Abstract
Intraluminal concentrations of bile acids are low in newborn infants and increase rapidly after birth, at least partly owing to increased bile acid synthesis rates. The expansion of the bile acid pool is critical since bile acids are required to stimulate bile flow and absorb lipids, a major component of newborn diets. The purpose of the present studies was to determine the mechanism responsible for the increase in bile acid synthesis rates and the subsequent enlargement of bile acid pool sizes (BAPS) during the neonatal period, and how changes in circulating hormone levels might affect BAPS. In the hamster, pool size was low just after birth and increased modestly until 10.5 days postpartum (dpp). BAPS increased more significantly (∼3-fold) between 10.5 and 15.5 dpp. An increase in mRNA and protein levels of cholesterol 7α-hydroxylase (Cyp7a1), the rate-limiting step in classical bile acid synthesis, immediately preceded an increase in BAPS. In contrast, levels of oxysterol 7α-hydroxylase (Cyp7b1), a key enzyme in bile acid synthesis by the alternative pathway, were relatively elevated by 1.5 dpp. farnesyl X receptor (FXR) and short heterodimeric partner (SHP) mRNA levels remained relatively constant at a time when Cyp7a1 levels increased. Finally, although simultaneous increases in circulating cortisol and Cyp7a1 levels occurred, precocious expression of Cyp7a1 could not be induced in neonatal hamsters with dexamethasone. Thus the significant increase in Cyp7a1 levels in neonatal hamsters is due to mechanisms independent of the FXR and SHP pathway and cortisol.
Keywords: neonate, development, FXR, SHP, hormones, FGF15, FGFR4
malabsorption of lipid occurs during the first month of life of full-term newborns and longer in preterm infants (16) concurrent with reduced intraluminal bile acid concentrations. Intraluminal bile acid concentrations, as well as bile acid pool sizes (BAPS), are lowest in the preterm infant, are greater in the term infant, and increase with postnatal age (6, 25). The pool expands as the result of increased bile acid synthesis rates and enhanced intestinal bile acid absorption (11, 24, 25, 37, 69, 70); elevated bile acid synthetic rates are the result of increases in enzyme levels of the bile acid synthetic pathways, and enhanced intestinal bile acid absorption is the result of increased ileal apical sodium-dependent bile acid cotransporter (ASBT).
Synthesis of bile acids occurs by two major pathways and includes several enzymatic steps (reviewed in Refs. 8, 53, 54). In the classical or neutral pathway, cholesterol is initially converted to 7-hydroxycholesterol (5-cholesten-3β,7α-diol). The enzyme responsible for the catalysis of this reaction is cholesterol 7α-hydroxylase (Cyp7a1). In the alternative or acidic pathway, cholesterol is initially hydroxylated at the 25 or 27 position by sterol 25-hydroxylase or sterol 27-hydroxylase (Cyp27a1), respectively. Oxysterol 7α-hydroxylase (Cyp7b1) then catalyzes the reaction whereby a 7α-hydroxyl group is added to 25- or 27-hydroxycholesterol. These di- and trihydroxylated sterols are converted to primary bile acids through modification of the ring structure and oxidation and shortening of the side chain. One other key enzyme involved in the synthesis of bile acids is sterol 12α-hydroxylase (Cyp8b1), which is responsible for the addition of a 12α-hydroxyl group to hydroxylated sterols. Activity of Cyp8b1 will lead to the formation of cholic acid (CA) whereas unhydroxylated sterols form chenodeoxycholic acid (CDCA).
Bile acids play a central role in the maintenance of whole body sterol balance and as such their synthesis rates are tightly regulated. It has been known for years that bile acids regulate their own synthesis rates (reviewed in Ref. 54). More recent studies have shown that the inhibition occurs at the level of negative feedback inhibition of transcription of Cyp7a1, the rate-limiting step of the classical pathway (47). Activation of the nuclear receptor farnesyl X receptor (FXR) by excess bile acids leads to an increase in transcription of the short heterodimeric partner (SHP) (17, 38). SHP in turn binds to the liver receptor homologue-1 (LRH-1)/α-fetoprotein transcription factor (FTF) which leads to a reduction in the transcription of Cyp7a1 and Cyp8b1 (13, 17). Activation of a second nuclear receptor, liver X receptor α (LXRα), increases bile acid synthesis in mice and rats (31, 34), though not in humans or hamsters (2, 7, 27). Cyp7a1 can also be regulated by SHP-independent pathways. Various nuclear receptors and growth factors can affect Cyp7a1, including the hepatocyte growth factor (HGF) (64) and intestinal fibroblast growth factor 15 (FGF15) acting as an intestinal hormone to signal the liver (30). Hormones, including insulin and glucocorticoids (9, 36, 49), and signaling cascades, including c-Jun NH2-terminal kinase (JNK) (19) and MAPK (65), also affect Cyp7a1 levels.
Bile acid synthesis can be modulated by substrate supply as well as expression levels of enzymes involved in the bile acid synthetic pathway. Synthesis of bile acids by the alternative pathway originates in the mitochondria as Cyp27a1 and is localized to the inner mitochondrial membrane. Thus substrate (cholesterol) must cross the mitochondrial membranes to be acted on by Cyp27a1. The steroidogenic acute regulatory protein (StAR) is responsible for the transport of sterol across the mitochondrial membrane (50, 51). Interestingly, elevated hepatic levels of StAR lead to increased bile acid formation via the alternative pathway, suggesting that bile acid synthesis may be dependent on presentation of substrate (50).
Although not the focus of these studies, a discussion of bile acid pool size is not complete without mentioning uptake of bile acids. Bile acids are taken up from the lumen of the ileum by enterocytes via ASBT and from the plasma by hepatocytes via Na+/taurocholate cotransporting polypeptide (NTCP). Both proteins are members of the SLC10 family of sodium/bile salt cotransporters (20), and expression of both increases with age (23, 60).
The relative activity of the classical and alternative biosynthetic pathways is not always constant and varies with age in humans. In healthy adult humans, the alternative pathway appears to play a less significant role than the classical pathway and thereby contributes only modest amounts of bile acids to the pool (15). In human neonates, however, the alternative pathway may play a more prominent role and is essential since a lack of CYP7B1 can lead to severe neonatal liver disease (59, 68). The goal of the present studies was to correlate BAPS and composition with the expression profiles of enzymes and transcription factors involved in bile acid synthesis during neonatal development. Studies were performed in the hamster because cholesterol does not affect Cyp7a1 levels in hamsters and humans (2, 7, 27, 31, 34). This is important when studying the neonatal period since hepatic sterol balance varies markedly as neonates age (75). In the present studies, BAPS was low after birth, increased modestly until 10.5 days postpartum (dpp), and then increased significantly between 10.5 and 15.5 dpp. The expansion of the pool size temporally followed the threefold increase in expression level of Cyp7a1 but not Cyp8b1 or Cyp7b1 and was independent of FXR activation.
MATERIALS AND METHODS
Animals and diets.
Nonpregnant female and male Golden Syrian hamsters (Mesocricetus auratus) weighing 110–120 g (Charles River, Kingston, NJ) were fed a pelleted chow diet (7102, Harlan Teklad, Madison, WI) and kept in a temperature- and humidity-controlled room with alternating light and darkness (14 h light-10 h dark). Female hamsters were mated as described previously (43). Pups were studied at 1.5, 5.5, 10.5, 15.5, and 20.5 dpp; once pups were 8 dpp, cages were changed every 2–3 days to reduce coprophagy. Adults were studied at ∼85 days after birth. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
BAPS and compositions.
BAPS and bile acid composition of the pools were measured as described previously (74). Briefly, the livers, including the gallbladder, were removed and placed in 100% ethanol. The small intestines were isolated from the animals with care so as not to lose any luminal contents and added to the liver sample. Samples were pooled when needed, depending on the age of the neonate, and bile acids were extracted. A portion of the sample was dried down under nitrogen with 5β-cholanic acid-7α, 12α-diol as an internal standard. The sample was filtered and 80 μl of sample plus internal standard was injected onto a 5-μm ODS HYPERSIL (C18) 4.6 × 250 mm analytical column (Keystone Scientific, Bellefonte, PA). A linear gradient mobile phase system was used. Bile acids were detected with an Evaporative Light Scattering Detector (ELSD; Alltech Associates, Deerfield, IL). Bile acids were identified on the basis of the retention time of known standards and quantified on the basis of the amount of internal standard added to the sample.
Livers, not including gall bladders when visible, were also collected. Samples were extracted in 90% ethanol. The amounts of bile acids in liver extracts were measured enzymatically (Trinity Biotech, Bray, Ireland).
Protein expression levels.
Livers were collected, and microsomal fractions were isolated as described and stored at −80°C until use (32). The distal third of the small intestines (ileum) were collected and stored at −80°C until homogenates were made and used. Protein concentrations were determined by the Lowry assay (40), and equal amounts of protein from samples from each litter at each age were pooled. Proteins were separated by SDS-PAGE by using a Bio-Rad 4–15% Tris·HCl Ready gel (Bio-Rad Laboratories, Hercules, CA) under denaturing conditions and then transferred to a polyvinylidene difluoride membrane. Membranes were blocked in Tris-buffered saline containing 0.1% Tween and 5% dry milk and then incubated with rabbit anti-Cyp7a1 (a gift from Dr. David Russell, University of Texas Southwestern Medical Center). After 1 h at 25°C, the membrane was incubated with a secondary antibody conjugated with peroxidase (donkey anti-rabbit IgG; GE Healthcare, Piscataway, NJ). Chemiluminescence from ECL Plus (GE Healthcare) was detected by a Storm 450 Phosphoimager. Blots were stripped with Restore Western Blot Stripping Buffer (Pierce, Rockford, IL) and reprobed with β-actin (Bioscience Research Reagents, Temecula, CA). Relative densities were determined by using NIH Image J software. Other gels were run under similar conditions by use of primary antibodies to Cyp7b1 (Santa Cruz Biotechnologies, Santa Cruz, CA), Cyp8b1 (Santa Cruz), and FGF15 (Santa Cruz); blots for Cyp7b1 were stripped and reprobed with Cyp8b1. Blots were stripped and reprobed with β-actin (liver). At least two blots on pooled samples were run for each protein.
mRNA levels.
Samples of livers and small intestines were collected and stored at −80°C until RNA isolation. Tissue RNA was isolated by use of TRIzol (Invitrogen, Carlsbad, CA) and stored in FORMAzol (Molecular Research Center, Cincinnati, OH). RNA was treated with RNase-free DNase I and reverse transcribed to cDNA by SuperScript II reverse transcriptase by using random hexamers. PCR assays were performed on a Bio-Rad iCycler iQ real-time PCR Detection System using SYBR Green as our fluorophore. A serial dilution of a randomly picked sample was used to generate a standard curve for each gene examined. This standard curve was used to calculate the relative levels of mRNA for the gene of interest and the reference/housekeeping gene (cyclophilin). Primers used were previously described for Cyp7a1 (5), Cyp8b1 (39), SHP (30), Cyp27a1 (42), and cyclophilin (76). The FXR primers were 5′ TCAGTTGCTGGGAATAGACTAAGG 3′ (forward) and 5′ GCTAACCTGAACCGGATTTTTCT 3′ (reverse) (personal communication from Dr. Joyce Repa, University of Texas Southwestern Medical Center); the StAR primers were 5′ TAAAGTGGTCCCTGATGTGGGCAA 3′ (forward) and 5′ TGCGGTCCACAAGTTCTGCATAGA 3′ (reverse); the HGF primers were 5′ GTTGAATGCATGACCTGCAACGGT 3′ (forward) and 5′ AAGAATTTGTGCCGGTGTGGTGTC 3′ (reverse); the FGFR4 primers were 5′ CGTGGCTGTGAAGATGCTGAAAG 3′ (forward) and 5′ ACGGAGGAATTCCCGAAGGTTT 3′ (reverse); and the PgP primers were 5′ TTTGGCCATCAGCCCTGTTCTT 3′ (forward) and 5′ TCTTCAGCAACTGCTCCAGCTT 3′ (reverse).
Hormone concentrations.
Plasma was collected from neonates. At 1.5 and 5.5 dpp, plasma from litters was pooled and at 10.5 and 20.5 dpp, plasma from one male and one female was pooled. Total cortisol (MP Biomedicals, Santa Ana, CA) and total corticosterone (MP Biomedicals) concentrations were measured by radioimmunoassays, and insulin concentrations (Linco Research, St. Charles, MO) were measured by Lincoplex multiplex kits.
Hormone treatment.
Neonates were treated with dexamethasone (0.4 μg/mg body wt sc) at 24 and 3 h prior to euthanasia (1, 63); 1–2 pups per litter were injected at each time. At 7.5 dpp, livers were collected from treated pups along with livers from untreated pups.
Statistics.
Data are presented as means ± SE. Initially, one male and one female from litters of pups 10.5, 15.5, and 20.5 dpp were analyzed separately. However, since no differences were observed between the sexes at these early ages, data from males and females were analyzed together. We tested data by three different statistical methods. First, data from sequential ages were compared with one another by unpaired t-tests. For example, values from animals at 1.5 dpp were compared with 5.5 dpp, 5.5 dpp with 10.5 dpp, etc. Using this method, we could determine whether values increased or decreased with age. We also compared adult male and adult female to 20.5 dpp neonates using unpaired t-tests or Mann-Whitney rank sum tests if the equal variance test failed. For the dexamethasone treatments, treatments were compared with no treatment. Significance for all t-tests was set at P < 0.025. The P value was more stringent since each age was compared with two different ages, the age prior to and the age subsequent to the age measured. The same stringency occurs for the dexamethasone treatments. In addition to t-tests, using ANOVA with age as a categorical variable, we determined whether age was significant for various parameters. Finally, the least squares of the means were calculated for each effect by age using Tukey-Kramer adjustment for multiple comparisons.
RESULTS
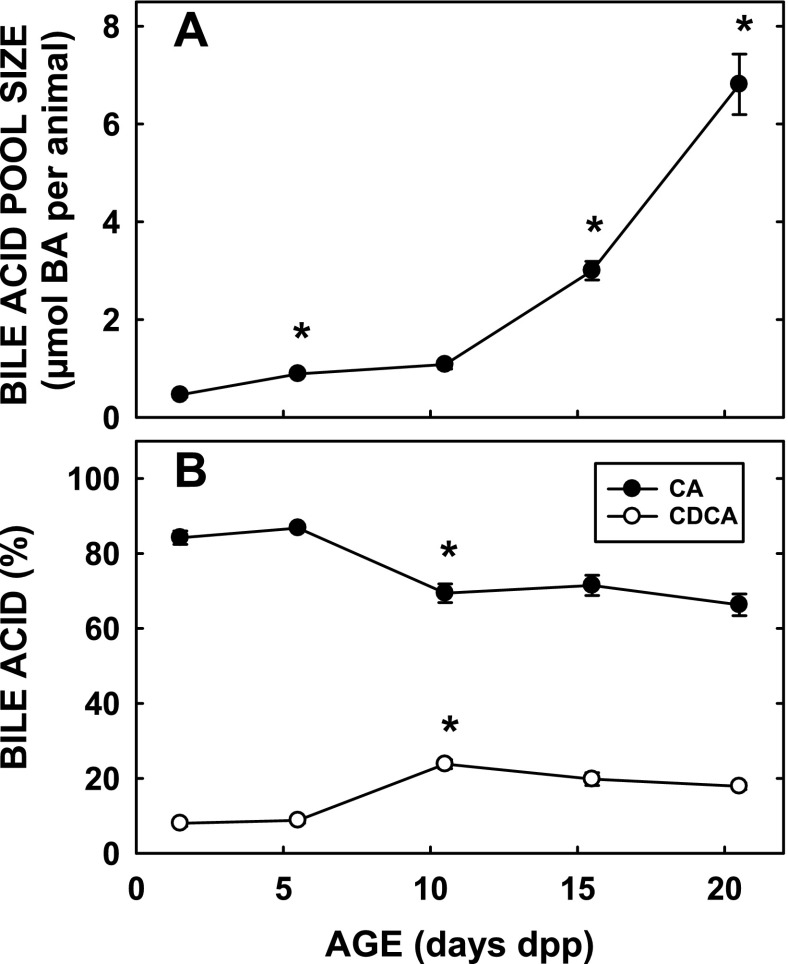
The BAPS and bile acid pool composition of hamsters were measured in neonates from 1.5 to 20.5 dpp. The pool size was lowest at 1.5 dpp (Fig. 1A). Pool size increased approximately twofold by 5.5 dpp (P < 0.001). Although there was no difference in size between 5.5 and 10.5 dpp, pool size increased approximately threefold between 10.5 and 15.5 dpp (P < 0.001). There was another doubling of pool size by 20.5 dpp (P < 0.001). The composition of the pool also varied with age (Fig. 1B). Pool size increased more dramatically compared with increases in liver masses of neonatal hamsters (75). Pool composition, specifically the percentages of CA and CDCA, were similar at 1.5 and 5.5 dpp with the ratio of CA to CDCA being ∼10. By 10.5 dpp, pool compositions had changed with a higher percentage of CDCA present (P < 0.001); the ratio CA/CDCA decreased to 3.7 at 10.5 dpp and remained unchanged with increasing age. Neonatal rats also demonstrated a decrease in the ratio CA/CDCA with aging (10). Hepatic bile acid concentrations were 0.40 ± 0.05, 0.75 ± 0.03, 0.80 ± 0.07, and 0.84 ± 0.08 μmol bile acid/g liver for 1.5, 5.5, 10.5, and 20.5 dpp. Using ANOVA with age as a categorical variable, age was significant (P < 0.01), and by least squares means, micromoles of bile acid per gram liver was least at 1.5 dpp compared with other ages (P < 0.04 for all comparisons).
Fig. 1.
Bile acid pool size and composition in neonatal hamsters. Females were mated and pups were born. At 1.5, 5.5, 10.5, 15.5, and 20.5 days postpartum (dpp), the liver, gallbladder, and small intestine of the animals were collected. Total bile acids were measured by HPLC and presented as the bile acid pool size (μmol bile acid/animal) (A). The percentages of the bile acid pools that were cholic acid (CA) or chenodeoxycholic acid (CDCA) were calculated and plotted as well (B). Data are presented as means ± SE (n = 4–9). *Significant differences between animals from previous ages studied (P < 0.025). By ANOVA with age as a categorical variable, age was statistically significant (P < 0.001) for bile acid pool sizes (BAPS) and percent CA or CDCA. By least squares means analyses, BAPS at 1.5, 5.5, and 10.5 were less than at 15.5 and 20.5 and BAPS at 15.5 was less than at 20.5 (P < 0.001 for all comparisons). Likewise, percent CA or CDCA was significantly less at 1.1 and 5.5 compared with older ages (P ≤ 0.01 for all comparisons).
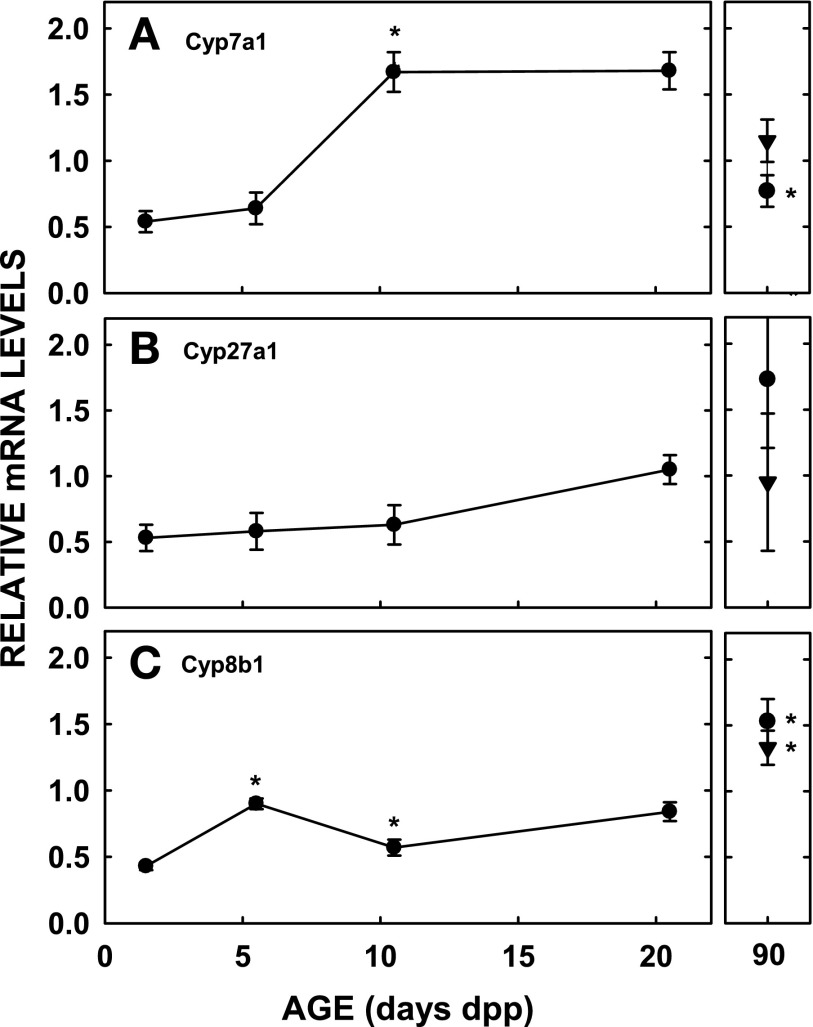
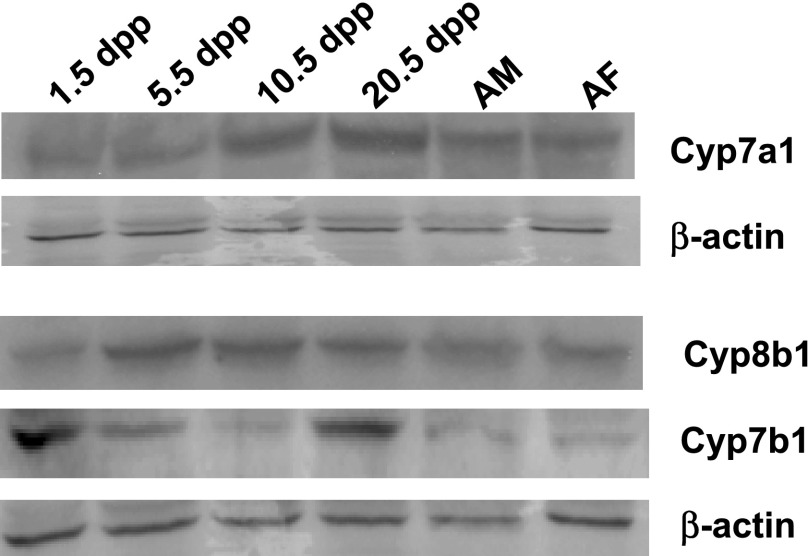
The BAPS is regulated by the balance of bile acid synthesis and excretion. Since the focus of the present studies was to study the role of bile acid-synthesizing enzymes in the expansion of the BAPS, mRNA and protein levels of key enzymes involved in the classical and neutral pathways of bile acid synthesis were measured. Cyp7a1 mRNA levels were relatively low until 10.5 dpp when mRNA levels increased approximately threefold (Fig. 2A) (P < 0.001). Adult females had greater mRNA levels than 20.5 dpp neonates (P = 0.021). Cyp7a1 protein levels followed the same pattern as that for mRNA (Fig. 3). Relative levels of mRNA for Cyp27a1 were fairly constant throughout the neonatal period, although age was a significant variable as seen by ANOVA (Fig. 2B) (P < 0.01). Relative levels of mRNA for Cyp8b1 increased approximately twofold between 1.5 and 5.5 dpp (P < 0.001) with a decline followed by an increase during the remainder of the neonatal period (Fig. 2C) (P < 0.016). Cyp8b1 protein levels increased between 1.5 and 5.5 dpp in parallel with the mRNAs and remained relatively unchanged throughout the remainder of the neonatal period (Fig. 3). mRNA levels were lower than in adulthood, however (P < 0.01). For Cyp7b1, only protein levels were measured since the sequence of this gene in the hamster has not been determined and primers for a homologous region of the rat and mouse did not result in a product when hamster mRNA was used. Protein levels of Cyp7b1 were relatively elevated by 1.5 dpp compared with other ages. Levels decreased in the midneonatal period and then increased by 20.5 dpp (Fig. 3).
Fig. 2.
mRNA levels of Cyp7a1, Cyp27a1, and Cyp8b1 in neonatal hamsters. Studies were performed as in Fig. 1. •, Females; ▾, males, at 90 dpp. On the day of the study, livers were collected. mRNA was isolated and stored at −80°C until use. mRNA levels for Cyp7a1 (A), Cyp27a1 (B), and Cyp8b1 (C) were determined by real-time PCR and with cyclophilin as the housekeeping gene. Data are presented as means ± SE (n = 3–8). *Significant differences between animals from previous ages studied (P < 0.025). By ANOVA, age was statistically significant for Cyp7a1 (P = 0.002). By least squares means, Cyp7a1 mRNA levels at 1.5 and 5.5 dpp were less than at ages 10.5 and 20.5 dpp (P < 0.04 for all comparisons). Although there was no age effect when compared directly, age was a significant variable (P < 0.02) for Cyp8b1. Using least squares means, mRNA at 1.5 dpp was lower than at 5.5 and 20.5 dpp and mRNA at 10.5 was lower than 5.5 dpp.
Fig. 3.
Expression levels of Cyp7a1, Cyp7b1, and Cyp8b1 in neonatal hamsters. Studies were performed as in Fig. 1. Protein levels were determined by immunoblotting on 3–4 pooled samples using β-actin as the loading control. AM, adult male; AF, adult female.
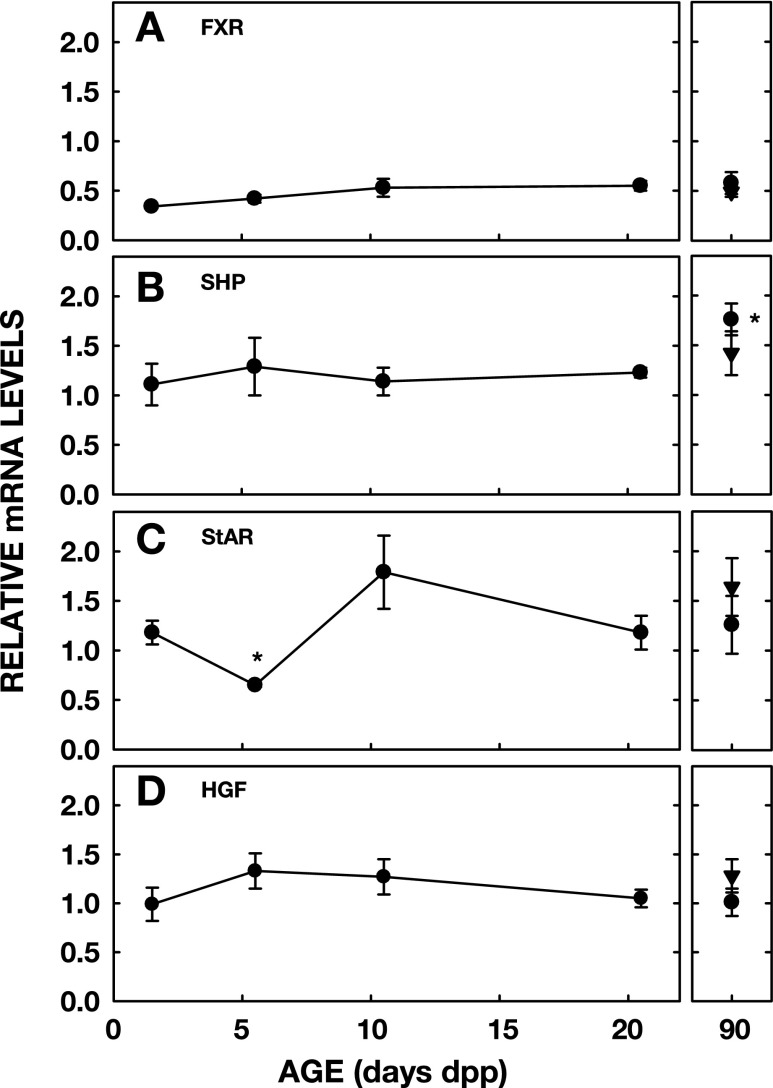
Several factors are known to regulate bile acid synthesis rates in mature animals. Since FXR is a primary regulator of bile acid synthesis rates, at least in the adult and cell culture, the levels of FXR mRNA were measured first. Although there appeared to be a modest increase with age of FXR mRNA (55% increase), there was no significant increase between any ages (Fig. 4A). mRNA levels for SHP, the downstream target of FXR (17, 38), remained relatively constant throughout the neonatal period (Fig. 4B) and were increased in the adult female (P = 0.016). StAR mRNA levels were measured to estimate the availability of sterol for the alternative pathway (50). StAR levels were elevated at 1.5 dpp compared with 5.5 dpp (P = 0.014) when Cyp7b1 was greatest (Fig. 4C). There was a trend toward an increase at 10.5 dpp (P = 0.07).
Fig. 4.
mRNA levels of farnesyl X receptor (FXR), short heterodimeric partner (SHP), steroidogenic acute regulatory protein (StAR), and hepatocyte growth factor (HGF) in neonatal hamsters. Studies were performed as in Fig. 1 and samples analyzed as in Fig. 2. mRNA levels of FXR (A), SHP (B), StAR (C), and HGF (D) throughout the neonatal period and in adulthood are presented. Data are presented as means ± SE (n = 3–8). *Significant differences between animals from previous ages studied (P < 0.025).
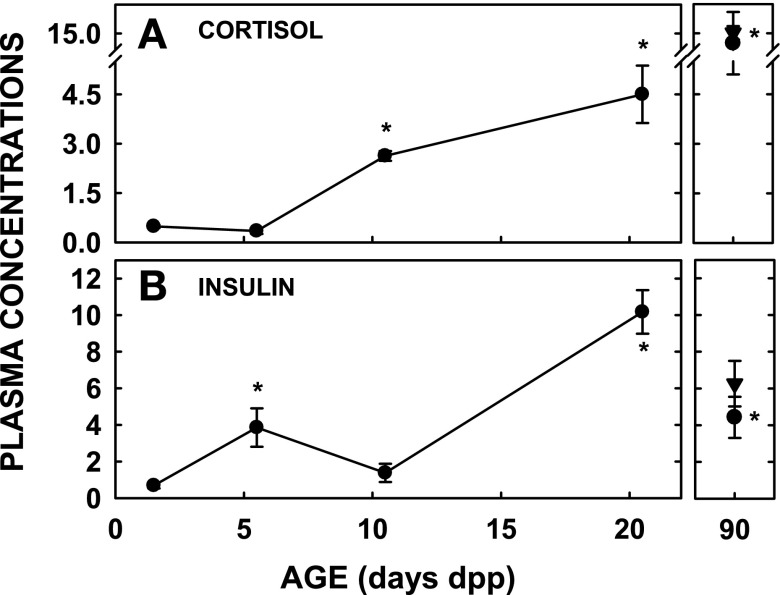
Since the increase in Cyp7a1 was not associated with a reduction in repression by the SHP-dependent pathway, we evaluated alternative modes of regulation. First, growth factors known to affect Cyp7a1 levels were examined. There was no effect of age on mRNA levels of HGF (Fig. 4D). FGF15 protein expression decreased markedly between 5.5 and 10.5 dpp when Cyp7a1 levels increased (Fig. 5A). Although levels increased again at 20.5 dpp, they were still lower than in adulthood. mRNA levels for FGFR4, the hepatic receptor for FGF15 (72), did not change much during the neonatal period but were lower in younger than older animals (Fig. 5B) (P < 0.025). Second, we studied potential roles of hormones since Cyp7a1 levels are affected in adults or cultured cells exposed to glucocorticoids (9, 28, 49) and insulin (12, 36). Plasma levels of both hormones were measured in neonates. Cortisol concentrations increased severalfold between 5.5 and 10.5 dpp (P < 0.001) and then approximately threefold between the neonatal and adult values (Fig. 6A); only the adult males demonstrated significant increases (P = 0.002) due to marked variation of levels in the females. Corticosterone concentrations, a second less abundant glucocorticoid in the hamster (67), remained relatively constant during the neonatal period (data not shown). Insulin levels increased between 1.5 and 5.5 dpp (P = 0.041) and then as neonates matured to 20.5 dpp (Fig. 6B) (P = 0.002). There was a modest decrease into adulthood.
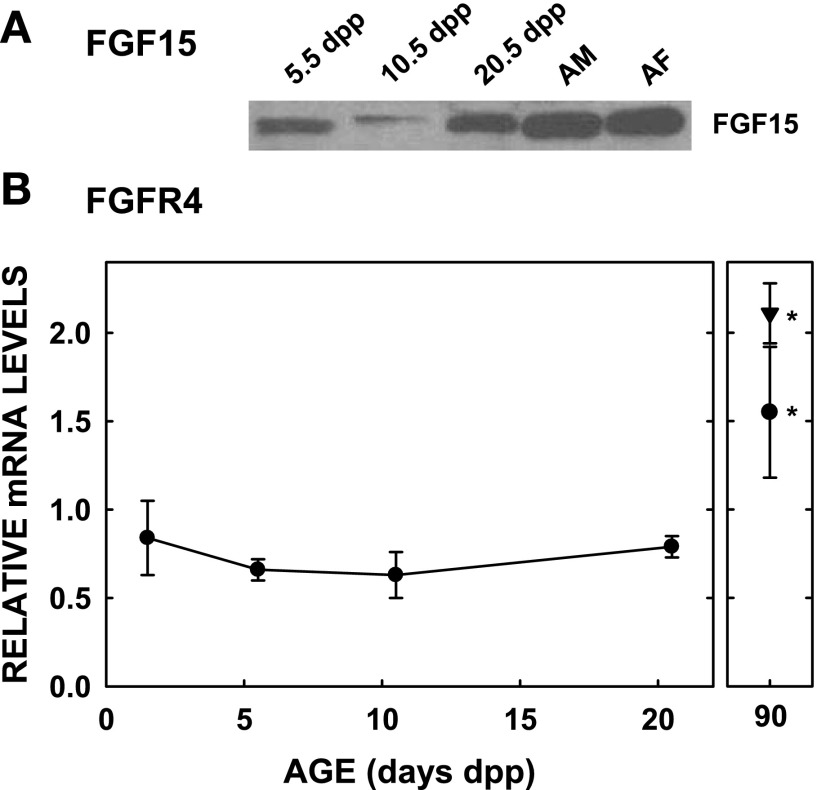
Fig. 5.
Expression levels of FGF15 and FGFR4 in neonatal hamsters. Studies were performed as in Fig. 1. Protein levels of FGF15 were determined by immunoblotting on 3–4 pooled samples (A). mRNA levels of FGFR4 were measured by use of cyclophilin as the housekeeping gene (B) (n = 3–6). Significance between 20.5 dpp and adulthood was determined by the Mann-Whitney rank sum test for the females.
Fig. 6.
Concentrations of hormones during development. Studies were performed as in Fig. 1. Hormones measured were cortisol (μg/dl; A) and insulin (ng/ml; B) (n = 3–4). •, Females; ▾, males. *Significant differences between animals from previous ages studied (P < 0.025). By ANOVA, age was statistically significant for insulin (P = 0.002) and cortisol (P = 0.001). By least squares mean, cortisol concentrations at 1.5 and 5.5 dpp were less than those at 10.5 and 20.5 dpp (P < 0.05 for all comparisons). For insulin, concentrations at 1.5, 5.5, and 10.5 dpp were less than concentrations at 20.5 dpp.
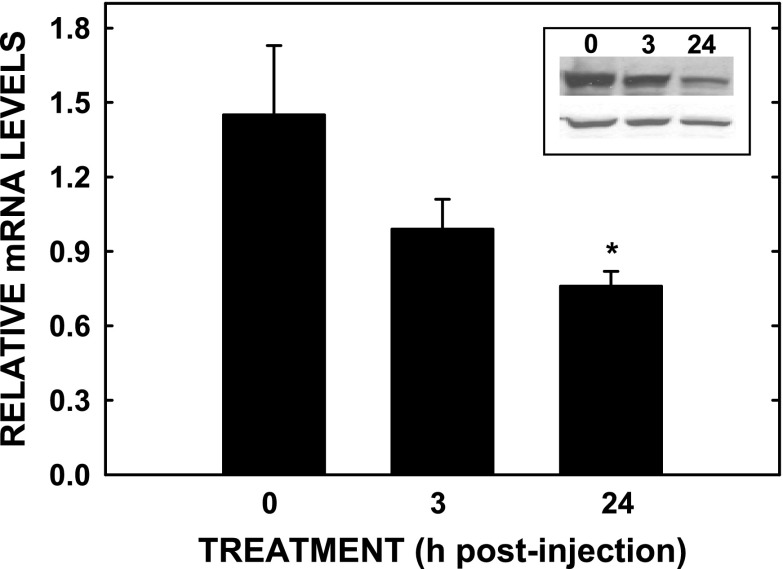
The increases in Cyp7a1 and cortisol occurred between 5.5 and 10.5 dpp. Thus neonates were treated with dexamethasone to determine whether precocious expression of Cyp7a1 could be induced. Neonates were treated at an age prior to when Cyp7a1 was markedly increased to study the effects of short-term as well long-term hormone-induced effects. Somewhat surprisingly, Cyp7a1 mRNA and protein levels tended to decrease at 3 h posttreatment (P = 0.175) and were suppressed by 24 h posttreatment (Fig. 7) (P = 0.03). Since dexamethasone can activate pregnane x receptor (PXR), another regulator of Cyp7a1 (66), mRNA levels for PgP, a downstream target of PXR (29) and the gene product of mdr-1a, were also measured. Although not significant, there was a trend for an increase between PgP mRNA levels of control hamsters (1.57 ± 0.15) and the neonates studied 3 h (2.17 ± 0.34; P = 0.16) and 24 h (2.19 ± 0.24; P = 0.09) after dexamethasone treatment.
Fig. 7.
Changes in Cyp7a1 mRNA and protein levels in hamster neonates exposed to dexamethasone. At −24 and −3 h, hamster neonates were injected subcutaneously with dexamethasone. At 0 h (7.5 dpp), livers were collected from treated and an additional 0-time sample was collected, and samples were pooled and analyzed for Cyp7a1 mRNA levels or protein levels (inset with β-actin as control, bottom) as described. mRNA data are presented as means ± SE (n = 7–8), and protein levels were obtained from pooled samples (n = 7–8). *Significant differences between treated and untreated animals (P < 0.025).
DISCUSSION
Bile acids are critical for lipid absorption, especially cholesterol and fat-soluble vitamins and to a lesser degree triglyceride. When BAPS are reduced and intraluminal bile acid concentrations fall below the critical micellar concentrations (71), cholesterol, triglyceride, and fat-soluble vitamin absorption decrease markedly (11, 52, 55, 57, 58). Adequate intraluminal bile acid concentrations are important early in life when the neonates consume diets high in lipids. Similar to that observed previously in rats and humans (6, 10, 25, 44, 69, 70), the hamster newborn has a small bile acid pool that increases with age, primarily after 10.5 dpp. An increase in pool size could be due to a change in the expression profile of enzymes in the bile acid biosynthetic pathway, a change in the supply of substrates for bile acids, or improved intestinal conservation. The focus of the present studies was to evaluate the changes in the ontogeny of bile acid biosynthetic enzymes in relation to changes in bile acid pool size and the ability to induce precocious expression of key enzymes.
Several overall conclusions could be drawn from the present studies. First, expansion of the BAPS most closely correlates to levels of Cyp7a1, the rate-limiting step of the classical bile acid synthesis pathway, and follows temporally with the increase in Cyp7a1 levels. The increases in enzyme levels appear to be the result of a change in transcription since both mRNA and protein levels increase. The rate of transcription and not the rate of mRNA degradation is the regulatory factor in most (8, 53), but not all (3), studies. These results are not unique to the hamster in that Cyp7a1 levels also increase in the neonatal rat (21, 41, 62) and bile acid pool size increases in the human at least partly due to an increase in bile acid synthesis (25).
What is the mechanism that might be responsible for the increase in Cyp7a1 mRNA levels by 10.5 dpp in the neonatal hamster? Increases in expression of proteins at various times during development are due to hard wiring of protein expression or are due to the presence or absence of various transcription factors or regulators, such as hormones (77). Thus our second overall conclusion is that the increase in Cyp7a1 in the neonatal period occurs by way of an SHP-independent pathway. SHP-dependent regulation of Cyp7a1 begins with activation of FXR. Our initial hypothesis was that FXR would be inactivated in the neonatal period, leading to increased Cyp7a1 levels because of failure of abundant bile acids to activate the receptor, as occurs when the enterohepatic circulation of bile acids is disrupted (11, 73). Inactivation of FXR would lead to less SHP and a derepression of Cyp7a1 expression. An indication of FXR activation is SHP transcription (17, 38). Since SHP mRNA levels were unchanged during the neonatal period, it appears that the increase in Cyp7a1 was independent of SHP. FXR activation did appear lower in the neonates than adults since SHP mRNA levels were greater in adulthood than in the neonatal period. The rat neonate differs from the hamster neonate in this regard since FXR appears to be activated to adult levels in the rat neonate early in the suckling period (7 dpp) (4, 10), whereas FXR activation was lower in neonatal hamsters. The differences in these two species could be related to a greater abundance of bile acids as FXR agonists in the rat neonates; cholesterol synthesis rates are very elevated in the rat vs. the hamster (14), and hepatic cholesterol will activate LXR and increase Cyp7a1 transcription in the rat (31, 34). As predicted by this reasoning, LXR is activated as well by 7 dpp in the rat (4). It would seem likely that the increase in bile acid synthesis in the neonatal rat is more dependent on LXR than FXR activation since activation of FXR will decrease Cyp7a1 expression.
It is not surprising that Cyp7a1 levels can be affected in an SHP-independent fashion since Cyp7a1 in mice lacking SHP can still be regulated (33). Although there are a number of possible SHP-independent regulators of Cyp7a1, we hypothesized that the effects might be related to growth factors and hormones. Levels of HGF, a known regulator of Cyp7a1, did not change with age. FGF15 levels decreased at an age when Cyp7a1 levels increased (10.5 dpp). The absence of a parallel reciprocal relationship between FGF15 levels and Cyp7a1 expression between 5.5 and 20.5 dpp raised doubt about its role in Cyp7a1 expression. However, the reciprocal relationship that occurs between 5.5 and 10.5 dpp may be an important factor for the initial Cyp7a1 increase. mRNA levels of FGFR4, the hepatic receptor for FGF15, were similar throughout the neonatal period. Interestingly, mice lacking FGFR4 have a greater increase in bile acid pool size in late gestation compared with wild-type mice, suggesting that the regulation of Cyp7a1 is complex and mediated by multiple factors, and one factor may be FGF15 (78). Interestingly, FGF15 and FGFR4 are both expressed at greater levels in adulthood compared with the neonate and could be part of the reason for the lower Cyp7a1 levels in the adult, especially in the female. The hormones we focused on were cortisol and insulin since their concentrations change with age, and both can regulate Cyp7a1. We did indeed find a parallel increase in cortisol and Cyp7a1 levels. Consequently, we treated neonates with dexamethasone prior to the postnatal increase in Cyp7a1 to try and induce precocious expression of Cyp7a1. Neonates actually had a decrease in Cyp7a1 expression levels when treated with dexamethasone. Thus it is likely we activated PXR, a known suppressor of Cyp7a1 (35, 66). It is possible that exogenously administered glucocorticoid may be able to induce Cyp7a1 expression if administered during gestation or at other times (70).
Our third overall conclusion is that during development, as in adulthood (46), the relative amounts of Cyp8b1 and Cyp7a1 can dictate the proportion of CA or CDCA in the bile acid pool. Cyp7a1 is the first enzyme in the biosynthetic pathway and can lead to synthesis of both CA and CDCA whereas Cyp8b1 adds a 12α-hydroxyl group to the hydroxylated sterol and leads to CA synthesis. The youngest neonates had proportionately more Cyp8b1 than Cyp7a1 and had proportionately more CA at this age, supporting a major role of Cyp8b1 and the ontogeny of pool composition. These results are consistent with the hypothesis that a relationship exists between Cyp8b1 and bile acid composition.
A fourth overall summary of the present studies is that it appears that the alternative pathway is active early in life of the hamster, like the human (59). For the alternative pathway to be active, the enzymes would need to be expressed and the sterol would need to be able to enter the subcellular compartment where the synthesis would occur. We found that Cyp7b1 is expressed at relatively higher levels than Cyp7a1 early in gestation suggesting that bile acids can be synthesized via the alternative pathway. These results differ from those in the mouse since appreciable levels of Cyp7b1 are not expressed until the time of weaning (55, 56). The presence of Cyp7b1 does not prove that the alternative pathway is active, however. The presence of elevated StAR early in life seems appropriate considering the fact that Cyp7b1 is present. Although Cyp27a1 is the first step in the alternative pathway, the expression of the enzyme was not greatest at 1.5 dpp when Cyp7b1 was relatively elevated. Thus perhaps StAR and Cyp7b1 are driving the alternative pathway during rapid growth. This idea would appear to be supported by studies in which StAR levels, more so than Cyp27a1 levels, drive bile acid synthesis (22, 48).
Even though it appears that a relationship between Cyp7a1 and BAPS was observed, a brief discussion of intestinal conservation on expansion of the pool size in the neonatal period is warranted. In adults, ASBT is responsible for maintenance of the bile acid pool (11, 45). When ASBT is eliminated, the pool sizes decreases over 80%, even in the face of an increase in bile acid synthesis rates (11). Expression of ASBT does not appear until late in the neonatal period in rats at ∼19 dpp (60). Thus, although it seems that the initial increase in pool size at 10.5 dpp occurs prior to an increase in ASBT and in parallel with the increase in Cyp7a1, we do not know whether ASBT is expressed earlier in the neonatal hamster than the rat; it seems unlikely that ASBT would be expressed by 10.5 dpp in the hamster since it appears early in animals that develop rapidly, such as the guinea pig (26).
The results of the present study have implications for the premature human infant. These infants have reduced bile acid synthesis and consequently have relatively small pool sizes and reduced lipid absorption (6, 16, 25, 61, 69, 70). Induction of Cyp7a1 earlier in life with attendant pool size expression could enhance growth rates and improve outcomes. Pool sizes could be enhanced by treatment of pregnant women with corticosteroid, a commonly used practice to ensure proper lung development and prevent respiratory distress syndrome in premature infants. Pregnant women given dexamethasone deliver infants with increased synthesis rates of bile acids or increased Cyp7a1 levels (70) and cortisol treatment of rat fetal hepatocytes increases taurocholate synthesis (18). The ability to enhance pool sizes with dexamethasone or other treatments known to affect Cyp7a1 transcription rates would be a facile way to ensure infants the ability to absorb nutrients more efficiently. It should be noted that the development of the pathways responsible for the catabolism of cholesterol and thereby the synthesis of bile acids in an animal with a large requirement for cholesterol (neonate) seems a contradiction within itself in the rapidly growing individuals. Why would cholesterol be catabolized in animals that need an abundant supply of sterol for membrane formation? It is possible that the ability to synthesize bile acids is not fully developed at birth and is not induced until there appears to be abundant sterol in the neonatal liver, such as between 5.5 and 10.5 dpp as denoted by reduced sterol synthesis rates (75). As bile acid synthesis is then induced, hepatic sterol synthesis rates once again increase (75).
GRANTS
This work was supported by funding from grants HD39419 (L. A. Woollett) and DK059630 (University of Cincinnati Mouse Metabolic Phenotype Center) of the National Institutes of Health.
Acknowledgments
We thank T. J. Jones for excellent technical support.
REFERENCES
- 1.Agbemafle BM, Oesterreicher TJ, Shaw CA, Henning SJ. Immediate early genes of glucocorticoid action on the developing intestine. Am J Physiol Gastrointest Liver Physiol 288: G897–G906, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Agellon LB, Brover VA, Cheema SK, Gbaguidi GF, Walsh A. Dietary cholesterol fails to stimulate the human cholesterol 7α-hydroxylase gene (CYP7A1) in transgenic mice. J Biol Chem 277: 20131–20134, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Baker DM, Wang SL, Bell DJ, Drevon CA, Davis RA. One or more labile proteins regulate the stability of chimeric mRNAs containing the 3′-untranslated region of cholesterol-7α-hydroxylase mRNA. J Biol Chem 275: 19885–19991, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Balasubramaniyan N, Shahid M, Suchy FJ, Ananthanarayanan M. Multiple mechanisms of ontogenic regulation of nuclear receptors during rat liver development. Am J Physiol Gastrointest Liver Physiol 288: G251–G260, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bilz S, Samuel V, Morina K, Savage D, Choi CS, Shulman GI. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab 290: E716–E722, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Boehm G, Braun W, Moro G, Minoli I. Bile acid concentrations in serum and duodenal aspirates of healthy preterm infants: effects of gestational and postnatal age. Biol Neonate 71: 207–214, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Chiang JY, Kimmel R, Stroup D. Regulation of cholesterol 7α-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRα). Gene 262: 257–265, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chiang JYL Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40: 539–551, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Crestani M, Stroup D, Chiang JYL. Hormonal regulation of the cholesterol 7α-hydroxylase gene (CYP7). J Lipid Res 26: 2419–2432, 1995. [PubMed] [Google Scholar]
- 10.Cuesta de Juan S, Monte MJ, Macias RIR, Waunthier V, Buc Calderon PB, Marin JJG. Ontogenic development-associated changes in the expression of genes involved in rat bile acid homeostasis. J Lipid Res 48: 1362–1370, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Dawson PA, Haywood J, Carddock AL, Wilson M, Tietjent M, Kluckman KD, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem 278: 33920–33927, 2003. [DOI] [PubMed] [Google Scholar]
- 12.De Fabiani E, Crestani M, Marrapodi M, Pinelli A, Golfieri V, Galli G. Identification and characterization of cis-acting elements conferring insulin responsiveness on hamster cholesteryl 7α-hydroxylase gene promoter. Biochem J 347: 147–154, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Castillo-Olivares A, Gil G. Role of FXR and FTF in bile acid-mediated suppression of cholesterol 7alpha-hydroxylase transcription. Nucleic Acids Res 28: 3584–3593, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res 34: 1637–1659, 1993. [PubMed] [Google Scholar]
- 15.Duane WC, Javitt NB. 27-hydroxycholesterol: production rates in normal human subjects. J Lipid Res 40: 1194–1199, 1999. [PubMed] [Google Scholar]
- 16.Fomon SJ, Ziegler EE, Thomas LN, Jensen RL, Filer LJJ. Excretion of fat by normal full-term infants fed various milks and formulas. Am J Clin Nutr 23: 1299–1313, 1970. [DOI] [PubMed] [Google Scholar]
- 17.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6: 517–526, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Graham TO, Van Thiel DH, Little JM, Lester R. Synthesis of taurocholate by rat fetal liver in organ culture: effects of cortisol in vitro. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E177–E184, 1979. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7α-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem 276: 15816–15822, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hagenbuch B, Dawson P. The sodium bile salt cotransport family SLC10. Pflügers Arch 447: 566–570, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Hahn P, Innis SM. Cholesterol oxidation and 7α-hydroxylation during postnatal development of the rat. Biol Neonate 46: 48–52, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Hall E, Hylemon PB, Vlahcevic R, Mallonee DH, Valerie K, Avadhani N, Pandak WM. Overexpression of CYP27 in hepatic and extrahepatic cells: role in the regulation of cholesterol homeostasis. Am J Physiol Gastrointest Liver Physiol 281: G293–G301, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Hardikar W, Ananthanarayanan M, Suchy FJ. Differential ontogenic regulation of basolateral and canalicular bile acid transport proteins in rat liver. J Biol Chem 270: 20841–20846, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Hardy KJ, Hoffman NE, Mihaly G, Sewell RB, Smallwood RA. Bile acid metabolism in fetal sheep; perinatal changes in the bile acid pool. J Physiol 309: 1–11, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heubi J, Balistreri W, Suchy F. Bile salt metabolism in the first year of life. J Lab Clin Med 100: 127–136, 1982. [PubMed] [Google Scholar]
- 26.Heubi J, Fondacaro J. Postnatal development of intestinal bile salt transport in the guinea pig. Am J Physiol Gastrointest Liver Physiol 243: G189–G194, 1982. [DOI] [PubMed] [Google Scholar]
- 27.Horton JD, Cuthbert JA, Spady DK. Regulation of hepatic 7α-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J Biol Chem 270: 5381–5387, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Hylemon PB, Gurley EC, Stravitz RT, Sitz JS, Pandak WM, Chiang JYL, Vlahcevic ZR. Hormonal regulation of cholesterol 7α-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J Biol Chem 267: 16866–16871, 1992. [PubMed] [Google Scholar]
- 29.Iizasa H, Genda N, Kitano T, Tomita M, Nishihara K, Hayashi M, Nakamura K, Kobayashi S, Nakashima E. Altered expression and function of P-glycoprotein in dextran sodium sulfate-induced colitis in mice. J Pharm Sci 92: 569–576, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Janowski BA, Willy PJ, Devi TR, Falch JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383: 728–731, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Jelinek DF, Andersson S, Slaughter CA, Russell DW. Cloning and regulation of cholesterol 7α-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem 265: 8190–8197, 1990. [PMC free article] [PubMed] [Google Scholar]
- 33.Kerr TA, Saeki L, Schneider M, Schaefer K, Berdy S, Redder T, Shan B, Russell DW, Schwartz M. Loss of nuclear receptor SHP impairs but does not eliminate negative feedback regulation of bile acid synthesis. Dev Cell 2: 713–720, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272: 3137–3140, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7α-hydroxylase gene transcription. Am J Physiol Gastrointest Liver Physiol 288: G74–G84, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Li T, Kong X, Wosley E, Ellis E, Strom S, Chiang JYL. Insulin regulation of cholesterol 7α-hydroxylase expression in human hepatocytes. Roles of forkhead box 01 and sterol regulatory element-binding protein 1c. J Biol Chem 281: 28745–28754, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little J, Lester R. Ontogenesis of intestinal bile salt absorption in the neonatal rat. Am J Physiol Gastrointest Liver Physiol 239: G319–G323, 1980. [DOI] [PubMed] [Google Scholar]
- 38.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6: 507–515, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Macias RIR, Serrano MA, Monte MJ, Jimenez S, Hernandez B, Marin JJG. Long-term effect of treating pregnant rats with ursodeoxycholic acid on the congenital impairment of bile secretion induced in the pups by maternal cholestasis. J Pharmacol Exp Ther 312: 751–758, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87: 206–210, 1978. [DOI] [PubMed] [Google Scholar]
- 41.Massimi M, Lear SR, Huling SL, Jones AL, Erickson SK. Cholesterol 7 α-hydroxylase (CYP7A): patterns of messenger RNA expression during rat liver development. Hepatology 28: 1064–1072, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki Y, Bouscarel B, Ikegami T, Honda A, Doy M, Ceryak S, Fukushima S, Yoshida S, Shoda J, Tanaka N. Selective inhibition of CYP27A1 and of chenodeoxycholic acid synthesis in cholestatic hamster liver. Biochim Biophys Acta 1588: 139–148, 2002. [DOI] [PubMed] [Google Scholar]
- 43.McConihay JA, Horn PS, Woollett LA. The effect of maternal hypercholesterolemia on fetal sterol metabolism in the Golden Syrian hamster. J Lipid Res 42: 1111–1119, 2001. [PubMed] [Google Scholar]
- 44.Morris AI, Little JM, Lester R. Development of the bile acid pool in rats from neonatal life through puberty to maturity. Digestion 28: 216–224, 1984. [DOI] [PubMed] [Google Scholar]
- 45.Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J Clin Invest 99: 1880–1887, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandak WM, Bohdan P, Franklund C, Mallonee DH, Eggersten G, Bjorkhem I, Gil G, Vlahcevic R, Hylemon PB. Expression of sterol 12α-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology 120: 1801–1809, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Pandak WM, Li YC, Chiang JY, Studer EJ, Gurley EC, Heuman DM, Vlahcevic ZR, Hylemon PB. Regulation of cholesterol 7 α-hydroxylase mRNA and transcriptional activity by taurocholate and cholesterol in the chronic biliary diverted rat. J Biol Chem 266: 3416–3421, 1991. [PubMed] [Google Scholar]
- 48.Pandak WM, Ren S, Marques D, Hall E, Redford K, Mallonee K, Bohdan P, Heuman DM, Gil G, Hylemon PB. Transport of cholesterol into mitochondria is rate-limiting for bile acid synthesis via the alternative pathway in primary rat hepatocytes. J Biol Chem 277: 48158–48164, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Princen HMG, Meijer P, Hofstee B. Dexamethasone regulates bile acid synthesis in monolayer cultures of rat hepatocytes by induction of cholesterol 7α-hydroxylase. Biochem J 262: 341–348, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren S, Hylemon PB, Marques D, Gurley EC, Bodham P, Hall E, Redford K, Gil G, Pandak WM. Overexpression of cholesterol transporter StAR increases in vivo rates of bile acid synthesis in the rat and mouse. Hepatology 40: 910–917, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Ren S, Hylemon PB, Marques D, Hall E, Redford K, Gil G, Pandak WM. Effect of increasing the expression of cholesterol transporters (StAR, MLN64, and SCP-2) on bile acid synthesis. J Lipid Res 45: 2123–2131, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Repa JJ, Lund EG, Horton JD, Leitersdorf E, Russell DW, Dietschy JM, Turley SD. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hypertriglyceridemia. Reversal by cholic acid feeding. J Biol Chem 275: 39685–39692, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Russell DW The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72: 137–174, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Russell DW, Setchell KDR. Bile acid biosynthesis. Biochemistry 31: 4737–4749, 1992. [DOI] [PubMed] [Google Scholar]
- 55.Schwarz M, Lund E, Setchell K, Kayden H, Zerwekh J, Bjorkhem I, Herz J, Russell D. Disruption of cholesterol 7 α-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7 α-hydroxylase. J Biol Chem 271: 18024–18031, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwarz M, Lund EG, Lathe R, Björkhem I, Russell DW. Identification and characterization of a mouse oxysterol 7 α-hydroxylase cDNA. J Biol Chem 272: 23995–24001, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz M, Russell DW, Dietschy JM, Turley SD. Alternate pathways of bile acid synthesis in the cholesterol 7α-hydroxylase knockout mouse are not upregulated by either cholesterol or cholestyramine feeding. J Lipid Res 42: 1594–1603, 2001. [PubMed] [Google Scholar]
- 58.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res 39: 1833–1843, 1998. [PubMed] [Google Scholar]
- 59.Setchell KDR, Schwarz M, O'Connell N, Lund EG, Davis DL, Lathe R, Thompson HR, Tyson RW, Sokol RJ, Russell DW. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7 α-hydroxylase gene causes severe neonatal liver disease. J Clin Invest 102: 1690–1703, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest 95: 745–754, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Signer E, Murphy G, Edkins S, Anderson C. Role of bile salts in fat malabsorption of premature infants. Arch Dis Child 49: 174–180, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith JL, Lear SR, Erickson SK. Developmental expression of elements of hepatic cholesterol metabolism in the rat. J Lipid Res 36: 641–652, 1995. [PubMed] [Google Scholar]
- 63.Solomon NS, Gartner H, Oesterreicher TJ, Henning SJ. Development of glucocorticoid-responsiveness in mouse intestine. Pediatr Res 49: 782–788, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Song KH, Ellis E, Strom S, Chiang JYL. Hepatocyte growth factor signaling pathway inhibits cholesterol 7α-hydroxylase and bile acid synthesis in human hepatocytes. Hepatology 46: 1993–2002, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7 α-hydroxylase gene expression. Hepatology 49: 297–305, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Standinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klassen CD, Brown KK, Reinhard J, Wilson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutano W, De Kloet ER. Species-specificity of corticosteroid receptors in hamster and rat brains. Endocrinology 121: 1405–1411, 1987. [DOI] [PubMed] [Google Scholar]
- 68.Ueki I, Kimura A, Nishyori A, Chen HL, Takei H, Nittono H, Kurosawa T. Neonatal cholestatic liver disease in an Asian patient with a homozygous mutation in the oxysterol 7α-hydroxylase gene. J Pediatr Gastroenterol Nutr 46: 465–469, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Watkins JB, Ingall D, Szczepanik PA, Klein PD, Lester R. Bile salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technique. N Engl J Med 188: 431–434, 1973. [DOI] [PubMed] [Google Scholar]
- 70.Watkins JB, Szczepanik P, Gould JB, Klein P, Lester R. Bile salt metabolism in the human premature infant. Preliminary observations of pool size and synthesis rate following prenatal administration of dexamethasone and phenobarbital. Gastroenterology 69: 706–713, 1975. [PubMed] [Google Scholar]
- 71.Woollett LA, Wang Y, Buckley DD, Yao L, Chin S, Granholm N, Jones PJH, Setchell KDR, Tso P, Heubi JE. Micellar solubilisation of cholesterol is essential for absorption in humans. Gut 55: 197–204, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A, Foster J, Liang J, Brush J, Gu Q, Hillan K, Goddard A, Gureney AL. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine 11: 729–735, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Xu G, Pan L, Erickson SK, Forman BM, Shneider BL, Ananthanarayanan M, Li X, Shefer S, Balasubramaniyan N, Ma L, Asoka H, Lear SR, Nguyen LB, Dussault I, Suchy FJ, Tint GS, Salen G. Removal of the bile acid pool upregulates cholesterol 7α-hydroxylase by deactivating FXR in rabbits. J Lipid Res 43: 45–50, 2002. [PubMed] [Google Scholar]
- 74.Yao L, Dawson PA, Woollett LA. Increases in biliary cholesterol-to-bile acid ratio in pregnant hamsters fed low and high levels of cholesterol. Am J Physiol Gastrointest Liver Physiol 284: G263–G268, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Yao L, Horn PS, Heubi JE, Woollett LA. The liver plays a key role in whole body sterol accretion of the neonatal Golden Syrian hamster. Biochim Biophys Acta 1771: 550–557, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao L, Jenkins K, Horn PS, Lichtenberg MH, Woollett LA. Inability to fully suppress sterol synthesis rates with exogenous sterol in embryonic and extraembyronic fetal tissues. Biochim Biophys Acta 1171: 1372–1379, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaylaoglu MB, Agbemafle BM, Oesterreicher TJ, Finegold MJ, Thaller C, Henning SJ. Diverse patterns of cell-specific gene expression in response to glucocorticoid in the developing small intestine. Am J Physiol Gastrointest Liver Physiol 291: G1041–G1050, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem 275: 15482–15489, 2000. [DOI] [PubMed] [Google Scholar]