Abstract
Sex hormones regulate cholangiocyte hyperplasia in bile duct-ligated (BDL) rats. We studied whether follicle-stimulating hormone (FSH) regulates cholangiocyte proliferation. FSH receptor (FSHR) and FSH expression was evaluated in liver sections, purified cholangiocytes, and cholangiocyte cultures (NRICC). In vivo, normal female and male rats were treated with FSH or immediately after BDL with antide (a gonadotropin-releasing hormone antagonist blocking FSH secretion) or a neutralizing FSH antibody for 1 wk. We evaluated 1) cholangiocyte proliferation in sections and cholangiocytes and 2) changes in secretin-stimulated cAMP (functional index of cholangiocyte growth) levels, and ERK1/2 and Elk-1 phosphorylation. NRICC were stimulated with FSH before evaluation of proliferation, cAMP/IP3 levels, and ERK1/2 and Elk-1 phosphorylation. To determine whether FSH regulates cholangiocyte proliferation by an autocrine mechanism, we evaluated the effects of 1) cholangiocyte supernatant (containing FSH) on NRICC proliferation and 2) FSH silencing in NRICC before measuring proliferation and ERK1/2 and Elk-1 phosphorylation. Cholangiocytes and NRICC express FSHR and FSH and secrete FSH. In vivo administration of FSH to normal rats increased, whereas administration of antide and anti-FSH antibody to BDL rats decreased 1) ductal mass and 2) secretin-stimulated cAMP levels, proliferation, and ERK1/2 and Elk-1 phosphorylation in cholangiocytes compared with controls. In NRICC, FSH increased cholangiocyte proliferation, cAMP levels, and ERK1/2 and Elk-1 phosphorylation. The supernatant of cholangiocytes increased NRICC proliferation, inhibited by preincubation with anti-FSH antibody. Silencing of FSH gene decreases cholangiocyte proliferation and ERK1/2 and Elk-1 phosphorylation. Modulation of cholangiocyte FSH expression may be important for the management of cholangiopathies.
Keywords: biliary epithelium, neuroendocrine hormones, MAP kinase, sex hormones
cholangiocytes are the epithelial cells lining the intrahepatic biliary epithelium (2). Constitutively, normal cholangiocytes have a low replicative activity (40), but they proliferate in a number of experimental models of cholestatis [e.g., following bile duct ligation (BDL)] (39) or they undergo apoptosis [e.g., after acute administration of carbon tetrachloride (CCl4)] (41). Cholangiocyte hyperplasia is regulated by a number of factors including gastrointestinal hormones (24, 46), cyclic adenosine 3′,5′-monophosphate (cAMP) (20), angiogenic growth factors [e.g., vascular endothelial growth factor (VEGF)] (17, 21) and sex hormones such as estrogens (8), prolactin (58), and progesterone (25). The cAMP/ERK1/2/Elk-1 signaling has been shown to play a key role in the regulation of cholangiocyte proliferation (18, 20, 24, 27, 37). For example, whereas increased cAMP levels sustain cholangiocyte growth (20, 24, 27, 37), decreased cAMP synthesis results in inhibition of cholangiocyte hyperplasia (24, 27, 37).
Follicle-stimulating hormone (FSH) is the central hormone of the mammalian reproduction (55). FSH, also called gonadotropin because it stimulates the gonads, is produced from the anterior pituitary gland of the brain (60). FSH is a large glycoprotein composed of alpha and beta subunits, similar to those of luteinizing hormone (LH), thyroid-stimulating hormone (TSH), and human chorionic gonadotropin (hCG) (1). The alpha subunits of FSH, LH, TSH, and hCG are identical, whereas the beta subunit is unique and endows each hormone with the ability to binds to its own receptor (60). FSH interacts with a receptor protein that has seven transmembrane-spanning domains; its binding is transduced into an intracellular signal via the heterotrimeric G proteins (61). Following coupling of the FSHR to Gαs adenylyl cyclase is stimulated, followed by production of cAMP and activation of PKA, which phosphorylates structural proteins, enzymes, and transcriptional activators (32, 66). Whether the FSH receptor is also linked to Gαi has not yet been completely defined. In fact, the treatment of Sertoli cells with pertussis toxin (PTX, a Gαi inhibitor) (29), an agent capable of removing the tonic inhibitory effects of Gαi, increases FSH-induced aromatase activity, suggesting that the inhibitory activity of Gαi may negatively modulate the PKA pathway of signal transduction (30).
Studies have demonstrated the role of sex hormones in the regulation of the growth of the biliary epithelium (8, 9, 25, 58). For example, in concert with growth factors, estrogens sustain cholangiocyte proliferation and reduce cholangiocyte apoptosis (8). Prolactin is expressed and secreted by cholangiocytes and regulates the growth of female cholangiocytes by activation of Ca2+/PKC-dependent signaling (58). Progesterone enhances the proliferative activity of female and male cholangiocytes via autocrine mechanisms, since cholangiocytes possess the enzymatic pathway for steroidogenesis (25). No information exists regarding the role of FSH and its receptors in the regulation of cholangiocyte functions. We tested the hypothesis that FSH regulates cholangiocyte growth by an autocrine mechanism by changes in the cAMP-dependent phosphorylation of ERK1/2 and Elk-1.
METHODS AND MATERIALS
Materials.
Reagents were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated. The monoclonal mouse antibody reacting with proliferating cellular nuclear antigen (PCNA), the goat polyclonal antibodies for the FSH receptor (FSHR), the FSH protein (FSHβ), and cytokeratin-19 (CK-19) were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The substrate for γ-glutamyl transpeptidase (γ-GT), N-(γ-l-glutamyl)-4-methoxy-2-naphthylamide was purchased from Polysciences (Warrington, PA). The RNeasy Mini Kit to purify total RNA from purified cholangiocytes was purchased from Qiagen, Valencia, CA. The RIA kits for the determination of intracellular cAMP (cAMP [125I] radioimmunoassay, Perkin Elmer Life and Analytical Sciences, Boston, MA) and d-myo-inositol 1,4,5-trisphosphate (IP3) [3H] Biotrak Assay System (TRK1000) levels in purified cholangiocytes were purchased from GE Healthcare (Piscataway, NJ).
Animals.
Female and male Fischer 344 rats (150–175 g) were purchased from Charles River (Wilmington, MA) and maintained in a temperature-controlled environment (20–22°C) with a 12:12-h light-dark cycle. Animals were fed standard rat chow ad libitum and had free access to drinking water. The experimental groups used in the studies are shown in Table 1. To evaluate the in vivo effect of FSH on cholangiocyte functions, normal female and male rats were treated by intraperitoneally implanted Alzet osmotic minipumps (Alzet, Palo Alto, CA) releasing 0.9% NaCl or FSH in 0.9% NaCl (1.0 μg/day) (51) for 1 wk. We also assessed the effect of chronic (1 wk) in vivo administration (by intraperitoneally implanted Alzet osmotic minipumps) of nonimmune serum, polyclonal neutralizing FSH antibody (300 μg/day) (47), or antide (4.8 μg/day) (57) to female or male BDL (immediately after surgery) (3) rats on cholangiocyte growth. Before each experimental procedure, animals were injected with pentobarbital sodium (50 mg/kg body wt ip) following the regulations of the panel on euthanasia of the American Veterinarian Medical Association and local authorities. All protocols were approved by the Institutional Animal Care and Use Committee of Texas A & M Health Science Center, Temple, TX.
Table 1.
Animal models
| Animals | Treatment |
|---|---|
| Normal female +0.9% NaCl | Normal rats were treated by intraperitoneal implanted Alzet osmotic minipump releasing 0.9% NaCl for 1 wk |
| Normal male +0.9% NaCl | |
| Normal female + FSH | Normal rats were treated by intraperitoneal implanted Alzet osmotic minipump releasing FSH (1 μg/day) for 1 wk |
| Normal male + FSH | |
| BDL female + nonimmune serum | Immediately after surgery, rats were treated by intraperitoneal implanted Alzet osmotic minipump releasing nonimmune serum for 1 wk |
| BDL male + nonimmune serum | |
| BDL female + Antide | Immediately after surgery, rats were treated by intraperitoneal implanted Alzet osmotic minipump releasing Antide (4.8 μg/day) for 1 wk |
| BDL male + Antide | |
| BDL female + anti-FSH antibody | Immediately after surgery, rats were treated by intraperitoneal implanted Alzet osmotic minipump releasing anti-FSH antibody (300 μg/day) for 1 wk |
| BDL male + anti-FSH antibody |
BDL, bile duct ligation; FSH, follicle stimulating hormone; NaCl, sodium chloride.
Purification of cholangiocytes and hepatocytes.
Pure cholangiocytes (by γ-GT histochemistry) (52) were isolated by immunoaffinity separation using a monoclonal antibody (IgM, kindly provided by Dr. R. Faris, Brown University, Providence, RI) that recognizes an unidentified antigen expressed by all intrahepatic cholangiocytes (33). Cell number and viability (greater than 97%) were assessed by standard Trypan blue exclusion. The in vitro experiments were performed in rat intrahepatic cholangiocyte cultures (NRICC) (4) from normal male rats; NRICC from female rats are not currently available. NRICC (which display morphological, phenotypic, and functional phenotypes of freshly isolated cholangiocytes) (4, 25) were maintained in culture as described (4). Pure hepatocytes were isolated by standard techniques (33).
Evaluation of follicle-stimulating hormone receptor expression.
The presence of FSHR was evaluated by 1) immunohistochemistry in liver sections (4–5 μm thick) from the groups of Table 1; 2) real-time PCR in total RNA (0.75 μg) from cholangiocytes, hepatocytes, or NRICC; and 3) immunofluorescence in smears of male NRICC. Liver sections were incubated overnight at 4°C with antibody for FSHR (1:50), washed in PBS, incubated for 20 min at room temperature with a secondary biotinylated antibody (Dako Cytomation LSAB Plus System-HRP, Glostrup, Denmark), then with Dako ABC for 20 min and developed with 3–3′ diaminobenzidine (Dako Cytomation Liquid DAB Plus Substrate Chromogen System). For all immunoreactions, negative controls were included. Immunohistochemical observations were taken by BX-51 light microscopy (Olympus, Tokyo, Japan) with a Videocam (Spot Insight; Diagnostic Instrument, Sterling Heights, MI) and processed with an Image Analysis System (IAS; Delta Sistemi, Rome, Italy). For immunofluorescence, NRICC were seeded on coverslips in a six-well plate (500,000 cells/well) and allowed to adhere overnight. Immunofluorescence was performed as described (15) by using a primary antibody for goat anti-FSHR (1:50) diluted in 1% BSA (in PBS-Tween 20). Images were visualized by use of an Olympus IX-71 confocal microscope. To evaluate the message expression of FSHR in purified cholangiocytes, hepatocytes, or male NRICC, we used the RT2 Real-Time assay from SABiosciences (Frederick, MD) as described by our laboratory (19). A ΔΔCT (delta delta of the threshold cycle) analysis was performed (19) using normal cholangiocytes as the control sample. The primer for FSHR (purchased from SABiosciences) was designed according to the NCBI GenBank Accession number NM 199237 (62). Data were expressed relative mRNA levels ± SE of FSHR to GAPDH ratio.
Evaluation of liver histology, cholangiocyte apoptosis, and proliferation.
We measured liver morphology, necrosis, and portal inflammation (58) in hematoxylin and eosin-stained paraffin-embedded liver sections (4–5 μm thick, 3 sections evaluated per group of animals) from the animals of Table 1. At least 10 different portal areas were evaluated for each parameter. Liver sections were examined in a coded fashion by BX-51 light microscopy (Olympus, Tokyo, Japan) equipped with a camera.
We evaluated cholangiocyte apoptosis by semiquantitative terminal deoxynucleotidyltransferase biotin-dUTP nick-end labeling (TUNEL) (37) kit (Apoptag; Chemicon International, Billerica, MA) in paraffin-embedded liver sections (4–5 μm thick, 3 sections evaluated per group of animals). Cholangiocyte proliferation was measured by 1) evaluating intrahepatic bile duct mass (BDM) and the number of PCNA-positive cholangiocytes in liver sections (4–5 μm thick, 3 sections evaluated per group of animals) and 2) immunoblots for PCNA protein expression in protein (10 μg) from whole cell lysate from cholangiocytes from the selected group of animals. Blots were normalized to β-actin (6). The intensity of the bands was determined by scanning video densitometry using the phospho-imager, Storm 860 (GE Healthcare, Piscataway, NJ) and the ImageQuant TL software version 2003.02 (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Measurement of cholangiocyte cAMP and IP3 levels and phosphorylation of ERK1/2 and Elk-1 in purified male cholangiocytes.
We evaluated basal and secretin-stimulated cAMP levels, a functional marker of cholangiocyte proliferation (26, 27, 40), and basal levels of IP3, a second messenger regulating cholangiocyte proliferation (19, 44), in purified male cholangiocytes from the groups of Table 1. The rationale for using only male cholangiocytes and NRICC (see below) in our studies is based on the facts that 1) the expression of FSHR and FSH is similar between female and male cholangiocytes; 2) the in vivo administration of FSH to normal rats and antide and anti-FSH antibody to BDL rats has similar in vivo effects on cholangiocyte apoptosis and proliferation in female and male animals (see results); and 3) female NRICC are not currently available.
Following incubation for 1 h at 37°C (7, 18, 20, 24, 40–42), cholangiocytes (1 × 105 cells) were stimulated at room temperature for 5 min (7, 20, 24, 40, 41) with 0.2% BSA (basal), or secretin (100 nmol/l in 0.2% BSA). Intracellular cAMP and IP3 levels were measured with commercially available kits (7, 20, 24, 40, 41). In cholangiocytes, we also measured protein expression of the phosphorylated form (expressed as ratio to total protein expression) of ERK1/2 and Elk-1 by immunoblots (19, 24). The intensity of the bands was determined by scanning video densitometry using the phospho-imager, storm 860 and the Image Quant TL software version 2003.02.
Effect of FSH on cAMP and IP3 levels, proliferation, and phosphorylation of ERK1/2 and Elk-1 in NRICC.
Following incubation for 1 h at 37°C (7, 19, 24, 27, 40, 41), NRICC were stimulated at room temperature with 1) 0.2% BSA (basal) or FSH (100 ng/ml with 0.2% BSA for 10 min) in the absence or presence of preincubation with PTX (a Gαi inhibitor, 1 μg/ml for 10 min) (29) before evaluation of cAMP (7, 19, 24, 27, 40, 41), or IP3 levels (19, 44) (see above).
After trypsinization, NRICC were seeded into 96-well plates (10,000 per well) in a final volume of 200 μl of growth medium and allowed to adhere to the plate overnight. Cells were stimulated with FSH (0.1 to 100 ng/ml) at 37°C for 24 h before evaluation of cell growth by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) proliferation assay (an index of changes in cell number) (19, 21). In separate experiments, NRICC were incubated with 0.2% BSA or FSH (100 ng/ml with 0.2% BSA) at 37°C for 24 h in the absence or presence of preincubation (1 h) with H-89 (a PKA inhibitor, 10 μM) (27), PD98059 (a MEK inhibitor, 10 nM) (20), or PTX (1 μg/ml) (29) before evaluation of cell growth by MTS assay. Absorbance was measured at 490 nm by a microplate spectrophotometer (Versamax, Molecular Devices, Sunnyvale, CA). Data was expressed as the fold change of treated cells compared with BSA-treated NRICC.
After trypsinization, NRICC were incubated at 37°C with 0.2% BSA or FSH (100 ng/ml with 0.2% BSA) for 30 and 60 min, 3 and 6 h before evaluation of proliferation by immunoblots for PCNA (normalized to β-actin) (23). In other experiments, NRICC were incubated at 37°C with 0.2% BSA or FSH (100 ng/ml with 0.2% BSA) for 6 h in the absence or presence of preincubation (1 h) with H-89 (10 μM), PD98059 (10 nM), or PTX (1 μg/ml) before measurement by immunoblots (20, 24) of protein expression for PCNA and phosphorylation of ERK1/2 and Elk-1.
Evaluation of expression and secretion of FSH in cholangiocytes.
We evaluated the expression of FSH by 1) immunohistochemistry (antibody dilution 1:50) in paraffin-embedded liver sections (4–5 μm thick, 3 slides were evaluated per group of animal); 2) real-time PCR in total RNA (0.75 μg) from cholangiocytes, hepatocytes, and male NRICC. The primer for FSHβ (purchased from SABiosciences) was designed according to the NCBI GenBank Accession number NM 001007597 (31); and 3) immunofluorescence (antibody dilution 1:50) in NRICC smears. FSH secretion was measured in short-term cultures (6 h at 37°C) of male NRICC, purified cholangiocytes from the groups of Table 1, and male hepatocytes by commercially available EIA kits (ALPCO Diagnostics, Salem, NH). To determine whether FSH (secreted by cholangiocytes into supernatant) stimulates biliary proliferation, we treated NRICC for 24 h at 37°C with the supernatant of normal and BDL female and male cholangiocytes (obtained after 6 h of incubation at 37°C) in the absence or presence of anti-FSH antibody (150 pg/ml of supernatant) before measuring cell growth by MTS assays (19).
Genetic knockdown of FSH in NRICC.
The role of FSH in the regulation of proliferation of NRICC was evaluated in subclones that have reduced expression of FSH. These cell lines were established by a method described previously (14) by using SureSilencing shRNA (short hairpin RNA) plasmids (SABiosciences) for rat FSH that also confers resistance to puromycin for the selection of stable transfected cells. Transfected cells were selected by the addition of 5 μg/ml puromycin into the media, and the selection process was allowed to continue for 3–4 days. Surviving cells (designated NRICC-FSH) were then assessed for the relative expression of FSH compared with the mock-transfected control cell line (NRICC-puro) by real-time PCR, and the clone with the greatest degree of knockdown was selected for subsequent experiments. The role of FSH in cell growth and apoptosis and cAMP-dependent signaling was determined in these cell lines by immunoblots for PCNA and Bax, a proapoptotic protein (50), and phosphorylated and total ERK1/2 and Elk-1 in protein (10 μg) from whole cholangiocyte lysate.
Statistical analysis.
All data are expressed as means ± SE. Differences between groups were analyzed by Student's unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
RESULTS
Cholangiocytes express the FSH receptor.
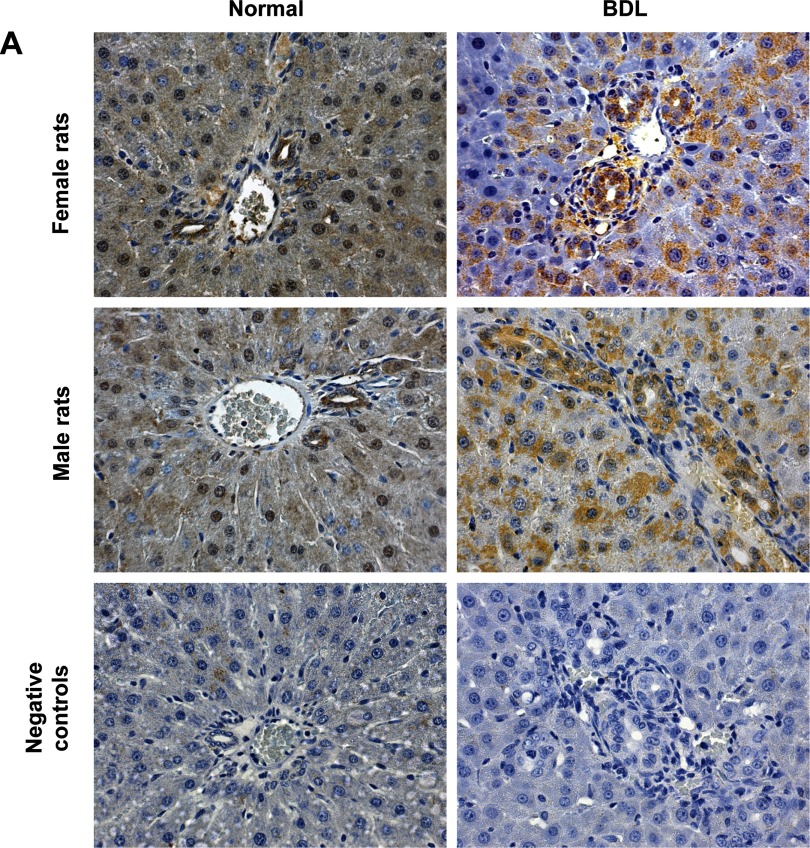
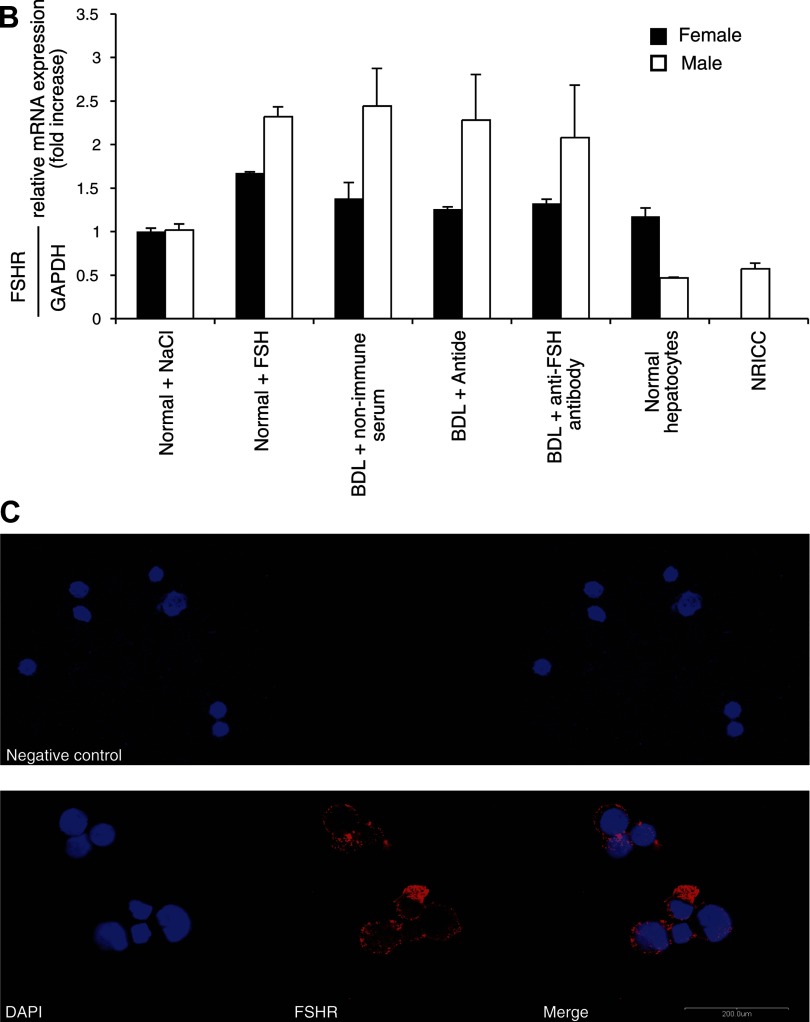
Immunohistochemistry in liver sections and real-time PCR in RNA from purified cholangiocytes and hepatocytes show that these two cell types express the protein (Fig. 1A and Table 2) and the message (Fig. 1B) for FSHR. Similarly, by immunofluorescence, NRICC express FSHR (Fig. 1C). The protein expression of FSHR 1) increased in cholangiocytes from normal female and male rats treated with FSH compared with normal cholangiocytes from animals treated with NaCl (Fig. 1, A and B and Table 2); and 2) decreased in cholangiocytes from female and male BDL rats treated with antide or anti-FSH antibody compared with BDL rats treated with nonimmune serum (Table 2).
Fig. 1.
A: representative immunohistochemistry for follicle-stimulating hormone receptor (FSHR) in liver sections (4–5 μm thick) from normal and bile duct-ligated (BDL) female and male rats. Intrahepatic bile ducts and hepatocytes express FSHR. For semiquantitative expression of FSHR see Table 2. Original magnification ×20. B: real-time PCR in purified cholangiocytes from 1) normal female and male rats treated with NaCl or FSH and BDL rats that immediately after surgery were treated with nonimmune serum, antide, or anti-FSH antibody for 1 wk; 2) normal female and male hepatocytes; and 3) NRICC. Data are means ± SE of 3 experiments. Cholangiocytes, hepatocytes, and NRICC express FSHR mRNA. C: immunofluorescence demonstrated that NRICC express FSHR. Specific immunoreactivity of 2 representative fields is shown in red and nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; blue). No staining was visible when primary antibodies were replaced with nonimmune serum, as shown in the negative control. Bar = 200 μm.
Table 2.
Measurement of BDM and the number of cholangiocytes positive for FSHR, PCNA, TUNEL, and FSH from all the selected experimental groups
| Parameters | Female |
Male | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal +0.9% NaCl | Normal + FSH | BDL + nonimmune serum | BDL +Antide | BDL +anti-FSH antibody | Normal +0.9% NaCl | Normal + FSH | BDL + nonimmune serum | BDL +Antide | BDL +anti- FSH antibody | |
| FSHR-positive cholangiocytes | 3.05±0.38 | 4.65±0.30* | 32.27±1.66* | 4.33±0.54† | 5.58±0.57† | 3.87±0.28 | 4.35±0.26 | 23.37±2.15* | 4.49±0.34† | 6.16±0.57† |
| BDM | 0.22±0.04 | 0.44±0.1* | 4.95±1.0* | 2.51±0.4† | 1.63±0.4† | 0.20±0.06 | 0.46±0.06* | 4.79±0.9* | 3.38±0.6† | 2.39±0.3† |
| PCNA-positive cholangiocytes | 7.83±3.2 | 35.8±8.3* | 46.13±5.7* | 31.60±7.7† | 25.80±6.3† | 6.17±2.6 | 34.00±7.9* | 45.50±5.9* | 30.00±11.1† | 26.60±9.8† |
| Apoptotic cholangiocytes | ND | ND | 4.02±2.53 | 37.50±7.58† | 43.50±4.8† | ND | ND | 5.50±1.94 | 39.33±8.24† | 45.33±6.4† |
| FSH-positive cholangiocytes | 6.70±0.46 | 8.24±0.39* | 42.57±2.54* | 10.53±1.25† | 21.43±1.91† | 6.13±0.39 | 7.50±0.31* | 39.98±1.94* | 9.19±0.9† | 20.65±1.46† |
BDM, bile duct mass; FSHR, FSH receptor; TUNEL, terminal deoxynucleotidyltransferase biotin-dUTP nick-end labeling. Values are means ± SE of cumulative values obtained from the evaluation of 10 portal tracts from 5 different slides for each group. ND, not detected.
P < 0.05 vs. corresponding values of normal rats treated with NaCl for 1 wk.
P < 0.05 vs. corresponding values of BDL rats treated with nonimmune serum for 1 wk.
Measurement of liver histology, portal inflammation, cholangiocyte apoptosis, and proliferation.
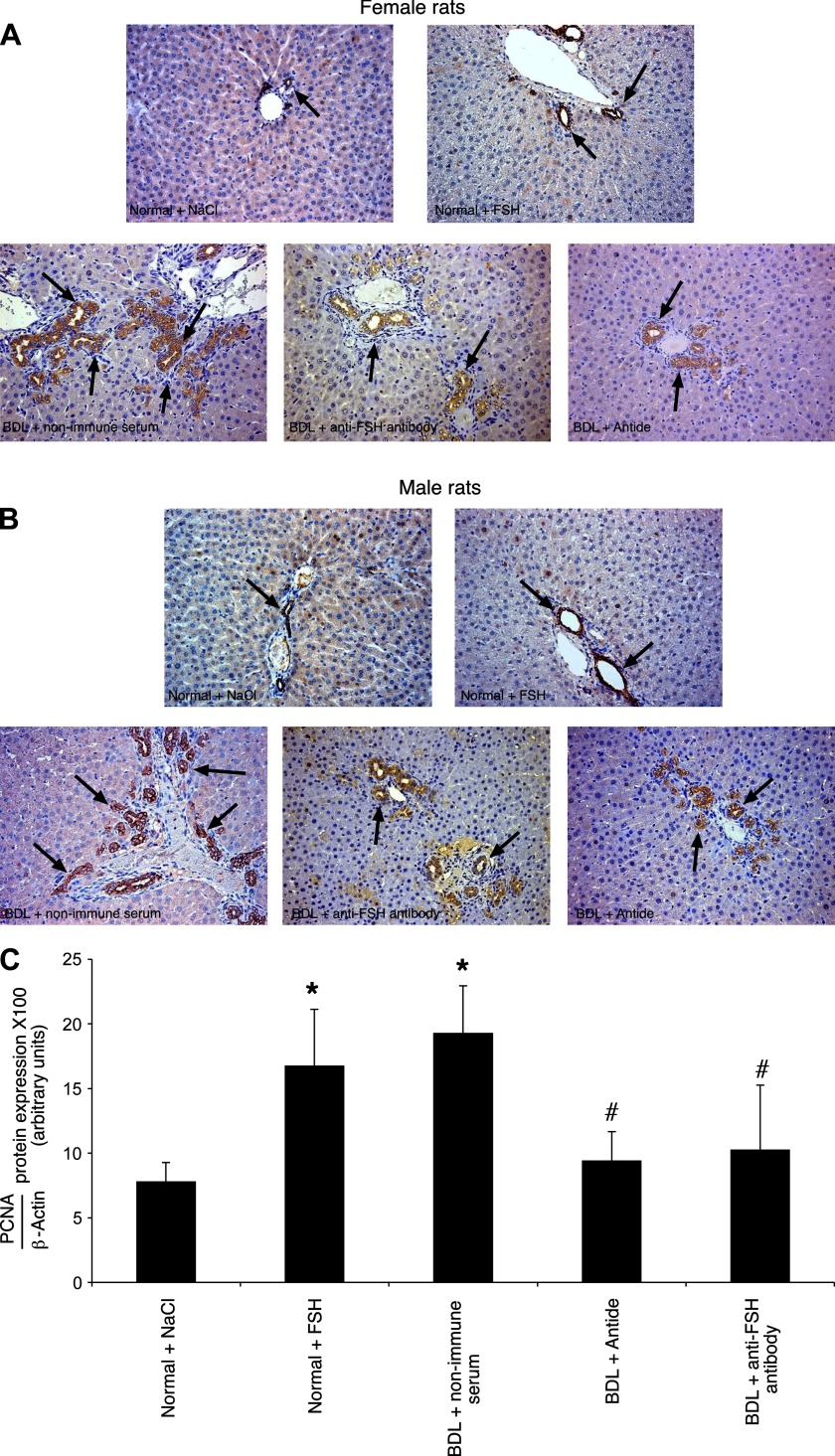
Hematoxylin and eosin staining of liver sections shows that there were no significant differences in the degree of necrosis, lobular damage, and portal inflammation among the groups of Table 1 (not shown). Administration of FSH to normal female and male rats increased BDM and the number of PCNA-positive cholangiocytes compared with their corresponding NaCl-treated rats (Fig. 2, A and B, and Table 2). The administration of antide or anti-FSH antibody to female or male BDL rats decreased BDM and the number of PCNA-positive cholangiocytes compared with their corresponding BDL rats treated with nonimmune serum (Fig. 2, A and B and Table 2). The decrease in BDL cholangiocyte proliferation (by antide or anti-FSH antibody) was associated with an increase in the number of TUNEL-positive cholangiocytes (Table 2). By immunoblots, PCNA protein expression increased in cholangiocytes from normal male rats treated with FSH compared with controls (Fig. 2C), whereas administration of antide and anti-FSH antibody to male BDL rats decreased PCNA protein expression compared with BDL rats treated with nonimmune serum (Fig. 2C).
Fig. 2.
A–B: representative immunohistochemistry for CK-19 in liver sections from the experimental groups of Table 1. Administration of FSH to normal female (A) and male (B) rats enhanced cholangiocyte proliferation as shown by the increased BDM compared with the corresponding value of NaCl-treated rats. The administration of anti-FSH antibody or antide to female (A) and male (B) BDL rats decreased BDM compared with the corresponding value of BDL rats treated with nonimmune serum for 1 wk. Original magnification ×20. Data are means ± SE of 10 cumulative values obtained from the 3 slides evaluated per each group of animals. C: by immunoblots we demonstrated that protein expression for PCNA increased in cholangiocytes from normal male rats treated with FSH compared with normal rats treated with NaCl, whereas the administration of anti-FSH antibody or antide to male BDL rats decreased PCNA protein expression compared with BDL rats treated with nonimmune serum. Data are means ± SE of 12 blots. *P < 0.05 vs. cholangiocytes from normal rats treated with NaCl for 1 wk. #P < 0.05 vs. cholangiocytes from BDL rats treated with nonimmune serum for 1 wk.
Measurement of cAMP and IP3 levels and phosphorylation of ERK1/2 and Elk-1 in purified male cholangiocytes.
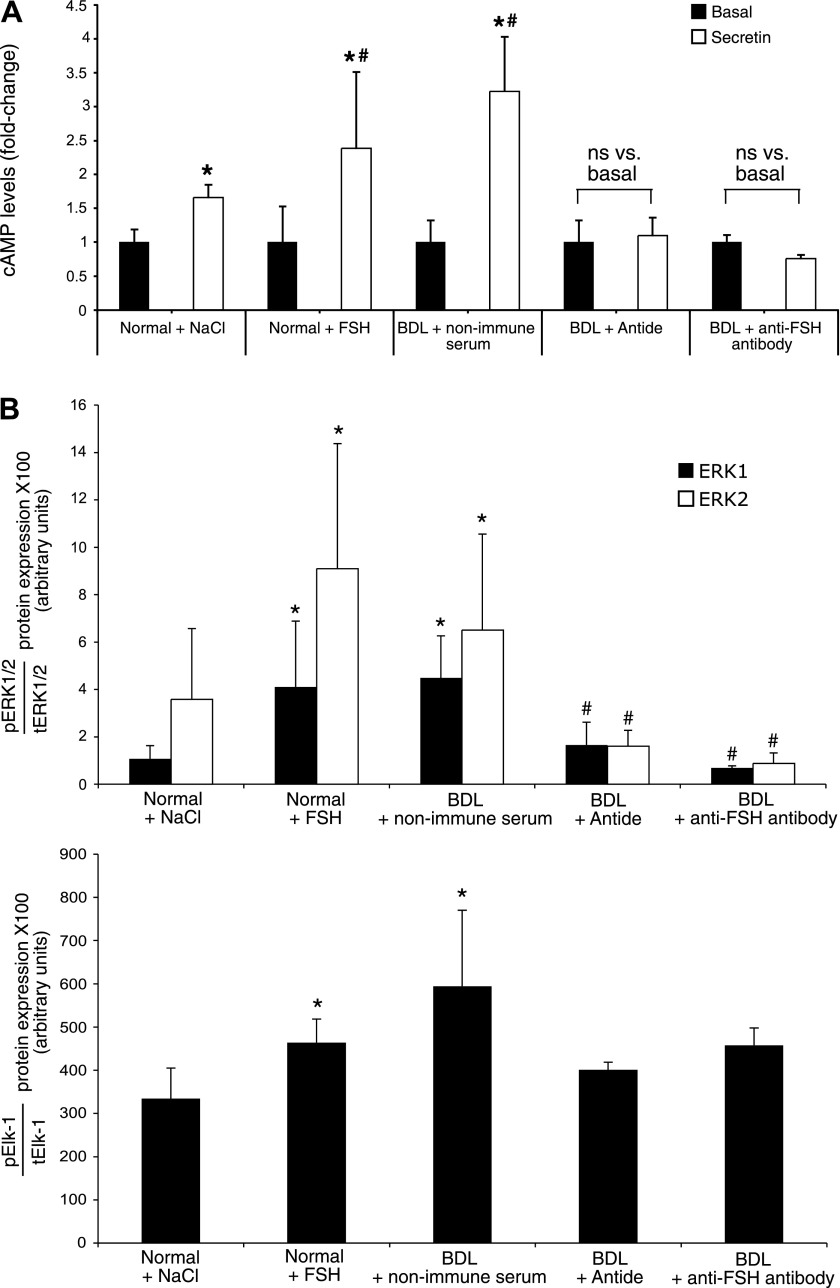
In normal male rats treated with FSH for 1 wk, secretin-stimulated cAMP levels of purified cholangiocytes were significantly greater than secretin-induced cAMP levels of cholangiocytes purified from normal rats treated with NaCl (Fig. 3A). In agreement with previous studies (24, 38), secretin significantly increased cAMP levels of cholangiocytes from BDL rats treated with nonimmune serum compared with its corresponding basal values (Fig. 3A). Parallel with decreased biliary proliferation, the administration of antide and anti-FSH antibody to BDL rats blocked secretin-stimulated cAMP levels of purified cholangiocytes (Fig. 3A). Consistent with the notion that FSH effects on cholangiocyte functions are mediated by cAMP signaling, basal IP3 levels were unchanged (not shown) among cholangiocytes purified from the experimental groups of Table 1.
Fig. 3.
A: measurement of basal and secretin-stimulated cAMP levels in cholangiocytes from 1) normal male rats treated with NaCl or FSH for 1 wk; and 2) BDL rats treated with nonimmune serum, antide, or anti-FSH antibody for 1 wk. In normal rats treated with FSH, secretin-stimulated cAMP levels of purified cholangiocytes were significantly greater than secretin-induced cAMP levels of cholangiocytes from normal rats treated with NaCl. Secretin significantly increased cAMP levels of cholangiocytes from BDL rats treated with nonimmune serum compared with its corresponding basal values. The administration of antide and anti-FSH antibody to BDL rats blocked secretin-stimulated cAMP levels of purified cholangiocytes compared with cholangiocytes from BDL rats treated with nonimmune serum. Data are means ± SE of 15 values. *P < 0.05 vs. the corresponding basal values. #P < 0.05 vs. secretin-stimulated cAMP levels of cholangiocytes from normal rats treated with NaCl for 1 wk. ns, not significant. B: evaluation of phosphorylation of ERK1/2 and Elk-1 in purified cholangiocytes. The administration of FSH to normal male rats increased the phosphorylation of ERK1/2 and Elk-1 compared with cholangiocytes from normal male rats treated with NaCl. When BDL male rats were treated with antide or anti-FSH antibody, there was a decrease in the phosphorylation of ERK1/2 and Elk-1 in cholangiocytes compared with cholangiocytes from BDL rats treated with nonimmune serum. Data are means ± SE of 6 blots. *P < 0.05 vs. the phosphorylation of ERK1/2 and Elk-1 of cholangiocytes from normal rats treated with NaCl for 1 wk. #P < 0.05 vs. the phosphorylation of ERK1/2 of cholangiocytes from BDL rats treated with nonimmune serum for 1 wk.
The administration of FSH to normal male rats increased the phosphorylation of ERK1/2 and Elk-1 compared with cholangiocytes from rats treated with NaCl (Fig. 3B). When BDL rats were treated with antide or anti-FSH antibody, the phosphorylation of ERK1/2 and Elk-1 decreased compared with normal cholangiocytes treated in vivo with FSH or to cholangiocytes from BDL rats treated with nonimmune serum (Fig. 3B).
Intracellular mechanisms of FSH regulation of cholangiocyte proliferation.
FSH increased intracellular cAMP (but not IP3) levels of NRICC compared with NRICC treated with BSA (basal) and with PTX (Fig. 4A). FSH-induced increase in cAMP levels was not prevented by preincubation with PTX (Fig. 4A); PTX alone did not change NRICC cAMP levels (Fig. 4A). FSH significantly increased the growth of NRICC in a time- and dose-dependent manner as shown by MTS assays (Fig. 4B) and PCNA expression (Fig. 4C), which was blocked by preincubation with H89 and PD98059 but not PTX (Fig. 4, B and C). FSH also increased the phosphorylation of ERK1/2 and Elk-1, which was ablated by preincubation with H89 and PD98059 (Fig. 4D).
Fig. 4.
A: measurement of cAMP [in the absence or presence of preincubation with pertussis toxin (PTX), 10 min at 1 μg/ml] or d-myo-inositol 1,4,5-trisphosphate (IP3) levels in NRICC stimulated with 0.2% BSA (basal) or FSH with 0.2% BSA for 10 min. FSH increased intracellular cAMP (but not IP3) levels of NRICC compared with NRICC treated with BSA. FSH-induced increase in cAMP levels was not prevented by preincubation with PTX. Data are means ± SE of 8 evaluations. *P < 0.05 vs. its corresponding basal value. B: evaluation of cholangiocyte proliferation by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay in NRICC treated with 0.2% BSA (basal) or FSH (0.1–100 ng/ml with 0.2% BSA) for 24 h. FSH significantly increased the growth of NRICC in a dose-dependent manner. FSH stimulation of NRICC proliferation (at 100 ng/ml of FSH) was blocked by preincubation with H89 (10 μM) and PD98059 (10 nM) but not PTX (1 μg/ml). Data are means ± SE of 7 experiments. *P < 0.05 vs. its corresponding basal value. C: evaluation of cholangiocyte proliferation (by immunoblotting analysis for PCNA) in NRICC stimulated with 0.2% BSA (basal) or FSH (100 ng/ml for 30 and 60 min and 3 and 6 h at 37°C) in the absence or presence of H89 (10 μM), PD98059 (10 nM), or PTX (1 μg/ml). FSH stimulation of NRICC proliferation was blocked by preincubation with H89 and PD98059 but not PTX. Data are means ± SE of 8 blots. *P < 0.05 vs. its corresponding basal value. D: measurement of ERK1/2 and Elk-1 phosphorylation in NRICC stimulated with 0.2% BSA (basal) or FSH (100 ng/ml) for 6 h (in the absence or presence of H89 and PD98059). FSH-induced increase in the phosphorylation of ERK1/2 and Elk-1 was blocked by H89 (10 μM) and PD98059 (10 nM). Data are means ± SE of 6 blots. *P < 0.05 vs. corresponding basal values.
Evaluation of FSH expression by cholangiocytes: role of FSH in the autocrine regulation of cholangiocyte growth.
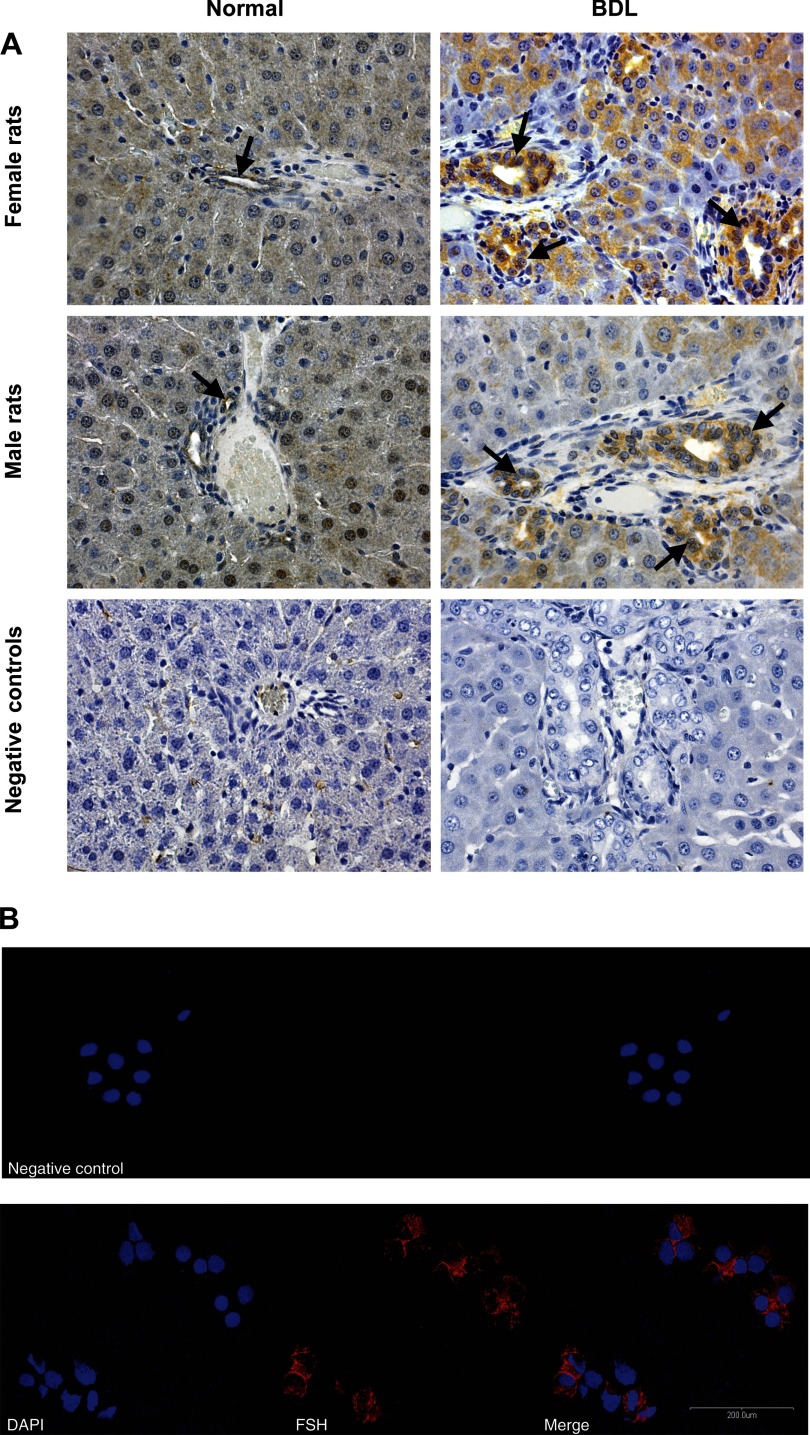
By semiquantitative immunohistochemistry in liver sections, real-time PCR in RNA cholangiocytes, and immunofluorescence in NRICC, we demonstrated that cholangiocytes express the message and protein for FSH (Figs. 5, A and B and 6, A and B); FSH mRNA was also expressed by hepatocytes (Fig. 6, A and B). FSH protein expression increased in cholangiocytes from normal female and male rats treated with FSH and from BDL female and male rats compared with normal rats (Fig. 5A and Table 2). The administration of antide or anti-FSH antibody to female and male BDL rats decreased cholangiocyte FSH expression compared with cholangiocytes from BDL rats treated with nonimmune serum (Table 2).
Fig. 5.
A: by immunohistochemistry in liver sections (4–5 μm) bile ducts from female and male normal and BDL rats express FSH. Original magnification ×20. B: by immunofluorescence, NRICC express FSH. Specific immunoreactivity is shown in red, and nuclei were stained with DAPI (blue). No staining was visible when primary antibodies were omitted, as shown in the negative control. Bar = 200 μm.
Fig. 6.
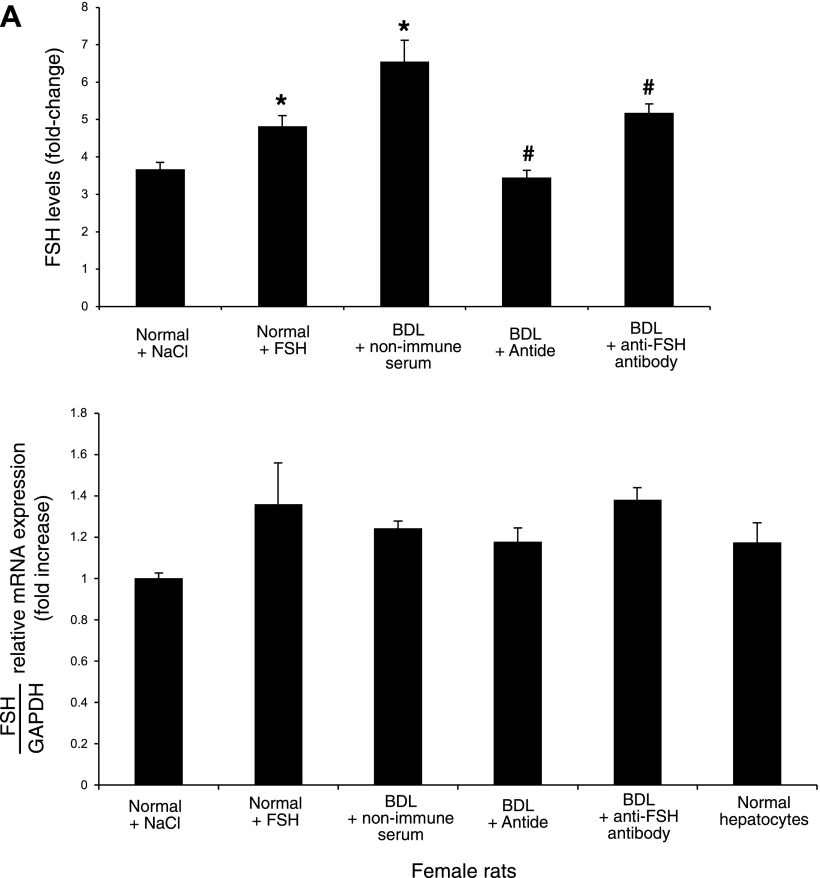
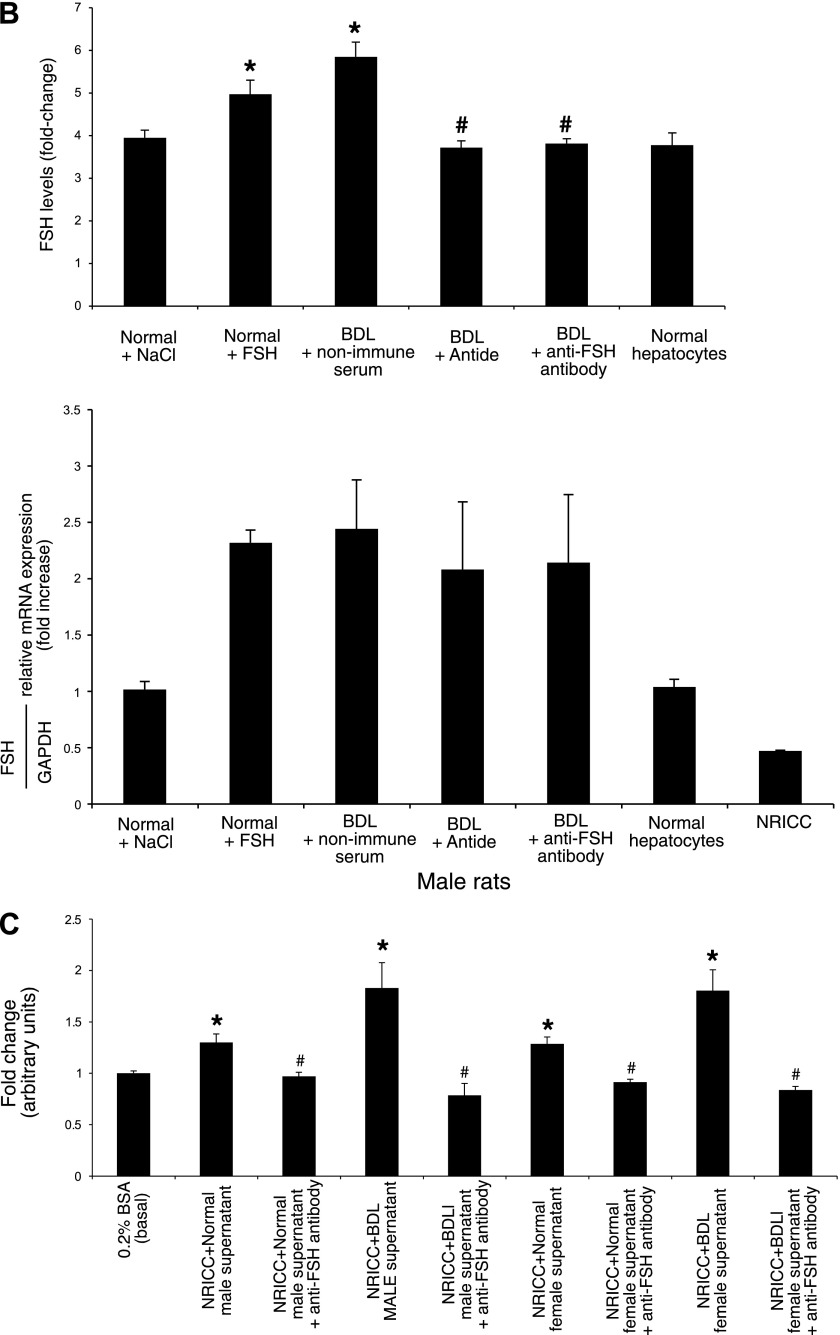
A: evaluation of FSH levels in the supernatant of short-term cultures (6 h) of cholangiocytes from normal and BDL female rats. Normal female cholangiocytes secrete FSH; FSH expression increased in cholangiocytes from normal female rats treated with FSH and BDL female rats compared with normal rats. The administration of antide or anti-FSH antibody to female BDL rats decreased cholangiocyte FSH secretion compared with cholangiocytes from BDL rats treated with nonimmune serum. In the second graph, it is represented the message for FSH expressed by female cholangiocytes and hepatocytes. Data are means ± SE of 7 evaluations. *P < 0.05 vs. FSH levels of female cholangiocytes from normal rats treated with NaCl. #P < 0.05 vs. FSH levels of female cholangiocytes from BDL rats treated with nonimmune serum. For real-time PCR, data are means ± SE of 3 experiments. B: the same measurement of FSH levels in the supernatant of short-term cultures (6 h) of cholangiocytes from normal and BDL male rats and NRICC. As noted previously, also normal male cholangiocytes secrete FSH and its expression increased in cholangiocytes from normal male rats treated with FSH and BDL male rats compared with normal rats. The administration of antide or anti-FSH antibody to male BDL rats decreased cholangiocyte FSH secretion compared with cholangiocytes from BDL rats treated with nonimmune serum. Bottom graph represents the message for FSH expressed by male cholangiocytes, hepatocytes, and NRICC. Data are means ± SE of 7 evaluations. *P < 0.05 vs. FSH levels of male cholangiocytes from normal rats treated with NaCl. #P < 0.05 vs. FSH levels of male cholangiocytes from BDL rats treated with nonimmune serum. For real-time PCR, data are means ± SE of 3 experiments. C: effect of cholangiocyte supernatant (containing FSH) on NRICC proliferation (evaluated by MTS assay after 24 h of incubation) in the absence or presence of anti-FSH antibody. When NRICC were incubated with the supernatant of BDL cholangiocytes, there was a significant increase in cholangiocyte proliferation, which was partially blocked by preincubation with neutralizing anti-FSH antibody; a similar but smaller effect was found with normal cholangiocyte supernatant (containing less FSH). Data are means ± SE of 7 experiments. *P < 0.05 vs. its corresponding basal value. #P < 0.05 vs. the corresponding value of NRICC treated with supernatant from female or male normal and BDL cholangiocytes.
We demonstrated that 1) after short-term culture (6 h), normal female and male cholangiocytes and NRICC secrete FSH (Figs. 6, A and B); and 2) FSH levels increased in the supernatant of female and male BDL cholangiocytes compared with their corresponding normal cholangiocytes (Fig. 6, A and B). FSH secretion increased in cholangiocytes from normal rats treated with FSH compared with normal cholangiocytes (Fig. 6, A and B). FSH secretion decreased in cholangiocytes from BDL rats treated with antide or anti-FSH antibody compared with cholangiocytes from BDL rats treated with nonimmune serum (Fig. 6, A and B). When the supernatant (after 6 h of incubation) of BDL female and male cholangiocytes was transferred into plates containing NRICC, we found a significant increase in cholangiocyte proliferation (by MTS), an increase that was partially blocked by preincubation with neutralizing anti-FSH antibody (Fig. 6C); a similar but smaller effect was found with normal cholangiocyte supernatant (Fig. 6C) (containing less FSH). The data support the concept that FSH modulates cholangiocyte growth by an autocrine mechanism.
To provide conclusive evidence for the key role of FSH in sustaining cholangiocyte growth, we specifically knocked down the expression of FSH to 56% of the parental cell line by stably transfecting an FSH shRNA construct (Fig. 7A). Over the time period studied, the NRICC-FSH cell line had reduced PCNA expression compared with NRICC-puro, further indicating that decreasing FSH expression reduces the proliferative capacity of the cells (Fig. 7B). Consistent with the concept that FSH is key in sustaining cholangiocyte proliferation, the NRICC-FSH cell line displayed a higher apoptotic activity compared with NRICC-puro cells as demonstrated by increased Bax protein expression (Fig. 7C). The phosphorylation of ERK1/2 and Elk-1 was reduced in the cell line knocked down compared with the control cell line (Fig. 7D). The data demonstrate the dependence of cholangiocyte proliferation, apoptosis, and signaling mechanisms on FSH biliary expression.
Fig. 7.
Specific knockdown of FSH expression slows the rate of cell proliferation. NRICC were stably transfected with FSH shRNA (short hairpin RNA) vectors. The expression of FSH was assessed in the mock-transfected cell line (NRICC-puro) and the cell lines containing the FSH shRNA (NRICC-FSH) by real-time PCR. A: data are expressed as average ± SE of 3 experiments after correction for GAPDH expression. The effect of FSH knockdown on the expression of PCNA (a marker of proliferative capacity) (B) and the apoptosis-related gene Bax (C). The silencing of FSH gene induced a decrease in the growth of NRICC and, in the other side, an increase in cellular apoptosis. Evaluation of phosphorylation and hence activation of Erk1/2 and Elk-1 (D) was assessed by immunoblotting. The ablation of this gene in cholangiocytes leads to impairment of the phosphorylation of ERK1/2 and Elk-1, two important proteins in the cAMP-dependent pathway. When appropriate, data are expressed as average ± SE of 9 experiments after correction for β-actin or total ERk1/2 or Elk-1 expression. *Statistical significance (P < 0.05) compared with the NRICC-puro cell line.
DISCUSSION
Our study demonstrated that 1) normal and BDL female and male cholangiocytes and polarized NRICC express FSH receptor, without significant differences in the expression of this receptor among the two sexes; 2) chronic in vivo administration of FSH to normal female and male rats induced an increase in cholangiocyte proliferation and secretin-stimulated cAMP levels (a functional index of cholangiocyte growth) (20, 24, 37, 40), an increase that may also be due to the enhanced expression of FSHR in the biliary epithelium following the administration of FSH; 3) cholangiocyte proliferation and secretin-stimulated cAMP levels induced by BDL (3, 24, 38) are decreased by the simultaneous administration of antide or a neutralizing anti-FSH antibody, decreases that are associated with increased cholangiocyte apoptosis and decreased FSHR expression; 4) FSH stimulation of cholangiocyte proliferation is associated with increased cAMP-dependent phosphorylation of ERK1/2 and Elk-1; 5) normal and BDL female and male cholangiocytes and NRICC transcribe and secrete FSH, which were upregulated following BDL both in female and male cholangiocytes; and 6) knockdown of FSH expression decreases cholangiocyte proliferation ERK1/2 and Elk-1 phosphorylation and induces increases in apoptosis.
In animal models of cholestasis as well as in human cholangiopathies, cholangiocytes proliferate or are damaged by apoptosis (3, 5, 9, 18, 21, 22, 24). In the BDL rat model, which is widely used for evaluating the mechanisms of cholangiocyte hyperplasia (3, 18, 21, 24, 37), there is an increase in ductal mass (3, 18, 24, 37) and secretin-stimulated cAMP levels (20, 24, 37, 40). Conversely, in rats treated with CCl4 there is damage of large, cAMP-responsive cholangiocytes and loss of secretin-stimulated cAMP levels (20, 24, 37, 40). In humans, cholangiocyte proliferation occurs in biliary obstruction, in chronic cholestatic liver diseases, and in many forms of liver injury (5, 9, 36). Cholangiopathies share common pathological features such as the damage of intrahepatic bile ducts and the proliferation of residual ducts (as a mechanism of compensatory repair to maintain biliary homeostasis), but they evolve toward ductopenia, which represents the terminal stage of the disease (5, 9, 36).
Information on the role of FSH in liver pathophysiology is limited (10, 28, 64). A study has demonstrated that liver cirrhosis is associated with endocrine dysfunction, notably in the gonadal axis (28). Patients with liver failure have low levels of LH and FSH (11). In fact, gonadotropin deficiency occurs with liver damage and in some patients with hemochromatosis (16). These findings suggest that the hypogonadism is primary in most patients with cirrhosis (10). The derangement of hypothalamic-pituitary function may play a role in the sexual dysfunction and changes in sex hormones in male patients with cirrhosis (64). No information exists regarding the role of FSH and FSHR in the regulation of the functions of the biliary epithelium.
We have shown for the first time that the biliary epithelium expresses FSHR and that FSH is a trophic factor for the biliary epithelium since chronic administration of FSH to rats increased cholangiocyte proliferation and intrahepatic ductal mass by cAMP-dependent phosphorylation of ERK1/2 and Elk-1. In support of the findings that chronic administration of FSH increases cholangiocyte FSH receptor expression, it has been demonstrated that FSH treatment induces follicular growth and ovulation, together with an increase in FSH binding and mRNA levels in ovaries (35). In addition, another study has demonstrated that 1) the inclusion of FSH in the rat granulosa cells culture medium prevented the decline in FSHR mRNA levels in a dose-dependent manner (59) and 2) treatment of these cells with FSH increases the levels of two FSH receptor mRNA transcripts (59).
A number of studies have shown that factors sustaining biliary proliferation (including acetylcholine and forskolin) increase cholangiocyte cAMP levels and cAMP-dependent signaling (20, 37). Conversely, inhibition of cholangiocyte proliferation by octreotide (46), RAMH (an H3 histamine receptor agonist) (18), interruption of cholinergic innervation by vagotomy (37), or adrenergic denervation (by 6-hydroxydopamine) (23) induces a decrease in cholangiocyte cAMP levels and cAMP-dependent signaling pathways (18, 23, 24, 37, 46). The key role of cAMP signaling in the regulation of cholangiocyte growth is further supported by the in vivo treatment of normal rats with the adenylate cyclase stimulator forskolin (20), which alone increases cholangiocyte proliferation and phosphorylation of PKA and ERK1/2 (20). Our present findings support the concept that activation of cAMP/ERK1/2/Elk-1 signaling is key in FSH stimulation of cholangiocyte proliferation, since FSH increases both in vivo and in vitro cAMP (but not IP3) levels. Parallel to our findings, studies in other cell types have shown that the effects of FSH are mediated by the activation of cAMP-dependent signaling pathways (13, 55, 56). In vitro, FSH-stimulated cAMP and proliferation were found to be PTX insensitive, supporting the coupling of FSH to Gαs. PTX catalyzes the ADP ribosylation of Gαi, causing the uncoupling of Gαi from G protein-coupled receptors (48). As expected, we found that FSH-induced cAMP levels and proliferation were PTX insensitive. These data confirm that the FSH receptor is coupled to Gαs in our studies. Although our findings differ from those of Gorczynska et al. (29) concerning the cross-talk between cAMP and Ca2+ in Sertoli cells after stimulation with FSH, we did not find alterations in IP3/Ca2+ signaling in the present studies. Thus we did not address the role of FSH in the interaction between Ca2+ and cAMP signaling, as shown in these studies in Sertoli cells (29), and our previous studies in cholangiocytes (after serotonin treatment) (45).
To better understand the role of FSH in the regulation of cholangiocyte functions, we administered in vivo a neutralizing anti-FSH antibody (47) to female and male BDL rats for 1 wk and demonstrated that this treatment reduces cholangiocyte mitosis and intrahepatic ductal mass concomitant with increased biliary apoptosis. These findings (demonstrating that circulating FSH levels are important in sustaining cholangiocyte proliferation) closely correlate with previous studies showing that the administration of neutralizing antibodies for progesterone, prolactin, nerve growth factor, and VEGF reduced cholangiocyte growth and increased cholangiocyte apoptosis (21, 22, 25, 58). These findings parallel previous studies showing that the survival of the specific germ cell types are dependent on FSH levels as demonstrated by the induction of apoptotic cell death by blockade of the gonadotropin action by immunoneutralization (54). Thus we treated BDL rats with antide, a third-generation Gonadotropin-releasing hormone (GnRH) antagonist (that inhibits gonadotropin secretion) (43) and demonstrated inhibition of cholangiocyte proliferation and increased apoptosis similar to what was observed with anti-FSH antibody. Parallel to our findings, antide has been shown to have antiproliferative effects by binding to GnRH receptor, which results in termination of receptor function (34). Furthermore, several in vitro studies in cell lines have shown inhibition of tumor cell growth by GnRH agonists and antagonists in a dose- and time-dependent manner (49, 53, 65).
We next performed studies to determine whether cholangiocytes express FSH and secrete FSH, thus regulating cholangiocyte growth by an autocrine mechanism. Indeed, we show that 1) both cholangiocytes and NRICC express and secrete FSH and 2) FSH secretion increased in female and male cholangiocytes from BDL rats compared with normal cholangiocytes. These findings support our previous studies demonstrating that cholangiocytes synthesize and secrete a number of growth factor such as nerve growth factor, VEGF, prolactin, and progesterone (21, 22, 25, 58). To shed light on the potential autocrine signaling mechanism, we demonstrated that supernatants of primary cultures of normal and BDL (containing more FSH than normal cholangiocytes) cholangiocytes stimulate NRICC proliferation. This proliferative response (induced by FSH present in the cholangiocyte supernatants) was partly blocked by a preincubation with an anti-FSH antibody. The partial blockage of proliferation is most likely due to other growth-promoting factors (e.g., nerve growth factor, NGF, VEGF, prolactin, and progesterone) secreted by cholangiocytes (21, 22, 25, 58). We observed that FSH levels increase in BDL supernatants compared with supernatants from normal cholangiocytes. The proliferative response induced by normal and BDL supernatants was partially blocked by anti-FSH, indicating that FSH is an important factor in cholangiocyte growth in vitro. As conclusive evidence that FSH plays a key role in sustaining cholangiocyte proliferation by an autocrine mechanism, we have knocked down the FSH gene in NRICC and demonstrated that lack of FSH in cholangiocytes leads to loss of proliferative capacity and enhanced apoptosis. In support of the growth-promoting effects of FSH on the biliary epithelium, other studies have shown that other pituitary hormones, such as growth hormone (12, 63) and prolactin (58), sustain cholangiocyte proliferation. Although FSH (expressed and secreted by hepatocytes) may modulate cholangiocyte growth by a paracrine mechanism, our studies support the novel concept that FSH is a key player in the autocrine loop regulating the balance between cholangiocyte proliferation and loss.
Our findings have important pathological implications since modulation of cholangiocyte expression and secretion of the trophic factor, FSH, may be important in the management of chronic cholestatic liver diseases regulating the balance between cholangiocyte growth and loss.
GRANTS
This work was supported partly by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, the Veterans Affairs (VA) Research Scholar Award, a VA Merit Award and National Institutes of Health (NIH) Grants DK58411, DK062975 and DK76898 to G. Alpini, an NIH K01 grant award (DK078532) to S. DeMorrow, and by University funds to P. Onori and PRIN 2007 and Federate Athenaeum funds from University of Rome “La Sapienza” to E. Gaudio.
REFERENCES
- 1.Alevizaki M, Huhtaniemi I. Structure-function relationships of glycoprotein hormones; lessons from mutations and polymorphisms of the thyrotrophin and gonadotrophin subunit genes. Hormones (Athens) 1: 224–232, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol 272: G1064–G1074, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 81: 569–578, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpini G, Phinizy JL, Glaser S, Francis H, Benedetti A, Marucci L, LeSage G. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 284: G1066–G1073, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Alpini G, Prall RT, and LaRusso NF. The pathobiology of biliary epithelia. In: The Liver: Biology and Pathobiology (4th ed.), edited by Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, and Shafritz DA. Philadelphia, PA: Williams & Wilkins, 2001, p. 421–435.
- 6.Alpini G, Ueno Y, Glaser S, Marzioni M, Phinizy JL, Francis H, LeSage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology 34: 868–876, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage G, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 272: G289–G297, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Alvaro D, Alpini G, Onori P, Perego L, Svegliati-Baroni G, Franchitto A, Baiocchi L, Glaser S, Le Sage G, Folli F, Gaudio E. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology 119: 1681–1691, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Baker HW, Burger HG, de Kretser DM, Dulmanis A, Hudson B, O'Connor S, Paulsen CA, Purcell N, Rennie GC, Seah CS, Taft HP, Wang C. A study of the endocrine manifestations of hepatic cirrhosis. Q J Med 45: 145–178, 1976. [PubMed] [Google Scholar]
- 11.Bell H, Raknerud N, Falch JA, Haug E. Inappropriately low levels of gonadotrophins in amenorrhoeic women with alcoholic and nonalcoholic cirrhosis. Eur J Endocrinol 132: 444–449, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Cai HH, Sun YM, Bai JF, Shi Y, Zhao HL, Miao Y. Relationship between the GH-IGFs axis and the proliferation of bile duct cancer cell line QBC939 in vitro. Hepatobiliary Pancreat Dis Int 7: 76–81, 2008. [PubMed] [Google Scholar]
- 13.Crepieux P, Marion S, Martinat N, Fafeur V, Vern YL, Kerboeuf D, Guillou F, Reiter E. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 20: 4696–4709, 2001. [DOI] [PubMed] [Google Scholar]
- 14.DeMorrow S, Francis H, Gaudio E, Ueno Y, Venter J, Onori P, Franchitto A, Vaculin B, Vaculin S, Alpini G. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol 294: G506–G519, 2008. [DOI] [PubMed] [Google Scholar]
- 15.DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem 282: 13098–13113, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Diamond T, Stiel D, Posen S. Osteoporosis in hemochromatosis: iron excess, gonadal deficiency, or other factors? Ann Intern Med 110: 430–436, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, Joplin RE, Okolicsanyi L, Strazzabosco M. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 43: 1001–1012, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, Gaudio E, Alvaro D, Fava G, Marzioni M, Vaculin B, Alpini G. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest 87: 473–487, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal DE, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CAMK I/CREB pathway. Am J Physiol Cell Physiol 295: C499–C513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Gigliozzi A, Alpini G, Svegliati-Baroni G, Marucci L, Metalli VD, Glaser S, Francis H, Mancino MG, Ueno Y, Barbaro B, Benedetti A, Attili AF, Alvaro D. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 127: 1198–1209, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, De Morrow S, Marzioni M, Mancino MG, Phinizy JL, Reichenbach R, Fava G, Summers R, Venter J, Alpini G. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol 290: G813–G826, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdhury U, Papa E, LeSage G, Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via d-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 32: 17–25, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Glaser S, DeMorrow S, Francis H, Ueno Y, Gaudio E, Vaculin S, Venter J, Franchitto A, Onori P, Vaculin B, Marzioni M, Wise C, Pilanthananond M, Savage J, Pierce L, Mancinelli R, Alpini G. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol 295: G124–G136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glaser S, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage G, Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 273: G1061–G1070, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Glaser S, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, Francis H, Dickerson IM, DiPette DJ, Supowit S, Alpini G. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest 87: 914–926, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Gonzales PH, Rhoden CR, Luz C, Correa G, Barbosa-Coutinho LM, Oliveira MC. Male gonadal function, prolactin secretion and lactotroph population in an experimental model of cirrhosis. Braz J Med Biol Res 40: 1383–1388, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Gorczynska E, Spaliviero J, Handelsman DJ. The relationship between 3′,5′-cyclic adenosine monophosphate and calcium in mediating follicle-stimulating hormone signal transduction in Sertoli cells. Endocrinology 134: 293–300, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Grasso P, Reichert LE Jr. Follicle-stimulating hormone receptor-mediated uptake of 45Ca2+ by cultured rat Sertoli cells does not require activation of cholera toxin- or pertussis toxin-sensitive guanine nucleotide binding proteins or adenylate cyclase. Endocrinology 127: 949–956, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB. Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-beta gene. Mol Endocrinol 19: 237–254, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Gromoll J, Simoni M, Nieschlag E. An activating mutation of the follicle-stimulating hormone receptor autonomously sustains spermatogenesis in a hypophysectomized man. J Clin Endocrinol Metab 81: 1367–1370, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology 97: 1236–1247, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Kakar SS Inhibition of growth and proliferation of EcRG293 cell line expressing high-affinity gonadotropin-releasing hormone (GnRH) receptor under the control of an inducible promoter by GnRH agonist (d-Lys6)GnRH and antagonist (Antide). Cancer Res 58: 4558–4560, 1998. [PubMed] [Google Scholar]
- 35.LaPolt PS, Tilly JL, Aihara T, Nishimori K, Hsueh AJ. Gonadotropin-induced up- and down-regulation of ovarian follicle-stimulating hormone (FSH) receptor gene expression in immature rats: effects of pregnant mare's serum gonadotropin, human chorionic gonadotropin, and recombinant FSH. Endocrinology 130: 1289–1295, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004. [DOI] [PubMed] [Google Scholar]
- 37.LeSage G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 117: 191–199, 1999. [DOI] [PubMed] [Google Scholar]
- 38.LeSage G, Alvaro D, Glaser S, Francis H, Marucci L, Roskams T, Phinizy JL, Marzioni M, Benedetti A, Taffetani S, Barbaro B, Fava G, Ueno Y, Alpini G. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology 40: 1116–1127, 2004. [DOI] [PubMed] [Google Scholar]
- 39.LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte proliferation. Liver 21: 73–80, 2001. [DOI] [PubMed] [Google Scholar]
- 40.LeSage G, Glaser S, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 111: 1633–1644, 1996. [DOI] [PubMed] [Google Scholar]
- 41.LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol 276: G1289–G1301, 1999. [DOI] [PubMed] [Google Scholar]
- 42.LeSage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 281: G182–G190, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Ljungqvist A, Feng DM, Hook W, Shen ZX, Bowers C, Folkers K. Antide and related antagonists of luteinizing hormone release with long action and oral activity. Proc Natl Acad Sci USA 85: 8236–8240, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, Reichenbach R, Mancino MG, Summers R, Alpini G, Glaser S. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol 168: 398–409, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, Venter J, Fava G, LeSage G, Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 128: 121–137, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Masyuk TV, Masyuk AI, Torres VE, Harris PC, LaRusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology 132: 1104–1116, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Meachem SJ, Wreford NG, Stanton PG, Robertson DM, McLachlan RI. Follicle-stimulating hormone is required for the initial phase of spermatogenic restoration in adult rats following gonadotropin suppression. J Androl 19: 725–735, 1998. [PubMed] [Google Scholar]
- 48.Moss J, Stanley SJ, Burns DL, Hsia JA, Yost DA, Myers GA, Hewlett EL. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J Biol Chem 258: 11879–11882, 1983. [PubMed] [Google Scholar]
- 49.Pati D, Habibi HR. Inhibition of human hepatocarcinoma cell proliferation by mammalian and fish gonadotropin-releasing hormones. Endocrinology 136: 75–84, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Pawlowski J, Kraft AS. Bax-induced apoptotic cell death. Proc Natl Acad Sci USA 97: 529–531, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ritter V, Thuering B, Saint Mezard P, Luong-Nguyen NH, Seltenmeyer Y, Junker U, Fournier B, Susa M, Morvan F. Follicle-stimulating hormone does not impact male bone mass in vivo or human male osteoclasts in vitro. Calcif Tissue Int 82: 383–391, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of γ-glutamyl transpeptidase activity. J Histochem Cytochem 17: 517–526, 1969. [DOI] [PubMed] [Google Scholar]
- 53.Segal-Abramson T, Kitroser H, Levy J, Schally AV, Sharoni Y. Direct effects of luteinizing hormone-releasing hormone agonists and antagonists on MCF-7 mammary cancer cells. Proc Natl Acad Sci USA 89: 2336–2339, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shetty J, Marathe GK, Dighe RR. Specific immunoneutralization of FSH leads to apoptotic cell death of the pachytene spermatocytes and spermatogonial cells in the rat. Endocrinology 137: 2179–2182, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 18: 739–773, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Suire S, Maurel MC, Guillou F. Follitropin action on the transferrin gene in Sertoli cells is mediated by cAMP-responsive-element-binding-protein and antagonized by chicken ovalbumin-upstream-promoter-transcription factor. Eur J Biochem 239: 52–60, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Sutton-Walsh HG, Whelly S, Cornwall GA. Differential effects of GnRH and androgens on Cres mRNA and protein in male mouse anterior pituitary gonadotropes. J Androl 27: 802–815, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Taffetani S, Glaser S, Francis H, DeMorrow S, Ueno Y, Alvaro D, Marucci L, Marzioni M, Fava G, Venter J, Vaculin S, Vaculin B, Lam IP, Lee VH, Gaudio E, Carpino G, Benedetti A, Alpini G. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol 7: 6, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tilly JL, LaPolt PS, Hsueh AJ. Hormonal regulation of follicle-stimulating hormone receptor messenger ribonucleic acid levels in cultured rat granulosa cells. Endocrinology 130: 1296–1302, 1992. [DOI] [PubMed] [Google Scholar]
- 60.Ulloa-Aguirre A, Uribe A, Zarinan T, Bustos-Jaimes I, Perez-Solis MA, Dias JA. Role of the intracellular domains of the human FSH receptor in G(alphaS) protein coupling and receptor expression. Mol Cell Endocrinol 260–262: 153–162, 2007. [DOI] [PMC free article] [PubMed]
- 61.Ulloa-Aguirre A, Zarinan T, Pasapera AM, Casas-Gonzalez P, Dias JA. Multiple facets of follicle-stimulating hormone receptor function. Endocrine 32: 251–263, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Vu Hai MT, Lescop P, Loosfelt H, Ghinea N. Receptor-mediated transcytosis of follicle-stimulating hormone through the rat testicular microvasculature. Biol Cell 96: 133–144, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Chen M, Zheng G, Dillard B, Tallarico M, Ortiz Z, Holterman AX. Transcriptional activation by growth hormone of HNF-6-regulated hepatic genes, a potential mechanism for improved liver repair during biliary injury in mice. Am J Physiol Gastrointest Liver Physiol 295: G357–G366, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang YJ, Wu JC, Lee SD, Tsai YT, Lo KJ. Gonadal dysfunction and changes in sex hormones in postnecrotic cirrhotic men: a matched study with alcoholic cirrhotic men. Hepatogastroenterology 38: 531–534, 1991. [PubMed] [Google Scholar]
- 65.Yano T, Pinski J, Radulovic S, Schally AV. Inhibition of human epithelial ovarian cancer cell growth in vitro by agonistic and antagonistic analogues of luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA 91: 1701–1705, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang SB, Dattatreyamurty B, Reichert LE Jr. Differential roles of high and low affinity guanosine 5′-triphosphate binding sites in the regulation of follicle-stimulating hormone binding to receptor and signal transduction in bovine calf testis membranes. Endocrinology 128: 295–302, 1991. [DOI] [PubMed] [Google Scholar]