Abstract
Osteopontin (OPN), a key component of the extracellular matrix, is associated with the fibrotic process during tissue remodeling. OPN and the cytokine interleukin (IL)-18 have been shown to be overexpressed in an array of human cardiac pathologies. In the present study, we determined the role of IL-18 in the regulation of cardiac OPN expression and the subsequent interstitial fibrosis and diastolic dysfunction. We demonstrated parallel increases in IL-18, OPN expression, and interstitial fibrosis in murine models of left ventricular pressure and volume overload. Exogenous recombinant (r)IL-18 administered for 2 wk increased cardiac OPN expression, interstitial fibrosis, and diastolic dysfunction. Stimulation of the T helper (Th)1 lymphocyte phenotype with a selective toll-like receptor (TLR)9 agonist induced cardiac IL-18 and OPN expression, which was associated with increased cardiac fibrillar collagen concentrations and interstitial fibrosis resulting in diastolic dysfunction. rIL-18 induced OPN expression and protein levels in primary of cardiac fibroblast cultures. Conditioned media from TLR9-stimulated T lymphocyte cultures induced IL-18 and OPN expression in cardiac fibroblasts, while blockade of the IL-18 receptor with a neutralizing antibody abolished the increase in OPN expression. Furthermore, a mutation in the transcriptional factor interferon regulatory factor (IRF)1 or IRF1 small interfering RNA (siRNA) resulted in the decreased expression of IL-18 and OPN in cardiac fibroblasts. With pressure overload, IRF1-mutant mice showed downregulation of IL-18 and OPN expression in cardiac tissue, reduced cardiac fibrotic development, and increased left ventricular function compared with wild type. These results provide direct evidence that the induction of IL-18 regulates OPN-mediated cardiac fibrosis and diastolic dysfunction.
Keywords: extracellular matrix, remodeling, T lymphocyte, interferon regulatory factor 1, toll-like receptor-9, lysyl oxidase
left ventricular remodeling in response to pressure and/or volume overload is associated with myocardial hypertrophy, fibroblast hyperplasia, and increased concentrations of fibrillar collagen in the extracellular matrix. Recent studies have shown that pathological myocardial fibrosis may cause diastolic dysfunction and heart failure (24), whereas myocyte hypertrophy in the absence of fibrosis is considered physiological remodeling (22, 46) if the mass-to-volume ratio is preserved (5). In this study, we investigated whether interleukin (IL)-18 regulates the gene and protein expression of the matricellular protein osteopontin (OPN), a component and regulator of cardiac extracellular matrix fibrillar collagen concentrations.
As reviewed by Okamoto (26), OPN also plays a critical role in the regulation of cellular proliferation and differentiation, interstitial fibrosis, arteriosclerosis, and angiogenesis. Although OPN is ubiquitously expressed in many tissues and is increased during stress-induced cardiac remodeling, it participates as a cytokine or humoral factor in the integration of intercellular networks during tissue remodeling (1, 40). In particular, OPN regulates tissue remodeling by controlling the tissue fibrotic process in cardiac remodeling and skin wound healing; this effect is reduced by the silencing or deletion of the OPN gene (8, 21, 23). Additionally, OPN has been recognized as a serum marker of myocardial injury, hypertrophy, and dysfunction in humans (14, 35, 36).
A key link between the cardiovascular and immune systems is OPN's role as a chemokine that activates T helper (Th)1 lymphocytes (2). OPN is, therefore, also termed early T lymphocyte activator-1 (Eta-1). Activated T lymphocytes contribute to the fibrotic process through elaboration of select cytokines as reviewed by Azouz et al. (3). Recent evidence has shown that toll-like receptors (TLRs) serve as pattern recognition receptors that control immune processes and are critical for cardiac remodeling in rodent models of myocardial infarction or pressure overload (19, 34, 37). TLRs govern the signaling pathways of downstream cytokines including IL-18 (27). As reviewed by Wang et al. (38), IL-18 can also be induced in fibroblasts by stress, neurohormones, and Th1 cytokines in the cardiac tissues. Moreover, IL-18 is significantly increased in the resident myocardial tissues and serum of heart failure patients (20, 41). Several studies have emphasized the role of IL-18 in the pathophysiology of myocardial inflammation and injury (6, 7, 9). However, the fibrotic regulation of OPN by IL-18 in the stressed heart has not been elucidated. Therefore, the goal of our study was to determine whether IL-18 regulates OPN expression and induces interstitial fibrosis using in vivo and in vitro models. Our work does not discount the contributions of neuroendocrine factors such as angiotensin II, endothelin-1, transforming growth factor-β, and aldosterone in cardiac remodeling; however, the immune system, via IL-18, is also a contributor to this pathological process. The results described here elucidate the regulatory role of IL-18 on OPN expression and OPN-mediated interstitial fibrosis and cardiac dysfunction.
METHODS
General protocol.
Female wild-type (WT) or interferon regulatory factor (IRF)1-mutant C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). The study was approved by the University of Arizona Animal Care Committee and conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Pub. No. 85-23, revised 1996). To characterize the relationship between OPN and IL-18, murine models including pressure overload, volume overload, administration of recombinant (r)IL-18, administration of CpG 1668 oligodeoxynucleotide (ODN), and IRF1 mutation were utilized as illustrated by Fig. 1. Cardiac function was evaluated with transthoracic echocardiography (echo) and in situ pressure-volume loop analysis. Subsequently, left ventricular tissues were harvested and analyzed for collagen concentrations and cross-linking by hydroxylproline assay, gene expression by real-time PCR, and protein concentrations of OPN and IL-18 with ELISA. The direct effect of IL-18 on OPN was determined in cardiac fibroblasts in vitro.
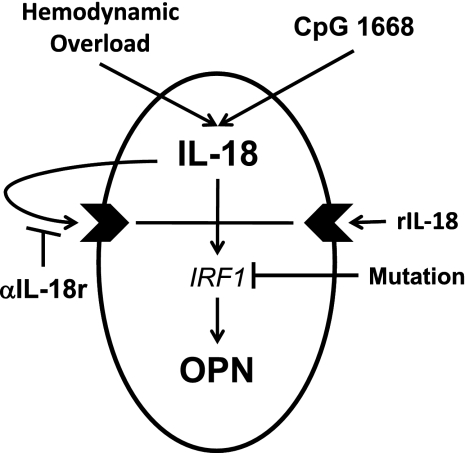
Fig. 1.
Study scheme. Five means were to used demonstrate the induction of osteopontin (OPN) by interleukin (IL)-18: hemodynamic overload with transverse aortic constriction (TAC) and aortocaval fistula (AVF), immunologic stimulation of IL-18 with CpG 1668, administration of recombinant (r)IL-18, neutralizing antibody against the IL-18 receptor (αIL-18r), and mutation of the transcriptional factor interferon regulatory factor-1 (IRF1).
Preparation of pressure/volume overload murine models.
Two-month-old WT or IRF1-mutant mice were randomly divided into the following groups: transverse aortic constriction (TAC) (n = 4 for day 1, n = 4 for day 7, and n = 8 for day 30), sham-operated TAC control (n = 8), aortocaval fistula (AVF) surgery (n = 4), and sham-operated AVF control (n = 4). With a TAC surgical technique according to Hu et al. (16), the postsurgical TAC survival rate was >85%, and only TAC mice in which the aortic velocity time integral was decreased by 30% were included in this study. Mouse AVF model studies limited to only 30 days after fistula were included to provide confirmation that comparable responses occur with these two types of hemodynamic overload. With an adaptation of the methods described by Garcia and Diebold (13), AVF was created in mice with a 27-gauge needle passed through the infrarenal abdominal aorta into the inferior vena cava. The AVF surgical procedure postsurgical survival rate was 100%, and those mice in which the 30-day cardiac output was increased by 50% as measured with echocardiography were included in the study.
IL-18 administration.
Mice were randomly divided into PBS and recombinant murine IL-18 (rIL-18) (R&D Systems, Minneapolis, MN) treatment groups. The rIL-18 administration dose was that described by Woldbaek et al. (39) at 25 μg·kg−1·day−1 subcutaneously for 2 wk (n = 8).
CpG 1668 administration.
The CpG 1668 ODN sequence, 5′ TCCATGACGTTCCTGATGCT 3′, synthesized by TIB Molbiol (Adelphia, NJ) was administered in vivo at a dose of 50 μg·mouse−1·wk−1 for 4 wk according to the protocol reported by Redecke et al. (29).
Quantification of left ventricular mechanics by echo and pressure-volume loop analysis.
At postsurgery week 4, at week 2 in the rIL-18 study, and at week 5 in the CpG 1668 study, the mice were anesthetized with 1.5% isoflurane and digital images were recorded at a frame rate of 200 frames/s with a linear 45-MHz probe (model 707B Scanhead, Visualsonics). Short-axis M mode was used to measure ejection fraction and fractional shortening. The mitral valve flow was measured with Doppler, using a four-chamber view to compute the E-to-A ratios. After echo analysis, pressure-volume loops were acquired with our previously described Millar conductance catheter system methods (42, 43).
Determination of hydroxylproline and collagen cross-linking.
Hydroxylproline and cross-linking assays have been described by Yu et al. (45). Hydroxylproline levels were quantified by comparison to a standard colorimetric curve of trans-hydroxylproline (Sigma). The data were calculated with the average hydroxylproline content of collagen (13.5%) and expressed as micrograms of collagen per milligram of dry heart weight. Collagen cross-linking was determined with cyanogen bromide (CNBr) digestion. The percentage of cross-linking was determined by comparing CNBr-nonsoluble hydroxylproline to total hydroxylproline.
Primary cardiac fibroblast isolation and treatment.
The isolation and culture of cardiac fibroblasts were performed as described by Yu et al. (45). At 80–90% confluence, cardiac fibroblasts were treated with 10 ng/ml rIL-18, 1:10 diluted supernatants from splenic T lymphocytes (described below), or 10 μg/ml CpG 1668. After 48 h of treatment, cells were collected for further assays.
Splenic T lymphocyte isolation.
Splenic T lymphocytes were isolated and cultured according to the methods reported by Yu et al. (45). After 48 h of treatment with 10 μg/ml CpG 1668, supernatants were collected as conditioned medium.
ELISA for OPN.
Mouse cardiac tissue OPN was extracted with CelLytic MT (Sigma) buffer with 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 10 μg/ml trypsin inhibitor. Extraction samples or cell culture supernatants were quantified with the TiterZyme EIA kit (Assay Designs) according to manufacturer's instructions.
RNA extraction and real-time PCR.
Messenger RNA from tissue or cells was quantified with real-time PCR SYBR Green (Qiagen) and the Rotor-Gene (RG)-6000 (Corbett Research), and the results were analyzed with Rotor-Gene 6 software (Corbett Research). Real-time PCR primers were based on GenBank sequences. The following candidate genes were investigated: collagen genes: pro-Collagen Iα1 (NM_007742) and pro-Collagen IIIα1 (AK079113); cross-linking enzyme genes: pro-lysyl oxidase (LOX, NM_010728), pro-lysyl oxidase-like protein 3 (LOXL3, NM_013586); matricellular gene: osteopontin (OPN, X16151); transcription factor gene: IRF1 (BC003821.1); and matrix metalloproteinase (MMP) and tissue inhibitor of MMP (TIMP) genes: pro-MMP-2 (NM_008610), pro-MMP-9 (NM_013599), pro-MMP-13 (NM_008607), TIMP-1 (NM_011593.1), TIMP-4 (NM_080639.2). All the candidate gene threshold cycle numbers were normalized to the relative amounts of the β-actin (BC040513) gene.
Cardiac tissue section histology.
Cardiac structure and fibrosis were evaluated by standard picrosirius red staining procedures performed by the Department of Pathology lab at the University of Arizona. The interstitial and perivascular fibrosis volume was quantified with polarized light, with six views per midregion of the left ventricular sections with Image J software (NIH).
Statistical analysis.
Statistical analyses of parameters of left ventricular function, gene expression, and ELISA data and results from all cell culture treatments were performed with ANOVA followed by Scheffé's F-test to assess specific group differences. Values are expressed as means ± SE. P < 0.05 was considered to indicate statistical significance.
RESULTS
Time-dependent correlation of cardiac IL-18 and OPN expression in pressure/volume overload murine models.
Previous studies demonstrate that OPN, a lymphocyte chemokine and matrix cellular protein, is essential for the development of cardiac interstitial fibrosis (2, 26). We therefore examined an immunologic mechanism regulating left ventricular OPN expression. The scheme of this investigation is illustrated by Fig. 1. The relationship between IL-18 and OPN expression in pressure and volume overload-induced left ventricular remodeling was compared with TAC and AVF murine models, respectively. Table 1 compares the morphological and hemodynamic data for TAC, AVF, and sham control groups. There was an increase in heart and lung mass in both ventricular overload models. Transthoracic echo demonstrated a 42% decrease in cardiac output in the TAC model, and the AVF model had an increase of 60%. Left atrial dimension and end-systolic (Ves) and end-diastolic (Ved) volumes were all increased compared with the sham control groups. These data support the idea that these models produced left ventricular hemodynamic overload.
Table 1.
Morphological and hemodynamic analysis of transverse aortic constriction and aortocaval fistula models
| Parameters | Sham | TAC | AVF |
|---|---|---|---|
| BW, g | 20.2±0.6 | 20.1±0.3 | 19.4±0.2 |
| LVW/BW, mg/g | 4.8±0.1 | 6.7±0.5 | 7.8±0.9 |
| LW/BW, mg/g | 8.3±0.1 | 12.7±0.9† | 12.6±1.1† |
| CO, ml/min | 10.8±0.8 | 6.3±0.4† | 17.3±1.4† |
| LAD, mm | 1.6±0.1 | 1.9±0.1* | 2.5±0.1† |
| Ves, μl | 12.9±1.1 | 21.7±4.6 | 34.7±0.6† |
| Ved, μl | 35.3±1.4 | 50.4±2.8† | 74.5±2.2† |
Values are means ± SE comparing transverse aortic constriction (TAC) and aortocaval fistula (AVF) groups with sham control groups at postsurgery day 30. There was an increase in left ventricular weight (LVW)-to-body weight (BW) ratio and lung weight (LW)-to-BW ratio in both TAC and AVF models. Transthoracic echocardiography demonstrated that cardiac output (CO) decreased in TAC and increased in AVF mice, and left atrial dimension (LAD) increased in both models compared with sham control groups. End-systolic volume (Ves) and end-diastolic volume (Ved) increased more in the AVF group than in the TAC group compared with the sham control groups (n = 8).
P < 0.05 compared with sham control group;
P < 0.01 compared with sham control group.
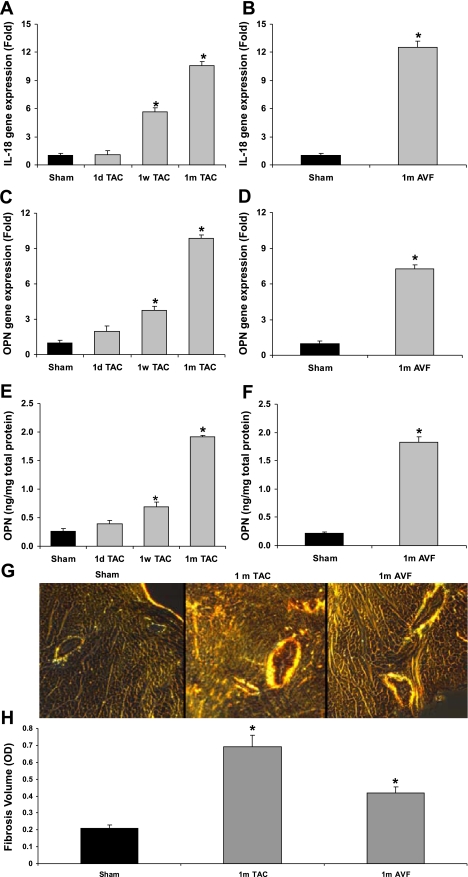
Cardiac pro-IL-18 gene expression increased time-dependently in the TAC model by 5.7-fold at week 1 to 10.6-fold at week 4 and increased by 12.5-fold in the AVF model at week 4 compared with the sham controls (Fig. 2, A and B). Moreover, cardiac gene expression of OPN increased in the TAC model by 3.7-fold at week 1 and 9.8-fold at week 4 and increased by 7.3-fold in the AVF model at week 4 compared with the sham controls (Fig. 2, C and D). There was a positive correlation between cardiac pro-IL-18 and OPN gene expression in the TAC hearts (r2 = 0.94, P < 0.01). Increased OPN protein expression findings supported the gene expression results in both TAC- and AVF-stressed hearts (Fig. 2, E and F), and in the same tissue cardiac IL-18 levels increased from control levels of 253 ± 35 to 1,443 ± 58 and 1,260 ± 17 pg/mg in TAC and AVF heart tissue, respectively (P < 0.001). Picrosirius red-stained images demonstrated increased cardiac fibrotic collagen in the TAC and AVF models at 1 mo (Fig. 2G). Fibrotic volume quantification showed increased fibrosis at 1 mo in TAC and AVF overloaded cardiac tissue compared with sham controls (Fig. 2H). These data reveal a temporal relationship among IL-18, OPN, and cardiac fibrosis during cardiac remodeling.
Fig. 2.
Time-dependent correlation of cardiac IL-18 and OPN expression in pressure /volume overload murine models. This experiment demonstrates that there is a temporal association among expression of IL-18, OPN, and fibrosis in 2 ventricular overload murine models. A: time-dependent gene expression of IL-18 in 1-day, 1-wk, and 1-mo pressure-overloaded cardiac tissue. B: time-dependent IL-18 gene expression in 1-mo volume-overloaded cardiac tissue. C: time-dependent OPN gene expression in 1-day, 1-wk, and 1-mo pressure-overloaded cardiac tissue. D: OPN gene expression in 1-mo volume-overloaded cardiac tissue. E: time-dependent OPN protein expression of IL-18 in 1-day, 1-wk, and 1-mo pressure-overloaded cardiac tissue. F: OPN protein expression in 1-mo volume-overloaded cardiac tissue G: images of picrosirius red (PSR) staining of fibrotic collagen in 1-mo pressure-/volume-overloaded cardiac tissue. H: fibrotic volume quantification of 1-mo pressure-/volume-overloaded cardiac tissue. *P < 0.05 compared with sham controls.
OPN expression in cardiac tissue of rIL-18-treated mice.
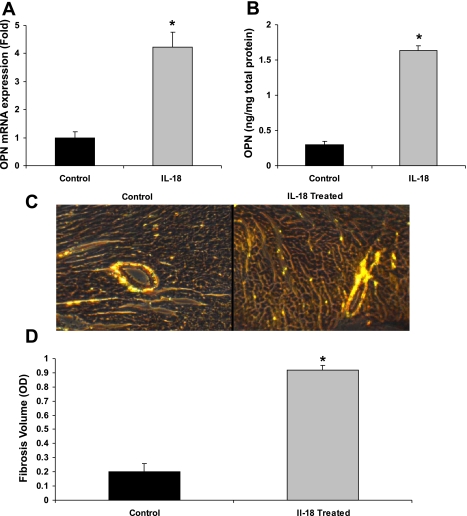
To further investigate the effect of IL-18 on OPN expression in vivo, rIL-18 was administered (25 μg·kg−1·day−1 sc) for 2 wk. An increase was seen in the expression of the cardiac OPN gene (4.2-fold, P < 0.05) as well as the OPN protein (5.5-fold, P < 0.01) (Fig. 3, A and B, respectively) at week 2 compared with sham controls. Histological analysis showed a 5.4-fold increase in interstitial fibrotic collagen accumulation compared with controls (Fig. 3, C and D). Thus the induction of OPN by IL-18 coincides with cardiac fibrillar collagen content.
Fig. 3.
OPN expression in cardiac tissue of rIL-18-treated mice. Since the expression of OPN coincides with IL-18 in hemodynamic overload, we determined whether exogenous rIL-18 would induce cardiac OPN and fibrosis in vivo. A: IL-18 administration in mice increased cardiac OPN gene expression by 4.2-fold. B: IL-18 administration in mice increased cardiac OPN protein levels by 5.5-fold. C: images of PSR staining of fibrotic collagen in IL-18-treated cardiac tissue. D: fibrotic volume quantification of IL-18-treated cardiac tissue. *P < 0.05 compared with sham controls or control cardiac fibroblasts.
IL-18, OPN expression, and cardiac diastolic dysfunction induced by CpG 1668 administration.
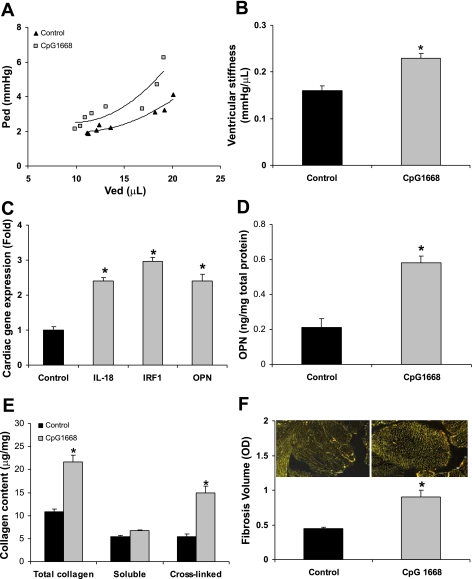
Ligation of TLR9 with single-stranded DNA (CpG 1668 ODN) induces IL-18 (15). We administered CpG 1668 (50 μg·mouse−1·wk−1 for 4 wk) according to Redecke et al. (29), and 7 days after the final CpG dose hemodynamic, gene expression, OPN, and collagen measurements were conducted on the left ventricular tissue. By conductance catheter pressure-volume loop analysis the maximum systolic pressure (Pmax), end-diastolic pressure (Ped), and arterial elastance (Ea) increased significantly, and by echo the ejection fraction and fractional shortening were decreased (Table 2). The reductions in Ved and Ves were suggestive of an impaired diastolic function. Further support of diastolic dysfunction is shown in Fig. 4A, where there was a leftward shift of the end-diastolic pressure-volume relationship that was characteristic of an abnormal diastolic filling phase in the cardiac cycle. Correspondingly, Fig. 4B shows an increase of 43% in the slope (β) of the end-diastolic pressure-volume relationship. Next, we examined the effect of CpG on cardiac matrix properties. Even at 5 wk from the initiation of the CpG, Fig. 4C shows an increase in the cardiac gene expression of pro-IL-18 by 2.4-fold, IRF1 by 3.0-fold, and OPN by 2.4-fold compared with controls. Cardiac OPN protein expression increased by 2.8-fold in CpG 1668-treated mice (Fig. 4D). Hydroxyproline was used to determine the total collagen and cross-linked collagen concentrations, and Fig. 4E shows that total ventricular collagen and cross-linked collagen increased by 2.0-fold and 2.8-fold, respectively. The percentage of cross-linked collagen increased from 49% to 68% in the CpG 1668 group compared with controls. Histological staining (Fig. 4F) further supports an increase in interstitial fibrosis (1.9-fold) induced by CpG 1668 administration. Collectively, chronic CpG 1668 administration increased IL-18, its transcriptional factor IRF1, and OPN and induced left ventricular diastolic dysfunction associated with the accumulation of cross-linked fibrillar collagen.
Table 2.
CpG 1668-induced cardiac and vascular functional changes
| Parameters | Control | CpG 1668 | P Value |
|---|---|---|---|
| BW, g | 19.6±0.4 | 18.8±0.2 | 0.12 |
| LVW, mg | 64.1±3.7 | 77.3±3.9 | 0.03 |
| LVW/BW, mg/g | 3.35±0.18 | 3.95±0.07 | 0.01 |
| HR, beats/min | 514.2±10.5 | 507.2±9.5 | 0.63 |
| CO, ml/min | 7.3±0.6 | 6.6±0.5 | 0.42 |
| Ved, μl | 18.9±0.9 | 14.6±0.8 | 0.006 |
| Ves, μl | 5.44±0.27 | 2.67±0.49 | 0.001 |
| EF, % | 75±1 | 59±2 | 0.0012 |
| FS, % | 43±1 | 31±2 | 0.0009 |
| Ped, mmHg | 4.07±0.41 | 6.86±1.03 | 0.05 |
| Pmax systolic, mmHg | 88.4±1.6 | 100.9±3.7 | 0.02 |
| Ea, mmHg/μl | 6.12±0.42 | 7.74±0.44 | 0.03 |
Values are means ± SE. The immune inducer of interleukin (IL)-18, CpG 1668, was administered at a dose of 50 μg/mouse weekly for 4 wk. Hemodynamic measurements were acquired at week 5 with in situ pressure-volume loop analysis. There was an increase in heart weight and left ventricular end-diastolic pressure and a decrease in Ves and Ved. There was an increase in arterial vascular resistance (arterial elastance, Ea) and maximal end-systolic pressures (Pmax systolic). HR, heart rate; EF, ejection fraction; FS, fractional shortening; Ped, end-diastolic pressure.
Fig. 4.
IL-18, OPN expression, and cardiac diastolic dysfunction induced by CpG 1668 administration. Immune challenge with CpG is known to stimulate IL-18, and therefore we determined whether CpG affects ventricular function, OPN expression, and remodeling of the extracellular matrix. A: end-diastolic pressure (Ped)-volume (Ved) relationship (EDPVR) demonstrates an increased ventricular stiffness. B: the parameter that defines the slope of EDPVR (β) was increased by 43%. C: CpG 1668 treatment in mice increased cardiac IL-18, IRF1, and OPN gene expression. D: CpG 1668 treatment increased cardiac OPN levels by 2.8-fold. E: CpG 1668 treatment increased total cardiac collagen by 2.3-fold and cross-linked collagen by 3.4-fold compared with controls. F: PSR image of CpG 1668-treated cardiac tissue. CpG 1668 treatment increased cardiac fibrotic volume by 3-fold. *P < 0.05 compared with controls.
Altered gene expression of cardiac extracellular matrix proteins by CpG 1668 administration.
Since immune stimulation through CpG 1668 administration resulted in increased cardiac fibrosis, we sought to determine whether the genes for other extracellular matrix-related proteins were affected (Table 3). The gene expression of pro-collagen type I increased by 2.5-fold; the collagen cross-linking enzyme pro-lysyl oxidase (LOX) increased by 2.1-fold, as did the pro-LOXL3 isoform by 9.1-fold compared with sham controls. These gene expression data were consistent with the above hydroxyproline data related to cardiac collagen content and fibrillar collagen cross-linking. Thus immune stimulation with CpG 1668 induces the expression of selected cardiac extracellular matrix genes including OPN, collagen, and lysyl oxidase.
Table 3.
Altered gene expression of cardiac extracellular matrix proteins by CpG 1668 administration
| Fold Change | P Value | |
|---|---|---|
| Pro-Col Iα1 | 2.46±0.10 | 0.009 |
| Pro-Col IIIα1 | 0.99±0.78 | 0.990 |
| Pro-MMP2 | 0.72±0.08 | 0.049 |
| Pro-MMP9 | 1.43±0.21 | 0.421 |
| Pro-MMP13 | 1.69±0.24 | 0.180 |
| TIMP1 | 1.65±0.17 | 0.071 |
| TIMP4 | 1.65±0.30 | 0.241 |
| Pro-LOX | 2.11±0.15 | 0.022 |
| Pro-LOXL3 | 9.06±0.16 | 0.001 |
Values are means ± SE comparing control vs. CpG 1668 (n = 8). CpG 1668 was administered at a dose of 50 μg/mouse weekly for 4 wk. Candidate genes related to the cardiac extracellular matrix were analyzed with real-time PCR at week 5. Pro-Col Iα1, pro-collagen type Iα1 chain; Pro-Col IIIα1, pro-collagen type IIIα1 chain; Pro-MMP 2, 9, 13 pro-matrix metalloproteinase 2, 9, 13; TIMP1, 4, tissue inhibitor of metalloproteinase 1, 4; LOX, lysyl oxidase; LOXL3, lysyl oxidase-like protein 3. Fold change was computed as 2 to the power of the difference between the control and CpG 1668 thresholds.
OPN expression in primary cardiac fibroblast cell culture induced by conditioned lymphocyte medium and IL-18.
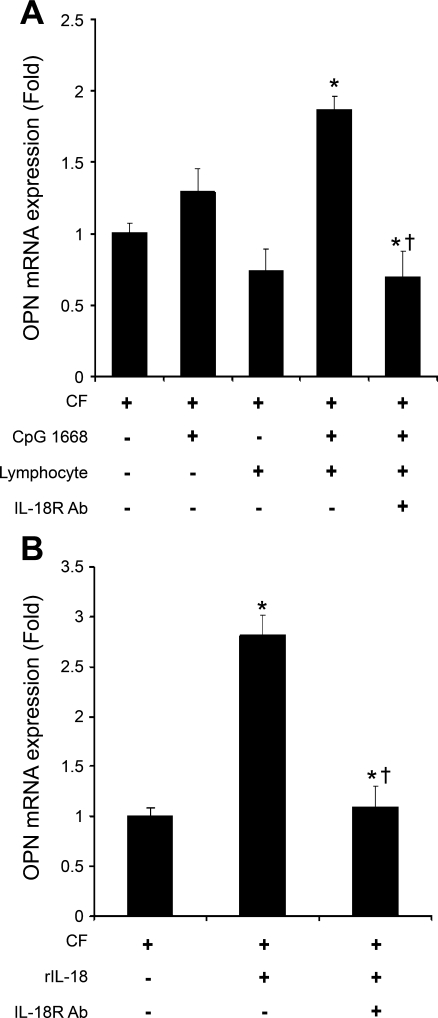
To characterize the direct effect of IL-18 on the induction of OPN expression in cardiac fibroblasts, we compared diluted lymphocyte conditioned medium (1:10) and rIL-18 in the absence or presence of an anti-IL-18 receptor neutralizing antibody. Figure 5A demonstrates that conditioned medium from CpG 1668 (10 μg/ml)-treated splenic T lymphocytes increased the gene expression of pro-OPN in cardiac fibroblasts without a direct effect from CpG 1668 or from medium from nonprimed splenic T lymphocytes. The full stimulation of OPN gene expression with conditioned medium was decreased by 63% with anti-IL-18 receptor antibody (5 μg/ml) (Fig. 5A). Furthermore, OPN gene expression was upregulated by 2.8-fold with rIL-18 (10 ng/ml) administration in vitro and was reduced by 60% with anti-IL-18 receptor neutralizing antibody in primary cardiac fibroblasts (Fig. 5B). The limitation of the above in vivo study is that CpG 1668 may induce numerous T lymphocyte-derived cytokines; however, this in vitro study provides support that IL-18 derived from CpG 1668-activated T lymphocytes induces OPN expression in cardiac fibroblasts.
Fig. 5.
OPN expression in primary cardiac fibroblast cell culture induced by conditioned lymphocyte medium and rIL-18. CpG and exogenous IL-18 appear to induce OPN in vivo; therefore we determined whether this observation could be detected in vitro. A: conditioned medium from CpG 1668 (10 μg/ml)-treated splenic T lymphocytes increased OPN gene expression from cardiac fibroblasts with no direct effect from CpG 1668; the enhancement of OPN gene expression was ablated by anti-IL-18 receptor neutralizing antibody (IL-18R Ab, 5 μg/ml). B: IL-18 (10 ng/ml) enhanced OPN gene expression by 2.4-fold in cardiac fibroblasts and IL-18R Ab abolished the effect of IL-18. *P < 0.05 compared with control cardiac fibroblasts; †P < 0.05 compared with conditioned medium from CpG 1668-treated splenic lymphocytes or IL-18-treated cardiac fibroblasts.
IRF1 mutation alters cardiac remodeling in response to pressure overload.
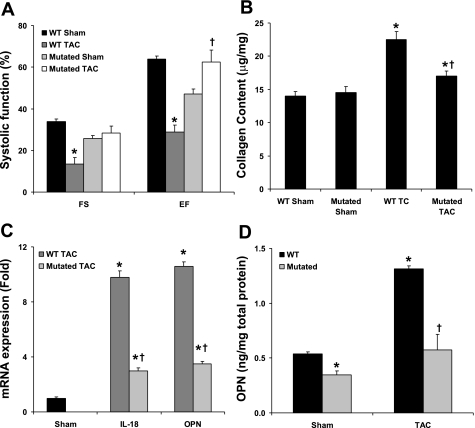
IRF1 is a transcriptional factor for the induction of pro-IL-18 and OPN. Using the TAC model with IRF1-mutant mice, we determined the effect of this mutation on cardiac remodeling, function, and OPN concentrations. Figure 6A demonstrates that the IRF1 mutation allowed for preserved normal left ventricular function after the TAC intervention. The cardiac collagen measured by hydroxyproline content revealed that the IRF1 mutation reduced the collagen accumulation in response to TAC by 35% compared with WT mice (Fig. 6B). The gene expression of cardiac IL-18 and OPN was also significantly reduced in the TAC IRF1-mutant group by 69% and 67%, respectively, compared with the TAC WT group (Fig. 6C). Compared with controls, the mice with the IRF1 mutation had a 36% decrease in basal cardiac OPN protein levels; compared with WT TAC mice, the IRF1-mutant TAC mice had a 56% reduction in myocardial OPN protein concentrations (Fig. 6D). Together, our in vivo studies indicate that IRF1 deficiency is associated with reduced cardiac remodeling and OPN expression concomitant with preserved left ventricular function.
Fig. 6.
IRF1 mutation alters cardiac remodeling in response to pressure overload. IRF1 is the transcriptional factor for IL-18 and OPN, and therefore we determined whether this mutation would affect left ventricular hemodynamic performance, extracellular matrix remodeling, IL-18, and OPN expression. A: comparison of left ventricular function in wild-type (WT) and IRF1-mutated pressure-overloaded mice. B: mutated IRF1 decreases cardiac collagen production during pressure overload. C: cardiac OPN gene expression from WT or IRF1-mutated pressure-overloaded mice. D: cardiac OPN protein from WT or IRF1-mutated pressure-overloaded mice. *P < 0.05 compared with respective controls; †P < 0.05 compared with WT pressure overload.
IRF1 mutation and silencing downregulates IL-18 and OPN expression in cardiac fibroblasts.
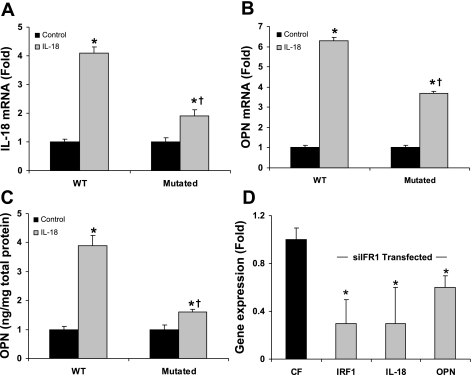
Our in vivo experiments indicate that the IRF1/IL-18 pathway is critical for the induction of OPN and cardiac remodeling. In cardiac fibroblasts isolated from IRF1-mutant mice treated with rIL-18 (10 ng/ml), the expression of IL-18 and OPN genes was suppressed by 53% and 47%, respectively, versus WT cardiac fibroblasts (Fig. 7, A and B). It appeared that the exogenous IL-18 induced cellular IL-18 synthesis (Fig. 7A), and therefore the difference between WT and IRF1-mutant IL-18-stimulated OPN expression by cardiac fibroblasts in Fig. 7B is related to the inhibition of endogenous IL-18 synthesis due to the IRF1 mutation. The stimulation of OPN protein levels with rIL-18 was reduced by 62% in IRF1-mutant cardiac fibroblasts (Fig. 7C). To confirm this, targeted IRF1 small interfering RNA (siRNA) treatment of cardiac fibroblasts significantly downregulated IRF1, pro-IL-18, and OPN gene expression compared with control cardiac fibroblasts (Fig. 7D). These data are consistent with our in vivo findings, which demonstrate the role of IRF1 in the expression of IL-18 and OPN in cardiac tissue remodeling.
Fig. 7.
IRF1 mutation and silencing downregulates IL-18 and OPN expression in cardiac fibroblasts. IRF1 mutation affected cardiac remodeling in vivo; therefore this experiment demonstrated a direct effect of rIL-18 treatment on IL-18 and OPN expression in vitro. A: mutation of IRF1 impaired IRF1 gene expression when stimulated by IL-18 in cardiac fibroblasts. B: mutation of IRF1 impaired OPN gene expression in cardiac fibroblasts. C: mutation of IRF1 decreased OPN protein secretion from cardiac fibroblasts. D: siRNA targeted IRF1 treatment downregulated the gene expression of IRF1, pro-IL-18, and OPN in cardiac fibroblasts. The siRNA sequence specific for IRF1 was AAGGUCUUCGGCUAUCUUCUU and scrambled CGATGTCTCTGGGTTC. CF, cardiac fibroblast. *P < 0.05 compared with respective controls; †P < 0.05 compared with WT mice.
DISCUSSION
The pathogenesis of diastolic dysfunction remains incompletely understood. However, prima facie evidence for the central role of the cardiac fibroblast in pathological cardiac fibrosis has been established by the observation that the differentiation to cardiac myofibroblasts results in the deposition of type I and III collagen and, second, the formation of fibrillar collagen cross-linking through fibroblast-derived lysyl oxidase. Recent studies indicate that OPN expression is obligatory for the formation of pathological myocardial fibrosis in rodents (21), strongly associated with left ventricular hypertrophy, and possibly related to recurrent ventricular arrhythmias in humans (14). In the study described here, we present results providing direct evidence for IL-18 as an inducer of OPN and the resulting pathological fibrosis that support the claim that cardiac fibrotic pathogenesis involves the interaction between the cardiac fibroblast and the adaptive immune system.
Our study develops the fundamental notion that when hearts are subjected to pressure overload there is a time-dependent upregulation of cardiac IL-18 and OPN. In addition, the activation of an immune pathway with the selective TLR9 agonist ODN CpG 1668 results in the induction of IL-18, leading to OPN expression that appears to be associated with cardiac interstitial fibrosis, ultimately resulting in diastolic dysfunction. Our data also suggest that mutation of the transcriptional factor IRF1 decreases the expression of IL-18 and thereby reduces cardiac OPN expression and protein concentrations. It is important to note that the comparison of WT mice with the IRF1 mutation in the TAC model shows preserved left ventricular function and fibrillar collagen concentrations in the IRF1-mutant mice. These studies indicate that administration of exogenous rIL-18 to primary cardiac fibroblasts induces IL-18 and that the IRF1 mutation reduces this rIL-18-mediated expression of IL-18. Consequently, this characterization of IL-18 provides an immunologic mechanism for the induction of OPN in cardiac tissues.
OPN is a multifunctional protein in the cardiovascular system, and this RGD-binding protein functions as an extracellular matrix protein providing structural integrity to the cardiac extracellular matrix. More specifically, as reviewed by Okamoto (24), OPN connects fibroblasts, cardiomyocytes, and the extracellular matrix through focal and fibrillar adhesions and initiates cell signaling cascades (26). OPN expression is substantially upregulated during remodeling in wound healing and myocardial injury (31). Importantly, as a mediator of tissue remodeling, OPN has many diverse cellular roles including regulation of cardiac fibroblast survival and differentiation (18), formation of a matrix to adhere or attract myocardial constituent fibroblasts and endothelial cells to the injury site, and promotion of scar formation as reviewed by Denhardt et al. (10). Evidence for the critical role of OPN during fibrosis development has been demonstrated in wound healing (23) and cardiac remodeling (8) models. Moreover, OPN has been reported as a serum marker of myocardial injury and dysfunction in humans (35, 36).
Alternatively, OPN is a key regulatory cytokine, as illustrated by its original description as early T cell activation gene-1 (Eta-1). In lymphocytes, the gene expression of OPN, like IFN-γ and IL-12Rβ2, is dependent on T-bet, a T-box transcriptional factor that is essential for Th1 polarization. In contrast, IL-18, also a Th1 cytokine, is T-bet independent (32). In OPN-deficient mice, there is an overexpression of IL-10, a prototypic Th2 cytokine (32). Therefore, OPN is a key factor governing the T lymphocyte phenotype. Specifically, OPN is coupled with TLR9 and induces dendritic cell maturation toward a Th1-prompting phenotype (30, 33). Thus our data link the fact that OPN is induced by IL-18 and provide direct evidence for the IL-18/OPN axis as an immunologic pathway of cardiac interstitial fibrosis.
Notably, IL-18 is a proinflammatory cytokine that has been shown to be intimately involved in the cardiac hypertrophy process (6, 7, 9). IL-18 activates the transcriptional factors AP-1 and NF-κB in T lymphocytes, which have been implicated in the upregulation of the transcription of the OPN gene (25, 32). Moreover, the regulatory role of IL-18 on extracellular matrix turnover could be the underlying mechanism for diastolic dysfunction and progressive heart failure since its serum levels have been shown to be significantly elevated in heart failure patients (20, 28, 44). Yamaoka-Tojo et al. (41) reported that there is an imbalance in the Th1-to-Th2 ratio in congestive heart failure and that IL-18 serum levels have a predictive value independent of β-blocker, angiotensin-converting enzyme (ACE) inhibitor, diuretic, and digitalis heart failure therapeutics. Furthermore, a cluster of secretory factors that are associated with cardiac remodeling, namely, aldosterone, endothelin-1, and angiotensin II, have been shown to induce IL-18 in cultured cardiomyocytes (11).
Therefore, to establish the concept that the induction of IL-18 facilitates cardiac OPN-mediated fibrosis, we employed pressure and volume overload models and found a significant correlation between IL-18 and OPN. The interstitial collagen accumulation is significantly increased at 1 mo in both pressure- and volume-overloaded hearts relative to sham controls. With the administration of rIL-18, we identified cardiomyocyte hypertrophy and cardiac dysfunction (data not shown), which is consistent with the report from Woldbaek et al. (39). Administration of rIL-18 also enhanced cardiac OPN expression with interstitial collagen accumulation. Also, we found the rIL-18 treatment of primary cardiac fibroblasts did not induce transforming growth factor-β1 (data not shown). However, since OPN expression can be stimulated by various cytokines and growth factors including IL-1β, TNF-α, and PDGF as reviewed by Denhardt et al. (10), we used a specific agonist for TLR9, CpG 1668, to induce Th1 lymphocyte responses. We confirm, as has been previously demonstrated in other studies (17), that in vivo treatment with CpG 1668 significantly increased Th1 cytokines including IL-12 and interferon-γ in splenic T lymphocytes (data not shown). In the left ventricular tissues of CpG 1668-treated mice, the expression of IL-18, IRF1, and OPN was significantly upregulated. Cardiac collagen content quantified by hydroxylproline and picrosirius red staining showed that CpG 1668 treatment increased collagen synthesis and fibrosis. Cardiac function, evaluated by both pressure-volume loop and transthoracic echo analysis, showed diastolic dysfunction. Since cardiac fibroblasts have been identified as the major source of extracellular matrix proteins and regulatory factors (4), we stimulated cardiac fibroblasts with CpG 1668-treated lymphocyte conditioned medium and found an overexpression of OPN that was inhibited with anti-IL-18 receptor neutralizing antibody. Consistent with others (37), our data indicate that Th1 type stimulation through the T lymphocyte alters the cardiac regulatory microenvironment including cytokines, growth factors, and remodeling enzymes, thereby affecting the cardiac extracellular matrix structure and modifying cardiac function.
IRF1 was first reported as a regulatory factor of interferons. Activation of IRF1 also increases IL-18 expression, while IRF1-knockout mice lose the ability to induce IL-18 (12). Given that induced pressure overload of IL-18−/− mice results in a maladaptive remodeling response (9), we investigated the effect of IRF1 mutation in response to pressure overload. With the TAC model, the IRF1-mutated mice were hemodynamically no different from the WT control mice and the concentration of left ventricular fibrillar collagen was significantly less than in the WT TAC mice. The proposed mechanism is that the IRF1 mutation suppressed both IL-18 and OPN expression. This is supported in isolated IRF1-mutated cardiac fibroblasts, where IL-18 and OPN expression were significantly reduced with challenge of rIL-18 compared with WT cardiac fibroblasts and inhibition of the IRF1 gene by siRNA showed downregulation of IRF1, IL-18, as well as OPN. Collectively, these data further demonstrate that IL-18 is a potent OPN inducer via IRF1. Moreover, reduction of OPN and fibrosis can be achieved by manipulating the transcription factor IRF1 for IL-18, thereby reducing stress-induced cardiac dysfunction.
In summary, the studies reported here conclusively demonstrate a regulatory role of the immune system, mediated via IL-18, in producing adverse remodeling of the cardiac extracellular matrix. The primary implication of our work, in the context of these studies, suggests that the IL-18/OPN axis may be a determinant in the pathophysiology of left ventricular diastolic dysfunction. Secondary findings that were outside the focus of this study include an increased expression of lysyl oxidase by IL-18, induction of hypertension with CpG 1668, and mutation of IRF-1 preserving cardiac function in the TAC model. In summary, the study contained here demonstrates the role of the immune system, namely IL-18, in cardiac extracellular matrix remodeling.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-079206-01, the Steinbronn Heart Failure Research Award, and the Sandra Katz and Diane Stephenson Hypertension Research Award to D. F. Larson.
REFERENCES
- 1.Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan TL, Hsueh WA. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest 98: 2218–2227, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287: 860–864, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Azouz A, Razzaque MS, El Hallak M, Taguchi T. Immunoinflammatory responses and fibrogenesis. Med Electron Microsc 37: 141–148, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Booz GW, Baker KM. Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res 30: 537–543, 1995. [PubMed] [Google Scholar]
- 5.Brower GL, Janicki JS. Contribution of ventricular remodeling to pathogenesis of heart failure in rats. Am J Physiol Heart Circ Physiol 280: H674–H683, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar B, Mummidi S, Claycomb WC, Mestril R, Nemer M. Interleukin-18 is a pro-hypertrophic cytokine that acts through a phosphatidylinositol 3-kinase-phosphoinositide-dependent kinase-1-Akt-GATA4 signaling pathway in cardiomyocytes. J Biol Chem 280: 4553–4567, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar B, Valente AJ, Freeman GL, Mahimainathan L, Mummidi S. Interleukin-18 induces human cardiac endothelial cell death via a novel signaling pathway involving NF-kappaB-dependent PTEN activation. Biochem Biophys Res Commun 339: 956–963, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol 43: 1698–1705, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Colston JT, Boylston WH, Feldman MD, Jenkinson CP, de la Rosa SD, Barton A, Trevino RJ, Freeman GL, Chandrasekar B. Interleukin-18 knockout mice display maladaptive cardiac hypertrophy in response to pressure overload. Biochem Biophys Res Commun 354: 552–558, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107: 1055–1061, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi T, Sakoda T, Akagami T, Naka T, Mori Y, Tsujino T, Masuyama T, Ohyanagi M. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H1279–H1287, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Fantuzzi G, Reed D, Qi M, Scully S, Dinarello CA, Senaldi G. Role of interferon regulatory factor-1 in the regulation of IL-18 production and activity. Eur J Immunol 31: 369–375, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res 24: 430–432, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Graf K, Do YS, Ashizawa N, Meehan WP, Giachelli CM, Marboe CC, Fleck E, Hsueh WA. Myocardial osteopontin expression is associated with left ventricular hypertrophy. Circulation 96: 3063–3071, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Gould MP, DeVecchio J, Canaday DH, Auletta JJ, Heinzel FP. CpG-induced IFNgamma expands TLR4-specific IL-18 responses in vivo. Cell Immunol 243: 75–82, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol 285: H1261–H1269, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA 93: 2879–2883, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res 102: 319–327, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Liu YY, Cai WF, Yang HZ, Cui B, Chen ZR, Liu HZ, Yan J, Jin W, Yan HM, Xin BM, Yuan B, Hua F, Hu ZW. Bacillus Calmette-Guerin and TLR4 agonist prevent cardiovascular hypertrophy and fibrosis by regulating immune microenvironment. J Immunol 180: 7349–7357, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Mallat Z, Heymes C, Corbaz A, Logeart D, Alouani S, Cohen-Solal A, Seidler T, Hasenfuss G, Chvatchko Y, Shah AM, Tedgui A. Evidence for altered interleukin 18 (IL)-18 pathway in human heart failure. FASEB J 18: 1752–1754, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 43: 1195–1201, 2004. [DOI] [PubMed] [Google Scholar]
- 22.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol 34: 255–262, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med 205: 43–51, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller-Brunotte R, Kahan T, Lopez B, Edner M, Gonzalez A, Diez J, Malmqvist K. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J Hypertens 25: 1958–1966, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa D, Stone JF, Takata Y, Blaschke F, Chu VH, Towler DA, Law RE, Hsueh WA, Bruemmer D. Liver x receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ Res 96: e59–e67, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto H Osteopontin and cardiovascular system. Mol Cell Biochem 300: 1–7, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA 105: 8067–8072, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, van Veldhuisen DJ, Tavazzi L, Mann DL, Capiaumont-Vin J, Li M, Hanriot D, Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail 14: 467–474, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol 172: 2739–2743, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin SF, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood 106: 946–955, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Satoh M, Nakamura M, Akatsu T, Shimoda Y, Segawa I, Hiramori K. Myocardial osteopontin expression is associated with collagen fibrillogenesis in human dilated cardiomyopathy. Eur J Heart Fail 7: 755–762, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci USA 102: 17101–17106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol 7: 498–506, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108: 2905–2910, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Stawowy P, Blaschke F, Pfautsch P, Goetze S, Lippek F, Wollert-Wulf B, Fleck E, Graf K. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur J Heart Fail 4: 139–146, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Tamura A, Shingai M, Aso N, Hazuku T, Nasu M. Osteopontin is released from the heart into the coronary circulation in patients with a previous anterior wall myocardial infarction. Circ J 67: 742–744, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res 102: 257–264, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Markel TA, Meldrum DR. Interleukin 18 in the heart. Shock 30: 3–10, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Woldbaek PR, Sande JB, Stromme TA, Lunde PK, Djurovic S, Lyberg T, Christensen G, Tonnessen T. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol 289: H708–H714, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension 44: 826–831, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Yamaoka-Tojo M, Tojo T, Inomata T, Machida Y, Osada K, Izumi T. Circulating levels of interleukin 18 reflect etiologies of heart failure: Th1/Th2 cytokine imbalance exaggerates the pathophysiology of advanced heart failure. J Card Fail 8: 21–27, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Yang B, Larson DF, Beischel J, Kelley R, Shi J, Watson RR. Validation of conductance catheter system for quantification of murine pressure-volume loops. J Invest Surg 14: 341–355, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol Heart Circ Physiol 277: H1906–H1913, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Yndestad A, Holm AM, Muller F, Simonsen S, Froland SS, Gullestad L, Aukrust P. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res 60: 141–146, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Yu Q, Watson RR, Marchalonis JJ, Larson DF. A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol 289: H643–H651, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007. [DOI] [PubMed] [Google Scholar]