Abstract
Bone marrow (BM) is the major reservoir for endothelial progenitor cells (EPCs). Postnatal neovascularization depends on not only angiogenesis but also vasculogenesis, which is mediated through mobilization of EPCs from BM and their recruitment to the ischemic sites. Reactive oxygen species (ROS) derived from Nox2-based NADPH oxidase play an important role in postnatal neovascularization; however, their role in BM and EPC function is unknown. Here we show that hindlimb ischemia of mice significantly increases Nox2 expression and ROS production in BM-mononuclear cells (BMCs), which is associated with an increase in circulating EPC-like cells. Mice lacking Nox2 show reduction of ischemia-induced flow recovery, ROS levels in BMCs, as well as EPC mobilization from BM. Transplantation of wild-type (WT)-BM into Nox2-deficient mice rescues the defective neovascularization, whereas WT mice transplanted with Nox2-deficient BM show reduced flow recovery and capillary density compared to WT-BM transplanted control. Intravenous infusion of WT- and Nox2-deficient BMCs into WT mice reveals that neovascularization and homing capacity are impaired in Nox2-deficient BMCs in vivo. In vitro, Nox2-deficient c-kit+Lin− BM stem/progenitor cells show impaired chemotaxis and invasion as well as polarization of actins in response to stromal derived factor (SDF), which is associated with blunted SDF-1–mediated phosphorylation of Akt. In conclusion, Nox2-derived ROS in BM play a critical role in mobilization, homing, and angiogenic capacity of EPCs and BM stem/progenitor cells, thereby promoting revascularization of ischemic tissue. Thus, NADPH oxidase in BM and EPCs is potential therapeutic targets for promoting neovascularization in ischemic cardiovascular diseases.
Keywords: NADPH oxidase, reactive oxygen species, angiogenesis, vasculogenesis, neovascularization, stromal derived factor, endothelial progenitor cells
Neovascularization is an important repair mechanism to rescue tissue from critical ischemia.1 It is a key process involved in normal development and wound repair as well as various pathophysiologies such as ischemic heart disease and peripheral artery disease. Postnatal new blood vessel formation involves not only angiogenesis but also vasculogenesis which is mediated through mobilization of endothelial progenitor cells (EPCs) and stem/progenitor cells from bone marrow (BM) as well as their homing to the ischemic tissues.1 Intravenous infusion of BM mononuclear cells (BMCs) and progenitor cells augments ischemia-induced neovascularization.2 BM microenvironment, which consists of macrophages, fibroblasts, and endothelial cells (ECs), is important for maintenance and mobilization of stem/progenitor cells.3–5 Understanding mechanisms by which EPCs and BM progenitor cells are mobilized and homed to the site of neovascularization in response to ischemia is essential for development of new therapeutic strategies for ischemic cardiovascular diseases.
Reactive oxygen species (ROS) such as superoxide anion (O2∙−) and hydrogen peroxide (H2O2) play an important role in normal cell growth, migration, differentiation, apoptosis, and senescence.6,7 Although excess amounts of ROS are toxic, low levels of ROS produced in response to tissue ischemia serve as intracellular signaling molecules to prevent tissue injury and promote angiogenesis.8,9 In ECs, NADPH oxidase is one of the major sources of ROS,10 and it consists of catalytic subunits (Nox1, Nox2, and Nox4), p22phox, p47phox, p67phox, and the small GTPase Rac1. The endothelial NADPH oxidase is activated by numerous stimuli including angiogenesis growth factors, cytokines, shear stress, hypoxia, and G protein– coupled receptor agonists.9 ROS derived from NADPH oxidase stimulate diverse redox signaling pathways leading to angiogenic-related responses in ECs.9 We previously reported that Nox2-derived ROS play an important role in VEGF signaling linked to EC migration and proliferation, as well as reparative angiogenesis in response to hindlimb ischemia in vivo.11,12 Increasing evidence suggests that ROS are generated by NADPH oxidase in EPCs and stem/progenitor cells,13–15 which are involved in differentiation, proliferation, senescence, or apoptosis depending on cell types and amount of ROS. However, the role of Nox2-based NADPH oxidase in BM and EPCs/progenitor cell function linked to postnatal vasculogenesis remains unknown.
Here we demonstrate that hindlimb ischemia of mice increases expression of Nox2-based NADPH oxidase and ROS production in BM. Mice lacking Nox2 show impaired ischemia-induced blood flow recovery and neovascularization, which is associated with reduction of ROS production in BM as well as number of EPCs in peripheral blood. Defective neovascularization in Nox2−/− mice is rescued by transplantation of BM from WT mice. Intravenous infusion of Nox2−/− BMCs show impaired homing ability to sites of neovascularization. Mechanically, chemotaxis and invasion as well as actin polymerization induced by chemokine, stromal cell derived factor-1α (SDF-1 α) are inhibited in Nox2−/− BM stem/progenitor cells, which is associated with blunted phosphorylation of Akt. These findings suggest that Nox2-derived ROS play an essential role in regulating redox state in BM which is required for reparative EPC and progenitor cells mobilization from BM as well as their homing and neovascularization capacity, thereby promoting revascularization and tissue repair in response to ischemic injury.
Materials and Methods
Isolation of BM mononuclear cells and BM stem/progenitor cells, mouse hindlimb ischemia model, immunocytochemical analysis of hindlimb tissues, measurement of ROS production in BM, BM transplantation, measurement of circulating EPCs and vascular progenitor cells levels using EPCs culture assay and FACS analysis, chemotaxis and invasion assay, phalloidin staining in BM progenitor cells, cell-matrix adhesion assay, Western blotting, and statistical analyses are described in the Material and Methods section in the online Data Supplement (available online at http://circres.ahajournals.org).
Results
ROS Levels and Nox2 Expression Are Increased in BMCs in Response to Hindlimb Ischemia
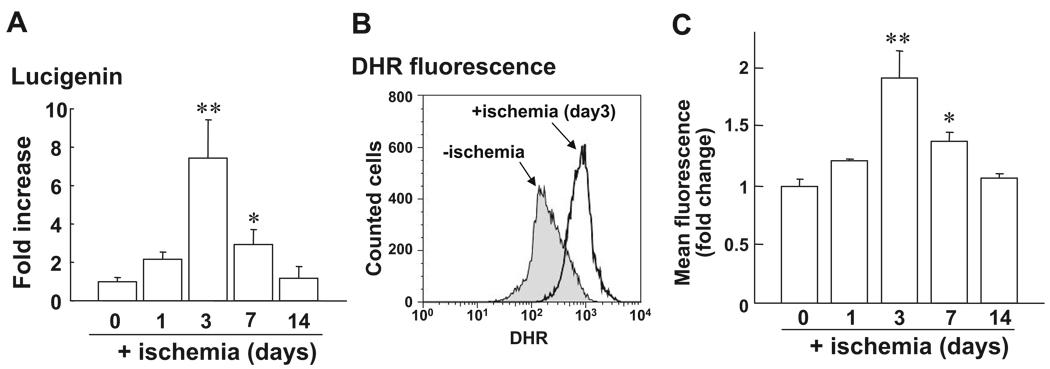
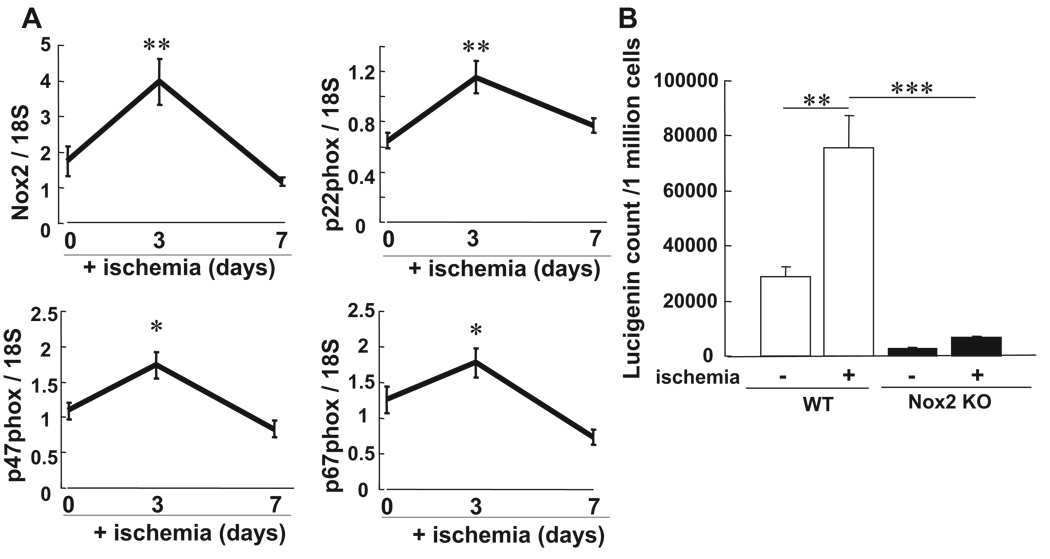
To assess the role of ROS in BM, we examined effects of hindlimb ischemia on ROS levels in BMCs. Figure 1 shows that ROS level in BMCs was markedly increased at 3 and 7 days after hindlimb ischemia peaking at day 3, as measured by lucigenin (Figure 1A) and dihidrorhodamine (DHR) fluorescence assays with FACS analysis (Figure 1B). Of note, lucigenin- and DHR-sensitive ROS production were abolished by the presence of superoxide (O2∙−) dismutase and catalase, respectively (data not shown), suggesting that both O2∙− and H2O2 are increased in BM after hindlimb ischemia. To assess the role of Nox2-based NADPH oxidase in BM, we next examined the expression of Nox2 and other NADPH oxidase components in BMCs with and without hindlimb ischemia using real-time PCR. Figure 2A shows that mRNAs for Nox2 and other components including p22phox, p47phox, and p67phox were significantly increased in BM with peak at 3 days after ischemia. Nox2 protein expression was also markedly increased in BMCs after hindlimb ischemia (data not shown). We found that Nox2 was the most highly expressed Nox isoforms compared to Nox1 and Nox4 in BMCs, as measured by real-time PCR. There was no significant increase of Nox1 and Nox4 mRNAs in BMCs in response to ischemia (data not shown). Moreover, basal and ischemia-induced O2∙− productions were almost completely abolished in Nox2 KO BMCs (Figure 2B). These results suggest that Nox2-based NADPH oxidase is the major source of ROS produced in BM in basal state and after hindlimb ischemia.
Figure 1.
Increase of ROS production in BMCs after hindlimb ischemia. Low-density BM-MNCs (BMCs) were separated by Histopaque 1077. A, O2∙− production in BMCs after hindlimb ischemia on postoperative days 0, 1, 3, 7, and 14, as measured by lucigenin-enhanced chemiluminescence technique (n=4 in each time point). B, Dihydrorhodamine 123 (DHR) fluorescence in BMCs after hindlimb ischemia is measured by FACS analysis. Upper panel shows the representative histogram of fluorescence intensity, expressed by the logarithum (x axis) on 0 and 3 days after ischemia. Lower panel shows the averaged data, expressed as fold change of fluorescence over basal (the ratio on day 0 was set to 1) on various postoperative days (n=4, **P<0.05, **P<0.01 vs day 0).
Figure 2.
Nox2-based NADPH oxidase is involved in ROS production in BMCs after hindlimb ischemia. A, The mRNA expression for Nox2 and its coupled NADPH oxidase components including p22phox, p47phox, and p67phox in BMCs at 0, 3, and 7 days after ischemia, as measured by real-time PCR. B, O2∙− production in BMCs at 0 and 3 days after hindlimb ischemia in WT and Nox2−/− mice, as measured by lucigenin-enhanced chemiluminescence technique (n=4 to 6, **P<0.01 vs baseline, ***P<0.001 vs WT).
Ischemia-Induced Increase in EPC-Like Cells in Peripheral Blood Is Inhibited in Nox2−/− Mice
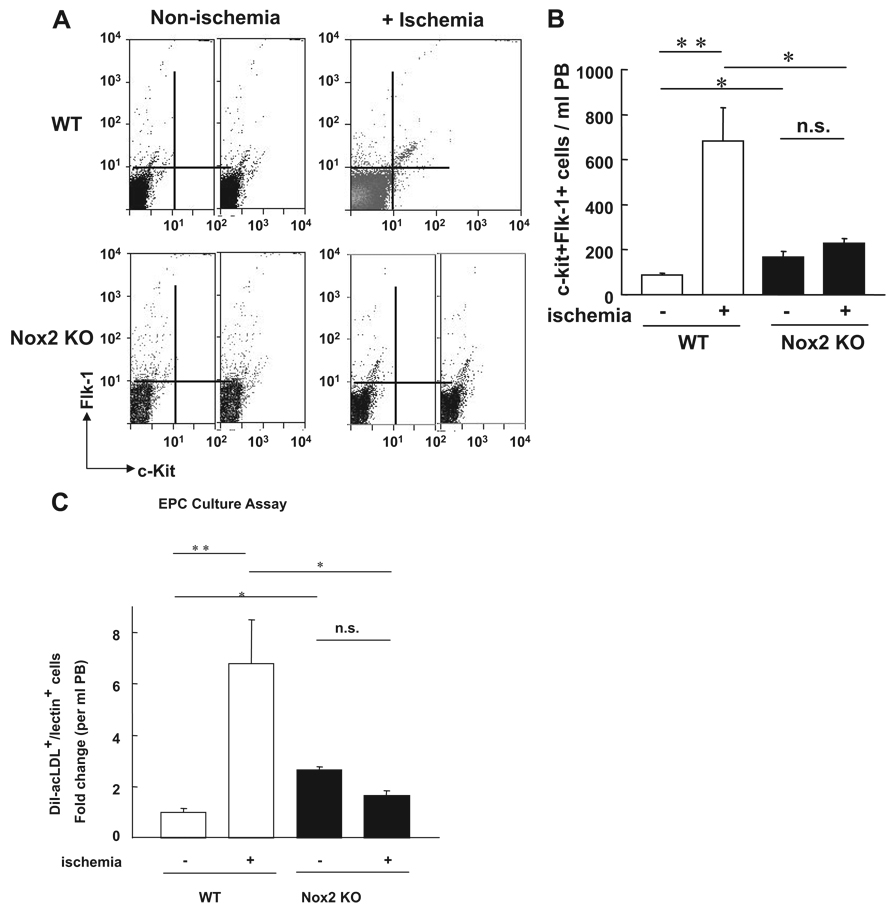
To examine whether Nox2-derived ROS are involved in EPC mobilization from BM, we analyzed the number of c-kit+Flk-1+ cells in peripheral blood (PB) in WT and Nox2−/− mice. FACS analysis shows a significant reduction in the number of c-kit+Flk-1+ cells in PB at 3 days after ischemia in Nox2−/− mice compared with WT mice (Figure 3A and 3B). This finding was further confirmed by the decrease of number of circulating Sca-1+Flk+ cells and Sca-1+Lin− cells after ischemia in Nox2−/− mice (supplemental Figure I). Moreover, number of DiI-acLDL and BS lectin double positive EPCs obtained by culture of PB-derived mononuclear cells after hindlimb ischemia was significantly decreased in Nox2−/− mice (Figure 3C and 3D). Of note, the number of EPC-like cells is increased in Nox2−/− mice under normoxic conditions presumably because of compensatory mechanism induced by the absence of Nox2. These results suggest that Nox2-based NADPH oxidase plays an important role in reparative mobilization of BM-derived EPCs and stem/progenitor cells in response to ischemia.
Figure 3.
The number of circulating EPC-like cells after hindlimb ischemia is inhibited in Nox2−/− mice. A, The number of EPC-like ckit+Flk1+ cells in peripheral blood (PB) mononuclear cell fraction at 0 (+ nonischemia) and 3 days (+ ischemia) after hindlimb ischemia in WT and Nox2−/− mice, as measured by FACS analysis. B, Statistical analysis of ckit+Flk1+ positive cells in PB (n=6, *P<0.05, **P<0.01). C, EPC culture assay. Quantitative analysis of the numbers of DiI-acLDL and BS lectin double positive EPCs measured at 4 days after culture of PB-derived MNCs obtained from WT and Nox2−/− mice at 0 and 3 days after ischemia. Data are expressed as fold change over basal (the ratio on day 0 in WT mice was set to 1). (n=4 to 6, *P<0.05, **P<0.01).
Nox2+/+ Bone Marrow Rescues Impairment of Neovascularization in Nox2−/− Mice
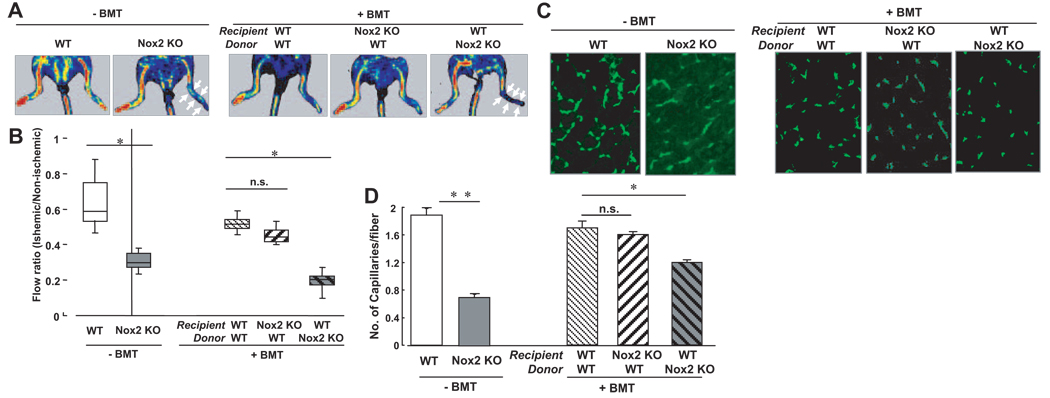
To assess the role of Nox2 in BM function involved in neovascularization in vivo, we performed BM transplantation between WT and Nox2−/− mice to make BM chimera mice. Figure 4 show that Nox2−/− mice without BM transplantation showed significant reduction of limb blood flow recovery and capillary density after ischemia compared with WT mice, which is consistent with our previous report.12 Transplantation of WT BMCs into the irradiated Nox2−/− mice rescued the impaired ischemia-induced blood flow recovery (Figure 4A and 4B) and reduced capillary formation in ischemic muscle (Figure 4C and 4D). In contrast, WT mice transplanted with Nox2−/− showed opposite effects (Figure 4). These data suggest that Nox2-based NADPH oxidase induced in BM after tissue ischemia plays a critical role in BM function, thereby regulating reparative neovascularization.
Figure 4.
Transplantation of BM from wild-type mice rescues the impaired neovascularization after hindlimb ischemia in Nox2−/− mice. The lethally irradiated recipient WT or Nox2−/− mice were transplanted with BMCs from WT or Nox2−/− mice and subjected to hindlimb ischemia at 6 to 8 weeks after BM transplantation. A, Representative picture of laser Doppler blood flow (LDBF) imaging at day 14 of ischemic (right) and nonischemic (left) limbs after ligation of femoral artery in WT and Nox2−/− mice without (−) or with (+) BM transplantation (BMT). Arrow indicates delayed blood flow recovery. B, Quantification of blood flow recovery, as determined by the ischemic/nonischemic LDBF ratio in each group (n=4 to 10, *P<0.05). C, Representative pictures of immunostaining of isolectin B4 positive cells which represent capillaries in ischemic tissues obtained from WT and Nox2−/− mice without or with BMT, as described above. D, Quantitative analysis of capillary density, expressed as the number of capillaries per fiber in each group (n=4, *P<0.05, **P<0.01).
Nox2 Is Involved in Homing and Neovascularization Capacity of BM-Derived Cells In Vivo
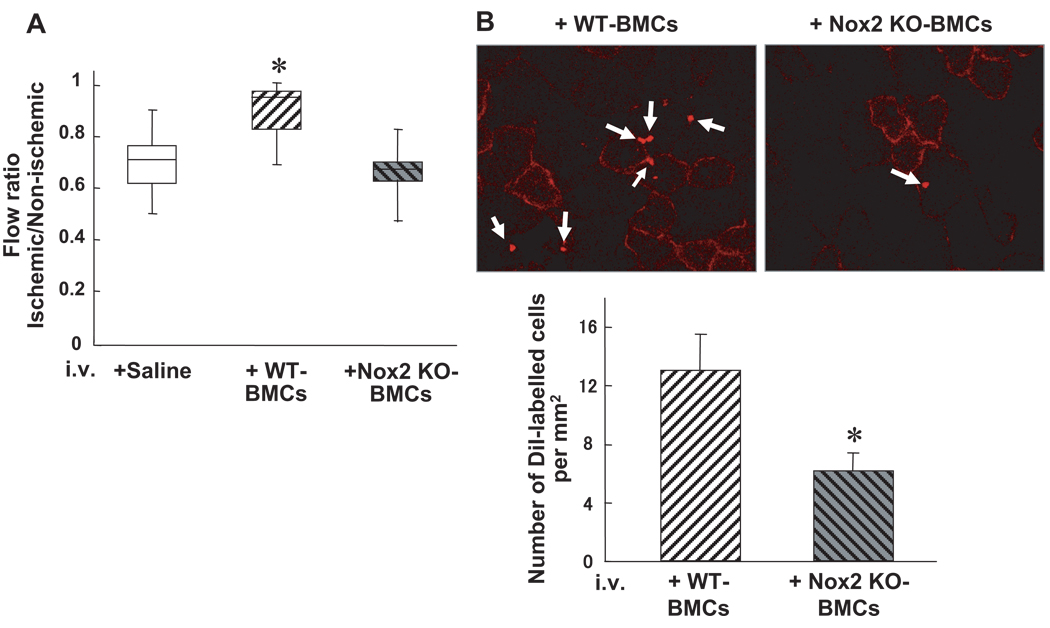
To examine the role Nox2 in neovascularization capacity of BM-derived cells, we performed intravenous infusion of BMCs derived from WT and Nox2−/− mice into WT mice at 1 day after induction of hindlimb ischemia. Figure 5A shows that WT-BMC injection into WT mice significantly augmented blood flow recovery at 14 days after operation, whereas Nox2−/− BMC infusion showed no significant effect. Of note, pretreatment of Nox2−/− BMCs with 5 µmol/L H2O2 rescued defective neovascularization capacity of Nox2−/− BMCs (supplemental Figure II). In addition, Nox2 deficiency did not affect the number of Sca-1+lk-1+cells, Sca-1+in− cells or expression of CXCR4+ cells in BMCs (data not shown). To examine the role of Nox2 in homing capacity of BMCs, we intravenously injected DiI-labeled WT and Nox2 KO BMCs at 1 day after ischemia. Figure 5B shows that Nox2−/− BMCs reduced ability to home to ischemic tissues in comparison to WT BMCs. These results suggest that Nox2 is required for proangiogenic and homing capacity of BMCs, thereby promoting neovascularization of ischemic tissue in vivo.
Figure 5.
Homing and neovascularization capacity are impaired in Nox2−/− BMCs in vivo. A, BMCs (3×106 cells) from WT or Nox2−/− mice, or saline control, were intravenously injected into WT mice at 24 hours after femoral artery ligation, and blood flow recovery, as assessed by the ischemic/nonischemic LDBF ratio, was measured at 14 days after hindlimb ischemia. (n=4, *P<0.05 vs control). B, Upper panel, representative photographs of accumulated intravenously injected Dil-labeled WT- and Nox2−/− BMCs in ischemic adductor muscles. Lower panel, quantitative analysis of the number of accumulated Dil-labeled WT- and Nox2−/− BMCs in ischemic border zones of adductor muscle (n=3 in each group, *P<0.05).
Nox2 Is Involved in Chemotaxis, Invasion, and Polarization of Actin in BM Progenitor Cells In Vitro
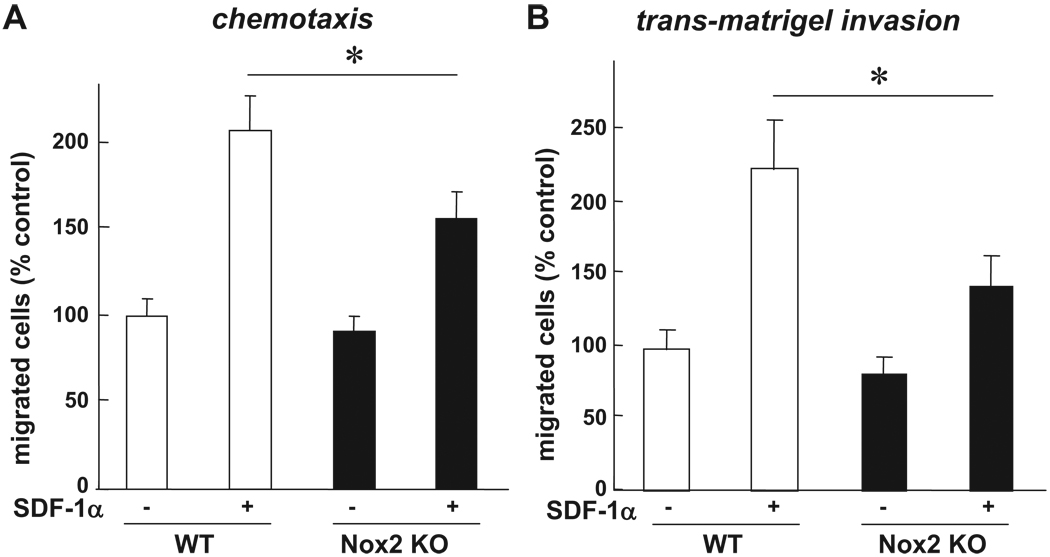
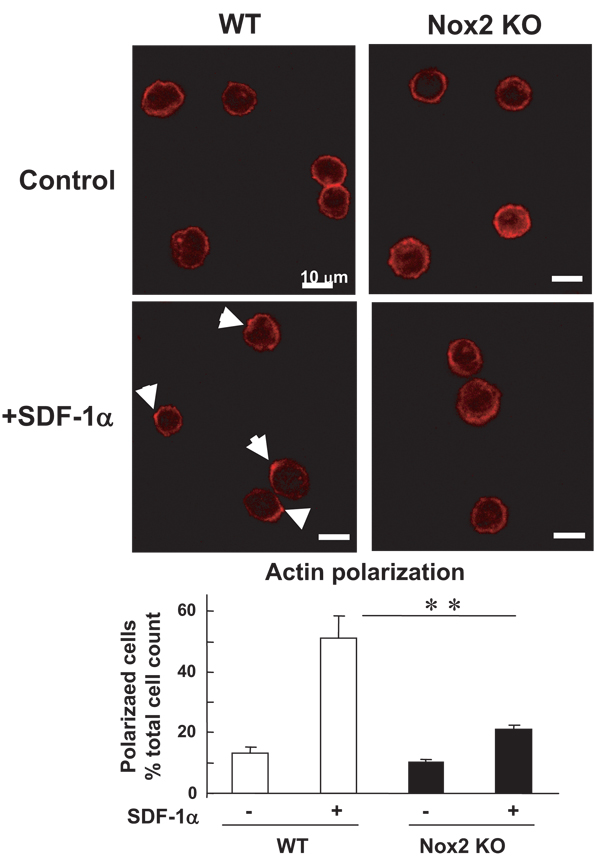
Because homing capacity of EPCs and stem/progenitor cells is regulated by various processes such as cell adhesion, chemotaxis, and invasion, we next examined whether Nox2 is involved in these responses using BM-MNCs and c-kit+Lin− BM progenitor cells which play an important role in revascularization of ischemic tissue.16 Adhesion capacity of c-kit+Lin− BM cells to various extracellular matrix proteins was not affected in Nox2−/− cells (supplemental Figure III). To determine the role of Nox2 in chemotaxis and invasion of BM progenitor cells, we performed transwell migration assay toward chemokine SDF-1α, an exclusive ligand of CXCR4 receptor, which is potent stimulator for migration and homing of stem/progenitor cells. Both migration (Figure 6A) and invasion capacity (Figure 6B) of ckit+Lin− BM cells toward SDF-1α were significantly inhibited in Nox2−/− cells. We confirmed that pretreatment of Nox2−/− BM c-kit+Lin− cells with 5 µmol/L H2O2 improved the impaired migration capacity of these cells, whereas it had no significant effect on WT-BM cells (supplemental Figure IV). SDF-1α–induced actin polymerization plays an important role in hematopoietic stem/progenitor cell migration.17,18 Furthermore, immuno-fluorescence analysis of ckit+Lin− BM cells revealed that SDF-1α–induced actin polarization, as visualized by FITC-phalloidin staining, was inhibited in Nox2−/− cells (Figure 7). These data suggest that Nox2 is necessary for chematoxis, invasion capacity at least in part by regulating actin polymerization, but not cell-matrix adhesion capacity of BM progenitor cells, in vitro.
Figure 6.
Chemotaxis and transmatrigel invasion induced by SDF-1α are impaired in Nox2−/− BM stem/progenitor cells in vitro. Quantification of SDF-1α–induced migration (chemotaxis; A) and invasion through matrigel (B) of WT-and Nox2−/− BM c-kit+Lin− progenitor cells, as measured by transwell migration assay. Cells were stimulated with or without SDF-1α (300 ng/mL) for 3 hours. Bar graph represents averaged data, expressed as fold change in number of migrated cells to lower chamber, over that in unstimulated WT cells (control). (n=4, *P<0.05).
Figure 7.
SDF-1α–induced F-actin polarization is impaired in Nox2−/− BM stem/progenitor cells in vitro. Upper panel, freshly isolated WT- and Nox2−/− BM c-kit+Lin− cells in suspension were stimulated with or without SDF-1α for 5 minutes, fixed, and then stained for Alexa Fluor 568 conjugated phalloidin, which is visualized by confocal microscopy. Arrows indicate the polarized F-actin. Original magnification is ×630 and bars show 10 µm. Lower panel, quantitative analysis of SDF-induced F-actin polarization in WT- and Nox2−/− BM c-kit+Lin− cells. Data are expressed as percentage of the total 100 cells from 4 randomly selected fields (×100; n=4, **P<0.01).
Nox2 Is Involved in Phosphorylation of Akt in BMCs
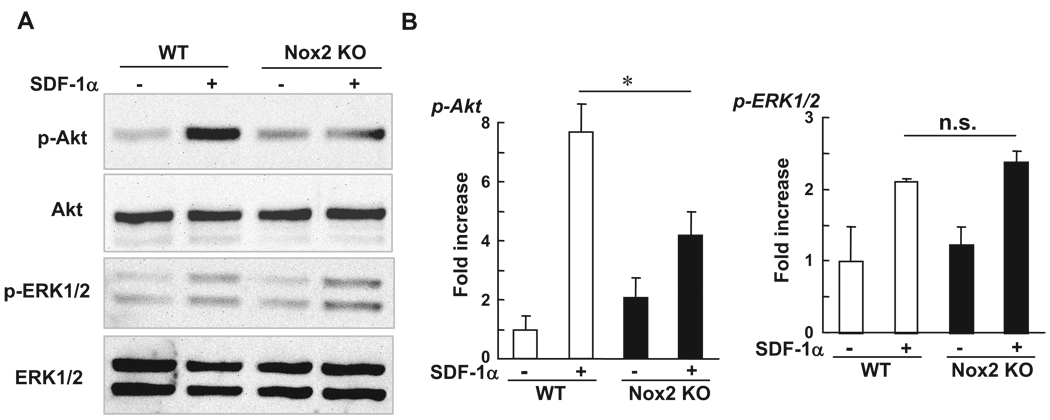
Akt plays an important role in migration of BM progenitor cells.19 To gain insight into the mechanism by which Nox2-derived ROS are involved in BMC migration, we examined whether Nox2 is involved in SDF-1α–stimulated Akt phosphorylation in BMCs. Figure 8 shows that phosphorylation of Akt in response to SDF-1α was significantly inhibited in Nox2−/− BMCs, whereas proliferation signal, ERK1/2 phosphorylation was not affected in Nox2−/− cells. This result suggests that function of CXCR4 is intact in Nox2−/− BMCs and that Nox2-derived ROS are involved in Akt phosphorylation in BM stem/progenitor cells, thereby promoting their homing capacity.
Figure 8.
Phosphorylation of Akt induced by SDF-1 is inhibited in Nox2−/− BMCs. Freshly isolated WT- and Nox2−/− BMCs were serum depleted for 4 hours and then stimulated with SDF-1α (300 ng/mL) for 5 minutes. Lysates were used for Western analysis with antiphospho-Akt (Ser473), phospho-ERK1/2, total Akt, or total ERK1/2 antibodies. Data are expressed as fold increase over the unstimulated WT cells (n=3, *P<0.05).
Discussion
We previously reported that Nox2-derived ROS are involved in reparative angiogenesis induced by hindlimb ischemia; however, their roles in postnatal vasculogenesis remains unclarified The present study extends our previous study by providing novel evidence that: (1) Hindlimb ischemia of mice significantly increases expression of Nox2-based NADPH oxidase and ROS production in BM, which is associated with an increase in circulating EPC-like c-Kit+Flk-1+ cells; (2) Mice lacking Nox2 show reduction of ischemia-induced increase in ROS production in BM as well as EPC mobilization; (3) Transplantation of WT-BM into Nox2−/− mice rescues the defective neovascularization; (4) Intravenous infusion of WT- and Nox2−/− BMCs into WT mice reveals impaired neovascularization and homing capacity of Nox2−/− BMCs in vivo; (5) In vitro SDF-stimulated chemotaxis and invasion as well as polarization of actins are inhibited in Nox2−/− BM stem/progenitor cells, which is associated with blunted phosphorylation of Akt.
EPCs and stem/progenitor cells are mobilized from BM in response to ischemic injury, which contributes to postnatal neovascularization.20–22 Using lucigenin assay, DHR fluorescence analysis, real-time PCR to detect expression of Nox2-based NADPH oxidase and Nox2−/− mice, here we demonstrate that hindlimb ischemia increases Nox2-dependent NADPH oxidase expression and ROS production in BM. Consistent with our data, previous reports show that NADPH oxidase components are expressed in various stem/progenitor cells including BM-derived human CD34+ cells,13,14 mouse embryonic stem cells,15 skeletal muscle precursor cells,23 and rat mesenchymal stem cells.24 In these studies, ROS derived from NADPH oxidase are shown to be involved in differentiation, proliferation, senescence, or apoptosis. We found that Nox2 is the most highly expressed Nox isoforms in normoxic BMCs, and that there is no significant increase of Nox1 and Nox4 mRNA expression in BMCs after ischemia. Mechanism by which Nox2 is increased in BMCs in response to hindlimb ischemia remains unclear. BMCs consist of heterogeneous populations including myeloid cells, immature lymphoid cells, and stem/progenitor cells. Thus, it is possible that Nox2 is increased in particular cell types or number of Nox2 expressing cells is increased attributable to differentiation, which may contribute to the increase in Nox2 expression in BMC after ischemia. Detailed analysis of mechanism of Nox2 induction in BMCs in response to ischemia is an objective of future study. In the present study, functional role of Nox2-based NADPH oxidase in BM is demonstrated by the observation that ischemia-induced increase in the numbers of EPC-like c-Kit+/Flk-1+ cells as well as DiI-acLDL and lectin double positive EPCs in PB, are significantly decreased in Nox2−/− mice which show defective neovascularization. Consistent with our result, number of circulating stem/progenitor cells induced by hematopoietic cytokine is reduced in Nox2−/− mice.25 Thus, Nox2 seems to play an important role in reparative mobilization of EPCs and stem/progenitor cells from BM in response to hindlimb ischemia.
The present study also demonstrates that transplantation of WT-BM into Nox2−/− mice rescue the defective neovascularization, whereas transplantation of Nox2−/− BM into WT mice shows opposite effects. This result suggests that Nox2-based NADPH oxidase expressed in BM is essential for BM function, which is required for maintenance and mobilization of EPCs as well as revascularization of ischemic tissues. Although excess amount of ROS is toxic to EPCs and stem/progenitor cells,26 our results are consistent with the notion that optimal levels of ROS, which are balanced by ROS-generating and antioxidant enzymes, are required for normal BM or EPC function involved in postischemic neovascularization. 27–29 Mechanisms by which hindlimb ischemia increases ROS levels in BM are unclear. It has been shown that ischemic injury of skeletal muscle increases production of hematopoietic cytokines, thereby stimulating stem/progenitor cells mobilization from BM.30 Of note, hematopoietic cytokines increase generation of ROS in progenitor cells, which in turn exits quiescent BM progenitor cells to promote cell-cycle progression.31 Thus, it is conceivable that induction of ischemia in skeletal muscles enhances circulating cytokine levels, which may stimulate Nox2-dependent ROS production in BM, thereby promoting reparative mobilization of EPCs from BM and revascularization of ischemic tissues.
EPCs and stem/progenitor cells are embedded in a local BM microenvironment, the so-called stem cell niche, and they are mobilized to the circulation in response to cytokines. 22,32 This process is dependent on EPCs and stem/progenitor cell proliferation, migration, and differentiation in BM. It remains unknown how Nox2-derived ROS regulate EPCs mobilization from BM. Most recent reports indicate that increased ROS in BM causes hematopoietic stem cells to exit from quiescent state, and drive to stimulate proliferation and differentiation33 during the early steps of commitment. 34 Stem cell mobilization is mediated by proteinases such as elastase, cathepsin G, and matrix metalloproteinase-9 (MMP-9).5 We found that there was no difference of MMP-9 expression and activity between WT and Nox2−/− BM before and after hindlimb ischemia (data not shown), suggesting that other targets of Nox2-derived ROS in the BM microenvironment are involved in mobilization of EPCs. Further studies are required to clarify the mechanism of how Nox2-derived ROS in BM can sense the ischemia-induced signals to promote EPCs and stem/progenitor cells mobilization.
BM-derived circulating EPCs and progenitor cells have the potential to home at the sites of ischemic tissues to contribute to revascularization. This homing is mediated through highly concerted mechanisms, which involve adhesion, chemotaxis, and invasion, followed by integration of cells into vascular structures.35 The present study with intravenous infusion of WT- and Nox2−/− BMCs demonstrates that in vivo homing capacity of Nox2−/− BMCs to sites of ischemia and their neovascularization capacity are significantly reduced compared to WT cells. Of importance, Nox2−/− BMCs pretreated with low-dose H2O2 rescued impaired proangiogenic capacity of these cells, confirming that phenotype of Nox2−/− BMCs is specifically attributable to the lack of Nox2-derived ROS. Consistent with our data, Kubo et al36 reported that intramuscular injection of BMCs pretreated with low-dose H2O2 enhances their angiogenic potency. By contrast, improvement by H2O2 in WT-BMCs was not observed in our study, presumably attributable to the possibility that WT-BMCs used in the present study have higher ROS levels and more active than those prepared by Kubo et al. These results suggest that Nox2-derived ROS are required for homing and angiogenic capacity of BMCs, thereby promoting neovascularization of ischemic tissues in vivo. Previous reports show that β2 integrin and phosphatidylinositol-3-kinase-γ (PI3Kγ) are involved in homing and neovascularization capacity of EPCs and BM progenitor cells by regulating adhesion of EPCs on fibronectin and ICAM-1.37,38 In the present study, adhesion of BMCs to fibronectin and ICAM-1 is not affected in Nox2−/− cells, suggesting that β2 integrin- and PI3Kγ-mediated EPC adhesion capacity is independent of Nox2-derived ROS.
SDF-1α, acting through CXCR4 receptor, is the most effective chemoattractant for hematopoietic progenitor cells and EPCs, and plays an important role in homing and engraftment of these cells.39,40 Our in vitro studies demonstrate that Nox2-deficiency impairs SDF-1–induced migration and invasion capacity of BM progenitor cells, which may contribute to defective homing and mobilization capacities of Nox2−/− BMCs in vivo. Nox2−/− BM progenitor cells pretreated with low-dose H2O2 improves the impaired migration capacity of these cells, further confirming that phenotype of Nox2−/− BMCs is attributable to lacking ROS levels. Mechanistically, SDF-1–induced actin polymerization and Akt phosphorylation, both of which are important for hematopoietic stem/progenitor cell migration,17,18 are significantly inhibited in Nox2−/− BMCs. Of note, SDF-induced ERK1/2 phosphorylation, which plays a minor role for SDF-1–induced migration,18 is not affected in Nox2−/− cells, suggesting that impairment of BMCs function is not attributable to the CXCR4 dysfunction. Thus, Nox2-derived ROS are selectively involved in the pathways linking CXCR4 to Akt, but not to ERK1/2, thereby promoting migration of BMCs and BM progenitor cells. To our knowledge, this is the first demonstration that Nox2-derived ROS are downstream mediators of CXCR4 signaling linked to migration of stem/progenitor cells. Consistent with our result, receptor tyrosine kinase activation by hematopoietic cytokines increases generation of ROS that are involved in cell proliferation of BM stem cells.31 PI3Kγ is involved in NADPH oxidase–mediated oxidant generation41 in ECs and Akt phosphorylation in EPCs.42 ROS derived from NADPH oxidase regulate actin polymerization43 and Akt phosphorylation44–46 in ECs. Thus, one may speculate that PI3Kγ may be upstream mediator for Nox2-based NADPH oxidase in BM progenitor cells, and it is crucial in ROS-dependent actin polymerization and Akt activation, thereby stimulating cell migration and homing of BMCs, which may contribute to postnatal neovascularization. This point requires further investigation in future study.
In summary, the present study demonstrates that Nox2-based NADPH oxidase expressed in BMCs and EPCs plays an essential role for mobilization of EPCs and stem/progenitor cells from BM and their homing capacity, which may contribute to revascularization of ischemic tissues. We also found that mobilization and homing defects of Nox2−/− BMCs are at least in part attributable to inhibition of chemotaxis and invasion function of BM progenitor cells by reduction of actin polarization and Akt phosphorylation in response to SDF-1α. Thus, NADPH oxidase in BM and EPCs is a potential therapeutic target for promoting neovascularization in ischemic cardiovascular diseases. Furthermore, our findings support the concept that ROS generated by NADPH oxidase serve as important signaling molecules to mediate EPCs and stem/progenitor cell function, which may contribute to promoting neovasculalization and tissue repair in response to injury.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by NIH R01 HL077524 (to M.U.-F.), HL70187 (to T.F.), HL079356 (to K.W), AHA Grant-In-Aid 0555308B (to M.U.-F.), 0755805Z (to M.U.-F.), MED FY08 UFRP Research Grant (to M.U.-F.), and NIH T32 GM070388 (to M.R.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Isner JM, Losordo DW. Therapeutic angiogenesis for heart failure. Nat Med. 1999;5:491–492. doi: 10.1038/8374. [DOI] [PubMed] [Google Scholar]
- 2.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 3.Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 4.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 5.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exper Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 6.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 8.Maulik N. Redox signaling of angiogenesis. Antiox Redox Signaling. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 9.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Babior BM. The NADPH oxidase of endothelial cells. IUBMB Life. 2000;50:267–269. doi: 10.1080/713803730. [DOI] [PubMed] [Google Scholar]
- 11.Ushio-Fukai M, Tang Y, Fukai T, Dikalov S, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91phox-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 12.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev NA, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 13.Piccoli C, D’Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem Biophys Res Comm. 2007;353:965–972. doi: 10.1016/j.bbrc.2006.12.148. [DOI] [PubMed] [Google Scholar]
- 14.Fan J, Cai H, Tan WS. Role of the plasma membrane ROS-generating NADPH oxidase in CD34+ progenitor cells preservation by hypoxia. J Biotechnol. 2007;130:455–462. doi: 10.1016/j.jbiotec.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Buggisch M, Ateghang B, Ruhe C, Strobel C, Lange S, Wartenberg M, Sauer H. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120:885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 16.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voermans C, Anthony EC, Mul E, van der Schoot E, Hordijk P. SDF-1-induced actin polymerization and migration in human hematopoietic progenitor cells. Exper Hematol. 2001;29:1456–1464. doi: 10.1016/s0301-472x(01)00740-8. [DOI] [PubMed] [Google Scholar]
- 18.Fuhler GM, Drayer AL, Olthof SG, Schuringa JJ, Coffer PJ, Vellenga E. Reduced activation of protein kinase B, Rac, and F-actin polymerization contributes to an impairment of stromal cell derived factor-1 induced migration of CD34+ cells from patients with myelodysplasia. Blood. 2008;111:359–368. doi: 10.1182/blood-2006-11-060632. [DOI] [PubMed] [Google Scholar]
- 19.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 22.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 23.Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, Quinn MT, Mayaki D, Petrof B, Hussain SN. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antiox Redox Signaling. 2008;10:559–574. doi: 10.1089/ars.2007.1792. [DOI] [PubMed] [Google Scholar]
- 24.Wang N, Xie K, Huo S, Zhao J, Zhang S, Miao J. Suppressing phosphatidylcholine-specific phospholipase C and elevating ROS level, NADPH oxidase activity and Rb level induced neuronal differentiation in mesenchymal stem cells. J Cell Biochem. 2007;100:1548–1557. doi: 10.1002/jcb.21139. [DOI] [PubMed] [Google Scholar]
- 25.van Os R, Robinson SN, Drukteinis D, Sheridan TM, Mauch PM. Respiratory burst of neutrophils is not required for stem cell mobilization in mice. Brit J Haematol. 2000;111:695–699. doi: 10.1046/j.1365-2141.2000.02374.x. [DOI] [PubMed] [Google Scholar]
- 26.Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol. 2006;7:101–108. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 27.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Anti-oxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 28.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, Fukai T. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 30.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 31.Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signaling and in cell cycle progression. Cellular Signalling. 2006;18:174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J Mol Cell Cardiol. doi: 10.1016/j.yjmcc.2008.01.004. In press. [DOI] [PubMed] [Google Scholar]
- 36.Kubo M, Li TS, Suzuki R, Ohshima M, Qin SL, Hamano K. Short-term pretreatment with low-dose hydrogen peroxide enhances the efficacy of bone marrow cells for therapeutic angiogenesis. Am J Physiol. 2007;292:H2582–H2588. doi: 10.1152/ajpheart.00786.2006. [DOI] [PubMed] [Google Scholar]
- 37.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavakis E, Carmona G, Urbich C, Gottig S, Henschler R, Penninger JM, Zeiher AM, Chavakis T, Dimmeler S. Phosphatidylinositol-3-kinase-gamma is integral to homing functions of progenitor cells. Circ Res. 2008;102:942–949. doi: 10.1161/CIRCRESAHA.107.164376. [DOI] [PubMed] [Google Scholar]
- 39.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–181. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- 42.Madeddu P, Kraenkel N, Barcelos LS, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gamma gene knockout impairs postischemic neovascularization and endothelial progenitor cell functions. Arterioscler Thromb Vasc Biol. 2008;28:68–76. doi: 10.1161/ATVBAHA.107.145573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res. 2006;71:236–246. doi: 10.1016/j.cardiores.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski L, Patrushev NA, Zhang L, Fukai T, Ushio-Fukai M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 45.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 46.Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol. 2006;291:H1563–H1572. doi: 10.1152/ajpheart.01081.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.