Abstract
Background
Clinical and epidemiologic evidence demonstrates sex differences in the prevalence and course of various psychiatric disorders. Understanding sex-specific brain differences in healthy individuals is a critical first step towards understanding sex-specific expression of psychiatric disorders. Here, we evaluate evidence on sex differences in brain structure, chemistry and function using imaging methodologies, including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), single photon emission computed tomography (SPECT) and structural magnetic resonance imaging (MRI) in mentally healthy individuals.
Methods
MEDLINE searches of English-language literature (1980-November 2006) using the terms sex, gender, PET, SPECT, MRI, fMRI, morphometry, neurochemistry and neurotransmission were performed to extract relevant sources.
Results
The literature suggests that while there are many similarities in brain structure, function and neurotransmission in healthy men and women, there are important differences that distinguish the male from the female brain. Overall brain volume is greater in men than women, yet, when controlling for total volume, women have a higher percentage of gray matter and men a higher percentage of white matter. Regional volume differences are less consistent. Global cerebral blood flow is higher in women than in men. Sex-specific differences in dopaminergic, serotonergic and GABAergic markers indicate that male and female brains are neurochemically distinct.
Conclusions
Insight into the etiology of sex differences in the normal living human brain provides an important foundation to delineate the pathophysiological mechanisms underlying sex differences in neuropsychiatric disorders and to guide the development of sex-specific treatments for these devastating brain disorders.
Keywords: sex, neuroimaging, morphometry, SPECT, PET, MRI
Introduction
Prior to the 1990s, few studies examined sex differences in symptoms, disease progression, or treatment of psychiatric disorders. Fortunately, in the last decade, research efforts have begun to include the examination of sex differences in studies on brain function and chemistry using animal models and in human studies. In this review, we use the Institute of Medicine definitions of sex and gender. Specifically, “sex” is used to describe distinctions in biology between men and women, whereas “gender is rooted in biology and shaped by environment,” and describes one’s self-representation as male or female (1).
Sex differences in brain chemistry historically have been evaluated preclinically. For example, they have been examined with autoradiography and histochemistry in animals and postmortem in human brain. A vast preclinical animal literature on sex differences in brain chemistry and structure exists. While these studies provide a necessary foundation for understanding sex differences in brain, it is not yet known how these findings generalize to humans. Postmortem studies also provide useful information, but are limited by a variety of methodological factors, such as agonal state at death and postmortem interval. Notably, advances in neuroimaging techniques have afforded the opportunity to evaluate differences in brain structure, function and chemistry in living men and women throughout the lifespan. In this review, we evaluate the literature on sex differences in brain structure, chemistry and function using in vivo imaging methodologies, including single photon emission computed tomography (SPECT), positron emission tomography (PET) and structural and functional magnetic resonance imaging (MRI and fMRI, respectively), as a foundation towards the future review and evaluation of brain sex differences in neuropsychiatric disorders (see (2; 3) for additional relevant reviews).
A major biological distinction between women and men is the menstrual cycle, which is associated with variations in female sex steroid hormones over a 28–32 day period. Estrogen has been widely studied in the preclinical and clinical literature, especially relating to cognition and neuroprotection, [for reviews see (4; 5)]. Estrogen is critically involved in the sexual differentiation of the brain (5), and thus likely contributes to sex differences in brain morphology and neurochemistry. Evaluation of brain structure, function and chemistry over the course of the menstrual cycle as well as across the lifespan in women is critical to understanding sex differences in both normal and aberrant behavior. However, in a majority of studies, menstrual cycle stage was not recorded. Given the paucity of information on menstrual cycle effects, this paper will review studies that evaluated differences between men and women regardless of whether the menstrual cycle was controlled, and will make note of menstrual cycle effects when known. Also, psychosocial factors such as expected gender roles that contribute to differences between men and women may affect sex-specific differences in the brain; but are outside the scope of the current review. Finally, in the emerging field of in vivo neuroimaging, there are between-study differences in variables and methodologies that may alter results and interpretations. Here, we review available neuroimaging studies from 1980–2006 to evaluate the evidence for the existence of sex differences in the living healthy human brain.
METHODS
MEDLINE searches were conducted of English-language literature (1980-November 2006) using the terms sex, gender, PET, SPECT, MRI, fMRI, morphometry, neurochemistry, and neurotransmission. Bibliographies of articles were reviewed to extract additional relevant sources. Studies that examined or reported analyses on sex differences, regardless of the direction of the finding, were selected. The emphasis was on studies seeking to directly examine a priori the effect of sex on brain structure, function or chemistry.
DATA SYNTHESIS
Imaging Techniques
Brain structure (also referred to as morphology) has been historically studied using computed tomography (CT). CT is an imaging technique that combines X-ray images into 2-dimensional cross sectional images of the brain. In CT, small amounts of x-ray radiation are passed through the body, and different tissues in the body absorb the radiation to different extents. Images are obtained in thin slices by x-ray tubes and detectors that circle the body. While CT is frequently used in clinical settings, brain structure is increasingly being examined using magnetic resonance imaging (MRI) both in clinical and research settings. MRI is a noninvasive technique for visualizing brain structures, and for differentiating between gray and white matter, and cerebrospinal fluid (CSF). MRI is superior to CT in that it provides significantly improved resolution for delineating different brain regions compared to CT. MRI uses tuned radiofrequency coils to detect radio signals given off by the excited nuclei in the brain as they are returning to equilibrium. The amount of contrast between tissues may be changed by varying the image acquisition sequence depending on the question being asked [See (6) for further technical details on MRI].
Brain function is commonly evaluated by measuring changes in regional brain activity that occur in response to a change in a cognitive or physical task or drug challenge. Regional brain activity is studied by measuring changes in the oxygenation of hemoglobin in blood (BOLD signal) using fMRI and also is measured using radiolabeled markers of blood flow and PET or SPECT cameras to detect the photons released during the radioactive decay of the compounds (7). For PET, brain regional activity is measured by monitoring glucose metabolism with [18F]fluorodeoxyglucose (FDG) uptake or by measuring changes in the rates of blood flow using [15O]H2O. For SPECT, blood flow is measured using 133Xe, which is an inert gas, that when inhaled is taken up in the blood and distributed throughout the cerebral cortex. [99mTc]HMPAO SPECT provides a measure of both blood flow and metabolism as it is a highly lipophilic compound with prolonged retention in brain (t1/2>6 h) that is converted into a hydrophilic compound in brain and hence is effectively trapped with little washout and redistribution over several hours after injection. 99mTc-ECD distributes similarly to HMPAO in the brain with shorter brain retention and greater stability.
Brain chemicals, including receptors and transporters present in low concentrations (nM-pM range) in brain, are measured using “trace” doses of highly specific radioactive drugs (called radiotracers) and imaged using a PET or SPECT camera. Currently there are only a few sites for which specific radiotracers exist, including the serotonin (5-HT) transporter, 5-HT1A receptor, and 5-HT2A receptor, dopamine (DA) D1 receptors, D2 receptors, the DA transporter, GABAA-benzodiazepine receptors, β2-containing nicotinic acetylcholine receptors and mu opioid receptors. Tracers are currently under development for other chemical sites in brain including, but not limited to, amyloid, metabotropic glutamate receptors 1–5, NMDA receptors, muscarinic receptors, 5-HT1B receptors, 5-HT6 and 5-HT7 receptors, and cannabinoid receptors that will be available soon to explore sex differences in brain neurochemistry. While a variety of radiotracers and methods exist to examine brain chemistry, relatively few have been used to examine sex differences. The majority of studies reporting sex differences in brain chemistry have focused on serotonin and dopamine systems and emerging evidence also suggests differences in GABA systems. Ideally, a radiotracer for use in vivo in living humans that targets the estrogen receptors will be developed, which will greatly advance our knowledge in the area of sex differences and hormonal influences in psychiatry.
Sex Differences in Brain Structure
It has been long known that women have smaller brain volumes than men, which is explained in part by their smaller stature. Average brain volumes (excluding cerebral spinal fluid (CSF), meninges, and other non-brain tissue) of 1,130 cc for females and 1,260 cc for males were reported based on normal subjects of European ancestry from a convergence of studies (8–10). Allen and colleagues (2002) reported that the whole brain and most major subdivisions (e.g., hemispheres, frontal and temporal lobes, left parietal lobe, insula and cerebellum) were significantly larger in men compared to women, but the proportional sizes of individual regions in relation to total hemisphere volume were similar. While men have greater brain volume (11), greater CSF volume or lateral ventricles (11–14), and greater sulcal volume (15) compared to women, ventricular volumes (12; 16) and intracranial areas corrected for differences in cranial size do not vary between sexes (13).
Gray and white matter volumes also vary by sex (17; 18). When covaried for intracranial volume, height and weight, women have a higher percentage of gray matter whereas, men have a higher percentage of white matter and CSF (15). The gray/white matter ratio was consistently higher in frontal, temporal, parietal, and occipital lobes, cingulate gyrus, and insula in women versus men (9; 15; 17; 19; 20). Thicker gray matter in the parietal cortex in women versus men has been consistently shown (17; 21; 22) and is evident across the life span (23). While the evidence for sex differences in brain morphometry is convincing, there are some studies that contradict these findings. For example, no sex difference was reported when controlling for brain size in total gray matter volume (10; 24) and one study reported greater gray matter volume as a percentage of total intracranial volume in men versus women (25).
Sex differences in the human brain are of increasing interest, mostly due to widely held beliefs about sex differences in cognitive abilities, namely better verbal skills in women and better spatial abilities in men. In men, IQ correlates with gray matter volume in the frontal and parietal lobes; whereas in women, IQ correlates with gray matter volume in the frontal lobe and Broca’s area, which is involved in language (20) suggesting that men and women use different brain areas to achieve a similar IQ. Thus, while differences in total brain size are less meaningful, size differences in smaller brain structures (19; 26; 27) are important for normal behaviors and diseases, and may reflect the prevalence or course of disease in men and women (See Table 1). Importantly, there is high variability between individuals in these studies that likely results from genetic and environmental influences, which are important considerations when evaluating sex differences.
Table 1.
Sex differences in whole brain and selected brain regions from in vivo MRI studies. These are findings from hypothesis-driven studies on sex differences in brain morphology with MRI selected from over 75 studies. All references controlled for total brain volume. M signifies male; F signifies female.
| Sex Difference | Brain Area | Sample Size (M/F) | Age Range | Reference |
|---|---|---|---|---|
| Men > Women | Total Volume | 23/23 | 22–49 | (8) |
| 10/10 | 17–37 | (26) | ||
| 34/35 | 18–80 | (11) | ||
| 69/48 | 19–41 | (9) | ||
| 79/37 | 1–80 | (10) | ||
| White Matter | 40/40 | 18–45 | (15) | |
| 10/10 | 17–37 | (26) | ||
| 13/30 | 24–82 | (103) | ||
| CSF | 40/40 | 18–45 | (15) | |
| 25/39 | 18–64 | (12) | ||
| Total Corpus Callosum | 23/23 | 22–49 | (8) | |
| Cerebellum | 77/113 | 18–81 | (104) | |
| 48/49 | 15–69 | (22) | ||
| Pons | 77/113 | 18–81 | (104) | |
| Caudate | 17/12 | 18–78 | (105) | |
| Amygdala | 27/21 | 25–52 | (19) | |
| Hypothalamus | 27/21 | 25–52 | (19) | |
| Frontomedial Cortex | 27/21 | 25–52 | (19) | |
| Women > Men | Gray Matter | 40/40 | 18–45 | (15) |
| 43/17 | 22–43 | (106) | ||
| Caudate | 10/10 | 17–37 | (26) | |
| Hippocampus | 10/10 | 17–37 | (26) | |
| Frontoorbital Cortex | 27/21 | 25–52 | (19) | |
| 57/59 | 18–49 | (107) | ||
| Superior Frontal and Lingual Gyri | 27/21 | 25–52 | (19) | |
| Men = Women | Pons | 51/49 | 20–85 | (108) |
| Hippocampus | 39/41 | 18–42 | (109) | |
| 57/59 | 18–49 | (107) | ||
| Thalamus | 32/25 | 21–82 | (27) | |
| 51/49 | 20–85 | (108) | ||
| Amygdala | 57/59 | 18–49 | (107) |
Age-related brain volume loss differs between men and women [see (28) for review]. Understanding of sex differences in the developing human brain is rapidly increasing (23; 29; 30). Young girls have larger hippocampal volume (26; 31) whereas the amygdala is larger in boys (14; 30). Interestingly, enzymes for estrogen synthesis (32) and estrogen receptor mRNA have been localized to the hippocampus (33; 34), whereas androgen receptors are more prevalent in the amygdala (35) [and see (36) for further discussion on estrogen receptor distribution and the relationship to psychiatry]. In adult men, volume loss in whole brain, frontal and temporal lobes increases with age, whereas in women, volume loss in hippocampus and parietal lobes increases with age (37). In adult men and women, global grey matter decreases linearly with age with a steeper decline in men (24; 38), a finding that has been confirmed postmortem (39). The reasons for these differences are not clear but may be related to the female sex steroids. There have been no studies evaluating the female sex steroids estrogen and progesterone and their receptor systems in the living human brain because the tools are currently unavailable. These questions will be addressed when PET or SPECT radioligands with specificity for estrogen, progesterone and androgen receptors are developed. Until the role of these important steroid hormones are understood in the living human brain, it is important that controls are well matched to the patient population by sex and by age. Since many brain disorders are associated with volume differences in regional brain structures, understanding the influences of sex along with age-specific developmental changes in sex steroid hormones, such as during puberty and menopause, are important. The relationship between regional brain volumes and the underlying neurochemical milieu in the healthy brain provides the crucial basis for understanding the pathophysiological mechanisms of neuropsychiatric disorders.
Sex Differences in Brain Function
A majority of studies have demonstrated that women consistently have higher global cerebral blood flow (CBF) compared to men during rest (40; 41) and cognitive activity (41–45) regardless of the brain imaging modality (See Table 2). Consistent with the findings of sex differences in cerebral blood flow, cerebral metabolic rate of glucose utilization (CMRglu) tends to be higher in women versus men (46), particularly in the orbital frontal area (47) although not consistently (48–50). However, global CMRglu may be inversely correlated with brain size such that individuals with smaller brains have higher CMRglu (48; 51), effectively negating sex differences in metabolism. Regional CMRglu varied significantly with menstrual cycle phase suggesting that there are acute hormonal effects on brain glucose metabolism (52). These findings cast doubt on the interpretations of studies that did not control for menstrual cycle phase or hormone levels and highlight the importance for future studies to control biological parameters known to affect cerebral blood flow, such as the menstrual cycle.
Table 2.
Data on sex differences in brain perfusion studies in healthy men and women. Studies using 99mTc, 133Xe and 123I are SPECT, whereas the ligands 18F and 15O are for PET; SPM is statistical parametric mapping; SVD is singular value decomposition; ROI is region of interest. M signifies male; F signifies female.
| Ligand | Sample Size (M/F) | Age Range | Main Finding | Reference |
|---|---|---|---|---|
| [99mTc]ECD | 43/46 | 20–81 | M>F cerebellum, L anterior temporal and orbitofrontal cortex F>M parietal cortex | (103) |
| [99mTc]ECD | 24/15 | 45–59 | F>M in R cerebellum | (104) |
| [99mTc]HMPAO | 20/25 | 46–60 | F>M in R cerebellum | (104) |
| [99mTc]HMPAO | 67/85 | 50–92 | F>M global perfusion, corpus callosum, inferior temporal and inferior parietal areas | (42) |
| 133Xe | 30/32 | 18–26 | F>M CBF | (41) |
| 133Xe | F>M CBF | (43) | ||
| 133Xe | 56/41 | 20–59 | F>M CBF | (40) |
| 123I | 7/7 | 20–30 | F>M CBF | (45) |
| [18F]FDG | 22/22 | 47–80 | M>F R insula, middle temporal gyrus, medial frontal lobe F>M hypothalamus, cerebellum M=F global metabolism | (105) |
| [18F]FDG | 15/13 | 36–52 | F>M cerebellum M=F global metabolism | (106) |
| [15O]H2O | 8/6 | 20–35 | F>M CBF | (44) |
| [18F]FDG | 37/24 | 20–34 | M=F global metabolism M>F cerebellum, temporal and cingulate regions | (107) |
| [18F]FDG | 21/18 | 19–35 | F>M CMRglu F>M orbital frontal area | (47) |
| [18F]FDG | 18/15 | 21–38 | M=F global metabolism | (49) |
| [18F]FDG | 29/29 | 21–81 | M=F global metabolism | (51) |
| [18F]FDG | 7/7 | 25–39 | F>M CMRglu | (46) |
| [18F]deoxyglucose | 47/33 | 18–67 | M=F global metabolism | (48) |
| [18F]FDG | 17/23 | 18–78 | M=F global metabolism | (50) |
The direct implications of sex differences in global cerebral blood flow on psychiatric disorders are unclear; however, increased blood flow in the brains of women may lead to a better distribution of psychotropic drugs in the brain. There is some evidence in postmenopausal women that estrogen increases regional cerebral blood flow (53; 54), thus estrogen may account for some of the variability in blood flow and metabolism between men and women. These differences may explain why some drugs are more effective for treating neuropsychiatric disorders in women versus men (55).
Hormones may modulate arousal circuitry in women. Circulating estrogen has been shown to reduce arousal in women via the hypothalamic-pituitary-adrenal axis (56). However, postmenopausal women had decreased arousal compared to premenopausal women in response to erotic videos which was reversed with the administration of estradiol (57). Men outperformed women (in the early follicular phase of the menstrual cycle when estrogen and progesterone levels are relatively low) at a task requiring response inhibition to obvious versus less obvious stimuli; however, no sex differences in neural activation were associated with different performance levels (58). A similar study determined that sex differences in performance on verbal and spatial cognitive tasks were not significantly related to endogenous hormone levels in men or in women during the early follicular phase of the menstrual cycle (59). This suggests that individual performance level may have a greater impact on brain activation patterns than sex, and that, in some studies, high individual variability in performance on cognitive tasks may preclude meaningful findings of sex differences. However, even when men and women are equally successful at a performance task, sex differences have been noted (60; 61).
Sex Differences in Brain Chemistry
Serotonin
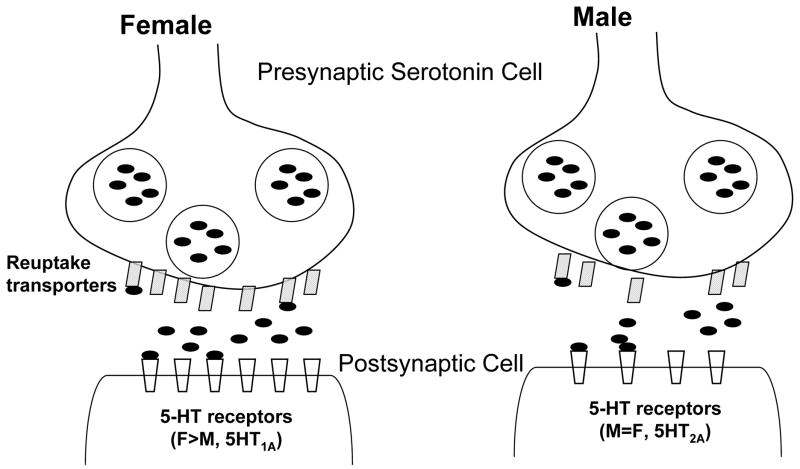
There is a wealth of preclinical and clinical evidence supporting sex differences in serotonin (5-HT) neurotransmission (62). Whole blood 5-HT levels are higher in women compared to men (63), which appears to be genetically determined (64), and men synthesize serotonin significantly faster than women (65). 5-HT functions to coordinate complex sensory and motor patterns during a variety of behavioral states and is implicated in the pathology of mood disorders, sleep and eating disorders, and schizophrenia. Sex differences in 5-HT function may underlie the known gender difference (women > men) in the prevalence of depression (66) and may impact pharmacological treatments that target 5-HT neurotransmission. Healthy women have higher 5-HT transporter availability in the diencephalon and brainstem compared to men (67) and 5-HT transporters are selectively decreased in an age-specific manner in depressed women but not in depressed men (55). Since the 5-HT transporter functions to regulate 5-HT neurotransmission, these findings suggest that baseline 5-HT function may be higher in women versus men and that dysregulation of this function in young depressed women may explain in part a unique, sex-specific pathophysiological mechanism underlying depressed mood in young women (55) (see Figure 1). It has been suggested that young women may be more responsive to selective serotonin reuptake inhibitors versus tricyclic antidepressants compared to older women and men (68). Indeed, in a placebo-controlled study, women receiving estrogen (vs. placebo) had an accelerated response to sertraline treatment (69). This is especially important as many antidepressants have a delayed onset of action (up to several weeks) that is not well understood.
Figure 1.
Proposed sex differences in a 5-HT synapse, with higher 5-HT reuptake transporters, neurotransmission, and 5HT1A receptors in women versus men.
Women have higher 5-HT1A receptor numbers than men in certain brain regions (70), a finding that has been both confirmed (71) and unsubstantiated (72) in previous postmortem studies. While a sex difference in 5-HT2A receptors has been reported (73), there is substantial evidence supporting no difference in receptor levels (74; 75) but a difference in radiotracer metabolism that may give the appearance of higher 5-HT2 receptor number (Adams et al., 2004). Interestingly, studies on the effects of exogenous sex steroids in postmenopausal women have demonstrated higher 5HT2A binding throughout the cerebral cortex in women treated with estradiol plus progesterone replacement (76) and higher 5HT2A receptors in the right prefrontal cortex of postmenopausal women receiving estrogen therapy (77). Additional clinical studies in premenopausal women, which take into account fluctuating hormones and radiotracer metabolism are needed to determine the mechanism by which hormones regulate the receptor and to determine implications (e.g., the effect of hormones on psychotropic medication such as SSRIs) for psychiatric disorders.
Dopamine
Dopaminergic function is also enhanced in women. DA is important for reward processes including the reinforcing effects of most drugs of abuse, and has been implicated in a variety of neuropsychiatric disorders including Parkinson’s Disease, which is more prevalent in men than women, and schizophrenia, for which sex differences exist in the onset and course of the disorder. Amphetamine-induced DA release in the right globus pallidus and right inferior frontal gyrus was higher in women compared to men (78). The DA transporter, which functions to regulate synaptic DA availability, is higher in women compared to men (67; 79; 80). The lack of difference between sexes in one study may be due to the large age range and inclusion of postmenopausal women (81). DA transporter availability does not appear to differ by menstrual cycle phase and fluctuating gonadal hormones (82).
Premenopausal women have higher striatal presynaptic DA synthesis than age-matched men (83). However, sex differences in D2 receptors are inconsistent (84; 85) (See Table 3). D2 receptor availability may vary with fluctuations in sex steroid hormones across the menstrual cycle (86) although the finding has not been confirmed (87). Larger sample sizes and repeated measures over the menstrual cycle are necessary to address this question in healthy living human subjects. Fluctuations in hormones in healthy premenopausal women may lead to larger variability in outcome measures compared to men and to postmenopausal women and, consequently, may preclude findings of sex differences. Together, these studies suggest that healthy women may have higher presynaptic dopaminergic tone in striatum and higher extrastriatal DA receptor density and availability compared to men. These results have implications for the many disease states in psychiatry, such as schizophrenia, that are associated with disturbances in DA neurotransmission and show differences by sex in rate and course. Specifically, higher dopaminergic tone in women may protect against the development of schizophrenia, alcoholism, and other diseases with established disturbances in DA function. In adults, alcoholism affects twice as many men as women and many studies have documented sex differences in the prevalence of and symptoms of schizophrenia (88–90). Notably, estrogen may possess neuroprotective qualities in its interaction with the DA system, especially with regard to schizophrenia (91). A second increase in the incidence of schizophrenia in women between the ages of 45–54 may be attributed to menopause and decreasing sex steroid hormone levels (92). If women have higher dopaminergic tone, antidopaminergic treatment drugs may be more effective in women versus men [See (93; 94) for reviews]. In early Parkinson’s Disease, a robust sex difference in prefrontal monoaminergic activity (95) may be associated with observed clinical sex differences, such that men have more severe parkinsonian motor problems than women (96), which DA likely underlies.
Table 3.
Summary of findings on sex differences in brain chemistry in healthy male and female subjects. M signifies male; F signifies female; MRS is magnetic resonance spectroscopy.
| Topic | Ligand | Sample Size (M/F) | Age Range | Brain Region | Main Finding | References |
|---|---|---|---|---|---|---|
| Serotonin Neurotransmission | ||||||
| 5-HT transporter | [123I]β-CIT | 9/12 | 29–51 | Brainstem | F>M | (67) |
| 5-HT1A receptor | [11C]WAY-100635 | 13/12 | 23–57 | Amygdala, Hippocampus, Cingulate, Medial and Orbital Prefrontal Cortex | F>M | (70) |
| 5-HT2A receptor | [18F]altanserin | 11/11 | 23–64 | Frontal, Cingulate Cortex | F>M | (73) |
| [18F]altanserin | 30/22 | 21–79 | Cortical areas | F=M | (74) | |
| [18F]setoperone | 11/15 | 19–43 | Cortical areas | F=M | (75) | |
| Dopamine Neurotransmission | ||||||
| DA transporter | [123I] β-CIT | 9/12 | 29–51 | Striatum | F>M | (67) |
| [123I]FP CIT | 23/22 | 18–83 | Striatum | F>M | (79) | |
| TRODAT-1 | 30/36 | 18–75 | Caudate Nucleus | F>M | (80) | |
| [123I] β-CIT | 70/52 | 18–88 | Striatum | F=M | (82) | |
| DA synthesis | [18F]fluorodopa | 23/12 | 20–60 | Caudate, Putamen | F>M | (83) |
| D2 receptor | [11C]raclopride | 33/21 | 19–82 | Striatum | F<M affinity | (84) |
| [11C]FLB 457 | 12/12 | 33–74 | Frontal Cortex | F>M binding potential | (85) | |
| GABA Neurotransmission | MRS | 11/7 | 28–49 | Occipital Cortex | F>M cortical GABA | (99) |
Other Receptor Systems
Although not as well studied, differences between men and women have been reported for other receptor systems. These include the cholinergic system, which is involved in memory and cognition; the GABAergic system, the major inhibitory neurotransmitter system involved in mood and memory; and the opioid system, which is involved in pain and reward processes. Imaging the vesicular acetylcholine transporter, a marker of cholinergic synaptic density, demonstrated that women with an early onset of menopause have higher concentrations of cholinergic synaptic terminals (97). Moreover, the length of hormone therapy in post-menopausal women was positively correlated with the concentrations of cholinergic synaptic terminals in cortical areas and the posterior cingulate suggesting that hormone therapy may positively influence the survival of cholinergic cells in postmenopausal women (97). Women also express higher numbers of cortical muscarinic acetylcholine receptors (98).
Women have higher cortical GABA levels than men as measured with magnetic resonance spectroscopy (MRS) (99). GABA levels in healthy and unmedicated women with premenstrual dysphoric disorder (PMDD) vary across the menstrual cycle (100) such that cortical GABA levels declined between the follicular and luteal phase in healthy women, and increased between the follicular and luteal phase in women with PMDD. This indicates that GABA neurotransmission in tightly regulated by the menstrual cycle.
Higher mu-opioid binding in women versus men has been reported throughout cortical and subcortical regions (101). Additionally, women in the follicular phase of their menstrual cycle appear to have a negative correlation between fluctuating estradiol and mu-opioid receptor availability in the amygdala and hypothalamus as measured with [11C]carfentanil, such that higher estradiol levels were associated with a lower mu-opioid receptor density (102).
More studies on the effects of menstrual cycle and sex in other major neurochemical systems are seriously needed to establish a foundation of brain neurochemistry necessary for delineating the neurochemical differences between men and women in neuropsychiatric disorders.
Conclusions
The development of MRI, PET, SPECT, fMRI, and MRS has afforded the opportunity to evaluate sex differences in brain morphology and chemistry in the living human brain. A review of the studies using these modalities over the last 26 years suggests that while brain structure, function and neurochemistry of healthy men and women are similar in many ways, there are important differences. While men have greater overall brain volume than women, relative to total volume, sex-specific regional differences exist. Men have a larger amygdala and hypothalamus, while women have a larger caudate and hippocampus. These regional differences may be related to the distribution of estrogen and androgen receptors. Global cerebral blood flow is consistently higher in women than in men, while global cerebral metabolism is equivalent. Differences in regional blood flow and metabolism are less well understood. Sex differences in the serotonin (5-HT transporter, 5-HT1A and 5-HT2A receptors), dopamine (DA transporter) and GABA (neurotransmitter levels) systems have been documented, with tendencies towards higher numbers in women compared to men. Importantly, these sex differences in brain morphology, function and neurochemistry likely impact normal and abnormal behavior and may increase vulnerability to certain types of neuropsychiatric disorders.
These sex-specific differences in the healthy brain highlight the need to evaluate sex differences in neuropsychiatric disorders especially those that differ in prevalence and symptoms between men and women. Unfortunately, many of the studies reported here were incidental findings and were not hypothesis driven, and thus often lacked critical variables, such as menstrual cycle phase, menstrual status (e.g., perimenopausal, menopausal), and hormonal contraceptive use, necessary to evaluate differences in brain between men and women in a scientifically meaningful manner.
It is our hope that this review encourages researchers to not only develop more hypothesis driven research studies to evaluate sex differences in the living human brain, but also to collect the necessary information about menstrual cycle status and phase in women to allow a thorough evaluation of the effects of sex on brain function in normal behavior and psychiatric disorders. Improving our knowledge about sex differences in the living human brain will enhance our ability to develop sex-specific treatments for neuropsychiatric brain disorders.
Acknowledgments
The authors wish to thank Drs. Robert M. Malison and Stephanie O’Malley for helpful comments on the manuscript. This work was supported by grants RO1 DA015577, P50 AA15632, KO1 DA020651, the Ethel F. Donaghue Women’s Health Investigator Program of Women’s Health Research at Yale and also by the NIMH Biological Sciences Training Program.
Footnotes
Financial Disclosures: The authors have no financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kelly P. Cosgrove, Assistant Professor of Psychiatry, Division of Psychiatry SPECT Imaging, Yale University School of Medicine and the VACHS.
Carolyn M. Mazure, Professor of Psychiatry, Associate Dean YSM Faculty Affairs, Director, Women’s Health Research, Yale University School of Medicine, carolyn.mazure@yale.edu.
Julie K. Staley, Associate Professor of Psychiatry and Diagnostic Radiology, Director, Psychiatry SPECT Imaging, Yale University School of Medicine and the VACHS, julie.staley@yale.edu.
References
- 1.Wizemann T, Pardue M-L. Does sex matter? Washington, D.C: Institute of Medicine; 2001. Exploring the biological contributions to human health. [PubMed] [Google Scholar]
- 2.Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–62. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- 3.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 4.Birzniece V, Backstrom T, Johansson IM, Lindblad C, Lundgren P, Lofgren M, et al. Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res Brain Res Rev. 2006;51:212–39. doi: 10.1016/j.brainresrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 6.Lim K, Rosenbloom M, Pfefferbaum A. In vivo structural brain assessment. In: Bloom F, Kupfer D, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd; 1995. pp. 881–893. [Google Scholar]
- 7.Malison R, Laruelle M, Innis R. Positron and single photon emission tomography. In: Bloom F, Kupfer D, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press, Ltd; 1995. pp. 865–879. [Google Scholar]
- 8.Allen J, Damasio H, Grabowski T. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. American Journal of Physical Anthropology. 2002;118:341–358. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- 9.Peters M, Jancke L, Staiger J, Schlaug G, Huang Y, Steinmetz H. Unsolved problems in comparing brain sizes in Homo sapiens. Brain Cognition. 1998;37:254–285. doi: 10.1006/brcg.1998.0983. [DOI] [PubMed] [Google Scholar]
- 10.Courchesne E, Chizum H, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis and in vivo MR imaging in healthy volunteers. Neuroradiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 11.Gur R, Mozley P, Resnick S, Gottlieb G, Kohn M, Zimmerman R, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant R, Condon B, Lawrence A, Hadley D, Patterson J, Bone I, et al. Human cranial CSF volumes measured by MRI: sex and age influences. Magnetic Resonance Imaging. 1987;5:465–468. doi: 10.1016/0730-725x(87)90380-8. [DOI] [PubMed] [Google Scholar]
- 13.Agartz I, Saaf J, Wahlund L-O, Wetterberg L. Quantitative estimations of cerebrospinal fluid spaces and brain regions in healthy controls using computer-assisted tissue classification of magnetic resonance images: relation to age and sex. Magnetic Resonance Imaging. 1992;10:217–226. doi: 10.1016/0730-725x(92)90482-f. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 15.Gur R, Turetsky B, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erdogan AR, Dane S, Aydin MD, Ozdikici M, Diyarbakirli S. Sex and handedness differences in size of cerebral ventricles of normal subjects. Int J Neurosci. 2004;114:67–73. doi: 10.1080/00207450490249428. [DOI] [PubMed] [Google Scholar]
- 17.Allen J, Damasio H, Grabowski T, Bruss J, Zhang W. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. NeuroImage. 2003;18:880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 18.Paus T, Otaky N, Caramanos Z, Macdonald D, Zijdenbos A, D’Avirro D, et al. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. Journal of Comparative Neurology. 1996;376:664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein J, Seidman L, Horton N, Makris N, Kennedy D, Caviness V, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 20.Haier R, Jung R, Yeo R, Head K, Alkire M. The neuroanatomy of general intelligence: sex matters. NeuroImage. 2005;25:320–327. doi: 10.1016/j.neuroimage.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 22.Carne RP, Vogrin S, Litewka L, Cook MJ. Cerebral cortex: an MRI-based study of volume and variance with age and sex. J Clin Neurosci. 2006;13:60–72. doi: 10.1016/j.jocn.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, et al. Sex Differences in Cortical Thickness Mapped in 176 Healthy Individuals between 7 and 87 Years of Age . Cereb Cortex. 2006 doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol. 1995;16:241–51. [PMC free article] [PubMed] [Google Scholar]
- 25.Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 26.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–60. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Werf Y, Tisserand D, Visser P, Hofman P, Vuurman E, Uylings H, et al. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. Cognitive Brain Research. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 28.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–92. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 29.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 30.Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–36. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 31.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, et al. lpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 5017a;101:865–70. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterlund MK, Overstreet DH, Hurd YL. The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol. Brain Res Mol Brain Res. 1999;74:158–66. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- 34.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 35.Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123:932–40. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- 36.Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64:251–67. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 37.Murphy D, DeCarli C, McIntosh A, Daly E, Mentis M, Pietrini P, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 38.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 39.Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129:386–98. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- 40.Devous M, Stokely E, Chehabi H, Bonte F. Normal distribution of regional cerebral blood flow measured by dynamic single-photon emission tomography. Journal of Cerebral Blood Flow and Metabolism. 1986;6:95–104. doi: 10.1038/jcbfm.1986.12. [DOI] [PubMed] [Google Scholar]
- 41.Gur R, Gur R, Obrist W, Hungerbuhler J, Younkin D, Rosen A, et al. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 42.Jones K, Johnson K, Becker J, Spiers P, Albert M, Holman B. Use of singular value decomposition to characterize age and gender differences in SPECT cerebral perfusion. J Nucl Med. 1998;39:965–973. [PubMed] [Google Scholar]
- 43.Slosman D, Chicherio C, Ludwig C, Genton L, Ribaupierre Sd, Hans D, et al. 133Xe SPECT cerebral blood flow study in a healthy population: determination of T-scores. J Nucl Med. 2001;42:864–870. [PubMed] [Google Scholar]
- 44.Esposito G, Horn JV, Weinberger D, Berman K. Gender differences in cerebral bloodflow as a function of cognitive state with PET. J Nucl Med. 1996;37:559–564. [PubMed] [Google Scholar]
- 45.Podreka I, Baumgartner C, Suess E, Muller C, Brucke T, Lang W, et al. Quantification of regional cerebral blood flow with IMP-SPECT. Stroke. 1989;20:183–191. doi: 10.1161/01.str.20.2.183. [DOI] [PubMed] [Google Scholar]
- 46.Baxter L, Mazziotta J, Phelps M, Selin C, Guze B, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Research. 1987;21:237–245. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 47.Andreason P, Zametkin A, Guo A, Baldwin P, Cohen R. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Research. 1993;51:175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 48.Hatazawa J, Brooks R, Di Chiro G, Campbell G. Global cerebral glucose utilization is independent of brain size: a PET study. Journal of Computer Assisted Tomography. 1987;11:571–576. doi: 10.1097/00004728-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Azari N, Rapoport S, Grady C, DeCarli C, Haxby J, Schapiro M, et al. Gender differences in correlations of cerebral glucose metabolic rates in young normal adults. Brain Research. 1992;574:198–208. doi: 10.1016/0006-8993(92)90817-s. [DOI] [PubMed] [Google Scholar]
- 50.Kuhl D, Metter E, Riege W, Phelps M. Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]fluorodeoxyglucose method. Journal of Cerebral Blood Flow and Metabolism. 1982;2:163–171. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]
- 51.Yoshii F, Barker W, Chang J, Loewenstein D, Apicella A, Smith D, et al. Sensitivity of cerebral glucose metabolism to age, gender, brain volume, brain atrophy, and cerebrovascular risk factors. Journal of Cerebral Blood Flow and Metabolism. 1988;8:654–661. doi: 10.1038/jcbfm.1988.112. [DOI] [PubMed] [Google Scholar]
- 52.Reiman E, Armstrong A, Matt K, Mattox J. The application of positron emission tomography to the study of the normal menstrual cycle. Human Reproduction. 1996;11:2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- 53.Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21:373–83. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 54.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–82. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 55.Staley J, Sanacora G, Tamagnan G, Maclejewski P, Malison RT, Berman RM, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2005;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Archer JS, Love-Geffen TE, Herbst-Damm KL, Swinney DA, Chang JR. Effect of estradiol versus estradiol and testosterone on brain-activation patterns in postmenopausal women. Menopause. 2006;13:528–37. doi: 10.1097/01.gme.0000188737.46746.cd. [DOI] [PubMed] [Google Scholar]
- 58.Halari R, Kumari V. Comparable cortical activation with inferior performance in women during a novel cognitive inhibition task. Behav Brain Res. 2005;158:167–73. doi: 10.1016/j.bbr.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 59.Halari R, Hines M, Kumari V, Mehrotra R, Wheeler M, Ng V, et al. Sex differences and individual differences in cognitive performance and their relationship to endogenous gonadal hormones and gonadotropins. Behav Neurosci. 2005;119:104–17. doi: 10.1037/0735-7044.119.1.104. [DOI] [PubMed] [Google Scholar]
- 60.Frings L, Wagner K, Unterrainer J, Spreer J, Halsband U, Schulze-Bonhage A. Gender-related differences in lateralization of hippocampal activation and cognitive strategy. Neuroreport. 2006;17:417–21. doi: 10.1097/01.wnr.0000203623.02082.e3. [DOI] [PubMed] [Google Scholar]
- 61.Bell EC, Willson MC, Wilman AH, Dave S, Silverstone PH. Males and females differ in brain activation during cognitive tasks. Neuroimage. 2006;30:529–38. doi: 10.1016/j.neuroimage.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 62.Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–75. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 63.Ortiz J, Artigas F, Gelpi E. Serotonergic status in human blood. Life Sci. 1988;43:983–90. doi: 10.1016/0024-3205(88)90543-7. [DOI] [PubMed] [Google Scholar]
- 64.Weiss LA, Abney M, Cook EH, Jr, Ober C. Sex-specific genetic architecture of whole blood serotonin levels. Am J Hum Genet. 2005;76:33–41. doi: 10.1086/426697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishizawa S, Benkelfat C, Young S, Leyton M, Mzengeza S, Montigny Cd, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 67.Staley J, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl J, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- 68.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–52. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 69.Rasgon NL, Dunkin J, Fairbanks L, Altshuler LL, Troung C, Elman S, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: A pilot study. J Psychiatr Res. 2006 doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Parsey R, Oquendo M, Simpson N, Ogden R, Heertum RV, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Research. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 71.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–33. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 72.Palego L, Marazziti D, Rossi A, Giannaccini G, Naccarato AG, Lucacchini A, et al. Apparent absence of aging and gender effects on serotonin 1A receptors in human neocortex and hippocampus. Brain Res. 1997;758:26–32. doi: 10.1016/s0006-8993(96)01415-1. [DOI] [PubMed] [Google Scholar]
- 73.Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, et al. Sex difference in 5HT2 receptor in the living human brain. Neuroscience Letters. 1996;204:25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- 74.Adams K, Pinborg L, Svarer C, Hasselbalch S, Holm S, Haugbol S, et al. A database of [18F]-altanserin binding to 5-HT2A receptors in normal volunteers: normative data and relationship to physiological and demographic variables. NeuroImage. 2004;21:1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Lewis R, Kapur S, Jone C, DaSilva J, Brown G, Wilson A, et al. Serotonin 5-HT2 receptors in schizophrenia: a PET study using [18F]setoperone in neuroleptic-naive patients and normal subjects. Am J Psychiatry. 1999;156:72–78. doi: 10.1176/ajp.156.1.72. [DOI] [PubMed] [Google Scholar]
- 76.Moses E, Drevets W, Smith G, Mathis C, Kalro B, Butters M, et al. Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: a PET study. Biological Psychiatry. 2000;48:854–860. doi: 10.1016/s0006-3223(00)00967-7. [DOI] [PubMed] [Google Scholar]
- 77.Kugaya A, Epperson C, Zoghbi S, Dyck Cv, Hou Y, Fujita M, et al. Increase in prefrontal cortex serotonin2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–1524. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 78.Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, et al. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163:1639–41. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- 79.Lavalaye J, Booij J, Reneman L, Habraken J, Royen Ev. Effect of age and gender on dopamine transporter imaging with [123I]FP-CIT SPET in healthy volunteers. Eur J Nucl Med. 2000;27:867–869. doi: 10.1007/s002590000279. [DOI] [PubMed] [Google Scholar]
- 80.Mozley L, Gur R, Mozley P, Gur R. Striatal dopamine transporters and cognitive functioning in healthy men and women. Am J Psychiatry. 2001;158:1492–1499. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 81.van Dyck C, Seibyl J, Malison R, Laruelle M, Wallace E, Zoghbi S, et al. Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CIT SPECT. J Nucl Med. 1995;36:1175–1181. [PubMed] [Google Scholar]
- 82.Best S, Sarrel P, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, et al. Striatal dopamine transporter availability with [123I]β-CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology. 2005;183:181–189. doi: 10.1007/s00213-005-0158-5. [DOI] [PubMed] [Google Scholar]
- 83.Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biological Psychiatry. 2002;52:759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- 84.Pohjalainen T, Rinne JO, Nagren K, SyvAlahti E, Hietala J. Sex Differences in the Striatal Dopamine D2 Receptor Binding Characteristics in Vivo. Am J Psychiatry. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- 85.Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. Sex Differences in Extrastriatal Dopamine D2-Like Receptors in the Human Brain. Am J Psychiatry. 2001;158:308–311. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- 86.Wong D, Broussolle E, Want G, Villemagne V, Dannals R, Links J, et al. In vivo measurement of dopamine receptors in human brain by positron emission tomography. Age and sex differences. Annals NY Acad Sci. 1988:203–214. doi: 10.1111/j.1749-6632.1988.tb32986.x. [DOI] [PubMed] [Google Scholar]
- 87.Nordstrom A–L, Olsson H, Halldin C. A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Research:Neuroimaging Section. 1998;83:1–6. doi: 10.1016/s0925-4927(98)00021-3. [DOI] [PubMed] [Google Scholar]
- 88.Walder DJ, Seidman LJ, Cullen N, Su J, Tsuang MT, Goldstein JM. Sex Differences in Language Dysfunction in Schizophrenia. Am J Psychiatry. 2006;163:470–477. doi: 10.1176/appi.ajp.163.3.470. [DOI] [PubMed] [Google Scholar]
- 89.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–71. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 90.Torgalsboen AK. Full recovery from schizophrenia: the prognostic role of premorbid adjustment, symptoms at first admission, precipitating events and gender. Psychiatry Res. 1999;88:143–52. doi: 10.1016/s0165-1781(99)00077-3. [DOI] [PubMed] [Google Scholar]
- 91.Rao ML, Kolsch H. Effects of estrogen on brain development and neuroprotection--implications for negative symptoms in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):83–96. doi: 10.1016/s0306-4530(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 92.Hafner H, Riecher-Rossler A, An Der Heiden W, Maurer K, Fatkenheuer B, Loffler W. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol Med. 1993;23:925–40. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- 93.Seeman MV. Gender differences in schizophrenia. Can J Psychiatry. 1982;27:107–12. doi: 10.1177/070674378202700204. [DOI] [PubMed] [Google Scholar]
- 94.Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–94. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- 95.Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, et al. Increased frontal [(18)F]fluorodopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–30. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- 96.Lyons KE, Hubble JP, Troster AI, Pahwa R, Koller WC. Gender differences in Parkinson’s disease. Clin Neuropharmacol. 1998;21:118–21. [PubMed] [Google Scholar]
- 97.Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:679–84. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida T, Kuwabara Y, Sasaki M, Fukumura T, Ichimiya A, Takita M, et al. Sex-related differences in the muscarinic acetylcholinergic receptor in the healthy human brain--a positron emission tomography study. Ann Nucl Med. 2000;14:97–101. doi: 10.1007/BF02988587. [DOI] [PubMed] [Google Scholar]
- 99.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 100.Epperson C, Haga K, Mason G, Sellers E, Gueorguieva R, Zhang W, et al. Cortical γ-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 101.Zubieta J–K, Dannals RF, Frost JJ. Gender and Age Influences on Human Brain Mu-Opioid Receptor Binding Measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 102.Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, et al. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metab. 1998;83:4498–505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]