Abstract
Recent studies have demonstrated common cortical activity regions associated with esophageal acidification and swallowing. The effect of sensory signals imparted on these regions by esophageal acidification on swallow-related brain activity has physiological and clinical ramifications. Our aim in this study was to determine the effect of prior, unperceived esophageal acid exposure on cortical activity associated with swallowing. Functional magnetic resonance imaging (fMRI) techniques monitored brain activity associated with volitional swallowing before and after subliminal esophageal acid stimulation. Studies were carried out in two phases. In phase I (15 healthy, right-handed subjects, age 21–49 yr, 7 female) using whole brain imaging, we documented the potentiating effects of esophageal acidification alone on swallow-related cortical activity. In phase II (10 healthy, right-handed subjects, age 20–54 yr, 5 female) using high-resolution fMRI, we measured swallow-induced regional brain activity within the cortical swallowing network before and after esophageal acidification. Unlike the phase I studies, we also tested the effect of saline perfusion alone on the cortical swallowing network in the phase II studies. Because of constraints imposed by high-resolution MRI for region-of-interest (ROI) analysis, we studied only the left hemisphere in this phase. None of the subjects developed heartburn during acid perfusion. In phase I, the number of swallow-induced activated voxels increased by 43% following esophageal acid stimulation (preacid, 44 ± 3 voxels; postacid, 63 ± 6 voxels; means ± SE, P < 0.05) In phase II, contrary to saline perfusion, ROI analysis showed significantly increased regional swallow-related fMRI activity volumes as well as percent maximum signal change after esophageal acid perfusion in cingulate, prefrontal, insula, and sensory/motor regions (P < 0.05). The precuneus showed no significant change. We concluded that subliminal esophageal acid stimulation has a potentiating effect on the cortical swallowing network in healthy individuals.
Keywords: subliminal, esophagus, acidification, deglutition, functional MRI
esophageal acid exposure activates a number of cerebral cortical subregions including the cingulate gyrus (11, 12, 15, 27), the prefrontal region (11, 12, 15), the sensory/motor region (11, 12, 15, 27), the insula (11, 12, 15, 27), and the precuneus (11, 12, 15). These same subregions have also been identified as members of the cortical swallowing network during volitional swallowing (2, 7, 8, 10, 13, 16–18, 22, 23, 30). However, the effect of esophageal acid exposure on swallow-related activity of these cortical subregions has not been systematically evaluated.
The aim of the present study was to test the hypothesis that the cortical activity within the swallowing network can be augmented by esophageal acid exposure. We therefore determined the effect of esophageal acid exposure on cerebral cortical functional magnetic resonance imaging (fMRI) activity volume and signal intensity changes in the previously defined cortical swallow network. Studies were performed in two phases. Phase I studies were done to verify the ability of fMRI to reliably detect the effect, if any, of esophageal acidification on the cortical swallowing network. Phase II studies were performed to determine these effects in each of the cortical subregions involved in swallowing. To avoid any of the confounding effects of cognitive processes such as inducement of heartburn or pain related to the esophageal acid perfusion, we used subliminal esophageal acid stimulation (11, 12).
METHODS
We studied a total of 25 healthy subjects. The Human Research Review Committee of the Medical College of Wisconsin approved the study, and all volunteers gave written informed consent before the study.
Phase I studies.
Fifteen healthy adult subjects were studied in this phase (age range 21–49 yr, 7 female). Subjects were recruited by advertisement. Following a detailed health questionnaire and interview with a physician, subjects were found to have no gastrointestinal symptoms, no diagnosis of gastroesophageal reflux disease, and no dysphagia.
All subjects were studied using paradigm-driven, fMRI scanning techniques. Gradient echo planar MRIs were acquired using a 3.0-tesla GE Signal System (General Electric Medical Systems, Waukesha, WI) equipped with a custom three-axes head coil designed for rapid gradient field switching and a shielded transmit/receive birdcage radiofrequency coil. The MR scanner and head coil were used to acquire a time course of echo planar images (EPI) over the whole brain volume. Eleven contiguous 12-mm thick sagittal slices, respectively, were acquired. One hundred twenty images were captured with an echo time of 41.6 ms and a repetition time of 1,000 ms. EPIs were 64 × 64 within slice pixel resolution over a 240-mm field of view (FOV). High-resolution spoiled gradient recalled acquisition at steady-state (GRASS) images were also obtained consisting of whole brain 1.2-mm-thick slices over a 240-mm FOV and 256 × 256 within slice pixel resolution. These high-resolution anatomical images were used for subsequent superposition of cortical activity regions derived from the lower-resolution echo planar blood oxygenation level-dependent (BOLD) contrast image data. All MRI data were stereotaxically transformed to the Talairach-Tournoux coordinate system for comparison and display purposes.
All fMRI signal analysis was carried out using the analysis of functional neuro-imaging (AFNI) software (written and maintained by Robert Cox, PhD, and the Scientific and Statistical Computing Core of the National Institute of Mental Health Intramural Research Program) (4). Subtle changes in head position during MRI scanning sessions were corrected using 3-dimensional (3D) volume registration that realigns motions of a few millimeters and rotations of a few degrees using first-order Taylor series at each point in the six motion parameters (3 shifts, 3 angles) and Fourier interpolation. General linear modeling (multiple regression and deconvolution) techniques that compute the hemodynamic response function from the magnetic signal time series in each voxel were used to detect cortical regions that exhibit BOLD changes and test whether the response function differs from the response associated with random Gaussian variation of the signal.
Subjects were intubated transnasally with a multilumen catheter such that an acid perfusion port was positioned 5 cm above the manometrically determined upper margin of the lower esophageal sphincter (LES). A Harvard Infusion pumping system was used to deliver acid or saline through the catheter to the distal esophagus at a controlled flow rate of 1 ml/min at desired times during the fMRI scanning sessions. After intubation, subjects were positioned in the scanner.
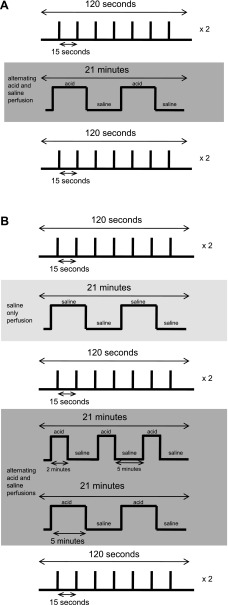
A schematic representation of the event-related experimental paradigm is shown in Fig. 1A. As seen, two scans of single-trial swallows were performed wherein swallows were separated by 15 s of rest. Each scan for the series of single-trial swallows was 2 min in total scanning time. Following the two initial swallowing scans, 1 ml/min perfusion of the distal esophagus with alternating 5-min intervals of 0.1 N HCl and saline solutions was performed over a 21-min time span. Acid was perfused at the catheter port 5 cm above the upper margin of the manometrically determined LES high-pressure zone. This intermittent form of esophageal acidification was used to simulate physiological acid reflux events. Two additional single-trial swallowing scans were completed after esophageal acid perfusion. All phase I subjects repeated the study protocol within 2 wk of their first study.
Fig. 1.
A: schematic representation of the event-related experimental paradigm from the phase I studies. Two functional magnetic resonance imaging (fMRI) scans of single-trial swallows were performed before and after esophageal acid perfusion. B: schematic representation of the event-related experimental paradigm from the phase II studies. Two fMRI scans of single-trial swallows were performed before and after esophageal acid perfusion. Various esophageal acid perfusion scenarios were used to simulate physiological reflux events.
Phase II studies.
Ten right-handed healthy subjects (mean age 44 ± 6 yrs) were studied. All participants completed a detailed health-related questionnaire and upper endoscopy to confirm absence of mucosal disease. Healthy subjects also underwent 24-h esophageal pH recording, which showed normal results for all tested subjects.
A double-lumen catheter was placed transnasally with infusion ports positioned 5 cm above the top of the LES. The subject was then placed supine in the General Electric 3T Scanner (as described in Phase I studies). A projection screen was placed at the foot of the bed. Anatomic scans were acquired using the spoiled-GRASS technique (256 × 256 pixel matrix over a 240-mm FOV). As seen schematically in Fig. 1B, paradigm-driven MRI data were acquired during 120-s scan sessions while subjects performed seven event-related dry swallows on visual cue, before and after distal esophageal infusion of saline solution alone and after alternating 0.1 N hydrochloric acid and normal saline infused at 1 m;/min. Two different acid perfusion schemes were performed in each subject, one 21-min session of 2 min of acid perfusion alternating with 5 min of saline perfusion and another session of 5 min of acid perfusion alternating with 5 min of saline perfusion. Both patterns of esophageal acidification were given followed by the fMRI swallowing scans; therefore, the effect of each individual pattern of esophageal acid exposure on the cortical swallowing network could not be evaluated using the protocol of the present study. These two methods of esophageal acid stimulation were designed to simulate physiological acid reflux episodes. Subjects were queried as to whether they experienced any changes in sensation during or after the perfusion scans.
Fifteen contiguous sagittal gradient high-resolution echo planar slices were obtained of the left hemisphere. MRIs were acquired at a repetition time of 1.2 s and an echo time of 40 ms. The left hemisphere was studied to facilitate using high-resolution EPI techniques. The slice thickness for our phase II studies was 4.5 mm, and a slice-wise pixel resolution of 96 × 96 pixels over a 240-mm FOV yielded a within-slice resolution of 2.5 × 2.5 mm. At these high-resolution EPI scanning specifications, computer memory and processing limitation make whole brain scanning untenable. Furthermore, there has been no conclusive evidence showing fMRI activity associated with volitional swallowing to be restricted to any one hemisphere. Studies from our research team (10, 13) as well as from other researchers (8, 16–18, 22) have shown bilateral activation of the cortical swallowing network with greater registration reported in the left hemisphere for all brain regions except the insula in which right hemispheric dominance has been reported in some studies and left hemisphere dominance has been reported in other studies. Because the limitation of the scanner technology constrains imaging of both hemispheres and all historical data point to either hemisphere as a viable target for measuring fMRI signal changes, the left hemisphere was arbitrarily chosen for our study scans.
All image data were mapped stereotaxically to the Talairach-Tournoux coordinate system for comparison purposes. Data analysis was performed using AFNI (4) software by the general linear model technique. The studied regions of interest were the cingulate gyrus, the insula, the prefrontal region, the precuneus, and the motor cortex. For the purposes of our analysis, the cingulate region was defined a priori as the portion of the cortex confined by the cingulate sulcus and consisting of Brodmanns areas (BA) 23, 24, 25, 29, 30, 31, 32, and 33. The prefrontal region was defined as predominantly the dorsolateral prefrontal cortex (BA 9) as well as BA 8, 10, 11, 46, and 47. The insula was defined as the region deep within the lateral fissure consisting of several long gyri parallel to the lateral fissure as well as five short gyri more rostral (BA 13). The precuneus region was defined as the division of the medial surface of the cortex between the cuneus and the paracentral lobule (BA7). The motor region was defined as the precentral gyrus on the lateral surface of the cortex extending into the longitudinal cerebral fissure medially (BA4) as well as the supplementary motor cortex and supplementary motor area (BA6).
All parametric statistical values are reported as means ± SE unless otherwise stated. Significance levels for all hypothesis testing were set at P < 0.05 corrected for multiple comparisons when appropriate.
RESULTS
All subjects tolerated the fMRI scanning and esophageal acid perfusion without complaint or complication. None of the subjects reported heartburn, pain, pressure, or dysphagia when queried after the scanning sessions.
Phase I studies.
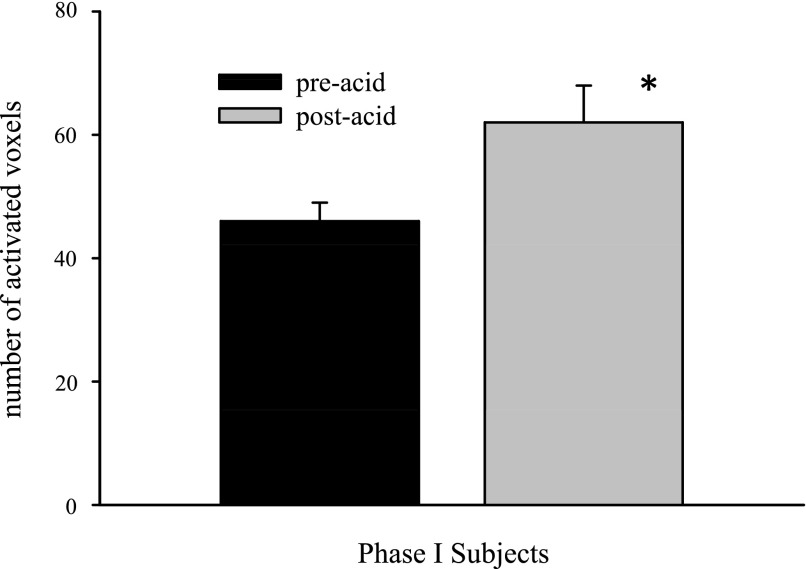
Swallowing was associated with a distributed network of fMRI activity, which included the anterior, mid- and posterior cingulate, the insula, the primary and secondary sensory/motor cortices, the prefrontal region, and the precuneus believed to comprise the cortical swallowing network. The volume of fMRI activity over the entire brain showed a significant increase associated with swallowing following esophageal acid stimulation (Fig. 2). Reproducibility of this finding between two different study days is shown in Fig. 3.
Fig. 2.
Volume of cerebral cortical fMRI activity over the entire brain across all subjects (n = 15) in phase I studies. Average swallow-related fMRI activity was significantly augmented after esophageal acid exposure (*P < 0.05).
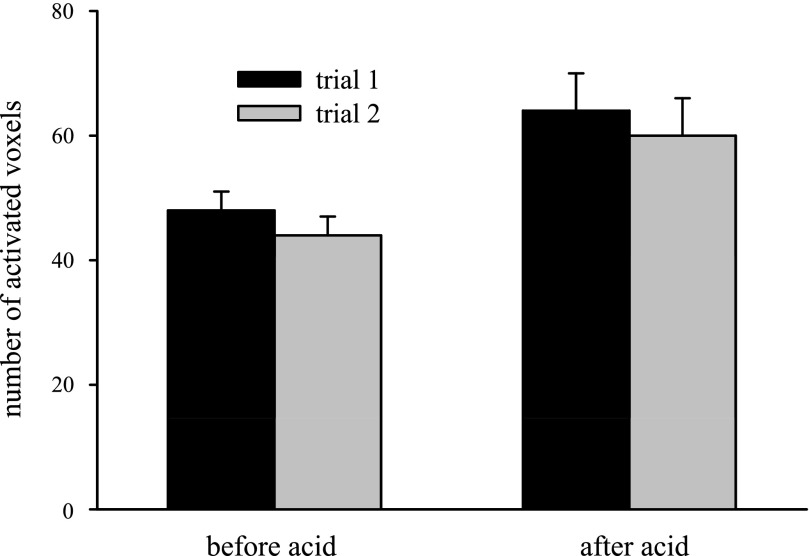
Fig. 3.
Comparison of the 2 swallowing trials showed no significant variability between trials both before and after esophageal acid exposure for all phase I studies. In both trials, esophageal acid stimulation resulted in significant increases in the average number of swallow-related fMRI activity voxels. Voxel counts were similar when comparing results from trials 1 and 2 within the pre- and postinfusion conditions.
Phase II studies.
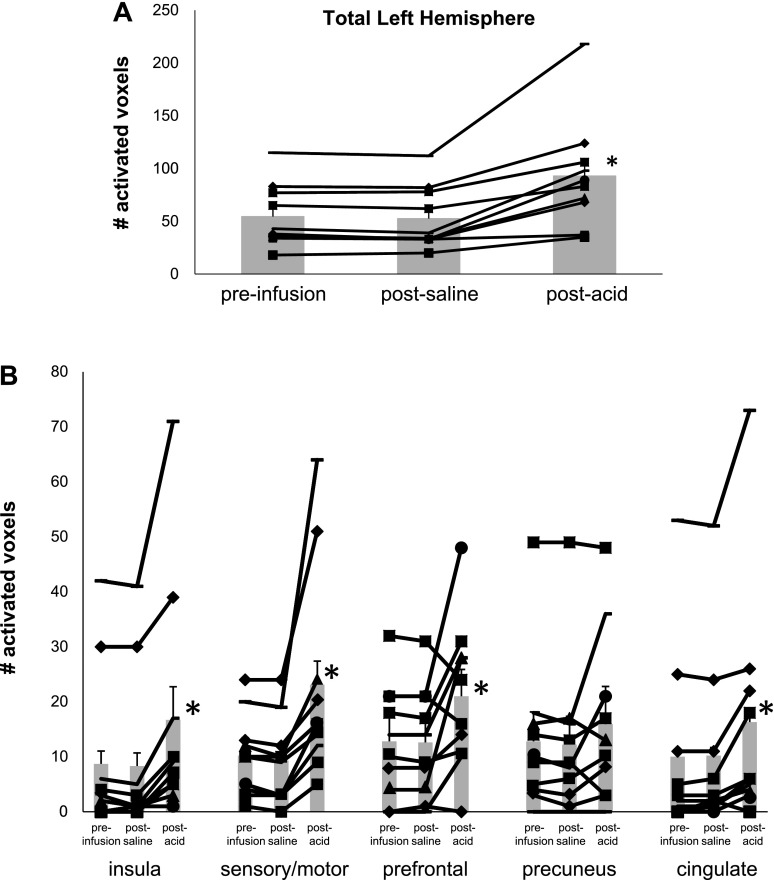
Comparisons of cortical fMRI activity volumes in the studied left hemisphere associated with swallowing before and after esophageal acid stimulation confirmed the findings of the phase I studies and showed accentuation of the cortical swallowing network following esophageal acid stimulation. A scatter plot of brain activity for each tested healthy control (Fig. 4A) shows that acid stimulation resulted in a significant increase in the number of swallow-induced activated voxels over the entire left hemisphere (P < 0.001). With the exception of the precuneus, region-of-interest (ROI) analysis showed significant increases in the number of fMRI-activated voxels in the cingulate gyrus as well as the prefrontal, insula, and sensory/motor regions (P ≤ 0.002). (Fig. 4B) In contrast, no differences were found when comparing presaline infusion to postsaline perfusion swallow-induced fMRI cortical activity volumes. Furthermore, comparison of the number of swallow-induced activated fMRI voxels showed significant differences between those activated following esophageal acid perfusion and those following esophageal saline perfusion (P ≤ 0.025) (Fig. 4).
Fig. 4.
Comparison of regional brain activity is shown by the number of activated voxels preinfusion and after saline and esophageal acid exposure in each healthy control subject. As seen, there were significant differences (*P < 0.05) in the insula, sensory/motor, and prefrontal and cingulate regions (B), as well as across the entire left hemisphere (A).
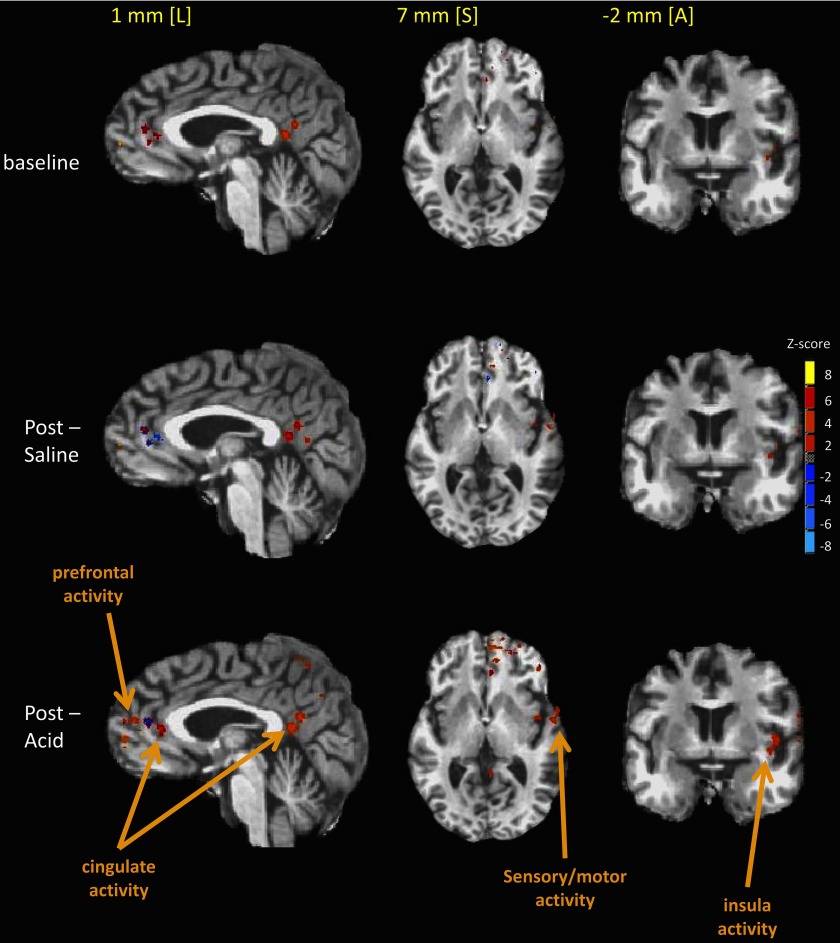
Locations of cerebral cortical swallow-related activity resulting from group analysis of all tested subjects are shown in Fig. 5. There is greater cortical recruitment and increased signal intensities for swallow-related fMRI activity in the cingulate, sensory-motor, prefrontal, and insular regions after esophageal acid stimulation (postacid) compared with after saline perfusion and preinfusion (baseline).
Fig. 5.
Locations of cerebral cortical swallow-related activity resulting from group analysis of all tested subjects are shown as colored areas superimposed on stereotaxic, high-resolution MR anatomical images. All data were transformed to the Talairach-Tournoux coordinate reference frame. Images are shown in the sagittal [1 mm left (L) in the stereotaxic coordinate frame], axial [7 mm superior (S)], and coronal [−2 mm anterior (A)] radiographic views. Different sagittal, axial, and coronal views are shown to illustrate all fMRI regional activity. As seen, there is greater cortical recruitment and increased signal intensities for swallow-related fMRI activity in the cingulate, sensory-motor, prefrontal, and insular region of interest after esophageal acid stimulation (postacid) compared with after saline perfusion and preinfusion (baseline).
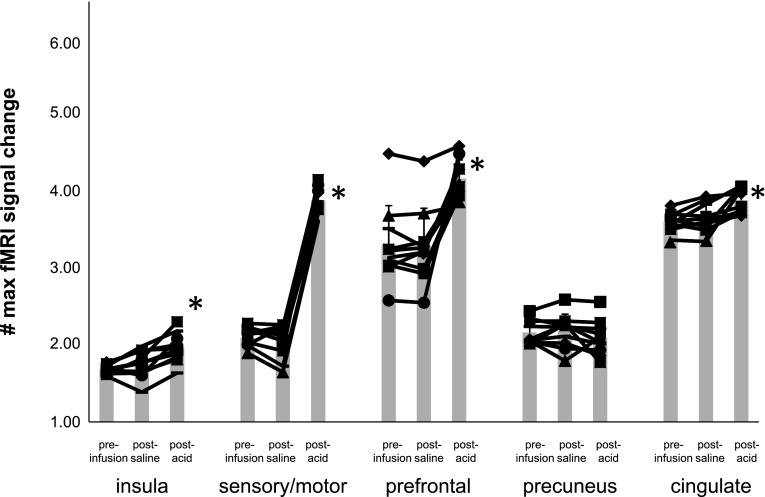
ROI analysis of the maximum fMRI signal change showed significant differences in maximum percent signal increase when comparing fMRI signals associated with swallowing after esophageal acidification to those signals before acidification in the insula, sensory/motor, prefrontal, and cingulate regions (P ≤ 0.002) but not in the precuneus (P = 0.22) (Fig. 6). In addition, similar differences in the same ROI were also found (P ≤ 0.0012) when comparing swallow-related fMRI activity after acid perfusion to activity following saline stimulation (Fig. 6). Esophageal perfusion with normal saline did not result in any significant change in fMRI activity volume nor percent maximum fMRI signal change when comparing preinfusion to postsaline perfusion data.
Fig. 6.
Comparison of regional brain activity is shown comparing the percent maximum fMRI signal change preinfusion and after saline and esophageal acid exposure in each healthy control subject. There were significant differences (*P < 0.05) in the insula, sensory/motor, prefrontal, and cingulate regions following acid exposure but not after saline infusion.
DISCUSSION
In this study, we documented the priming effect of subliminal esophageal acid stimulation on the cortical swallowing network in healthy individuals.
The findings of the present study confirm previous reports that the cortical regions associated with swallowing form a distributed network including the cingulate gyrus, insula, prefrontal cortex, precuneus, and primary sensory/motor cortex (2, 7, 8, 10, 13, 16–18, 22, 23, 30). Our study findings indicate that chemical stimulation of the esophagus at subliminal levels increases the swallow-related fMRI activity volume as well as signal intensity change in all but one of these structures, suggesting priming of the cortical swallowing network.
Earlier studies have shown increases in the esophageal distension-induced fMRI activity of the cingulate and insula following esophageal acid exposure (15). Considering these findings together, it seems that the effect of esophageal acid exposure on the brain-gut axis extends beyond the visceral sensory domain and includes volitional motor functions such as swallowing.
Since components of the cortical swallowing network participate in other functions in addition to swallowing, it is conceivable that the priming effect of esophageal acid exposure may extend even beyond swallowing. The cingulate, for example, is an executive and evaluation center for coupling painful stimulus to fearful responses (5). The midcingulate and posterior cingulate contain the functional component of the cingulate motor areas (31, 32), the midcingulate playing a role in skeletomotor regulation and response selection (32). The posterior cingulate also plays a role in evaluating body orientation in space as well as processing self-relevant emotional and nonemotional information (32). In addition, the posterior cingulate is activated during volitional external anal sphincter contraction (9). The insula, with its many interconnections with limbic and other paralimbic structures as well as sensory association areas, has been implicated as an integrative element in sensory and motor function (1). Esophageal acid exposure also amplified the swallow-related fMRI activity in the prefrontal and sensory/motor regions. Because the experimental paradigm of the present study involved volitional swallowing, the observed amplification of activity may reflect the role of the prefrontal region for planning and activation of the motor region for execution of the muscular events of swallowing. Enhancement of cortical recruitment in the prefrontal regions in healthy controls after esophageal acid exposure may represent a facilitatory function to promote swallowing and thereby clear the esophagus of the acidic material.
Augmentation of neural activity associated with motor processes by afferent sensory stimulation is not without precedent. Nociceptive visceral stimulation in the form of colorectal distension in a rat model has shown modulation of cerebellar Purkinje cell activity (26). Given the direct role that the cerebellum has on motor activity, these reports support the notion of visceral stimulation modulating motor responses (26).
The effects of visceral esophageal acidification on brain-gut activity associated with esophageal sensation such as mechanical and electrical stimulation of the esophagus have been previously reported (6b, 6c, 19, 25, 26, 28, 29, 33–35). These studies clearly document sensitization of the brain-gut axis by esophageal acid stimulation. The cortical manifestation of this sensitization has been reported in two studies (6b, 15).
Studies of the cortical manifestation of esophageal acid exposure on swallow-related activity are limited (3, 14, 24). Cerebral cortical activity can be evaluated by fMRI in terms of changes in fMRI signal intensity and activity volume. Previous studies of the effect of esophageal acid stimulation on swallowing cortical activity evaluated the relative change in fMRI signal intensity (24). In the present study, we determined both the regional changes in fMRI activity volume as well as maximum signal intensity. Findings of the present study with regard to signal intensity changes are at variance with those of a previous report (24), which suggested increases in the signal intensity in some cortical regions and signal intensity decreases in others. These differences could be attributable to differences in sample size, analysis technique, and, perhaps most importantly, experimental design. In the aforementioned study, the esophageal perfusion rate was eight times the perfusion rate of the present study. Such relatively high perfusion rates may stimulate neural responses not activated by the lower perfusion rates of the present study. In addition, acid infusion occurred over a 30-min period, thus introducing 240 ml of acid representing a substantially larger acid load than the 16 ml of acid introduced into the esophagus in the present study. These design differences may introduce additional variables in the previous study that were not a part of our study.
Since our experimental protocol involves repeated exposure of esophageal mucosa to acid, habituation to esophageal acidification is a possible confounding factor in our study. However, there is little evidence of habituation to esophageal acidification as evidenced by the results of the phase I studies wherein subjects returned after 2 wk to repeat the study protocol, resulting in similar findings.
The mechanistic and physiological explanation of our finding that esophageal acid stimulation increases swallow-related activity within the cortical swallowing network can be offered in two different ways. One interpretation is that the increase in swallow-related brain activity after esophageal acid exposure represents priming of the swallow network to facilitate deglutition. This interpretation has some physiological appeal in that a facilitated swallowing network could result in improved esophageal acid clearance. In this way, the priming of the cortical swallowing network would work as a protective mechanism after esophageal acid exposure such as that which occurs during gastroesophageal reflux. Alternatively, increased effort for swallowing can theoretically explain the increased fMRI activity of the swallowing cortical network because prior studies of the cortical representation of external anal sphincter squeeze (10) and finger tapping (6, 6a) have clearly shown that increased levels of effort in performing volitional tasks are associated with increased fMRI cortical activity. However, the reason or potential explanation for the requirement of such increased effort in performing swallowing following unperceived esophageal acid stimulation is unclear and purely speculative. Nevertheless, prior studies have shown increases in esophageal contractile pressure following acid exposure (6b). Reconciling this phenomenon, which mostly affects the smooth muscle segment of the esophagus with the volitional aspect of swallowing as recorded by fMRI, is difficult. Altogether and irrespective of explanation, it seems clear that unperceived esophageal acid exposure (and possibly perceived acid stimulation) enhances the activity of the cortical swallowing network. The physiological consequence of this enhancement remains to be systematically determined.
In conclusion, subliminal acid-induced esophageal sensory input enhances the activity of the cortical swallowing network in healthy individuals. This finding indicates that the effect of esophageal acid exposure on the brain-gut axis extends beyond the visceral sensory domain.
GRANTS
This work was supported in part by NIH Grant 5R01DK025731-27.
REFERENCES
- 1.Augustine JR Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 22:229–244, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp 7: 106–114, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chai K, Kern M, Kounev V, Shaker R. Subliminal esophageal acid stimulation sensitizes the cortical swallowing network in healthy individuals but not GERD patients (Abstract). Neurogastroenterol Motil 18: 680, 2006. [Google Scholar]
- 4.Cox RW AFNI software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126: 2139–2152, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Dai TH, Lui JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional-MRI brain activation. Exp Brain Res 140: 290–300, 2001. [DOI] [PubMed] [Google Scholar]
- 6a.Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 74: 802–808, 1995. [DOI] [PubMed] [Google Scholar]
- 6b.Drewes AM, Reddy H, Staahl C, Pedersen J, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Sensory-motor responses to mechanical stimulation of the esophagus after sensitization with acid. World J Gastroenterol 11: 4367–4374, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c.Drewes AM, Pedersen J, Reddy H, Rasmussen K, Funch-Jensen P, Arendt-Nielsen L, Gregersen H. Central sensitization in patients with non-cardiac chest pain: a clinical experimental study. Scand J Gastroenterol 41: 640–649, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20: 135–144, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol Gastrointest Liver Physiol 277: G219–G225, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Kern M, Arndorfer RC, Hyde JS, Shaker R. Cerebral cortical representation of external anal sphincter contraction: effect of effort. Am J Physiol Gastrointest Liver Physiol 286: G304–G311, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 280: G531–G538, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Kern MK, Birn RM, Jaradeh S, Jesmanowicz A, Cox RW, Hyde JS, Shaker R. Identification and characterization of cerebral cortical response to esophageal mucosal acid exposure and distention. Gastroenterology 115: 1353–1362, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Kern MK, Hofmann C, Hyde J, Shaker R. Characterization of the cerebral cortical representation of heartburn in GERD patients. Am J Physiol Gastrointest Liver Physiol 286: G174–G181, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280: G354–G360, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kern MK, Lawal A, Sanjeevi A, Jesmanowicz A, Shaker R. Esophageal acid stimulation sensitizes the swallow-related insular but not cingulate gyrus neural circuitry. Gastroenterology 128, Suppl 2: A373–A374, 2005. [Google Scholar]
- 15.Lawal A, Kern M, Sanjeevi A, Antonik S, Mepani R, Rittmann T, Hussaini S, Hofmann C, Tatro L, Jesmanowicz A, Verber M, Shaker R. Neurocognitive processing of esophageal central-sensitization in the insula and cingulate gyrus. Am J Physiol Gastrointest Liver Physiol 294: G787–G794, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Martin R, Barr A, MacIntosh B, Smith R, Stevens T, Taves D, Gati J, Menon R, Hachinski V. Cerebral cortical processing of swallowing in older adults. Exp Brain Res 176: 12–22, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Martin RE, Goodyear BG, Gati Jus Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Martin RE, Macintosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol 92: 2428–2443, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Medda BK, Sengupta JN, Lang M, Shaker R. Response properties of the brainstem of the cat following intra-esophageal acid-pepsin infusion. Neuroscience 135: 1285–1294, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am J Neuroradiol 20: 1520–1526, 1999. [PMC free article] [PubMed] [Google Scholar]
- 23.Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 109: 1417–1423, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Paine PA, Hamdy S, Chitnis X, Gregory LJ, Giampietro V, Brammer M, Williams S, Aziz Q. Modulation of activity in swallowing motor cortex following esophageal acidification: a functional magnetic resonance imaging study. Dysphagia 23: 146–154, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Reddy H, Staahl C, Arendt-Nielsen L, Gregersen H, Mohr Drewes A, Funch-Jensen P. Sensory and biomechanical properties of the esophagus in non-erosive reflux disease. Scand J Gastroenterol 42: 432–440, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Saab CY, Willis WD. Nociceptive visceral stimulation modulates the activity of cerebellar Purkinje cells. Exp Brain Res 140: 122–126, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Sami SAK, Rossel P, Dimcevski G, Dremstrup Nielsen K, Funch-Jensen P, Valeriani M, Arendt-Nielsen L, Drewes AM. Cortical changes to experimental sensitization of the human esophagus. Neuroscience 140: 269–279, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar S, Hobson AR, Furlong PL, Woolf CJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol 281: G1196–G1202, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar S, Hobson AR, Hughes A, Growcott J, Woolf CJ, Thompson DG, Aziz Q. The prostaglandin E2 Receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology 124: 18–25, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Toogood JA, Barr AM, Stevens TK, Gati JS, Menon RS, Martin RE. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp Brain Res 161: 81–90, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 18: 3134–3144, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29: 452–466, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willert RP, Delaney C, Kelly K, Sharma A, Aziz Q, Hobson AR. Exploring the neurophysiological basis of chest wall allodynia induced by experimental oesophageal acidification- evidence of central sensitization. Neurogastroenterol Motil 19: 270–278, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson David G, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-d-aspartate receptor. Gastroenterology 126: 683–692, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Yang M, Li ZS, Xu XR, Fang DC, Zou DW, Xu GM, Sun ZX, Tu ZX. Characterization of cortical potentials evoked by oesophageal balloon distention and acid perfusion in patients with functional heartburn. Neurogastroenterol Motil 18: 292–299, 2006. [DOI] [PubMed] [Google Scholar]