubMed] [Google Scholar]
- 54.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol 274: H178–H186, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Wilson JX, Dixon SJ, Yu J, Nees S, Tyml K. Ascorbate uptake by microvascular endothelial cells of rat skeletal muscle. Microcirculation 3: 211–221, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Wu F, Cepinskas G, Wilson JX, Tyml K. Nitric oxide attenuates but superoxide enhances iNOS expression in endotoxin- and IFNγ-stimulated skeletal muscle endothelial cells. Microcirculation 8: 415–425, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am J Physiol Regul Integr Comp Physiol 285: R50–R56, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Xia J, Duling BR. Electromechanical coupling and the conducted vasomotor response. Am J Physiol Heart Circ Physiol 269: H2022–H2030, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Xin D, Bloomfield SA. Effects of nitric oxide on horizontal cells in the rabbit retina. Vis Neurosci 17: 799–811, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol 535: 181–195, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]