Abstract
High-conductance apical K+ (BK) channels are present in surface colonocytes of mammalian (including human) colon. Their location makes them well fitted to contribute to the excessive intestinal K+ losses often associated with infective diarrhea. Since many channel proteins are regulated by phosphorylation, we evaluated the roles of protein kinase A (PKA) and phosphatases in the modulation of apical BK channel activity in surface colonocytes from rat distal colon using patch-clamp techniques, having first increased channel abundance by chronic dietary K+ enrichment. We found that PKA activation using 50 μmol/l forskolin and 5 mmol/l 3-isobutyl-1-methylxanthine stimulated BK channels in cell-attached patches and the catalytic subunit of PKA (200 U/ml) had a similar effect in excised inside-out patches. The antidiarrheal peptide somatostatin (SOM; 2 μmol/l) had a G protein-dependent inhibitory effect on BK channels in cell-attached patches, which was unaffected by pretreatment with 10 μmol/l okadaic acid (an inhibitor of protein phosphatase type 1 and type 2A) but completely prevented by pretreatment with 100 μmol/l Na+ orthovanadate and 10 μmol/l BpV (inhibitors of phosphoprotein tyrosine phosphatase). SOM also inhibited apical BK channels in surface colonocytes in human distal colon. We conclude that cAMP-dependent PKA activates apical BK channels and may enhance colonic K+ losses in some cases of secretory diarrhea. SOM inhibits apical BK channels through a phosphoprotein tyrosine phosphatase-dependent mechanism, which could form the basis of new antidiarrheal strategies.
Keywords: BK channels, colon, patch clamp, potassium secretion
although the apical K+ permeability and K+ secretory capacity of normal human colon are relatively small (15, 24), recent studies suggest that the increased activity and/or expression of high-conductance apical K+ (BK) channels may play an important role in mediating enhanced colonic K+ secretion in several diseases. Thus increased colonic K+ secretion in patients with end-stage renal disease is associated with increases in both the barium-sensitive luminal (apical) K+ permeability and apical BK channel expression along the surface-crypt axis (24). Furthermore, severe K+ secretory diarrhea resulting in profound hypokalemia has been described in colonic pseudo-obstruction (38, 46). Some enteric pathogens may also enhance colonic K+ secretion by activating kinase-regulated apical K+ channels. Activation of cAMP-dependent protein kinase A (PKA) in acute cholera is likely to stimulate colonic K+ secretion, in addition to small intestinal Cl− and water secretion (41). Similar PKA-dependent increases in colonic K+ secretion probably occur in other enteric infections, including Clostridium difficile colitis (13, 20, 26). cAMP-mediated colonic K+ secretion may therefore contribute to excessive stool K+ losses in infective diarrhea, and high-conductance apical K+ (BK) channels in surface cells of human colon (31) are a possible target for PKA-mediated protein phosphorylation.
The level of channel protein phosphorylation reflects a balance between protein kinase and phosphoprotein phosphatase activities. In rat distal colon, inhibition of phosphoprotein tyrosine kinase prevents electrogenic Cl− secretion and attenuates the K+ conductance elicited by carbachol (10). On the other hand, stimulation of phosphoprotein tyrosine phosphatase (possibly linked to inhibitory G protein) is a critical event in the antiproliferative effect of somatostatin (SOM) in both pancreatic and colonic cancer cells (21, 27, 47). A similar mechanism may underlie the antisecretory action of SOM. SOM and its synthetic analog octreotide, which is used clinically as an antidiarrheal agent, suppress basolateral intermediate conductance, Ca2+-dependent (IK) channel activity (a critical component of the Cl− secretory process) in human colonic crypts in an inhibitory G protein-dependent manner (32). This raises the question of whether SOM has a similar effect on apical BK channel-mediated K+ secretion in human colon and, if so, whether it is mediated by a phosphoprotein phosphatase.
There is increasing evidence that apical BK channels have a pivotal role in K+ secretion in colonic epithelia. Distal colonic K+ secretion is abolished by iberiotoxin, a specific BK channel blocker (25), and is entirely absent in BK channel knockout mice (34). Immunohistochemical studies have localized BK channels to the apical membrane in crypt cells (34) and surface cells (12, 29) in mouse colon, as well as surface cells in human colon (31). Patch-clamp techniques have also identified BK channels at low abundance in the apical membrane of surface cells from rat (8) and human (31) distal colon under basal conditions, and here we describe their use to evaluate the regulation of apical BK channels in these epithelia by PKA and SOM.
METHODS
Isolation of rat surface colonocytes.
Previous studies have shown that apical BK channels are present at low abundance in normal rat distal colon, whereas the prevalence of these channels is greatly increased when animals are fed a K+-enriched diet (8). Since the fundamental characteristics of the BK channels were unaffected by chronic dietary K+ loading, we used this model of enhanced apical BK channel expression throughout the present study.
Male Wistar rats weighing 200–250 g were fed a paste chow diet supplemented with KCl (20 g per day; 1.6 mmol K+/g; “K+-loaded” animals) for 10–14 days and allowed access to tap water ad libitum. Animals were killed by inducing CO2 narcosis prior to dislocation of the neck. These animal procedures were approved by the UK Home Office. Segments of distal colon (4 cm) were removed from just above the pelvic brim. Single-surface colonocytes were isolated from distal colon by a modified Ca2+ chelation technique (42). Colonic segments were flushed with an ice-cold solution containing (in mmol/l) 154 NaCl, 10 glucose, and 0.5 dithiothreitol, opened longitudinally, and incubated (30 min at room temperature) in 30 ml of a solution containing (in mmol/l) 30 NaCl, 5 Na2EDTA, 8 HEPES, and 0.5 dithiothreitol, buffered to pH 7.6 with 1 mol/l Trizma base. Colonocytes were released by gentle shaking at 10 min intervals, isolated by centrifugation (600 g for 5 min), and resuspended for 5 min in 25 ml of high-K+ solution containing (in mmol/l) 135 KCl, 1.2 CaCl2, 1.2 MgCl2, 5 Na+ butyrate, 5 glucose, and 10 HEPES, buffered to pH 7.4 with 1 mol/l KOH, and supplemented with 1 mg/ml collagenase Type 1A. Cells were recentrifuged and resuspended in 20 ml high-K+ solution, and the protocol was repeated three times before finally cells were resuspended in 5 ml of high-K+ solution kept on ice. Histology of residual mucosa confirmed that Ca2+ chelation removed surface cells and occasionally cells in the upper 25% of the crypts.
Isolation of human colonic crypts.
With written, informed consent, four or five biopsies of sigmoid colonic mucosa were obtained from patients undergoing colonoscopy for the investigation of altered bowel habit or abdominal pain after 24 h bowel cleansing with Klean-Prep (Norgine). Histology was subsequently shown to be normal in all cases. The study was approved by Leeds Health Authority Ethics Committee. Intact crypts were isolated by Ca2+ chelation as previously described (22), suspended in a storage solution containing (mmol/l): 100 K+ gluconate, 30 KCl, 20 NaCl, 1.25 CaCl2, 1 MgCl2, 10 HEPES, 5 glucose, 5 Na+ pyruvate, 5 Na+ butyrate, supplemented with 1 g/l of bovine serum albumin, buffered to pH 7.4 with 1 mol/l KOH, and kept on ice until required.
Patch-clamp recording.
Single-channel recordings were obtained in cell-attached and excised inside-out configurations (14) from the cell membrane of isolated surface colonocytes (rat colon) and from the apical membrane of surface cells around crypt openings (human colon). Although isolated colonocytes were nonpolarized, previous studies have shown that dietary K+ increases the abundance of BK channels originating from the apical pole of the cell (8). In all of the protocols described, data from each experiment were obtained from a different animal or patient.
Fiber-filled borosilicate patch pipettes were prepared to give tip and membrane seal resistances of 5–10 MΩ and 10–15 GΩ, respectively (8). The bath solution contained (in mmol/l) 140 NaCl, 4.5 KCl, 1.2 CaCl2, 1.2 MgCl2, 5 glucose, 5 Na+ butyrate, and 10 HEPES buffered to pH 7.4 with 1 mol/l NaOH. The pipette solution contained (in mmol/l) 145 KCl, 1.2 CaCl2, 1.2 MgCl2, and 10 HEPES buffered to pH 7.4 with 1 mol/l KOH. Experiments were done at 20–22°C rather than at 37°C to maintain viability (43). Membrane patches were voltage clamped via the patch-clamp amplifier (EPC-7, List Electronics). Single-channel currents were recorded for 30 s and stored on videotape after pulse-code modulation (PCM 701ES, Sony). Stored currents were low-pass filtered at 750 Hz and loaded into computer memory via a DigiData 1200 interface system (Axon Instruments) at a sampling frequency of 2.5 kHz by use of pClamp software (version 5.1, Axon Instruments). Single-channel open probability (Po) was determined by using an analysis program written in Quick Basic 4.0 (Microsoft). Transitions between the fully closed and fully open current levels occurred when the current crossed a threshold set midway between these two states. Single-channel Po was calculated as Po = (∑ntn)/N, where N is the maximum number of channels seen to be open simultaneously during 30 s of recording under a specific set of experimental conditions (verified by the number of peaks on current amplitude histograms generated during single-channel analyses), n represents the state of the channels (0, closed; 1, one channel open, etc.) and tn is the time spent in state n.
Reagents.
Forskolin, 3-isobutyl-1-methylxanthine (IBMX), and PKA catalytic subunit (isolated from the regulatory subunit of the enzyme in bovine heart) were from Calbiochem. ATP, SOM, pertussis toxin (PTX), okadaic acid (a potent and specific inhibitor of type 1 and type 2A protein phosphatases), sodium orthovanadate (an inhibitor of phosphoprotein tyrosine phosphatase), and potassium bisperoxo(1,10-phenanthroline) oxovanadate(V) (BpV, a 1,000-fold more potent inhibitor of phosphoprotein tyrosine phosphatase than sodium orthovanadate) were from Sigma-Aldrich. All but the PKA catalytic subunit were dissolved in dimethylsulfoxide (DMSO) and kept as stock solutions at −20°C. The final concentration of DMSO in the bath solution was <0.01%. The PKA catalytic subunit was stored at −70°C and added directly to the bath.
Statistical analysis.
Results are shown as means ± SE. Comparisons were made using Student's t-test for paired data and ANOVA where appropriate, P < 0.05 indicating a statistical difference between means.
RESULTS
BK channel activation by cAMP in rat colonocytes.
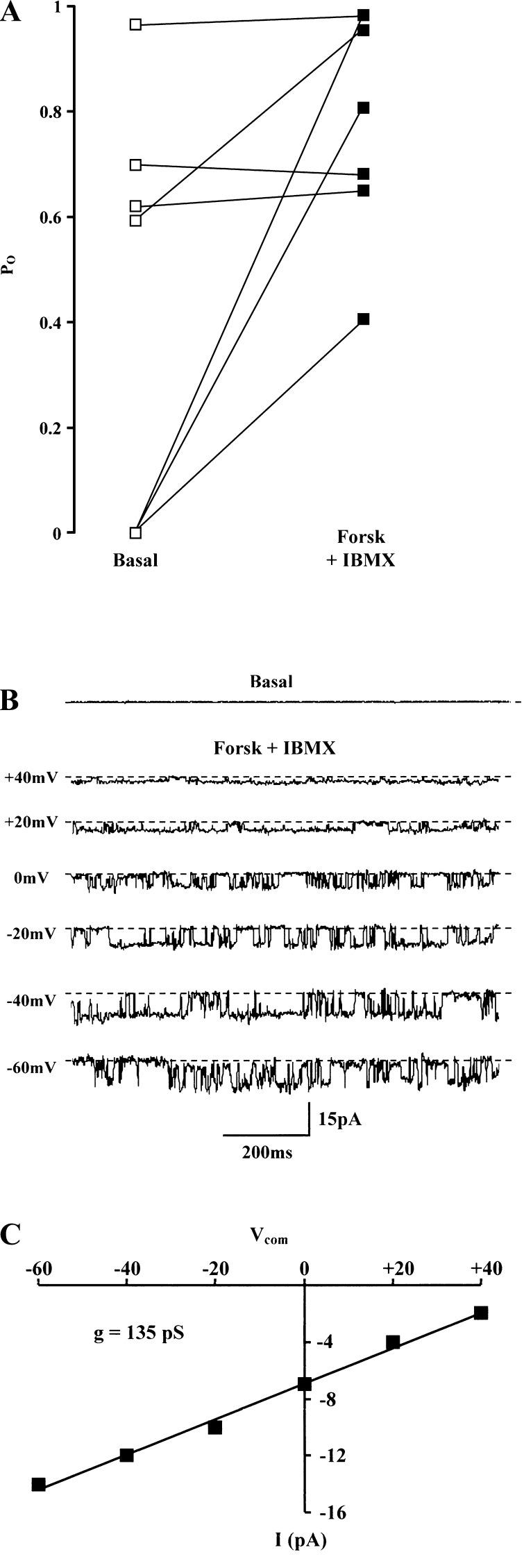
The effect of PKA on BK channel activity was studied in cell-attached patches using 50 μmol/l forskolin and 5 mmol/l IBMX to increase intracellular cAMP concentration. This elicited variable changes in channel activity after 10 min (Fig. 1A). Pooled data from seven patches indicated that forskolin and IBMX increased Po from 0.41 ± 0.15 to 0.79 ± 0.08 (P < 0.05). Figure 1B shows an example of BK channel activation in an initially quiescent patch, whereas no response was seen in other quiescent patches not exposed to forskolin and IBMX (data not shown). The linear current-voltage relationship of this activated channel indicated a single-channel conductance of 135 pS (Fig. 1C). After excision of this patch into the inside-out configuration and changing the bath solution to the high-K+ pipette solution, the estimated single-channel conductance was 210 pS, as previously reported (8).
Fig. 1.
Effect of 50 μmol/l forskolin (Forsk) and 1 mmol/l IBMX on high-conductance apical K+ (BK) channel activity. A: responses in 7 cell-attached patches in surface colonocytes after 10-min exposure [140 mmol/l NaCl in bath and 145 mmol/l KCl in pipette, command voltage (Vcom) = 0 mV]. B: single experiment from A showing a quiescent patch under basal conditions (command voltage = 0 mV), with stimulated channel activity at different command voltages. In this figure and Figs. 3, 5, and 6, dashed lines indicate closed channel current and downward deflections denote inward K+ current flow from pipette to cell. C: current-voltage relationship from experiment shown in B, indicating a single-channel conductance (g) of 135 pS.
BK channel activation by PKA catalytic subunit in rat colonocytes.
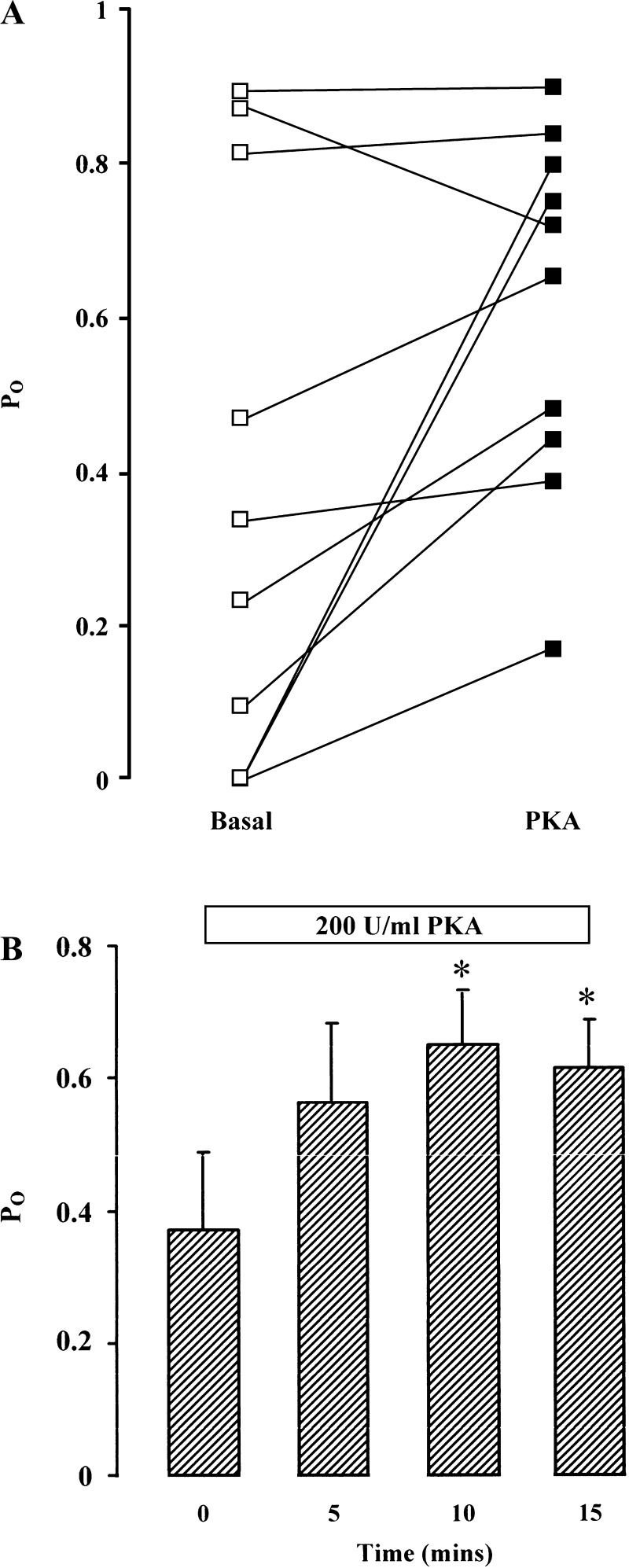
To further elucidate the regulatory role of PKA on BK channels, PKA catalytic subunit (final concentration 200 U/ml) and ATP (final concentration 100 μmol/l) were added to the “cytosolic” side of excised inside-out patches from single colonocytes. There was a variable response in BK channel activity after 10-min exposure to the PKA catalytic subunit (Fig. 2A), similar to that seen when forskolin and IBMX was added to cell-attached patches. Pooled data from 10 patches (Fig. 2B) indicated that the PKA catalytic subunit stimulated BK channel activity after 5 min, although the increase in Po from a basal level of 0.37 ± 0.11 was only significant after 10 min (to 0.64 ± 0.08, P < 0.05) and remained so after 15 min. Together with the response to forskolin and IBMX in cell-attached patches, these results support the view that cAMP-dependent PKA has a direct stimulatory effect on apical BK channels present in surface colonocytes from rat distal colon.
Fig. 2.
Effect of PKA catalytic subunit on BK channel activity. A: responses in 10 excised inside-out patches in surface colonocytes after 15-min exposure to PKA catalytic subunit (200 U/ml) and 100 μmol/l ATP (140 mmol/l NaCl in bath and 145 mmol/l in pipette, command voltage = 0 mV). B: summary of the data from 10 excised inside-out patches showing time course of stimulation of channel activity by PKA catalytic subunit. Po, single-channel open probability.
BK channel inhibition by SOM in rat colonocytes.
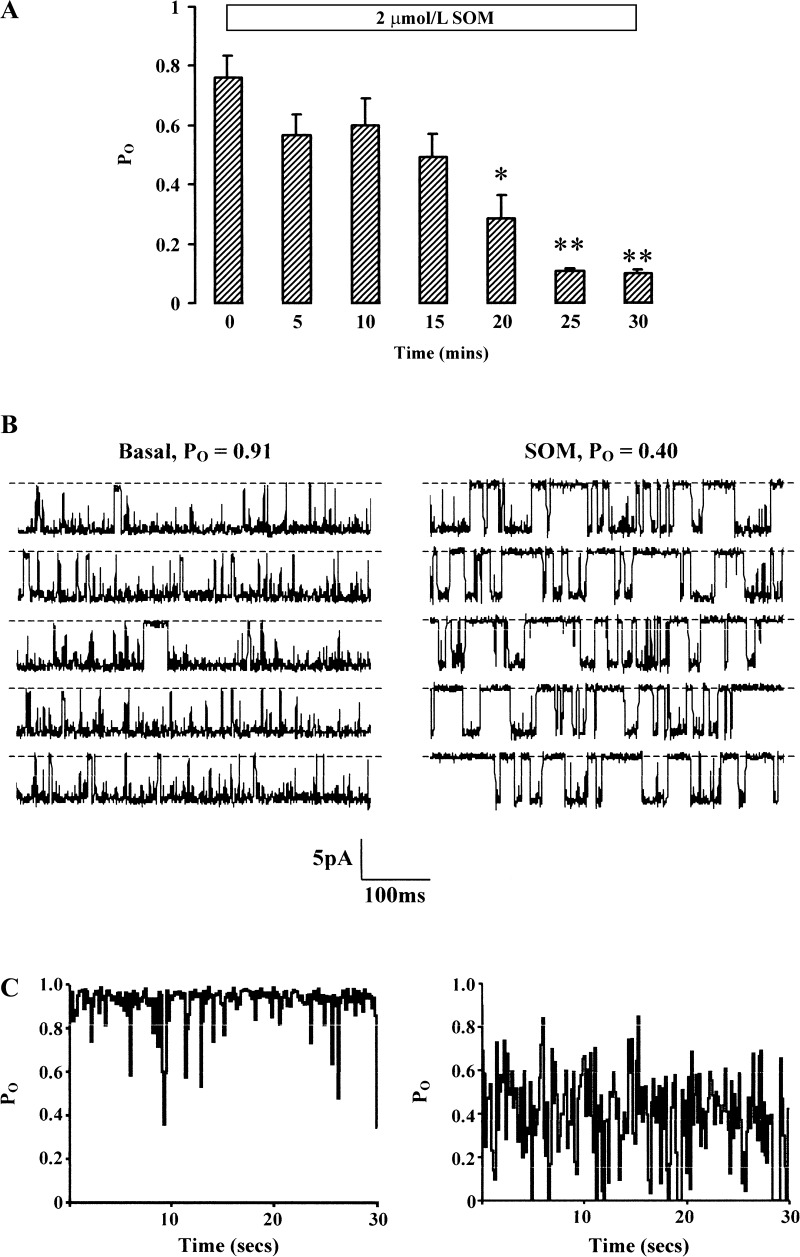
Adding 2 μmol/l SOM to the bath inhibited BK channels in seven cell-attached patches, Po decreasing from 0.76 ± 0.08 to 0.10 ± 0.01 after 30 min (P < 0.001; Fig. 3A). Half-maximal inhibition occurred after ∼17 min (Fig. 3A) and maximal inhibition occurred after 25 min; data from a representative experiment are shown (Fig. 3, B and C).
Fig. 3.
Effect of 2 μmol/l somatostatin (SOM) on BK channel activity. A: time course of SOM-induced inhibition of channel activity in 7 cell-attached patches in surface colonocytes (140 mmol/l NaCl in bath and 145 mmol/l in pipette, command voltage = 0 mV). Mean data ± SE are shown. *P < 0.02 and **P < 0.001 compared with t = 0 by ANOVA. B: representative 3-s segments from 30-s recordings from single patch containing 1 spontaneously active BK channel in the basal state (left) and after 25-min exposure to SOM. C: sustained inhibition of channel activity (Po) produced by SOM during the entire 30-s recordings represented in B.
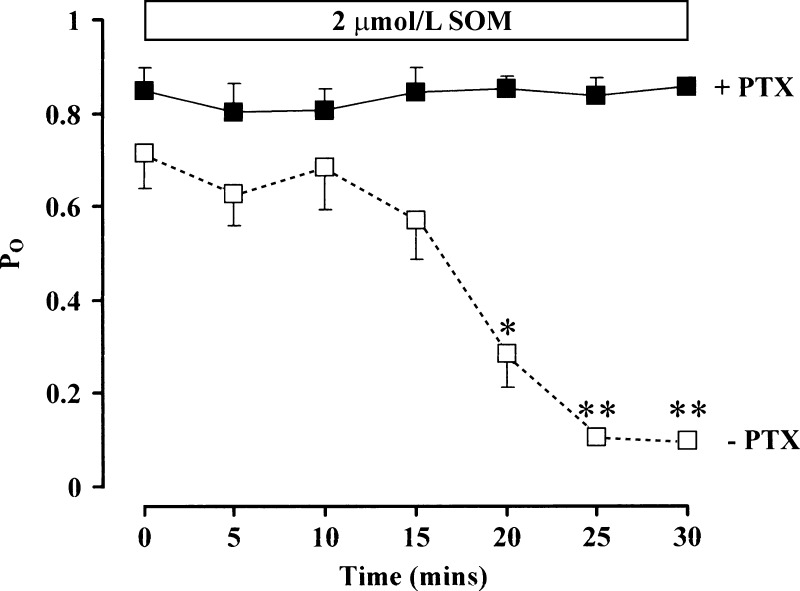
Most of the effects of SOM involve its interaction with G protein-coupled receptors. The bacterial toxin PTX promotes ADP-ribosylation of a cysteine residue on the G protein α-subunit, uncoupling it from its receptor and preventing its activation (49). Colonocytes were preincubated with PTX (200 ng/ml) for 18–24 h. Whereas PTX had no effect on basal (t = 0) BK channel activity [Po = 0.85 ± 0.05 with PTX compared with 0.76 ± 0.08 without PTX, P = not significant (NS)], it completely prevented the inhibitory effect of SOM on channel activity seen in colonocytes not pretreated with PTX (n = 5 in both groups; Fig. 4), indicating the critical role of G protein-coupled receptors.
Fig. 4.
Effect of pertussis toxin (PTX) on SOM-induced inhibition of BK channel activity. Summary of channel activity before (t = 0) and at 5-min intervals after addition of 2 μmol/l SOM with (▪) and without (□) PTX pretreatment (200 ng/ml) for 18–24 h (n = 5 both groups, 140 mmol/l NaCl in bath and 145 mmol/l in pipette, command voltage = 0 mV). Mean data ± SE are shown. *P < 0.02 and **P < 0.001 compared with t = 0 by ANOVA.
Role of serine/threonine protein phosphatases in SOM-induced BK channel inhibition in rat colonocytes.
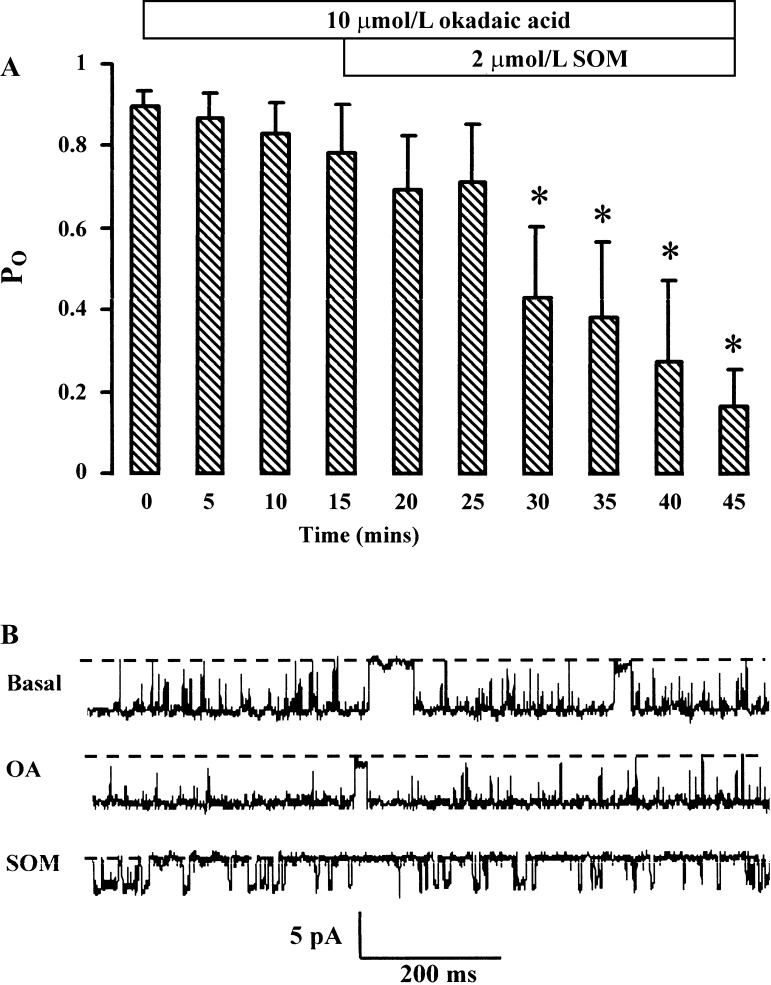
In rat pituitary cells, SOM activates BK channels by stimulating protein phosphatase 2A (48). In rat colonocytes, where SOM inhibited BK channels, it is feasible that SOM stimulated protein phosphatase activity, resulting in channel protein dephosphorylation. This was examined by using okadaic acid, a potent and specific inhibitor of serine/threonine protein phosphatases type 1 and type 2A (6). In six cell-attached patches tested, pretreatment with 10 μmol/l okadaic acid for 15 min had no effect on basal (t = 0) BK channel activity (Po = 0.78 ± 0.12 with okadaic acid compared with 0.89 ± 0.04 without okadaic acid, P = NS; Fig. 5A). Addition of 2 μmol/l SOM in the presence of okadaic acid decreased Po to 0.16 ± 0.09 (P < 0.05) after 30 min (Fig. 5A), the magnitude and time course of this inhibition being similar to that with SOM alone (Fig. 3A). Recordings from a representative experiment are shown (Fig. 5B). Thus, in rat colonocytes, serine/threonine protein phosphatases type 1 and type 2A play little or no part in the regulation of basal BK channel activity or in the mediation of SOM's inhibitory effect on these channels.
Fig. 5.
Effect of 10 μmol/l okadaic acid (OA) on SOM-induced inhibition of BK channel activity. A: results from 6 cell-attached patches in surface colonocytes indicating that okadaic acid had no effect on SOM-induced inhibition of channel activity (140 mmol/l NaCl in bath and 145 mmol/l in pipette, command voltage = 0 mV). Mean data ± SE are shown. *P < 0.05 compared with t = 0 by ANOVA. B: representative recordings from a patch containing 1 spontaneously active BK channel in the basal state (top trace, Po = 0.87), after 15-min pretreatment with okadaic acid (middle trace, Po = 0.94), and 30 min after subsequent addition of SOM (bottom trace, Po = 0.10).
Role of phosphoprotein tyrosine phosphatase in SOM-induced BK channel inhibition in rat colonocytes.
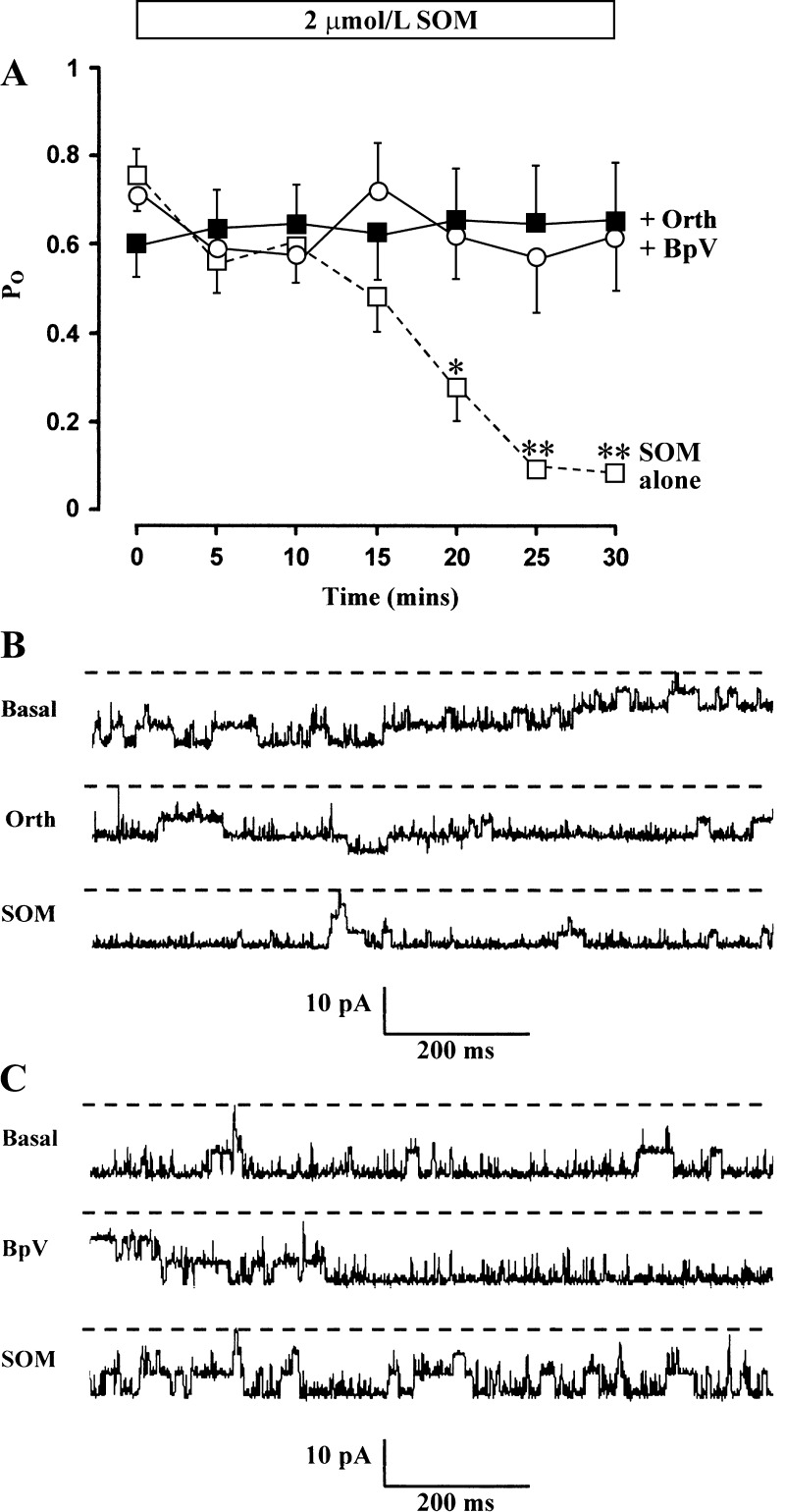
A complex interplay between protein tyrosine kinases and phosphoprotein tyrosine phosphatases regulates ion channels by modulating the phosphorylation state of specific tyrosine residues in channel proteins (37). Increased phosphoprotein tyrosine phosphatase activity has been implicated in the antiproliferative action of SOM (21, 23). The possibility that SOM activated phosphoprotein tyrosine phosphatases, thereby dephosphorylating the BK channel or an associated regulatory protein, was addressed using Na+ orthovanadate, a nonspecific inhibitor of phosphoprotein tyrosine phosphatase activity (44). In all six cell-attached patches, pretreatment with 100 μmol/l Na+ orthovanadate for 15 min had no effect on basal (t = 0) BK channel activity (Po = 0.61 ± 0.08 with Na+ orthovanadate compared with 0.53 ± 0.09 without Na+ orthovanadate, P = NS; Fig. 6A). However, pretreatment with Na+ orthovanadate prevented SOM's inhibitory effect on BK channel activity (Fig. 6A), and recordings from a representative experiment are shown (Fig. 6B). Additional experiments with 10 μmol/l BpV, which has a 1,000-fold higher potency than Na+ orthovanadate (5), indicated that in all six cell-attached patches, pretreatment with 10 μmol/l BpV for 15 min had no effect on basal (t = 0) BK channel activity (Po = 0.72 ± 0.1 with BpV compared with 0.70 ± 0.11 without BpV, P = NS; Fig. 6A) but prevented the inhibitory effect of SOM on these channels (Fig. 6, A and C). Thus, although phosphoprotein tyrosine phosphatases appear to have no role in regulating basal BK channel activity in surface colonocytes, they are required for SOM's inhibitory effect, which strongly suggests that tyrosine dephosphorylation of the channel or an associated regulatory protein is an important component of SOM's mode of action.
Fig. 6.
Effect of 100 μmol/l Na+ orthovanadate (Orth) and 10 μmol/l potassium bisperoxo(1,10-phenanthroline) oxovanadate(V) (BpV) on SOM-induced inhibition of BK channel activity. A: results from cell-attached patches in surface colonocytes (140 mmol/l NaCl in bath and 145 mmol/l in pipette, command voltage = 0 mV) indicating that Na+ orthovanadate (▪; n = 6) and BpV (ο; n = 6) completely prevented the inhibition of channel activity produced by SOM alone (□; n = 4). Mean data ± SE are shown. *P < 0.05 and **P < 0.002 compared with t = 0 by ANOVA. B: representative recordings from a patch containing 4 spontaneously active BK channels in the basal state (top trace, Po = 0.69), after 15-min pretreatment with Na+ orthovanadate (middle trace, Po = 0.70), and 30 min after subsequent addition of SOM (bottom trace, Po = 0.92). C: representative recordings from a patch containing 3 spontaneously active BK channels in the basal state (top trace, Po = 0.93), after 15-min pretreatment with BpV (middle trace, Po = 0.96), and 30 min after subsequent addition of SOM (bottom trace, Po = 0.95).
Apical BK channel inhibition by SOM in human colon.
We have previously identified BK channels at low abundance in cell-attached patches on the apical membrane of surface cells surrounding the openings of intact crypts from human colon (31). These apical BK channels have similar characteristics to those in the apical membrane of surface cells in rat colon (8). We therefore performed experiments to determine whether SOM had an inhibitory effect on apical BK channels in human colon that was similar to that seen in rat colonocytes. The addition of 2 μmol/l SOM to the bath solution decreased Po from 0.75 ± 0.06 to 0.41 ± 0.13 after 15 min (P < 0.02) in five cell-attached patches by using crypts from five different patients. In three patches where it was possible to maintain a high-resistance membrane seal, the inhibitory effect of SOM was reversed by washout. Thus the magnitude and time course of SOM's inhibitory effect on apical BK channels in human surface colonocytes was comparable not only to its effect on BK channels in rat surface colonocytes but also to its inhibitory effect on basolateral IK (23 pS) channels in human colonic crypt cells (32). It seems extremely unlikely that the inhibitory effect of SOM on apical BK channels in human and rat colonocytes reflected inhibition of basal adenylate cyclase activity, since previous studies in human colonic crypts have shown that SOM had no effect on the basal intracellular cAMP level and produced only a modest (∼11%) decrease in the maximal level of intracellular cAMP in forskolin-treated crypts (32).
DISCUSSION
The apical BK channels we describe are normally present mainly in surface cells in rat and human distal colon at relatively low density, but their basic biophysical properties (unitary conductance, K+:Na+ selectivity, Po) are identical in both epithelia (8, 31). Interestingly, there appear to be significant species-related differences in the distribution of colonic BK channels. For example, in rabbit distal colon, surface cells were shown to contain a high number of BK channels, whereas they were virtually absent in crypt cells (16). Furthermore, BK channels were present in both the apical and basolateral membranes, immunostaining indicating an apparently denser apical localization owing to the relatively small area of the apical membrane (16). Studies directed at localizing apical BK channels in mouse distal colon have provided conflicting results; in one, apical BK channels were located in crypt cells but not surface cells (34), whereas others have identified apical BK channels in surface cells but not crypt cells (12, 29). Nevertheless, hyperaldosteronism secondary to dietary K+ enrichment enhances electrogenic K+ secretion across mouse distal colon and increases the expression of both BK channel α- and β2-subunit mRNAs and apical BK channel protein in colonic crypts (40). Because apical BK channels are likely to contribute to enhanced intestinal K+ losses in secretory diarrheal diseases (1, 7), our aim was to study the effect of increasing intracellular cAMP (a potent intestinal secretory agonist) and SOM (a potent inhibitor of intestinal secretion) on apical BK channel activity. We facilitated data collection by first feeding animals a K+-enriched diet, which increased the abundance of apical BK channels without altering their biophysical properties (8).
cAMP-dependent PKA activates colonic BK channels.
Apical BK channel activation by forskolin and IBMX provided the initial clue that cAMP-dependent phosphorylation of BK channel protein (or an associated protein) might be an important regulatory step. Stimulation of BK channels in excised inside-out patches by the PKA catalytic subunit strongly suggests that channel activation involves direct protein phosphorylation of either BK channel protein per se or accessory protein(s). Although the variable responses to PKA could reflect different degrees of phosphorylation under basal conditions, alternative mRNA splicing in the mouse BK channel α-subunit generates different splice variants that are either activated (e.g., ZERO, e20, e22) or inhibited (e.g., STREX) by cAMP-dependent PKA (9). BK channel regulation by PKA may also be affected by the differential expression of BK channel β-subunits, since PKA stimulated channel activity in HEK293 cells expressing the human BK channel α-subunit but decreased channel activity when the α- and β1-subunits were coexpressed (11). The existence of β1-, β3-, and β4-subunits in whole colonic tissue (4) and distinct splice variants of the α-subunit, as well as other regulatory proteins (50), raises the possibility that BK channels with varying composition and responsiveness to PKA-mediated phosphorylation may be present in colonic epithelia.
Role of phosphatases in SOM-induced BK channel inhibition.
SOM inhibits basolateral IK channels in human colonic crypt cells, thereby reducing electrogenic Cl− secretion (32). We now show for the first time that SOM also inhibits apical BK channels in rat and human surface colonocytes. This is in direct contrast to the effect of SOM on rat pituitary neurosecretory cells, where it stimulates BK channels (48). Our experiments with PTX indicate that SOM receptors are coupled to at least one of the inhibitory G proteins in rat colonocytes, and G proteins have been identified in the apical membrane of HT-29cl.19A colon cells (45). To identify a possible link between inhibitory G proteins and the apical BK channels in rat colonocytes, we used okadaic acid to inhibit serine/threonine protein phosphatases type 1 and type 2A. Okadaic acid has been shown to completely reverse PTX-sensitive SOM activation of BK channels in rat pituitary cells (48), but we found that okadaic acid had no effect on either the basal or the SOM-induced decrease in BK channel activity, which suggests that neither serine/threonine protein phosphatase type 1 nor type 2A plays a part in SOM's inhibitory effect on BK channels in rat colonocytes.
The antiproliferative effects of SOM on human pancreatic cancer cells are linked to a substantial rise in phosphoprotein tyrosine phosphatase activity (21). SOM's antiproliferative effect did not occur in the presence of the nonhydrolyzable guanine nucleotide analog GDP-β-S and was abolished by pretreatment with PTX, implying involvement of G proteins (27). Furthermore, vanadate (a phosphoprotein tyrosine phosphatase inhibitor) prevented the antiproliferative action of SOM (27), and similar results were obtained in colonic carcinoma cell lines (47). Our studies show that Na+ orthovanadate (a nonspecific inhibitor of phosphoprotein tyrosine phosphatase) had no effect on basal BK channel activity but completely abolished the inhibitory effect of SOM on BK channels in rat colonocytes. However, at the concentration used (100 μmol/l), Na+ orthovanadate also inhibits H+-K+-ATPase (31), Na+-K+-ATPase (37), adenylyl cyclase (2), and some serine/threonine kinases and phosphatases (39). In addition, vanadate mimics the effect of PTX on the G protein transducin (GT), by dissociating the GαT and GβγT subunits, thereby preventing GT interaction with rhodopsin and inhibiting GDP-GTP exchange (18). GαT is also a member of the PTX-sensitive G protein family, and it is possible that Na+ orthovanadate could have a similar inhibitory effect on SOM receptor-coupled G protein, and consequently on SOM's ability to downregulate colonic BK channels. In additional experiments, we therefore evaluated BpV, a synthetic peroxovanadium derivative with a specific and 1,000-fold more potent inhibitory effect on phosphoprotein tyrosine phosphatase activity (28), which is readily transported across the cell membrane and is ideal for use in cell-attached patches (39). Although BpV also regulates other enzyme systems (e.g., extracellular signal-regulated kinase, ERK; mitogen-activated protein kinase phosphatase-1, MKP-1; insulin receptor kinase, IRK) (3, 28), we found that the effects of BpV were identical to those of Na+ orthovanadate, which provides a strong indication that phosphoprotein tyrosine phosphatase has a critical role in the inhibition of colonic BK channels by SOM.
If SOM inhibits colonic BK channels by stimulating phosphoprotein tyrosine phosphatase activity, it seems reasonable to assume that BK channel protein is tyrosine phosphorylated under basal conditions. Consistent with this view, the protein tyrosine kinase inhibitor genistein markedly decreased the carbachol-enhanced whole-cell K+ conductance in rat distal colonic crypts (10). Since the stimulatory effect of carbachol on whole-cell K+ conductance probably reflected the activation of apical BK channels and basolateral Ca2+-activated IK channels (17), protein tyrosine kinase(s) may phosphorylate and upregulate the activity of one or both of these types of K+ channel. As a corollary, it seems likely that phosphoprotein tyrosine phosphatase-mediated dephosphorylation is a critical event in SOM's inhibitory effect on apical BK channels and basolateral IK channels in colonic epithelia. It should be noted that at least five different SOM receptor isoforms exist in the rat gastrointestinal tract (19, 36), and further studies are required to determine which isoforms are involved in the SOM-induced inhibition of apical BK channels in rat and human colon.
BK channels and K+ losses in secretory diarrheal diseases.
Most cases of severe secretory diarrhea reflect the movement of Cl− ions via cAMP-activated apical Cl− channels (with the obligatory movement of water) into the intestinal lumen. In addition, high-volume diarrhea driven by excessive colonic K+ losses has been reported in colonic pseudo-obstruction (38, 46). In one case, K+ movement into the colonic lumen was deemed to reflect an active secretory process based on the Nernst equation, using the lumen-negative transmucosal electrical potential difference of −13.9 mV and the high average fecal K+ concentration of 154 mmol/l (46). In the other case, in which the fecal K+ concentration was also high (143 mmol/l), immunostaining indicated massive overexpression of apical BK channels throughout the surface-crypt axis (38), consistent with increased active K+ secretion. Although cases such as these are uncommon, patients with active ulcerative colitis also exhibit increased apical BK channel expression along the entire surface-crypt axis (in contrast to noninflamed colon, where apical BK channels are mainly restricted to surface cells), which may at least partly account for the excessive K+ losses that often occur in this disease (31). In cases of infective diarrhea, hypokalemia when it occurs reflects K+ losses across the “leaky” small intestinal epithelium, K+ moving passively into the lumen with the bulk flow of water. The results of our study suggest that increased intestinal K+ losses in some common enteric infections may also have a significant colonic component and reflect cAMP-dependent PKA-mediated activation of apical BK channels (26, 41). Furthermore, our data suggest that SOM peptides inhibit colonic apical BK channels through G protein-dependent, phosphoprotein tyrosine phosphatase-mediated dephosphorylation. If they inhibit basolateral IK channels in a similar manner (32), the discovery of alternative phosphoprotein tyrosine phosphatase inhibitors may provide a new method for minimizing intestinal K+ as well as Cl− losses in patients with secretory diarrhea.
GRANTS
This research was supported in part by a grant from the Wellcome Trust.
REFERENCES
- 1.Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 107: 548–571, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Aiton JF, Cramb G. The effects of vanadate on rabbit ventricular muscle, adenylate cyclase and sodium pump activities. Biochem Pharmacol 34: 1543–1548, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Band CJ, Posner BI, Dumas V, Contreres JO. Early signalling events triggered by peroxovanadium [bpV(phen)] are insulin receptor kinase (IRK)-dependent: specificity of inhibition of IRK-associated protein tyrosine phosphatase(s) by bpV(phen). Mol Endocrinol 11: 1899–1910, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Behrens R, Nolting A, Reimann F, Schwarz M, Waldschütz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett 474: 99–106, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bevan AP, Burgess JW, Yale JF, Drake PG, Lachance D, Baquiran G, Shaver A, Posner BI. In vivo insulin mimetic effects of pV compounds: role for tissue targeting in determining potency. Am J Physiol Endocrinol Metab 268: E60–E66, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Bialojan C, Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J 256: 283–290, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder HJ, Sandle GI, Rajendran VM. Colonic fluid and electrolyte transport in health and disease. In: The Large Intestine: Physiology, Pathophysiology, and Disease, edited by Phillips SF, Pemberton JH, Shorter RG. New York: Raven, 1994, p. 141–168.
- 8.Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol 503: 537–547, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Tian L, MacDonald S, McClafferty H, Hammond MS, Huibant JM, Ruth P, Knaus HG, Shipston MJ. Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Diener M, Hug F. Modulation of Cl− secretion by genistein, a protein kinase inhibitor. Eur J Pharmacol 299: 161–170, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang CP, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci 16: 4543–4550, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores CA, Melvin JE, Fiqueroa CD, Sepúlveda FV. Abolition of Ca2+-mediated intestinal anion secretion and increased stool dehydration in mice lacking the intermediate conductance Ca2+-dependent K+ channel Kcnn4. J Physiol 583: 705–717, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrant RL, Ganguly U, Casper G, Moore EJ, Pierce NF, Carpenter CC. Effect of Escherichia coli on fluid transport across canine small bowel. Mechanism and time-course with enterotoxin and whole bacterial cells. J Clin Invest 52: 1707–1714, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- 15.Hawker PC, Mashiter KE, Turnberg LA. Mechanisms of transport of Na, Cl, and K in the human colon. Gastroenterology 74: 1241–1247, 1978. [PubMed] [Google Scholar]
- 16.Hay-Schmidt A, Grunnet M, Abrahamse SL, Knaus HG, Klaerke DA. Localization of Ca2+-activated big-conductance K+ channels in rabbit distal colon. Pflügers Arch 446: 61–68, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Heinke B, Horger S, Diener M. Mechanisms of carbachol-induced alterations in K+ transport across the rat colon. Eur J Pharmacol 362: 199–206, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Kanaho Y, Chang PP, Moss J, Vaughan M. Mechanism of inhibition of transducin guanosine triphosphatase activity by vanadate. J Biol Chem 263: 17584–17589, 1988. [PubMed] [Google Scholar]
- 19.Krempels K, Hunyady B, O'Carroll AM, Mezey E. Distribution of somatostatin receptor messenger RNAs in the rat gastrointestinal tract. Gastroenterology 112: 1948–1960, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Lauritsen K, Laursen LS, Bukhave K, Rask-Madsen J. In vivo profiles of eicosanoids in ulcerative colitis, Crohn's colitis, and Clostridium difficile colitis. Gastroenterology 95: 11–17, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Liebow C, Reilly C, Serrano M, Schally AV. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci USA 86: 2003–2007, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomax RB, Warhurst G, Sandle GI. Characteristics of two basolateral potassium channel populations in human colonic crypts. Gut 38: 243–247, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez F, Esteve JP, Buscail L, Delesque N, Saint-Laurent N, Theveniau M, Nahmias C, Vaysse N, Susini C. The tyrosine phosphatase SHP-1 associates with the sst2 somatostatin receptor and is an essential component of sst2-mediated inhibitory growth signaling. J Biol Chem 272: 24448–24454, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Mathialahan T, MacLennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 206: 46–51, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J. Role of cholinergic-activated KCa 1.1 (BK), KCa 31 (SK4) and KV 7.1 (KCNQ1) channels in mouse colonic Cl− secretion. Acta Physiol (Oxf) 189: 251–258, 2007. [DOI] [PubMed] [Google Scholar]
- 26.McDonel JL In vivo effects of Clostridium perfringens enteropathogenic factors in the rat ileum. Infect Immun 10: 1156–1162, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan MG, Florio T, Stork PJ. G protein activation of a hormone-stimulated phosphatase in human tumor cells. Science 256: 1215–1217, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhang-Sun G, Fantus IG, Ng JB, Hall DA, Lum BS, Shaver A. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem 269: 4596–4604, 1994. [PubMed] [Google Scholar]
- 29.Puntheeranurak S, Schreiber R, Spitzner M, Ousingsawat J, Krishnamra N, Kunzelmann K. Control of ion transport in mouse proximal and distal colon by prolactin. Cell Physiol Biochem 19: 77–88, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Rumora L, Hadzija M, Maysinger D, Zanic-Grubisic T. Positive regulation of ERK activation and MKP-1 expression by peroxovanadium complex bpV (phen). Cell Biol Toxicol 20: 293–301, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Sandle GI, Perry MD, Mathialahan T, Linley JE, Robinson P, Hunter M, MacLennan KA. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol 212: 66–73, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Sandle GI, Warhurst G, Butterfield I, Higgs NB, Lomax RB. Somatostatin peptides inhibit basolateral potassium channels in human colonic crypts. Am J Physiol Gastrointest Liver Physiol 277: G967–G975, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Sangan P, Thevananther S, Sangan S, Rajendran VM, Binder HJ. Colonic H-K-ATPase alpha- and beta-subunits express ouabain-insensitive H-K-ATPase. Am J Physiol Cell Physiol 278: C182–C189, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Searle BM, Higashino H, Khalil F, Bogden JD, Tokushige A, Tamura H, Kino M, Aviv A. Vanadate effect on the Na,K-ATPase and the Na-K pump in in vitro-grown rat vascular smooth muscle cells. Circ Res 53: 186–191, 1983. [DOI] [PubMed] [Google Scholar]
- 36.Schäfer J, Meyerhof W. sst1 mRNA is the prominent somatostatin receptor mRNA in the rat gastrointestinal tract: reverse transcription polymerase chain reaction and in situ-hybridization study. Neuropeptides 33: 457–463, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Siegelbaum SA Channel regulation. Ion channel control by tyrosine phosphorylation. Curr Biol 4: 242–245, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Simon M, Duong JP, Mallet V, Jian R, MacLennan KA, Sandle GI, Marteau P. Over-expression of colonic K+ channels associated with severe potassium secretory diarrhoea after haemorrhagic shock. Nephrol Dial Transplant 23: 3350–3352, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Sims C, Chiu J, Harvey RD. Tyrosine phosphatase inhibitors selectively antagonize β-adrenergic receptor-dependent regulation of cardiac ion channels. Mol Pharmacol 58: 1213–1221, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Sørensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger J. Aldosterone increases KCa 1.1 (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speelman P, Butler T, Kabir I, Ali A, Banwell J. Colonic dysfunction during cholera infection. Gastroenterology 91: 1164–1170, 1986. [DOI] [PubMed] [Google Scholar]
- 42.Stieger B, Marxer A, Hauri HP. Isolation of brush-border membranes from rat and rabbit colonocytes: is alkaline phosphatase a marker enzyme? J Membr Biol 91: 19–31, 1986. [DOI] [PubMed] [Google Scholar]
- 43.Sträter J, Wedding U, Barth TF, Koretz K, Elsing C, Möller P. Rapid onset of apoptosis in vitro follows disruption of β1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology 110: 1776–1784, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Swarup G, Cohen S, Garbers DL. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Comm 107: 1104–1109, 1982. [DOI] [PubMed] [Google Scholar]
- 45.Tilly BC, Kansen M, van Gageldonk PG, van den Berghe N, Galjaard H, Bijman J, de Jonge HR. G-proteins mediate intestinal chloride channel activation. J Biol Chem 266: 2036–2040, 1991. [PubMed] [Google Scholar]
- 46.Van Dinter TG, Fuerst FC, Richardson CT, Santa CA, Polter DE, Fordtran JS, Binder HJ. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology 129: 1268–1273, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Vantus T, Csermely P, Teplan I, Keri G. The tumor-selective somatostatin analog, TT2–32 induces a biphasic activation of phosphotyrosine phosphatase activity in human colon tumor cell line, SW620. Tumour Biol 16: 261–267, 1995. [PubMed] [Google Scholar]
- 48.White RE, Schonbrunn A, Armstrong DL. Somatostatin stimulates Ca2+ activated K+ channels through protein dephosphorylation. Nature 351: 570–573, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev 75: 865–885, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Xia X, Hirschberg B, Smolik S, Forte M, Adelman JP. dSlo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci 18: 2360–2369, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]