Abstract
Members of the Kv7 voltage-gated K+ channel family are important determinants of cardiac and neuronal membrane excitability. Recently, we and others have shown that Kv7 channels are also crucial regulators of smooth muscle activity. The aim of the present study was to assess the Kv7 expression in different parts of the murine gastrointestinal (GI) tract and to assess their functional roles by use of pharmacological agents. Of KCNQ/Kv7 members, both KCNQ4/Kv7.4 and KCNQ5/Kv7.5 genes and proteins were the most abundantly expressed Kv7 channels in smooth muscles throughout the GI tract. Immunohistochemical staining also revealed that Kv7.4 and Kv7.5 but not Kv7.1 were expressed in the circular muscle layer of the colon. In segments of distal colon circular muscle exhibiting spontaneous phasic contractions, the nonselective Kv7 blockers XE991 and linopirdine increased the integral of tension. Increases in the integral of tension were also observed under conditions of neuronal blockade. Similar effects, although less marked, were observed in the proximal colon. As expected, the Kv7.1-selective blocker chromanol 293B had no effect in either type of segment. These data show that Kv7.x especially Kv7.4 and Kv7.5 are expressed in different regions of the murine gastrointestinal tract and blockers of Kv7 channels augment inherent contractile activity. Drugs that selectively block Kv7.4/7.5 might be promising therapeutics for the treatment of motility disorders such as constipation associated with irritable bowel syndrome.
Keywords: colon, voltage-gated K+ channel, KCNE
the movements of the contents of the gastrointestinal (GI) tract rely on the coordinated contractions of the smooth muscles that surround each specialized region. Contractility of GI tissues is dependent on several governing factors and is different between each anatomically defined segment. The coordinated response of the smooth muscle depends on the influences, in each region, of the enteric nervous system, the intrinsic pacemaker cells [interstitial cells of Cajal (ICC)], endocrine and paracrine mediators, and the intrinsic spontaneous excitability of the smooth muscle cells. Much is now known about the ionic conductances responsible for the formation of electric slow waves and the maintenance of the resting membrane potential of smooth muscle and ICC (2, 14, 30, 37, 40–42). However, there is still a lack of information about the fundamental K+ channels suppressing gastrointestinal smooth muscle (GISM) contractility although Kv1.2/Kv1.5 [4-aminopyridine (4-AP) sensitive] and Kv2 [tetraethylammonium (TEA) sensitive] contribute significantly to delayed rectifier K+ currents (5, 13, 24, 25, 29, 33).
Recently, K+ channels encoded by KCNQ genes have been identified as major regulators of vascular contractility (4, 10, 16, 18, 23, 50). Kv7.1-Kv7.5, encoded by KCNQ1-5 genes, are voltage-gated K+ channels that exhibit minimal inactivation and a negative activation threshold (15, 28, 31), whose pharmacological and biophysical characteristics are modulated by association with KCNE gene products (20). The physiological role of KCNQ gene products has been studied extensively in cardiac myocytes, neurons, and epithelia cell and their importance in regulating membrane excitability is highlighted by mutations in several members of KCNQ and KCNE underling inherited diseases such as cardiac long QT syndrome, epilepsy, and deafness (15, 28). In vascular smooth muscle cells, the expression of KCNQ1, -4, and -5 dominates, but functional experiments with a range of pharmacological tools suggest that Kv7.4 and Kv7.5 have a greater physiological impact. In contrast to the recent studies on vascular smooth muscle, there is relatively little information on the expression and functional impact of KCNQ genes in visceral tissues although K+ currents suppression by phospholipase C-linked receptors, analogous to neuronal M-channels, has been observed in toad stomach (36). The expression and functional role of KCNQ-encoded potassium channels has been determined in murine myometrium (19), and initial evidence of KCNQ gene expression in gastrointestinal smooth muscles was reported in rat gastric antrum smooth muscle (22). However, no further studies have been conducted since that report, when less was known about the diversity of Kv7 channels.
The aim of the present study is to determine the molecular identities and potential functional role of each of the known Kv7 channels in murine GISMs. To address this issue, RT-PCR (real-time PCR and cell-based RT-PCR) and immunochemical (Western blotting, immunohistochemistry) examinations were performed to identify the molecular components of Kv7 and KCNE members in murine GISMs. Finally, we establish the functional role of these channels for the first time by examining the effects of Kv7 channel modulating agents.
MATERIALS AND METHODS
Dissection of smooth muscle layers and isolation of smooth muscle cells.
Adult BALB/c mice were killed by cervical dislocation in accordance with the protocols approved by the Institutional Animal Care and Use Committees of Nagoya City University, University of London, and University of Nevada. Segments of tissues were pinned in a dissecting dish with the mucosa facing upward. The mucosa and submucosa were removed by sharp dissection. Small portions of the circular smooth muscle tissue were placed on the Ca2+-free Hanks' solution containing (in mM) 125 NaCl, 5.36 KCl, 15.5 NaOH, 0.336 Na2HCO3, 0.44 KH2PO4, 10 glucose, 2.9 sucrose, and 11 HEPES. Strips of tissue were incubated in a Ca2+-free Hanks' solution containing collagenase (Sigma-Aldrich, St. Louis, MO), fatty acid-free bovine serum albumin, and trypsin inhibitor (Sigma-Aldrich) at 37°C for 8–12 min. Resulting isolated smooth muscle cells were differentiated by their characteristic morphology and were collected through applied suction by aspirating them into a wide-bore patch-clamp pipette (borosilicate glass). Approximately 50 smooth muscle cells were collected, flash frozen in liquid nitrogen, and stored at −80°C until use for cell-based RT-PCR.
Total RNA extraction and cell-based RT-PCR.
As previously reported (23), total RNA was extracted from tissues and isolated smooth muscle cells with the use of a TRIzol RNA purification kit (Invitrogen, Carlsbad, CA) and a SNAP total RNA isolation kit (Invitrogen), respectively. cDNA synthesis was performed by using the SuperScript II RNase H− (Invitrogen), and 500 μg/μl of oligo(dT) primer were used to reverse transcribe the RNA sample. The resulting cDNA product was amplified with gene-specific primers by PCR. The amplication profile for these primer pairs were follows: a 10-s denaturation step at 94°C, a 10-s annealing step at 60°C, and a 30-s primer extension step at 72°C. Since several reports have identified spliced variants of KCNQ1 (23), KCNQ2 (21), and KCNQ5 (49), we designed PCR primers from the conserved region of their respective spliced variants.
For cell-based RT-PCR, the amplification was performed for 40 (for KCNQ) and 37 (for KCNE) cycles. The amplified products were separated by electrophoresis on a 2% agarose/1 × TAE (Tris-acetic acid-EDTA) gel, and the DNA bands were visualized by ethidium bromide staining. Specific primers were designed for nonquantitative, cell-based RT-PCR to determine relative levels of expression of KCNQ4, KCNQ5, and KCNE4, as follows: KCNQ4 (GenBank accession number AF249747): 18–582, amplicon = 565 bp; KCNQ5 (AF263836) 112–606, 495 bp; KCNE4 (NM_021342) 197–581, 385 bp. The no-template control (NTC) was a PCR amplification for which the template was not added, controlling for nonspecific amplification and spurious primer-dimer fragments. Negative controls were subjected to a second round of amplification to assure specificity of the reaction and the quality of the reagents. β-Actin primers were used to confirm that the products generated were representative of RNA (498 bp) and not contaminated with genomic DNA (intron containing 708 bp band) because these primers were designed to span an intron as well as two exons. Each amplified product was sequenced by the chain termination method with an ABI PRIZM (model 310) (Applied Biosystems, Foster City, CA).
Quantitative, real-time PCR.
Specific primers were designed for quantitative, real-time PCR to determine relative levels of expression of KCNQ and KCNE subtypes in GI smooth muscles. Quantitative, real-time PCR performed with the use of SYBR Green chemistry on an ABI 5700 sequence detector (Applied Biosystems) as previously reported (23). Regression analysis of the mean values of six multiplex RT-PCRs for the log10 diluted cDNA was used to generate standard curves. Unknown quantities relative to the standard curve for a particular set of primers were calculated, yielding transcriptional quantitation of KCNQ and KCNE gene products relative to the endogenous standard (β-actin). The reproducibility of the assay was tested by ANOVA comparing repeat runs of samples, and mean values generated at individual time points were compared by Student's t-test.
Western blot.
Membrane protein fractions were prepared from murine GI layers using similar procedures as previously reported (23), and proteins (50 μg/lane) were subjected to SDS-PAGE (10%). The blots were incubated with rabbit anti-KCNQ4 (Kv7.4) (H-130) and anti-KCNQ5 (Kv7.5) (H-170) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and then incubated with anti-rabbit horseradish peroxidase-conjugated IgG (Chemicon International, Temecula, CA). An enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ) was used for the detection of the bound antibody. Resulting images were analyzed by a LAS-1000 device (Fujifilm, Tokyo, Japan).
Immunohistochemical analyses.
Segments of distal colon where fixed in cold 4% paraformaldehyde for 1 h at 4°C. The preparations were subsequently washed several times in phosphate-buffered saline (PBS) and then cryoprotected overnight in PBS containing 10% sucrose and 0.01% sodium azide. Segments were then embedded in Tissue-Tek OCT compound (Sakura Finetek, Zoeterwoude, Netherlands) and gradually frozen at −80°C. Frozen sections (4 μm) were collected on polylysine-coated slides and stored at −20°C for future use. Sections that had been allowed to air dry at room temperature for 1 h were washed in PBS (5 min) to remove residual OCT compound. Endoperoxidase and serum blocking steps were then performed, per the manufacturers' instructions for the immunostaining kits used (ImmunoCruz; Santa Cruz Biotechnology). Primary antibodies, diluted in the appropriate serum block, were applied to the sections for 18 h at 4°C. After a wash in PBS (5 min), a biotinylated secondary antibody was applied (30 min) and the sections were washed in PBS (5 min). A solution of streptavidin-conjugated horseradish peroxidase (HRP) was applied to the sections (30 min), prior to a final wash in PBS. Finally, a diaminobenzidine-HRP (DAB-HRP) reaction was performed until such time as immunolocalization was apparent. Negative controls were performed by incubating sections in the appropriate control serum alone and run in parallel for each experiment. A number of primary antibodies were tested. Those that produced the most specific staining with the least amount background staining were: anti-Kv7.1 (1:50; Alomone Labs, Jerusalem, Israel), anti-Kv7.4 (1:200; Abcam, Cambridge, UK) and anti-Kv7.5 (1:200; Millipore, Billerica, MA).
Measurements of contractile responses.
Adult BALB/c mice were killed by cervical dislocation and the colon was removed and transferred to a Krebs' solution (composition in mM: 119 NaCl, 5.4 KCl, 2.5 CaCl2, 0.6 KH2PO4, 1.2 MgSO4, 25 NaHCO3, and 11.7 glucose). After gently flushing any fecal pellets from the lumen, 3- to 4-mm segments taken from the first centimeter after the cecum, (termed “proximal”) and the last centimeter before the anus (termed “distal”) were carefully mounted on two stainless steel supports in a myograph (Danish Myo Technology, DMT, Aarhas N, Denmark) for recording of isometric force production. The myograph chamber was filled with Krebs, maintained at 37°C and continuously bubbled with 95% O2-5% CO2. After equilibrating at 37°C, an initial passive tension of 12 mN was applied to the preparation, followed by several washes in Krebs. In many preparations, spontaneous contractile activity subsequently developed and stabilized over a period of 1 h. In some preparations, particularly those which returned to a low resting tension, passive tension (12 mN) was reapplied. Spontaneous contractile activity generally developed in such preparations. Responses to different agents were quantified by measuring the integral of tension over a 5-min period immediately before the application of a drug (control) and 30 min after drug application.
Solutions and reagents.
XE991 was purchased from Ascent Scientific (Weston Super Mare, UK), linopirdine from Sigma (Poole, UK), and ω-conotoxin MVIIC and tetrodotoxin from Calbiochem (Nottingham, UK). Retigabine was a gift from Dr. Schwake, University of Kiel, Kiel, Germany.
RESULTS
Quantitative determination of the transcriptional expression of KCNQ and KCNE subtypes in murine gastrointestinal smooth muscles.
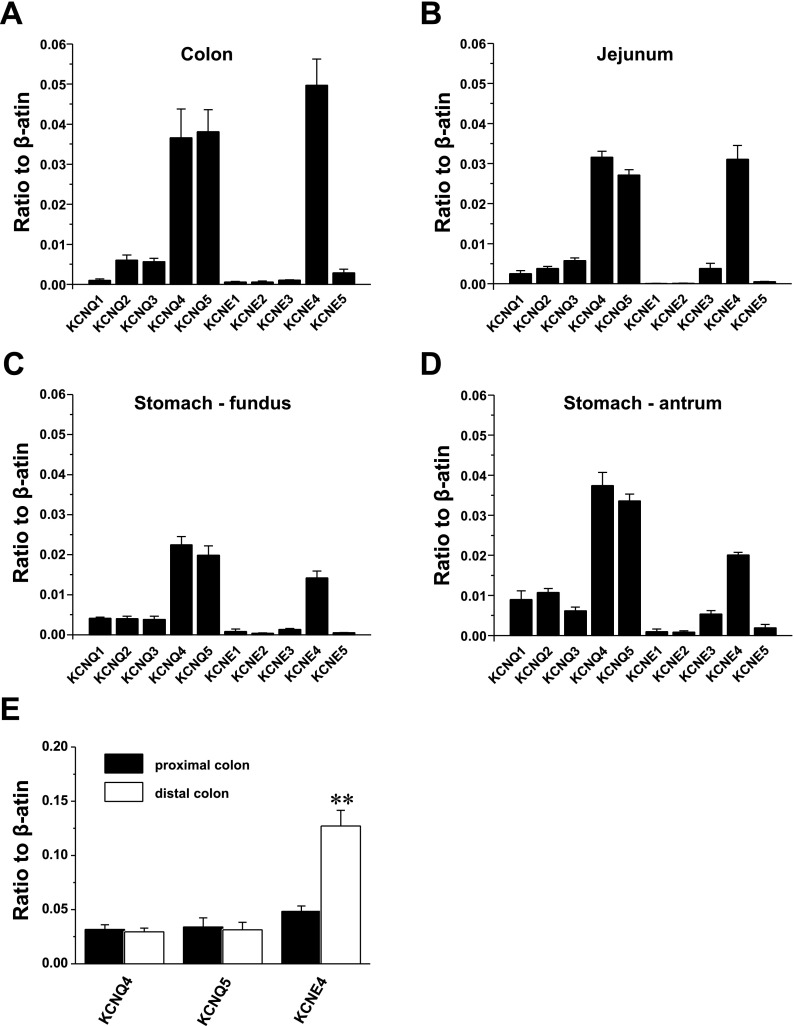
Recent studies have shown that KCNQ genes are expressed and have a functional impact in various types of smooth muscles (10). In the present study, we first examined the relative transcriptional expression of KCNQ and KCNE subtypes to β-actin in colon, jejunum, stomach fundus, and stomach antrum by quantitative, real-time PCR analysis (Fig. 1, A–D). Of the five KCNQ subtypes both KCNQ4 and KCNQ5 were expressed abundantly in all GISMs examined (Fig. 1, A–D). In contrast, KCNQ1-3 expression was negligible in GISM (less than 0.009 relative to β-actin in all GI smooth muscles examined, n = 5 for each). As positive controls, expression of KCNQ1, KCNQ2, and KCNQ3 was 0.019 ± 0.001 (in heart), 0.069 ± 0.004 (in brain), and 0.024 ± 0.006 (in brain), respectively (n = 5 for each). Yeung et al. (49) revealed that vascular smooth muscles expressed an exon 9-lacking spliced isoform of KCNQ5 (KCNQ5Δexon9). Similar to vascular smooth muscle GISM also expressed the KCNQ5Δexon9 isoform (not shown). Of five KCNE subtypes (KCNE1-5), KCNE4 alone was abundantly expressed in all GISMs examined (Fig. 1, A–D) and was more abundantly expressed in the distal compared with proximal colon (Fig. 1E). In contrast, KCNE1, -2, -3, and -5 expression was less than 0.005 in all GISMs examined (n = 5 for each). As positive controls, their expression in brain was 0.014 ± 0.005 (KCNE1), 0.065 ± 0.009 (KCNE2), 0.021 ± 0.005 (KCNE3), and 0.063 ± 0.004 (KCNE5) (n = 5 for each).
Fig. 1.
Quantitative, real-time PCR detection of KCNQ and KCNE subtype transcript expressions relative to β-actin in murine gastrointestinal smooth muscles (GISMs) (A, colon; B, jejunum; C, stomach fundus; D, stomach antrum). Values are shown for KCNQ (KCNQ1-5) and KCNE (KCNE1-5) subtypes' steady-state transcripts relative to β-actin in the same preparation. Note that the scale is kept consistent to allow comparison of relative expression level between tissues. E: expression of KCNQ4, KCNQ5, and KCNE4 in proximal (solid bar) and distal (open bar) colon smooth muscle layers. All expression data are expressed as means ± SE (n = 5 for each).
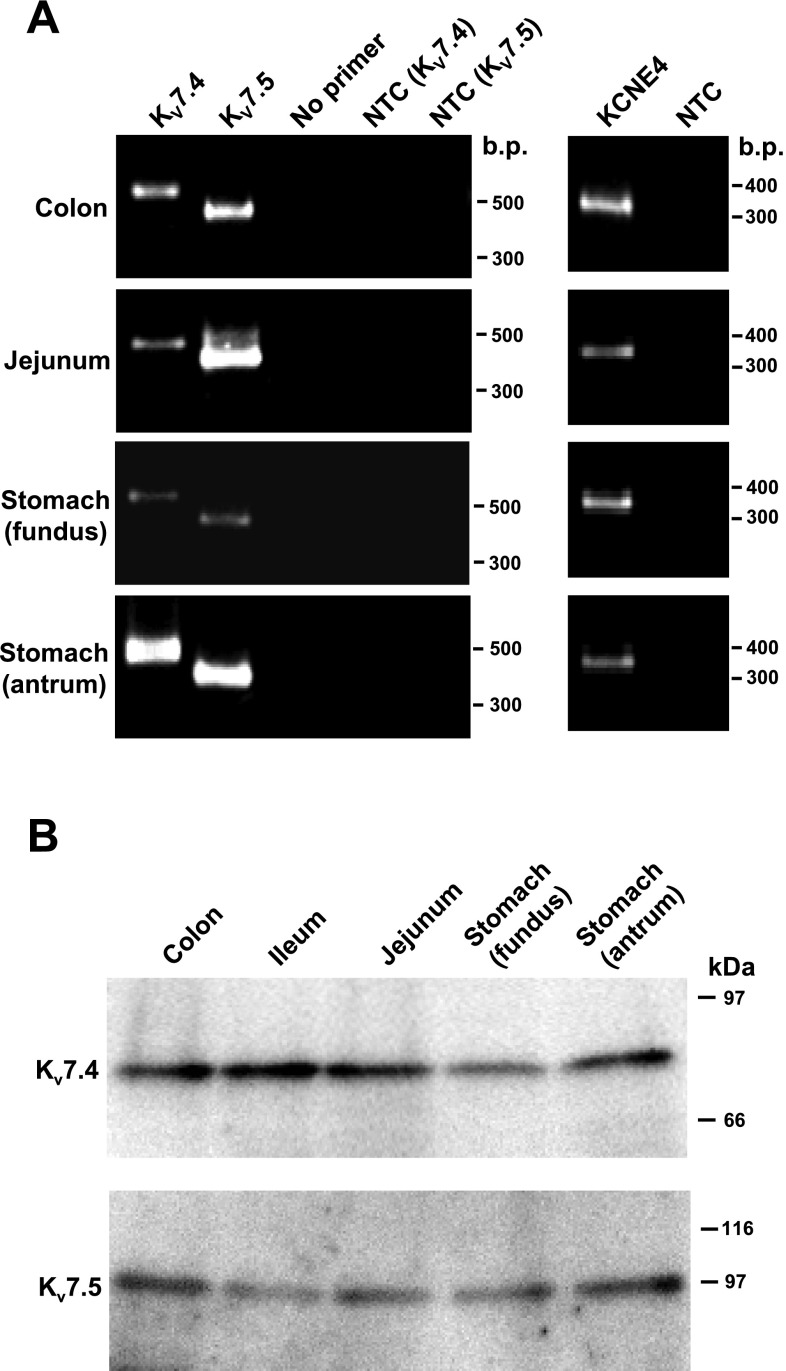
To confirm that the smooth muscle cells expressed KCNQ4 and 5, cell-based RT-PCR analyses were performed on freshly isolated murine GISM cells (GISMCs). As shown in Fig. 2A, KCNQ4, KCNQ5, and KCNE4 signals were easily detected in all GISMCs examined, whereas no detectable signals were observed in negative controls [without primers (no primers) and without template cDNAs (NTC)]. These results indicate that the transcripts of KCNQ4, KCNQ5, and KCNE4 are present in murine gastrointestinal smooth muscle cells. These results suggest that homo- and/or heterotetramer of Kv7.4 and/or Kv7.5 may contribute to outward currents in murine GISMCs, and KCNE4 may also be a significant regulatory component of outward currents.
Fig. 2.
Cell-based RT-PCR and Western blot analyses in murine GISMs. A: PCR products were generated through the use of gene-specific primers for KCNQ4, KCNQ5, and KCNE4 for 45 (KCNQ) and 40 cycles (KCNE). Amplified products were separated on 2.0% agarose gels and were identified by ethidium bromide staining. A 100-bp molecular weight ladder was used to estimate the size of the amplicon and the migration is shown on the right. Similar results were obtained from 3 separate experiments. B: the membrane fractions extracted from GI smooth muscles were immunoblotted with anti-Kv7.4 (top) and anti-Kv7.5 (bottom) antibodies (at 1:200). Molecular mass standards are shown in kilodaltons at right.
Protein expressions of Kv7.4 and Kv7.5 in murine GI smooth muscles.
We next determined the protein expressions of Kv7.4 and Kv7.5 using Western blot and immunohistochemical techniques. As shown in Fig. 2B, Western blot analysis revealed single bands specific for anti-Kv7.4 and anti-Kv7.5 antibodies with molecular weights of ∼80 and 100 kDa in plasma membrane fractions of murine GISMs: colon, jejunum, stomach fundus, and stomach antrum. These signals were not apparent when the primary antibody was preabsorbed with excess antigen (not shown).
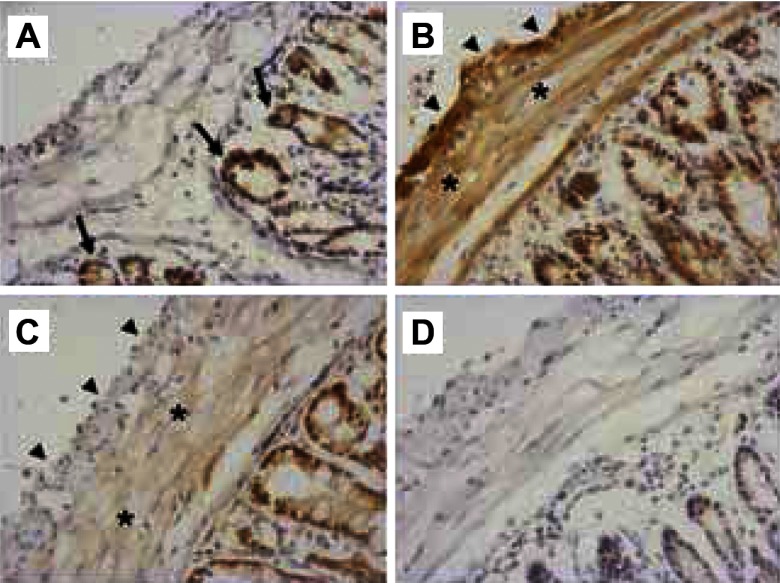
Since GI tissues comprise a number of different functional layers, immunohistochemical experiments were undertaken to identify the location of Kv7 proteins in slices of mouse colon. Similar to previous work (35) antisera raised against Kv7.1 stained the colonic crypts epithelium intensely but was completely absent from the muscle layers (Fig. 3A). Antisera raised against Kv7.4 and 7.5 also stained the colonic crypt cells but immunoreactivity for these proteins was also apparent in the muscle layers (Fig. 3, B and C). Unlike Kv7.1 staining Kv7.4 was immunodetected in both the longitudinal and circular smooth muscle (Fig. 3B) with the former appearing more intense. Staining for Kv7.5 was obvious in the circular layer of muscle (Fig. 3C) and was also found in the myenteric region between the circular and longitudinal muscle layers, possibly due to labeling of myenteric neurons or ICC (data not shown). Similar staining was obtained with matched control sections exposed to control rabbit or goat serum were indistinguishable from one another, with only very faint staining of the epithelium apparent (Fig. 3D). These data revealed that colonic smooth muscle express only Kv7.4 and Kv7.5.
Fig. 3.
Immunohistochemical staining of murine distal colon sections. A: although enterocytes are intensely stained by use of an antibody raised against Kv7.1 (arrows), no immunoreactivity is observed in the smooth muscle layers. B: an antibody raised against Kv7.4 stains both the longitudinal (arrowheads) and circular (asterisks) layers of smooth muscle. The longitudinal layer consistently stained more intensely. The epithelium is also immunoreactive for Kv7.4. C: although the epithelium also contains immunoreactivity for Kv7.5, only faint staining of the circular muscle layer is apparent. D: a representative time-matched section incubated in control serum reveals only very faint false-positive staining of the epithelium only.
Functional experiments.
After a period of equilibration all segments of mouse colon exhibited spontaneous contractile activity. The proximal segments only displayed a single type of high-frequency contraction whereas in segments of distal colon two distinct types of contraction were observed: low-amplitude, high-frequency contractions (LAHFs), and high-amplitude, low-frequency contractions (HALFs). HALFs were observed in 53% of segments before any drugs were added whereas LAHFs were always observed. In control conditions, HALFs had an average amplitude of 22.12 ± 2.02 mN and a mean duration of 29.0 ± 1.27 s and occurred at an average frequency of 0.003 ± 0.0003 Hz (n = 12 animals). In comparison, LAHFs in control conditions had an average amplitude of 0.77 ± 0.17 mN and were apparent at a frequency of 0.23 ± 0.02 Hz (n = 20). In many preparations in which HALF contractions were dominant, each large contraction was followed by a few minutes of suppressed LAHF activity.
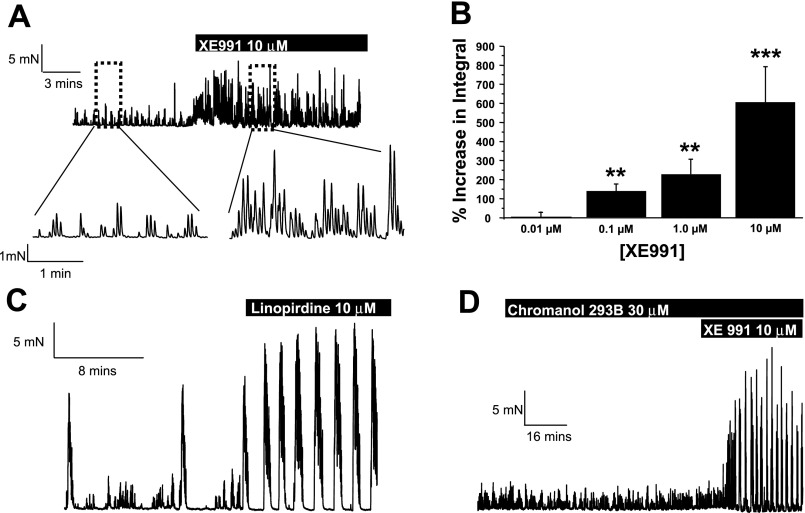
A functional role for Kv7 channels in the colon was assessed by using different pharmacological tools. All Kv7 channels are inhibited by the nonspecific K+ channel blocker TEA to varying degrees (IC50 3–30 mM) (12), as well as the selective agents XE991 and linopirdine at low micromolar concentrations (28, 44, 50) but are relatively unaffected by 4-AP up to 5 mM (28). Kv7.1 channels, but not Kv7.2–7.5, are also blocked by chromanol 293B (IC50 ∼30 μM) (17). In contrast, retigabine activates Kv7.2–7.5 with EC50s ∼5 μM (32, 39) but has no stimulatory effect on Kv7.1 channels. Application of XE991 to segments of distal colon caused an increase in spontaneous contractile activity (Fig. 4A), which was concentration dependent (Fig. 4B). Table 1 shows the effect of XE991 on individual contractile parameters for both HALFs and LAHFs. Linopirdine (10 μM) also increased the spontaneous contractile activity (Fig. 4C) with the mean integral of tension increasing by 222 ± 36% (n = 10, P < 0.05). The effects of XE991 were compared with those of 4-AP. Application of 1 mM 4-AP evoked an increase in the mean integral of tension by 32.6 ± 13.7% (n = 7), which was less than that produced by XE991 (increase was 223 ± 86% in paired tissues). Subsequent addition of 10 μM XE991 in the presence of 1 mM 4-AP increased the mean integral of tension by a further 63.5 ± 20.1% (n = 4). The effect of XE991 and linopirdine was not mirrored by the Kv7.1-selective blocker chromanol 293B (30 μM; Fig. 4D), which had no significant effect on the mean integral of tension (mean integral was reduced by 36 ± 13% of control, n = 4), suggesting that Kv7.1 does not have major functional impact in this tissue.
Fig. 4.
Effect of Kv7 blockers in segments of murine distal colon. A: effect of the Kv7 blocker XE991 (10 μM) in a segment of distal colon exhibiting only low-amplitude, high-frequency contraction (LAHF) activity. Bottom traces show an amplified section of the contractility in the absence (left) and presence (right) of XE991. B: concentration-effect relationship for the change in integral contractile activity in response to XE991 (**P < 0.01, ***P < 0.005 compared with control). C: effect of 10 μM linopirdine on contractile activity in a segment of distal colon only exhibiting LAHFs and high-amplitude, low-frequency contractions (HALFs). D: a representative trace of the lack of effect of chromanol 293B (30 μM) in the distal colon. Note the stimulatory effect of XE991 (10 μM) applied after chromanol 293B.
Table 1.
Effect of XE991 on individual contractile parameters
| LAHFs |
HALFs | |||||
|---|---|---|---|---|---|---|
| Amplitude, mN | Duration, s | Frequency, Hz | Amplitude, mN | Duration, s | Frequency, Hz | |
| Control | 0.53 (0.17–0.95) | 5.47 (2.62–9.98) | 0.24 (0.1–0.38) | 23.3 (15.1–35.5) | 25.4 | 0.002 |
| 1 μM XE991 | 1.02 (0.49–2.00) | 4.28 (3.00–6.06) | 0.25 (0.17–0.33) | 23.4 (10.9–31.9) | 57.1 (48.9–67.6) | 0.007 (0.0061–0.0072) |
| P = 0.040 | P = 0.230 | P = 0.424 | P = 0.221 | P = 0.006 | P = 0.010 | |
| Control | 0.93 (0.12–2.05) | 5.61 (3.55–12.0) | 0.21 (0.08–0.28) | 25.08 (13.82–34.46) | 33.22 (28.9–38.13) | (0.00056–0.0044) |
| 10 μM XE991 | 2.63 (0.94–8.35) | 4.13 (2.87–6.00) | 0.26 (0.17–0.35) | 28.19 (17.43–36.12) | 46.58 (37.12–61.78) | 0.0093 (0.0078–0.011) |
| P = 0.057 | P = 0.090 | P = 0.026 | P = 0.063 | P = 0.035 | P = 0.0014 | |
Each value is the mean of 4 experiments with the range shown in parentheses. LAHFs, low-amplitude, high-frequency contractions; HALFs, high-amplitude, low-frequency contractions. P values represent paired Student's t-test.
In contrast to the marked effects in the distal colon, application of XE991 or linopirdine had minimal effects in segments of proximal colon (data not shown). Hence, no stimulatory effect was observed with 0.01–1 μM XE991 although 10 μM XE991 increased the mean integral of tension by 217 ± 119% (n = 4, *P < 0.05); 10 μM linopirdine also had a small but significant effect on contractile activity (n = 3). Overall these data show that blockade of Kv7 channels other than Kv7.1 increased contractility markedly in segments of distal colon and had a small effect on the less active proximal segments.
Involvement of nerves.
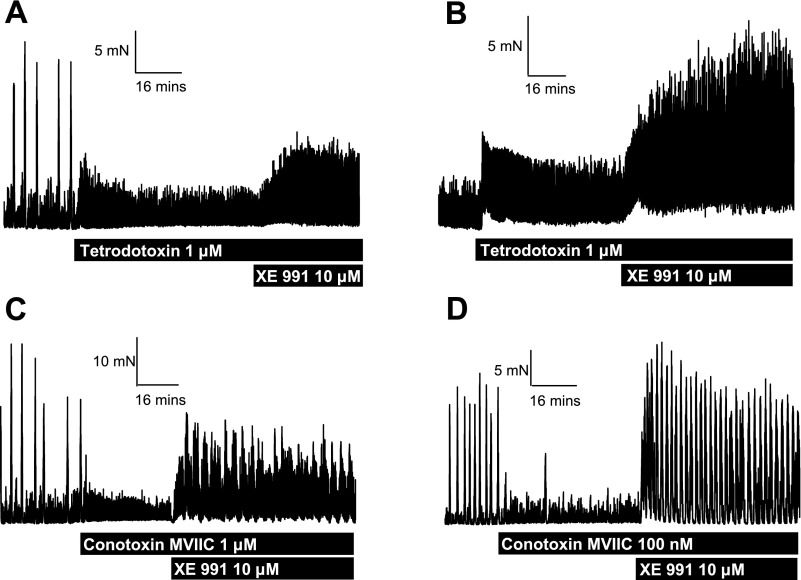
It is possible that the increase in contractile activity produced by the Kv7 channel inhibitors was due to blockade of Kv7 channels located on myenteric nerve terminals. Consequently, experiments were undertaken in the presence of two different neurotoxins. The fast sodium channel blocker tetrodotoxin (1 μM) and the N-, P-, and Q-calcium channel blocker ω-conotoxin MVIIC (1 μM) completely inhibited any spontaneous HALF activity in the distal colon and frequently increased baseline LAHF activity. In the presence of 1 μM tetrodotoxin or 1 μM ω-conotoxin MVIIC, application of XE991 (1 and 10 μM) elicited a generalized increase in LAHF activity (Fig. 5, A–C) but did not result in HALFs reappearing. Interestingly, application of a lower concentration of ω-conotoxin MVIIC (100 nM), which was sufficient to abolish HALF contractions, did not prevent restoring HALF activity in the presence of 10 μM XE991, and the frequency and amplitude of contractions are rather increased compared with control conditions (Fig. 5D). Chromanol (30 μM) had no stimulatory effect on contractile activity in the presence of ω-conotoxin MVIIC (mean effect was a reduction of 26 ± 6%, n = 3). These data suggest that the excitatory effect of XE991 was not due to blockade of neuronal Kv7 channels and a subsequent increase in neurotransmitter release.
Fig. 5.
Effect of XE991 in distal colon segments in the presence of neurotoxins. A: tetrodotoxin (1 μM) abolishes HALF activity and increases baseline LAHF activity. Addition of XE991 (10 μM) causes an additional increase in LAHF activity. B: in a segment exhibiting LALF activity only, tetrodotoxin increases LAHF activity, and addition of XE991 causes a further increase in contractility. C: a concentration of ω-conotoxin MVIIC (1 μM) that is sufficient to block all prejunctional neuronal calcium channels has a similar effect as tetrodotoxin on LAHF and HALF activity. Addition of XE991 caused an increase in LAHF activity in these conditions. D: a lower concentration of ω-conotoxin MVIIC (100 nM) inhibited HALF activity, without modifying LAHF contractions. Under these conditions, addition of XE991 appeared to restore HALF contractions and increase their frequency compared with those observed under control conditions.
Effect of the Kv7 activator retigabine.
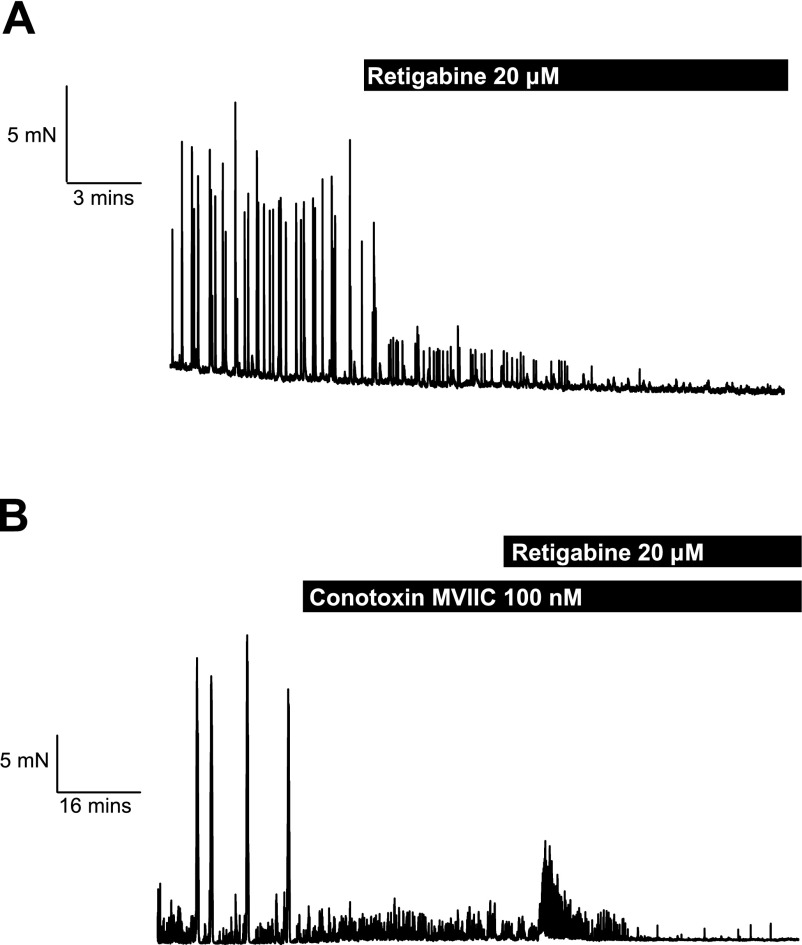
To consolidate the work with Kv7 blockers experiments were performed with the Kv7.2-Kv7.5 activator retigabine (32, 38, 39, 47). Application of 20 μM retigabine, a concentration shown to augment Kv7 activity (20 μM) and to relax precontracted blood vessels (50), inhibited ongoing HALFs or LAHF activity (Fig. 6). Irrespective of the contractile pattern exhibited by the tissues, retigabine (20 μM) inhibited spontaneous contractile activity by 68.9 ± 6.7% of the integral of tension (n = 4, P < 0.05). The inhibitory effect was not reversed by application of the nonselective Kv channel blocker 4-AP (1 mM), but 10 μM XE991 increased the integral of tension significantly (577 ± 252%, n = 4, P < 0.01). In tissues exhibiting LAHFs, inhibition by retigabine resulted in an initial slight increase in HALF activity (Fig. 6) as the suppressive events associated with LAHF discharge were removed, followed by a progressive reduction in HALF contractions. These observations suggested that retigabine influenced myenteric nerves as well as smooth muscle activity. Consequently, experiments were undertaken in the presence of ω-conotoxin MVIIC to remove nerves' input. Under these conditions retigabine produced a rapid suppression of HALF activity with the mean integral of tension being reduced by 68.7 ± 6.4 (n = 3, P < 0.05). These data are consistent with Kv7 channels other than Kv7.1 having a crucial role in the distal colon.
Fig. 6.
Effect of the Kv7 activator retigabine on contractile activity on murine distal colon. A: a representative trace of retigabine (20 μM) on a segment of distal colon displaying LAHFs and HALFs. B: effect of retigabine (20 μM) in the presence of ω-conotoxin MVIIC (100 nM).
DISCUSSION
Kv7 channels are emerging as major regulators of smooth muscle contractility (4, 10) in addition to their accepted roles in the heart and neurons. To date most research on smooth muscle KCNQ gene expression and Kv7 channel function has been undertaken on vascular myocytes (16, 18, 23, 48–51). Consequently, the present study represents the first extensive characterization of KCNQ expression in the GI tract and a determination of a functional role for the expression products in regulating colonic activity. The data clearly show that the most abundant transcripts throughout the GI tract are KCNQ4 and KCNQ5 as well as the auxiliary subunit KCNE4. Moreover, Kv7.4 and Kv7.5 proteins were apparent in the muscle layers as well as the epithelium whereas Kv7.1 was localized in the epithelium only. This finding suggests that in addition to the accepted role of Kv7.1/KCNE3 in colonic epithelium (35), Kv7.4 and 7.5 may also be key regulators of colonic fluid balance.
Because Kv7 channel expression was highest in the colon, we focused our attention on the potential functional roles for these channels in this tissue using pharmacological tools. XE991 blocks heterologously expressed Kv7 channels with an IC50 ∼1 μM (43) except for Kv7.5, which is less sensitive (IC50 ∼60 μM) (34, 49). Linopirdine is slightly less potent than XE991 (IC50 values ∼4–8 μM) (44). At the concentrations used in the present study (0.01–10 μM) neither XE991 nor linopirdine has any known effect on any conductance other than Kv7 channels (see Refs. 10 and 50 for more complete discussion). Similarly, retigabine has only been shown to activate Kv7.2–7.5 channels (32, 39, 47) and to augment XE991-sensitve K+ currents in vascular myocytes (51) at the concentrations used in the present study. With the above caveats in mind, we now show that increasing Kv7 opening probability with retigabine or blockade with XE991/linopirdine had marked inhibitory and excitatory effects on spontaneous colonic motility, respectively. However, careful consideration must be given to the cellular target of these compounds. The activity of GI preparations in vitro is determined by the complicated balance of the influences of pacemaker ICC, the inherent spontaneous property of smooth muscle cells and the enteric nervous system (14, 30). As in numerous other studies of preparations of mouse colon (3, 8, 26, 27), these preparations generally exhibited spontaneous activity. This consisted of either LAHF or HALF contractions. HALF activity was consistently inhibited by agents that interfere with neurotransmission (tetrodotoxin and ω-conotoxin MVIIC), strongly suggesting that this activity is regulated by the myenteric plexus in some way and may represent the motor complexes observed to migrate regularly in longer segments of this preparation (26). The LAHF activity that persisted in the presence of neuronal blockade was an intrinsic property of the smooth muscle layer and/or ICC network. Our data point to a direct effect of Kv7 blockers and retigabine, and hence an involvement of Kv7 channels, on this intrinsic activity. Thus, although XE991 produced an increase in HALF activity in preparations already exhibiting these contractions, it never evoked HALF contractions in preparations not undergoing this kind of spontaneous activity. Indeed, in preparations demonstrating baseline LAHF activity, XE991 caused an obvious increase in frequency and amplitude of these contractions. Furthermore, the effect of XE991 was similar in preparations in which HALF activity had been abolished by neuronal blockade, leaving only the LAHF contractions. Finally, we observed no Kv7 immunoreactivity in myenteric ganglia nor nerve terminals in the smooth muscle layer to suggest that XE991 had an indirect action on smooth muscle cells within the preparation. Our combined molecular, biochemical, and pharmacological findings point overwhelmingly to a role for Kv7.4/7.5 channels in the ICC or smooth muscle layer of the colon.
It is difficult to dissect the relative contributions of the intrinsic myogenic activity of colonic smooth muscle, the influence of pacemaker ICC and the enteric nervous system. A previous study showed an abundance of transcripts for KCNQ5 in intramuscular interstitial cells of Cajal in the small intestine [ICC associated with the deep muscular plexus (ICC-DMP) in that organ] (6), and some discreet labeling with anti-Kv7.5 was observed in the myenteric region between the circular and longitudinal muscle layers in the present study. Our working model for the activity of the mouse colon is one in which ICC generate the pattern for HALF activity but are rigidly controlled by the influence of the enteric nervous system. Therefore, under conditions of neuronal blockade, only the influence of unstimulated ICC, or indeed the intrinsic contractility of the smooth muscle cell layer alone, is apparent. Since the ICC network and smooth muscle layer are a functional syncytium (31), it is impossible to determine from the present experiments whether XE991 depolarized ICC or the smooth muscle layer to elicit an excitatory effect. The simplest explanation consistent with findings in vascular smooth muscle (50) is that the Kv7 channels expressed on GISM are the major target of Kv7 blockers to increase colonic motility in this preparation. This does not discount the possibility that a significant fraction of the expression of these channels might be localized to ICC. This is interesting since Kv7 channels are involved in neurotransmission in the central nervous system (15), and the intramuscular ICC in the GI tract make synaptic contacts with enteric motor neurons and mediate postjunctional responses to both nitrergic and cholinergic neurotransmission (45, 46). Ongoing work in our laboratories aims to determine the cell type-specific expression of Kv7 channels and whether our present findings are applicable to other species, including human.
In the presence of low concentrations of ω-conotoxin MVIIC (100 nM) sufficient to suppress HALF activity, XE991 restored HALF contractions. This concentration of ω-conotoxin MVIIC blocks excitatory neurotransmission but was probably insufficient to inhibit inhibitory motor drive to the smooth muscle, since it did not mimic the effects of TTX or a high concentration of ω-conotoxin. Basal inhibitory motor activity is known to dominate in this preparation, where TTX increases contractility and/or causes smooth muscle depolarization (1, 7, 9, 37). By increasing smooth muscle sensitivity to excitatory stimuli, XE991 appeared to be able to restore the partial paralysis of HALF activity induced by low concentrations of ω-conotoxin MVIIC. The logical corollary to this is that blockade of Kv7 channel in the smooth muscle by XE991 amplified the cellular effect of reduced transmitter release, highlighting the key role of Kv7 channels as suppressors of colonic contractility. It would be of considerable interest to determine whether XE991 has a similar effect in models of GI disease that have been shown to cause alterations in muscle activity and sensitivity to neurotransmitters. Thus, on the basis of our data, it would appear that Kv7-blocking drugs might be valuable therapeutics in human disease, where similar changes are observed.
Interestingly, XE991 was a far more effective stimulant in the distal compared with proximal colon. We postulated that this reflected a difference in the levels of KCNQ gene expression between the two regions. However, there was no difference in the relative abundance of KCNQ4 or -5 transcripts in these regions but there was a significant disparity in KCNE4 expression. The protein encoded by this gene suppresses Kv7.1 channel activity but augments Kv7.4 activity (11). It is tempting to speculate that the functional impact of the Kv7 channels in the colon may be regulated by the expression of KCNE proteins, which allows the level of functional control to be finely tuned.
In conclusion, the results of the present study strongly suggest that Kv7 channels encoded by KCNQ genes are important in limiting contractile activity in the gastrointestinal tract, and particularly in the colon. Our findings suggest the Kv7.4 and Kv7.5 may be the specific members of this family that play a role in these tissues. The excitatory effect of Kv7 activators suggests that similar compounds with increased selectivity for these members of the Kv7 channel family may be useful drugs in the treatment of chronic constipation associated with irritable bowel syndrome and similar motility diseases.
GRANTS
This work was supported by Grant-in-Aid for Young Scientists from Japan Society for the Promotion of Science, Nagai International Foundation, and the Pharmacological Research Foundation, Tokyo (SO). T. A. Jepps was supported by a grant from the St George's Hospital Charitable Foundation. Work in the laboratory of I. A. Greenwood is supported by the British Heart Foundation. The initial expression studies for this study were begun in the laboratory of Dr. Burton Horowitz via funding from DK41315 from the National Institute of Digestive Diseases and Kidney.
REFERENCES
- 1.Alberti E, Jimenez M. Effect of 4-aminopyridine (4-AP) on the spontaneous activity and neuromuscular junction in the rat colon. Pharmacol Res 52: 447–456, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Benham CD, Bolton TB. Patch-clamp studies of slow potential-sensitive potassium channels in longitudinal smooth muscle cells of rabbit jejunum. J Physiol 340: 469–486, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brierley SM, Nichols K, Grasby DJ, Waterman. Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol 132: 507–517, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, Byron KL. Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H1352–H1363, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carl A Multiple components of delayed rectifier K+ current in canine colonic myocytes. J Physiol 484: 339–353, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Ordög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics 31: 492–509, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil 9: 99–107, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Fontaine J, Lebrum P. Pharmacological analysis of the effects of Bay K 8644 and organic calcium antagonists on the mouse isolated distal colon. Br J Pharmacol 94: 1198–1206, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez A, Sarna SK. Neural regulation of in vitro giant contractions in the rat colon. Am J Physiol Gastrointest Liver Physiol 281: G275–G282, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood IA, Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol 156: 1196–1203, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunnet M, Jespersen T, Rasmussen HB, Ljungstrøm T, Jorgensen NK, Olesen SP, Klaerke DA. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol 542: 119–130, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br J Pharmacol 129: 413–415, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart PJ, Overturf KE, Russell SN, Carl A, Hume JR, Sanders KM, Horowitz B. Cloning and expression of a Kv1.2 class delayed rectifier K+ channel from canine smooth muscle. Proc Natl Acad Sci USA 90: 9659–9663, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol 61: 19–43, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Jentsch TJ Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res 7: 31, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lerche C, Bruhova I, Lerche H, Steinmeyer K, Wei AD, Strutz-Seebohm N, Lang F, Busch AE, Zhorov BS, Seebohm G. Chromanol 293B binding in KCNQ1 (Kv7.1) channels involves electrostatic interactions with a potassium ion in the selectivity filter. Mol Pharmacol 71: 1503–1511, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther 325: 475–483, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCallum LA, Greenwood IA, Tribe RM. Expression and function of Kv7 channels in murine myometrium throughout oestrous cycle. Pflügers Arch 457: 1111–1120, 2009. [DOI] [PubMed] [Google Scholar]
- 20.McCrossan ZA, Abbott GW. MinK-related peptides. Neuropharmacology 47: 787–821, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Watanabe H, Kubo Y, Yokoyama M, Matsumoto T, Sasai H, Nishi Y. KQT2, a new putative potassium channel family produced by alternative splicing. Isolation, genomic structure, and alternative splicing of the putative potassium channels. Receptors Channels 5: 255–271, 1998. [PubMed] [Google Scholar]
- 22.Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional expression of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol 282: G277–G287, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Ohya S, Sergeant GP, Greenwood IA, Horowitz B. Molecular variants of KCNQ1 channels expressed in murine portal vein myocytes: a component of delayed rectifier current. Circ Res 92: 1016–1023, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Ohya S, Tanaka M, Watanabe M, Imaizumi Y. Diverse expression of delayed rectifier K+ channel subtype transcripts in several types of smooth muscles of the rat. J Smooth Muscle Res 36: 101–115, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Overturf KE, Russell SN, Carl A, Vogalis F, Hart PJ, Hume JR, Sanders KM, Horowitz B. Cloning and characterization of a Kv1.5 delayed rectifier K+ channel from vascular and visceral smooth muscles. Am J Physiol Cell Physiol 267: C1231–C1238, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Porcher C, Horowitz B, Ward SM, Sanders KM. Constitutive and functional expression of cyclooxygenase 2 in the murine proximal colon. Neurogastroenterol Motil 16: 785–799, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Powell AK, Fida R, Bywater RAR. Motility in the isolated mouse colon: migrating motor complexes, myoelectric complexes and pressure waves. Neurogastroenerol Motil 15: 257–266, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Robbins J KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 90: 1–19, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Russell SN, Overturf KE, Horowitz B. Heterotetramer formation and charybdotoxin sensitivity of two K+ channels cloned from smooth muscle. Am J Physiol Cell Physiol 267: C1729–C1733, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Sanders KM Ionic mechanisms of electrical rhythmicity in gastrointestinal smooth muscles. Annu Rev Physiol 54: 439–453, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and mink (IsK) proteins to form cardiac IKs potassium channel. Nature 384: 80–83, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Schenzer A, Friedrich T, Pusch M, Saftig P, Jentsch TJ, Grötzinger J, Schwake M. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci 25: 5051–5060, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmalz F, Kinsella J, Koh SD, Vogalis F, Schneider A, Flynn ERM, Kenyon JL, Horowitz B. Molecular identification of a component of delayed rectifier current in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 274: G901–G911, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem 275: 24089–24095, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Sims SM, Walsh JV Jr, Singer JJ. Substance P and acetylcholine both suppress the same K+ current in dissociated smooth muscle cells. Am J Physiol Cell Physiol 251: C580–C587, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Spencer NJ, Bywater RAR, Taylor GS. Evidence that myoelectric complexes in the isolated mouse colon may not be of myogenic origin. Neurosci Lett 250: 153–156, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Streng T, Christoph T, Andersson KE. Urodynamic effects of the K+ channel (KCNQ) opener retigabine in freely moving, conscious rats. J Urol 172: 2054–2058, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed K.CNQ potassium currents, and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci 21: 5535–5545, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thornbury KD, Ward SM, Sanders KM. Outward currents in longitudinal colonic muscle cells contribute to spiking electrical behavior. Am J Physiol Cell Physiol 263: C237–C245, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Thornbury KD, Ward SM, Sanders KM. Participation of fast-activating, voltage-dependent K currents in electrical slow waves of colonic circular muscle. Am J Physiol Cell Physiol 263: C226–C236, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Tomita T Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Smooth Muscle: an Assessment of Current Knowledge, edited by Bulbring E, Brading AF, Jones AW, Tomita T. London: Arnold, 1981, p. 127–156.
- 43.Wang HS, Brown BS, McKinnon D, Cohen IS. Molecular basis for differential sensitivity of KCNQ and IKs channels to the cognitive enhancer XE991. Mol Pharmacol 57: 1218–1223, 2000. [PubMed] [Google Scholar]
- 44.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282: 1890–1893, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol 576: 675–682, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol 573: 147–159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wuttke TV, Seebohm G, Bail S, Maljevic S, Lerche H. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol Pharmacol 67: 1009–1017, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Yeung SY, Greenwood IA. Electrophysiological and functional effects of the KCNQ channel blocker XE991 on murine portal vein smooth muscle cells. Br J Pharmacol 146: 585–595, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeung SY, Lange W, Schwake M, Greenwood IA. Expression profile and characterisation of a truncated KCNQ5 splice variant. Biochem Biophys Res Commun 371: 741–746, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Yeung SY, Pucovský V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA. Molecular expression and pharmacological identification of a role for Kv7 channels in murine vascular reactivity. Br J Pharmacol 151: 758–770, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeung SY, Schwake M, Pucovský V, Greenwood IA. Bimodal effects of the Kv7 channel activator retigabine on vascular K+ currents. Br J Pharmacol 155: 62–72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]