Abstract
The disorazoles comprise a family of 29 closely related macrocyclic polyketides isolated in 1994 from the fermentation broth of the gliding myxobacterium Sorangium cellulosum. Disorazoles A1, E and C1 have shown exceptional biological activities toward inhibiting the proliferation of human cancer cell lines in picomolar and nanomolar concentrations through the disruption of microtubule polymerization. This review gives a brief introduction describing the biosynthesis and the significance of the disorazoles as a new class of microtubulin disruptors. Another portion of the review focuses on the biology of the disorazoles, specifically disorazole A1 and C1, and their antiproliferative efficacy against animal and human tumor cell lines, as well as the available SAR data. The majority of the discussion addresses synthetic efforts, including partial syntheses of various disorazoles and a summary of the total synthesis of disorazole C1.
1 Introduction
Natural products present us with a treasure trove of structurally novel and mechanistically inspiring biologically active agents and, occasionally, even therapeutic drugs. The disorazoles comprise a new class of microtubule disrupting cytotoxic macrodiolides isolated from the fermentation broth of the myxobacterium Sorangium cellulosum So ce12. These secondary metabolites show exceptional subnanomolar activity against a panel of cancer cell lines and have a mode of action related to but not identical to that of vinca alkaloids. The blend of intriguing biological potency along with the complex and unusual molecular architecture of the disorazoles has triggered the curiosity of biologists and synthetic chemists alike.
In this review, the isolation and current information regarding the biosynthesis and biological target of the disorazoles will be discussed. Biological data for the disorazoles are summarized and compared to known anti-cancer agents such as the vinca alkaloids, dolastatins, and epothilones. In addition, synthetic efforts including partial syntheses and the total synthesis of disorazole C1 will be addressed in detail. A brief overview of published efforts toward the syntheses of disorazoles A1 and D1 will also be presented.
2 Isolation, structure and biosynthesis
The disorazoles comprise a family of 29 macrodiolides first isolated in 1994 by Jansen, Irschik, Reichenbach, Wray and Höfle from the fermentation broth of the gliding bacteria (myxobacteria) strain Sorangium cellulosum So ce12.1 Myxobacteria exhibit features of both unicellular and multicellular organisms and have a history of producing a rich collection of biologically active secondary metabolites,2 including the epothilones,3 chondramide B,4 apicularen,5 gephyronic acid,6 rhizopodin,7 and tubulysins.8 These “slime bacteria” travel by gliding, or creeping, in waves across the surface of a host and are found in soil, rotting plant material, animal dung, and on the bark of living and dead trees.9
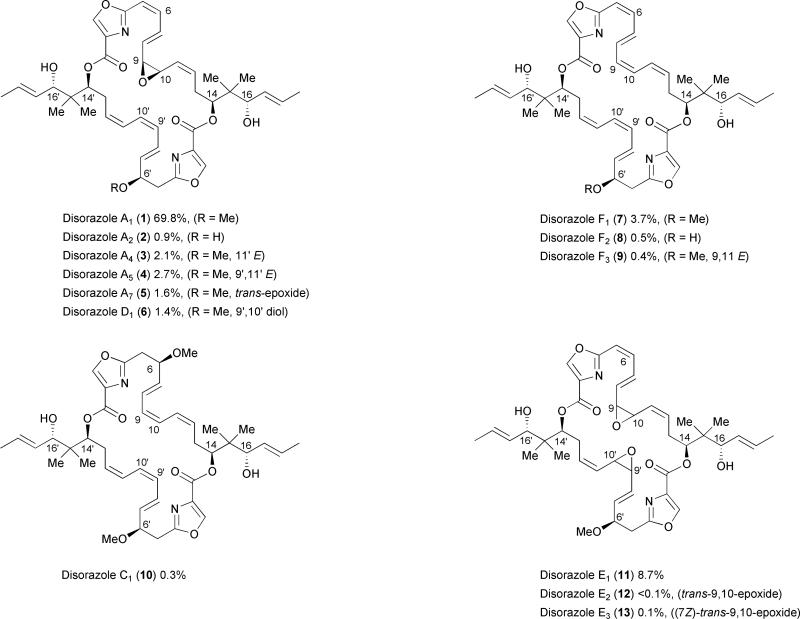
The disorazoles were isolated from the original fermentation broth by solvent partition and chromatographic separation. Disorazole A1 was identified as the major component, comprising 69.8% of the relative mass amount compared to the remaining 28 disorazoles (Fig. 1). Disorazole A1 was also used as the basis for the structure elucidation of the disorazoles. Its molecular formula was determined by negative and positive ion FAB mass spectrometry, high-resolution mass spectrometry, and elemental analysis. Major structural subunit determinations and further structure refinements were achieved using 1D and 2D NMR techniques. The isolation group reported that the disorazoles were stable during the purification process, and, most importantly, that disorazole A1 (1) was not contaminated with disorazole D1 (6) after chromatography with aqueous methanol, indicating that the epoxide of disorazole A1 was not readily opened to form the diol group in disorazole D1.1 However, the possibility that several of the minor members of this family are artefacts derived from side reactions such as olefin isomerization or epoxide solvolysis during the isolation and purification process cannot be completely excluded.
Fig. 1.
Selected members of the disorazole family. Percentages correspond to relative amounts isolated from So ce12.
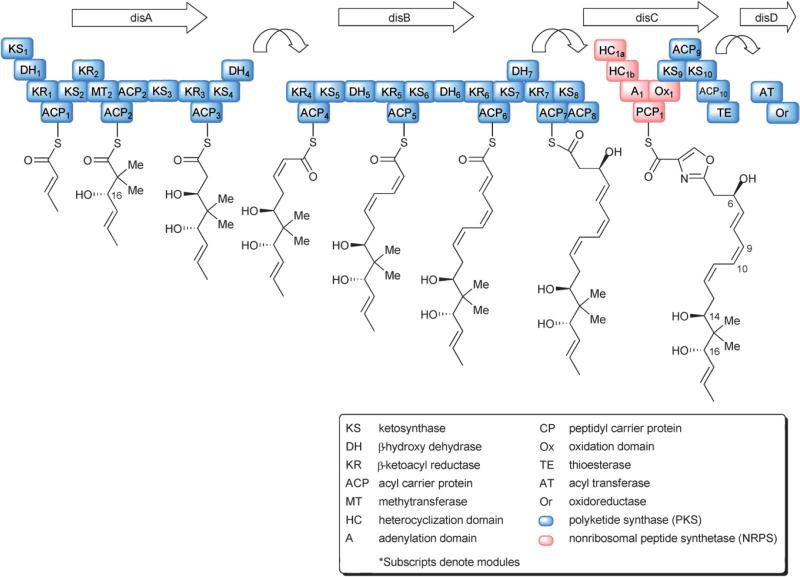
The potential therapeutic value of the disorazoles was highlighted by the identification of the biosynthetic gene cluster by an industrial group in 2005.10,11 Interestingly, the DisA gene does not contain a loading domain to transfer acetyl-CoA or malonyl-CoA to an acyl carrier protein (ACP) (Fig. 2). The most likely scenario to explain the initiation of the biosynthesis involves a direct transfer of an acetyl group from CoA onto the ketosynthase (KS) module.10,11 The geminal methyl groups at C-15 and C-15′ are derived from S-adenosyl methionine based on 13C-labelled methionine feeding studies,1 and are consistent with the presence of a methyltransferase (MT). The purpose of the second ACP in module 2 is not clear as it lacks the active site serine for 4′-phosphopantetheine addition. The β-hydroxyl group at C-14 and C-14′ is installed at module 3 via the KS, β-ketoreductase (KR), and ACP domains. Chain extension occurs at the DisB site through a series of KS, β-hydroxydehydrase (DH), KR, and ACP domains to afford the conjugated polyene unit observed in the disorazoles. The point at which the hydroxyl group at C-6 is installed remains a subject of debate. The DH domain at module 7 may remain either continuously active to produce an intermediate containing four conjugated double bonds, with the hydroxyl group being installed during post PKS synthetic steps, or, alternatively, the DH domain at module 7 may function only half of the time. Obviously, the latter scenario is only one out of many alternative reasons for the formation of unsymmetrical macrodiolide products from two non-identical seco-acids. Ultimately, cyclization may be driven by conformational factors and/or ring strain, thus only allowing some combinations of seco-acids to cyclize. An interrupted process controlled by the seemingly unnecessary extension units might account for the diversity in seco-acid combinations. Similar mechanisms have been discussed in cyclopeptide biosynthesis. However, in the absence of any specific evidence to the contrary, the most likely hypothesis is the formation of a yet-to-be-identified symmetric macrodiolide that is subjected to post-PKS diversifications. Formation of an oxazolidine ring occurs at the DisC site via two heterocyclization (HC) domains. The first HC domain of the unusual dual HC domain may serve to incorporate serine, while the second HC domain promotes cyclization to the oxazoline. Alternatively, both steps may be catalyzed by the first HC domain while the second site remains inactive.10 Oxidation of the oxazoline to the final oxazole heterocycle by the oxidation domain (Ox) of the DisC site completes the synthesis of the monomeric disorazole backbone. Modules 9 and 10 of DisD appear to be unnecessary for the synthesis of disorazole, and therefore the monomer could be released from the chain by the thioesterase (TE). Whether the TE of DisD participates in the dimerization of the biosynthetic monomer or the dimerization occurs via an alternative enzyme has yet to be determined. Further structural modifications required to construct the various members of the disorazole family are believed to occur post PKS.
Fig. 2.
Proposed biosynthetic pathway for the disorazoles.
3 Biological function
Soon after the initial isolation of the disorazoles in 1994,1 biological follow-up studies revealed the exceptional cytotoxicity of these secondary metabolites in cell cultures as well as their antifungal effects. In contrast, disorazoles do not appear to possess potent antiviral or antibacterial activity.12 To date, disorazole A1 (1) remains the most extensively studied member of the disorazole family with regard to its biological activity and mode of action.12-14 Mainly, this preference is due to its relatively large natural abundance in comparison to other disorazoles.1 In 2004, Elnakady, Sasse, Lünsdorf and Reichenbach demonstrated that the antimitotic properties of disorazole A1 originated from its tubulin polymerization inhibitory activity. They provided evidence that picomolar concentrations of disorazole A1 were sufficient for destabilizing microtubule assembly, blocking mitosis, and inducing apoptosis. Additional studies supported that the disorazoles derived their tubulin depolymerization activity from binding to or near the vinca domain, thus resulting in an irreversible perturbation of the microtubule network as well as cell cycle arrest at the G2/M checkpoint, and triggering of the apoptotic cell death cascade.14
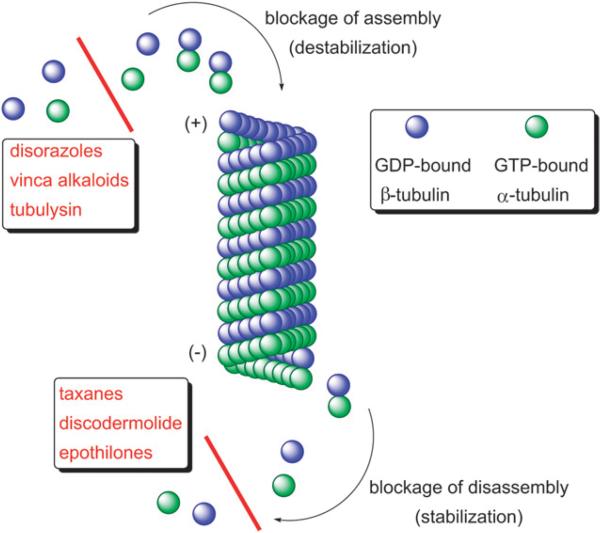
The exposure of cells to agents that perturb the dynamic polymerization–depolymerization equilibrium of tubulin α,β-heterodimers results in dysfunctional mitotic spindles, which are critical for proper sister chromatid segregation, mitosis and the cytokinesis of dividing cells.15 The potent bioactivity of the disorazoles mainly originates from their ability to disrupt microtubule formation; accordingly they share a mechanism of action found for the vinca alkaloids and other anticancer drugs, such as the lipophilic peptides dolastatin 1516 and tubulysin A,17 as well as the autumn crocus secondary metabolite colchicine.18 In contrast, the clinically used taxanes19 and epothilones,20 as well as (+)-discodermolide,21 dictyostatin,22 laulimalide,23 and other natural products,24 derive their activity from the stabilization of microtubules (Fig. 3). Among the secondary metabolites isolated from myxobacteria, the disorazoles represent the third distinct class of compounds (in addition to epothilones and tubulysins) that interfere with microtubule dynamics.
Fig. 3.
Stabilization and destabilization of microtubule formation by anti-cancer agents.
Disorazole A1 exhibits a concentration-dependent growth inhibition of the leukemia cell line L929 with an exceptionally potent IC50 value of 3 pM.12,14 This disorazole has also shown remarkable inhibition properties towards numerous other human cancer cell lines. Compared to epothilone B and vinblastine, the antiproliferative efficacy of disorazole A1 was superior in all cell-based studies by at least an order of magnitude.14 Remarkably, disorazole A1 retained its potency against the multi-drug resistant cell line KB-V1 which overexpresses the P-glycoprotein (Pgp) efflux pump (Table 1). Pgp is believed to function as an ATP-linked transporter for hydrophobic xenobiotics, and high expression levels of Pgp in tumors are often associated with a poor response to chemotherapeutic drugs, resulting in grave tumor prognosis.25 While it remains to be established if the disorazoles share a mechanism-based neurotoxicity with other tubulin (de)stabilizing agents, their activity in a multidrug resistant cell line bodes well for potential future use in cancer therapy.
Table 1.
Activity comparison of disorazole A1, epothilone B, and vinblastine against established animal and human cancer cell lines (data taken from refs 12 and 14). IC50 values refer to antiproliferative activities
| IC50 (nM) |
||||
|---|---|---|---|---|
| Cell Line | Origin | Disorazole A1 (1) | Epothilone B | Vinblastine |
| A549 | Human lung carcinoma | 0.0023 ± 0.0005 | 0.26 ± 0.14 | 5.9 ± 0.5 |
| PC-3 | Human prostate adenocarcinoma | 0.0071 ± 0.0012 | 2.0 ± 0.3 | 0.82 ± 0.06 |
| SK-OV-3 | Human ovary adenocarcinoma | 0.0049 ± 0.0001 | 0.64 ± 0.07 | 1.4 ± 0.1 |
| A-498 | Human kidney carcinoma | 0.016 ± 0.004 | 4.3 ± 3.6 | 46 ± 12 |
| U-937 | Human histiocytic lymphoma | 0.002 ± 0.001 | 0.09 ± 0.01 | 0.43 ± 0.13 |
| K-562 | Human myelogenous leukemia | 0.006 ± 0.001 | 0.69 ± 0.03 | 8.7 ± 1.8 |
| KB-3.1 | Human cervix carcinoma | 0.0025 ± 0.0003 | 1.6 ± 0.6 | 8.6 ± 0.3 |
| KB-V1 | Human cervix carcinoma (multi-drug resistant) | 0.042 ± 0.008 | 0.57 ± 0.03 | 114 ± 31 |
| L929 | Mouse fibroblasts | 0.0038 ± 0.0002 | 1.3 ± 0.6 | 28 ± 7 |
Comparatively few biological data are available for other disorazoles. Wipf, Graham, Vogt, Sikorski, Ducruet, and Lazo have demonstrated that disorazole C1 (10) was an effective cytotoxic agent with IC50 values ranging from 1.6−6.9 nM in various human cancer cell lines (Table 2).26 These results were comparable to IC50 values of the clinically used vinblastine and vincristine. Additionally, a high-content analysis of mitotic arrest in HeLa cells treated with disorazole C1, vincristine, vinblastine, colchicine, and paclitaxel provided IC50 values of 14.6, 6.9, 0.3, 23.9, and 6.7 nM, respectively.27 Remarkably, while the absence of the highly electrophilic vinyl oxirane at carbons 9 and 10 in disorazole C1 reduced its cytotoxicity by 50−100 fold compared to disorazole A1, the simplified macrodiolide is still an exceedingly potent agent. Furthermore, disorazole C1 was recently shown to induce premature senescence and may bind to a site distinct from the vinca alkaloids.28
Table 2.
Activity comparison of disorazole C1, vincristine, and vinblastine against established animal and human cancer cell lines (data taken from ref. 26). IC50 values refer to antiproliferative activities
| IC50 (nM) |
||||
|---|---|---|---|---|
| Cell Line | Origin | Disorazole C1 (10) | Vincristine | Vinblastine |
| A549 | Human lung carcinoma | 2.21 ± 0.23 | 21.62 ± 2.68 | 1.52 ± 0.09 |
| PC-3 | Human prostate adenocarcinoma | 1.57 ± 0.10 | 4.68 ± 0.29 | 0.86 ± 0.08 |
| MDA-MB-231 | Human breast epithelial adenocarcinoma | 3.53 ± 0.19 | 7.16 ± 0.37 | 1.34 ± 0.21 |
| 2008 | Human ovarian carcinoma | 1.91 ± 0.23 | 21.81 ± 2.92 | 2.24 ± 0.16 |
| Quiescent WI-38 | Normal lung fibroblast | >100 | N/D | >100 |
| HCT-116 WT | Human colorectal carcinoma | 1.09 ± 0.41 | 5.62 ± 0.33 | 1.40 ± 0.07 |
| HCT-116 p53 −/− | Human colorectal carcinoma | 2.25 ± 0.71 | 5.42 ± 0.47 | 2.17 ± 0.35 |
| DC3F WT | Chinese hamster lung cancer fibroblasts | 5.55 | 17.53 | |
| VCRD-5L | Chinese hamster lung cancer fibroblasts (multi-drug resistant) | 6.77 | N/A | |
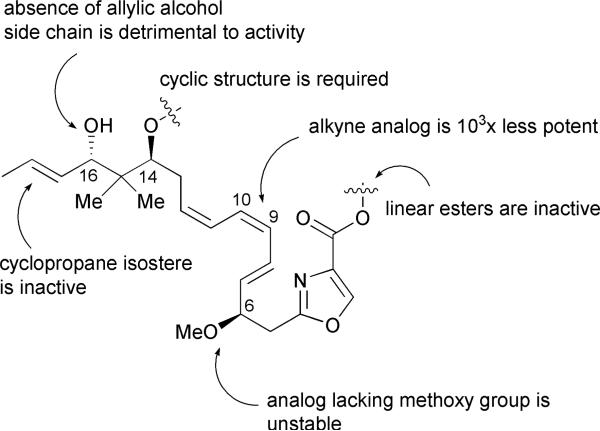
Structure–activity relationship (SAR) studies performed on disorazole C1 revealed that truncation or alteration of the backbone functionality was highly detrimental to the activity of the macrodiolide (Fig. 4).26 The absence of the allylic alcohol side chain or incorporation of a cyclopropane alkene isostere destroyed all activity. Additionally, replacement of the triene unit with a dienyne yielded an analog exhibiting three orders of magnitude lower potency. Subunits of disorazole C1 were completely inactive, and compounds lacking a methoxy group at C-6 were too unstable to isolate. Based on this information, it appears that the side chain functionalities as well as the distinct triene backbone are essential for adequate binding to the biological target, and necessary to exhibit potent antiproliferative activity. It is quite feasible that disorazole C1 represents the minimum pharmacophore of the disorazole family. The final resolution of this puzzle will likely require the elucidation of the molecular mode of interaction of the disorazoles with the α,β-tubulin heterodimer.
Fig. 4.
Summary of disorazole C1 (10) SAR studies.
4 Synthetic strategies
4.1 Meyers’ partial synthesis of disorazole C1
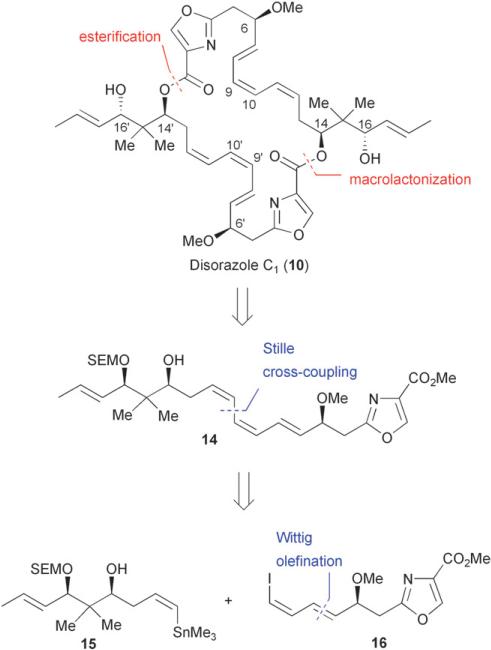
The disorazoles are a structurally novel class of macrocyclic polyketide microtubule disruptors. The presence of both heterocyclic and labile polyene linkages within the macrocyclic scaffold present the synthetic chemist with intriguing challenges. The C2-symmetrical disorazole C1 remains the simplest of the currently known disorazoles and was the first to be featured in synthetic studies by the Meyers group in 2000.29 At the start of these investigations, neither the relative nor the absolute configuration of the disorazoles had been reported, resulting in an arbitrary initial selection of the stereochemical features of the target molecule.
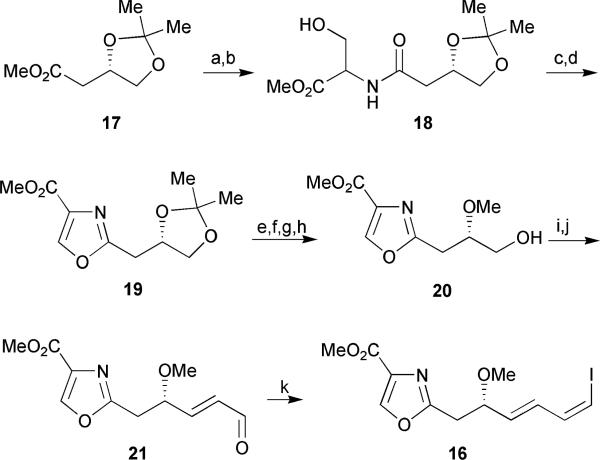
Meyers’ retrosynthetic approach involved a disconnection of the dimeric macrolactone to the triene monomer 14 which could be constructed through the joining of oxazole 16 and diol 15 via a Stille cross-coupling at carbons 10 and 11 (Fig. 5). The construction of the oxazole C-1 through C-10 segment began with the hydrolysis of the readily available ester 17 followed by coupling with a racemic serine methyl ester to afford the hydroxy amide 18 (Scheme 1). Subsequent diethylaminosulfurtrifluoride (DAST) mediated cyclodehydration followed by oxidation30 provided oxazole 19 in 79% yield over two steps. Successive protecting group manipulations followed by Parikh–Doering oxidation of the resulting primary alcohol 20 afforded the desired aldehyde which was immediately submitted to a Wittig olefination to generate enal 21. Finally, a Stork–Zhao olefination31 in the presence of hexamethylphosphoramide (HMPA) provided the (Z)-vinyl iodide fragment 16 (Z/E = 18: 1) in 11 steps from ester 17.
Fig. 5.
Meyers’ retrosynthetic analysis of disorazole C1 (10).
Scheme 1.
Meyers’ oxazole segment synthesis. Reagents and conditions: (a) 2N LiOH, THF; (b) D,L-Ser·OMe, 1,1′-CDI, THF, 67% (over 2 steps); (c) DAST, CH2Cl2, −78 °C; (d) DBU, BrCCl3, 0 °C to rt, 79% (over 2 steps); (e) Dowex-H+, MeOH; (f) TIPSOTf, 2,6-lutidine, CH2Cl2 (0.05M), −78 °C, 74% (over 2 steps); (g) MeI, Ag2O, CH3CN, Δ; (h) TBAF, THF, 75% (over 2 steps); (i) SO3·Pyr, DMSO, Et3N, CH2Cl2; (j) Ph3P=CH2CHO, benzene, Δ, 62% (over 2 steps); (k) I−Ph3P+CH2I, NaHMDS, HMPA, THF, −78 °C, 71%.
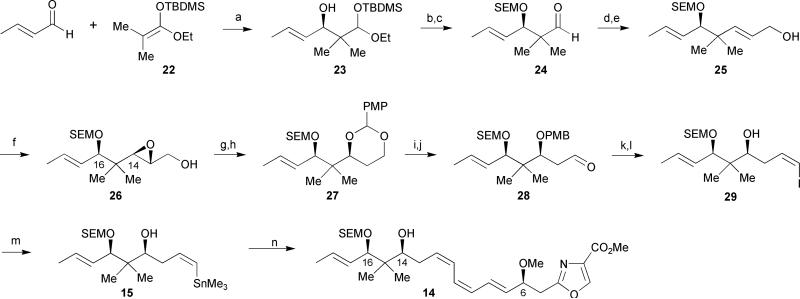
The synthesis of the C-11 through C-19 diol fragment 15 commenced with an enantioselective Mukaiyama aldol reaction between (E)-crotonaldehyde and the O-silyl ketene acetal 22 under Kiyooka's conditions32 to afford acetal 23 in 73% yield and 92−93% ee based on Mosher ester analysis (Scheme 2). Silyl protection of the secondary alcohol 23 followed by acetal hydrolysis in the presence of 80% AcOH revealed the desired aldehyde 24. Horner–Wadsworth–Emmons olefination of this aldehyde effectively installed an (E)-α,β-unsaturated ester that was immediately reduced in the presence of DIBAL-H to generate the allylic alcohol 25. The remaining stereocenter at C-14 was installed via a Sharpless asymmetric epoxidation in a 15: 1 dr and in 95% yield with epoxide 26 representing the major product. Subsequent selective epoxide opening with Red-Al followed by diol protection afforded the acetal 27 in 83% yield over 2 steps. A regioselective ring opening of acetal 27 with DIBAL-H and Dess–Martin oxidation of the resulting primary alcohol gave aldehyde 28. Stork–Zhao olefination31 of aldehyde 28 followed by oxidative PMB cleavage in the presence of DDQ successfully installed the (Z)-olefin geometry at carbons 11 and 12. The authors observed that the PMB protecting group had to be removed prior to the generation of the vinyl stannane due to a DDQ-mediated destannylation. Palladium-catalyzed deiodination/stannylation of vinyl iodide 29 followed by Stille coupling of the resultant vinyl stannane to the oxazole segment 16 completed the synthesis of the monomeric unit 14. Unfortunately, the authors were unable to continue with the synthesis and effect the dimerization of 14, possibly due to the instability of the labile triene unit.
Scheme 2.
Meyers’ 1st generation synthesis of the southern fragment of disorazole C1. Reagents and conditions: (a) BH3·THF, N-Ts-L-Val, CH2Cl2, −78 °C, 73%; (b) SEMCl, Hünig's base, CH2Cl2, −78 °C; (c) 80% AcOH, 79% (over 2 steps); (d) (EtO)2P(O)CH2CO2Et, NaH, toluene/THF, 95%; (e) DIBAL-H, CH2Cl2, −78 °C, 76%; (f) D-(−)-DIPT, t-BuOOH, Ti(Oi-Pr)4, CH2Cl2, −30 °C, 95%; (g) Red-Al, THF, −20 °C: (h) p-methoxybenzylidene dimethyl acetal, PPTS, CH2Cl2, 83% (over 2 steps); (i) DIBAL-H, CH2Cl2, −78 °C, 92%; (j) Dess–Martin periodinane, pyridine, t-BuOH, CH2Cl2, 83%; (k) I−Ph3P+CH2I, NaHMDS, HMPA, THF, −78 °C, 67%; (l) DDQ, CH2Cl2, H2O, 79%; (m) PdCl2(PPh3)2, (Me3Sn)2, Li2CO3, THF, 74%; (n) PdCl2(CH3CN)2, 16, DMF, 76%.
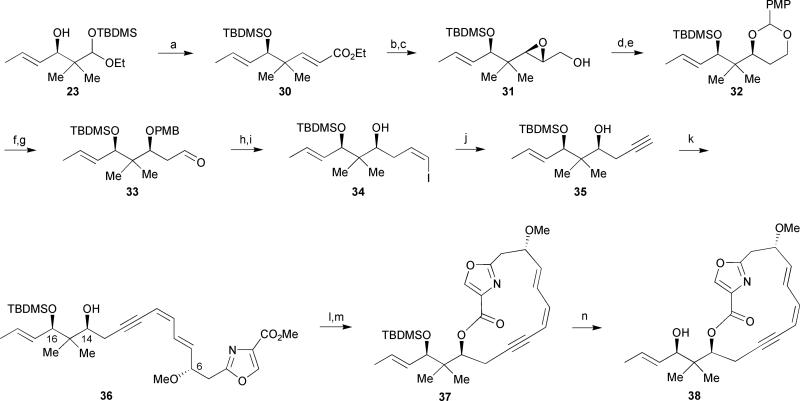
In order to overcome the propensity of the triene unit at C-7 through C-12 to isomerize, Meyers investigated a second generation approach by masking the (Z)-alkene at C-11/C-12 as an alkyne (Scheme 3).33 An approach similar to the one described in Scheme 2 was adopted to construct the requisite alkyne from acetal 23. The authors found that by simply treating acetal 23 with 2.0 equivalents of NaH in the presence of triethylphosphonoacetate, direct access to the α,β-unsaturated ester 30 was achieved in 72% yield via an initial collapse of the acetal and a concurrent 1,5 O→O silyl migration,34 resulting in the exclusive formation of the aldehyde (Scheme 3). This aldehyde was then free to participate in the subsequent in situ Horner–Wadsworth–Emmons olefination. Subjecting the α,β-unsaturated ester 30 to a similar series of reactions described in Scheme 2 (vide supra) provided (Z)-vinyl iodide 34 which smoothly underwent elimination in the presence of NaHMDS to afford the terminal alkyne 35 in 93% yield. Sonogashira cross-coupling with oxazole segment 16 provided dienyne 36 in 87% yield. However, upon hydrolysis of the methyl ester and treatment of the crude carboxylic acid with dipyridyl thionocarbonate (DPTC) in the presence of a catalytic amount of DMAP in toluene at reflux afforded exclusively the intramolecular cyclization product macrolactone 37. Furthermore, changes in the reactant concentration did not influence the outcome of this reaction and no attempts to employ alternative macrolactonization conditions were made. The structural assignments of the synthetic intermediates were confirmed by a single crystal X-ray analysis of alcohol 38.
Scheme 3.
Meyers’ 2nd generation approach. Reagents and conditions: (a) (EtO)2P(O)CH2CO2Et, NaH (2.0 equiv), THF, −78 °C to rt, 72%; (b) DIBAL-H, CH2Cl2, −78 °C; (c) D-(−)-DIPT, t-BuOOH, Ti(Oi-Pr)4, CH2Cl2, −30 °C, 80% (over 2 steps); (d) Red-Al, THF, −20 °C to 0 °C; (e) p-methoxybenzylidene dimethyl acetal, PPTS, CH2Cl2, 77% (over 2 steps); (f) DIBAL-H, CH2Cl2, −78 °C; (g) Dess–Martin periodinane, pyridine, t-BuOH, CH2Cl2, 90% (over 2 steps); (h) I−Ph3P+CH2I, NaHMDS, HMPA, THF, −78 °C; (i) DDQ, CH2Cl2, H2O, 67% (over 2 steps); (j) NaHMDS (2.0 equiv), THF, −78 °C to rt, 93%; (k) 16, PdCl2(PPh3)2, CuI, Et3N, CH3CN, −20 °C to rt, 87%; (l) 1N LiOH, THF; (m) DPTC, DMAP, toluene, Δ, 46% (over 2 steps); (n) HF·Pyr, CH3CN, 45%.
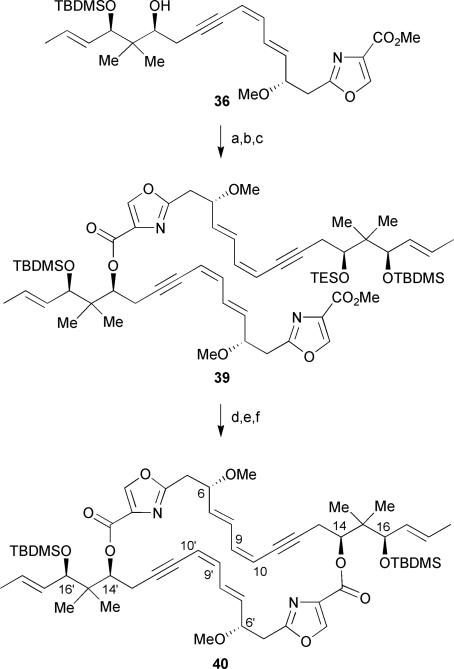
The authors addressed the dimerization problem by adopting a stepwise coupling approach (Scheme 4). An orthogonal silyl protection of the secondary alcohol at C-14 of dienyne 36 followed by hydrolysis of the methyl ester afforded the corresponding carboxylic acid. Coupling to a second equivalent of alcohol 36 in the presence of DPTC and a catalytic amount of DMAP in toluene at reflux gave exclusively ester 39. Selective removal of the secondary TES protecting group in the presence of TFA/H2O (1: 1) in THF followed by saponification and Yamaguchi macrolactonization led to the desired bislactone 40 in a low 24% yield over the final two steps. Formation of a large amount (67%) of the lactone monomer 37 was also observed due to a non-selective ester hydrolysis of 39. Attempts to overcome the problematic dimerization step were unsuccessful. Unfortunately, desilylation at C-16 and C-16′ of 40 was also unsuccessful and resulted in significant amounts of transesterification and decomposition products that were inseparable by column chromatography. Furthermore, partial reduction of the triple bonds at C-11/C-12 and C-11′/C-12′ was ineffective. In contrast, partial reduction of monomer 36 in the presence of Zn–Cu–Ag alloy in MeOH/H2O at 80 °C for 48 h in a sealed tube afforded the triene in 91% yield accompanied by some olefin isomerization products (not shown).
Scheme 4.
Meyers’ dimerization approach. Reagents and conditions: (a) TESOTF, 2,6-lutidine, CH2Cl2, −78 °C, 69%; (b) 1N LiOH, THF; (c) 36, DPTC, DMAP, toluene, Δ, 65% (over 2 steps); (d) TFA, H2O, THF, 65%; (e) 1N LiOH, THF; (f) 2,4,6-trichlorobenzoyl chloride, DMAP, toluene, 24% (over 2 steps).
4.2 Hoffmann's synthesis of tetradehydrodisorazole C1
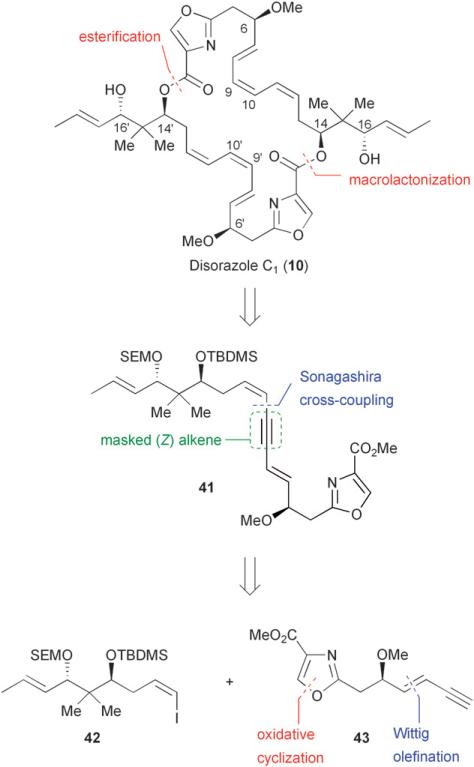
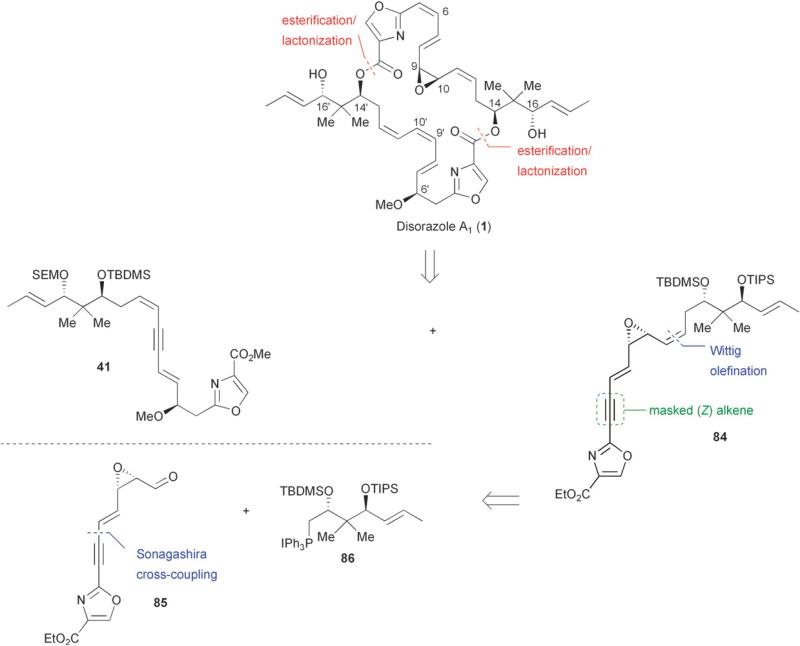
In 2000, the absolute and relative configuration of the disorazoles was assigned by Höfle through degradation studies of disorazole A1 (1).35 The stereochemical information gained from these studies was undoubtedly unavailable to Meyers and co-workers at the time of their work on disorazole C1.29,33 In 2002, Hoffmann and co-workers first reported the synthesis of a masked fragment of disorazole C1 with the correct absolute and relative configurations at all stereocenters.36 Hoffmann adopted a synthetic strategy that utilized a strategically placed alkyne along the C-7/C-12 backbone to mask the labile triene moiety of disorazole C1, thereby allowing for a more efficient cyclodimerization of dienyne 41 (Fig. 6).
Fig. 6.
Hoffmann's retrosynthetic analysis of disorazole C1 (10).
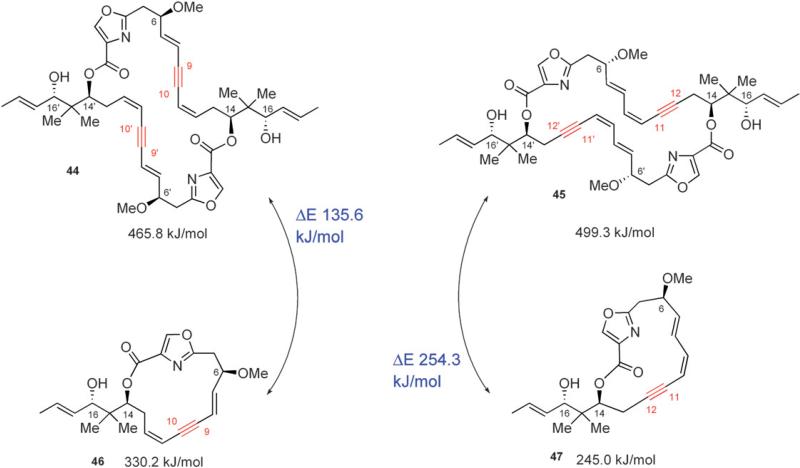
The appropriate placement of an alkyne was expected to induce sufficient ring strain to suppress an intramolecular lactonization to the undesired 15-membered macrolactone. Force field energy minimizations revealed that positioning the alkyne at C-9/C-10 versus C-11/C-12 resulted in greater ring strain for the undesired 15-membered lactone (330.2 kJ/mol vs 245.0 kJ/mol, respectively) and a lower energy in the corresponding 30-membered lactone (465.8 kJ/mol vs 499.3 kJ/mol, respectively) (Fig. 7).36 Additionally, positioning the alkyne at C-9/C-10 did not significantly affect the relative energies of tetradehydrodisorazole C1 (44) and disorazole C1 (465.8 kJ/mol vs 463.3 kJ/mol, respectively).
Fig. 7.
Hoffmann's estimates of ring strain of 15- versus 30-membered lactones based on molecular mechanics energy minimizations.
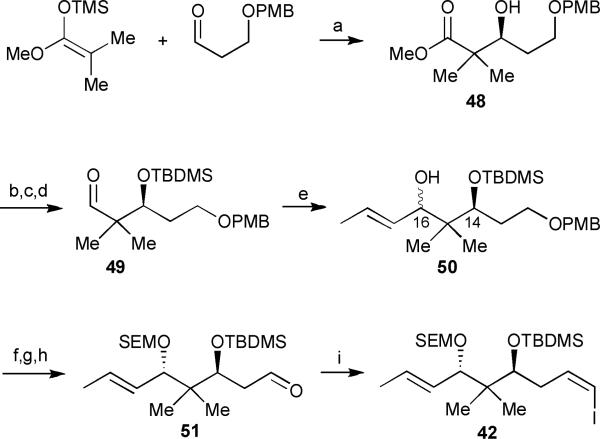
The synthesis of vinyl iodide 42 commenced with an asymmetric Mukaiyama aldol reaction under Kiyooka's conditions32 to afford β-hydroxy ester 48 in 96% yield and 88% ee (Scheme 5). Protection of the secondary alcohol followed by sequential ester reduction and re-oxidation in the presence of Dess–Martin periodinane generated the desired aldehyde 49. An attempted stereoselective addition of lithiated trans-bromopropene to aldehyde 49 proved problematic, resulting in a 1.1: 1.0 syn: anti mixture of separable diastereomers at C-16. Nonetheless, the anti-diol was carried forward an additional 4 steps to provide the orthogonally protected diol fragment 42.
Scheme 5.
Hoffmann's 1st generation C-11 to C-19 segment synthesis. Reagents and conditions: (a) BH3·THF, N-Ts-D-Val, CH2Cl2, −78 °C, then K2CO3, MeOH, 96%; (b) TBDMSOTf, 2,6-lutidine, DMAP, CH2Cl2, 99%; (c) DIBAL-H, toluene, −78 °C, 94%; (d) Dess–Martin periodinane, CH2Cl2, 0 °C to rt, 83%; (e) trans-1-bromopropene, t-BuLi, Et2O/THF, −95 °C, 99% (combined yield of both diastereomers); (f) SEMCl, Hünig's base, Bu4NI, CH2Cl2; (g) DDQ, CH2Cl2/H2O, 0 °C, 98% (yield over 2 steps, based on the (5S)-diastereomer of 50); (h) SO3·Pyr, Et3N, CH2Cl /DMSO, 0 °C, 80%; (i) I−Ph3P+CH2I, NaHMDS, HMPA, THF, −78 °C, 82%.
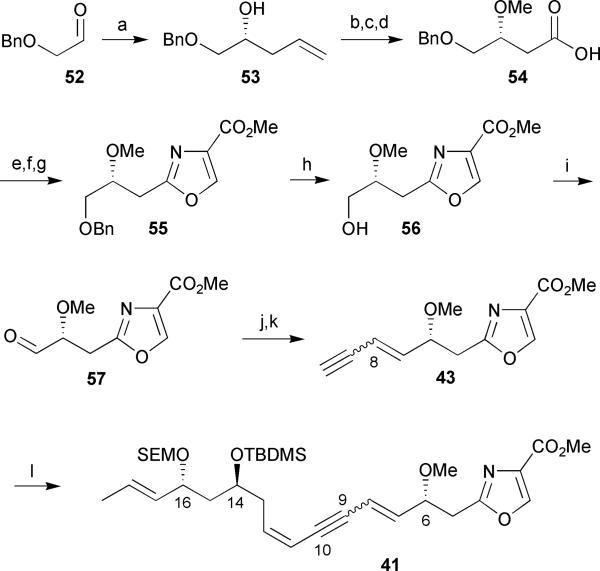
Oxazole 43 was constructed beginning with a Keck allylation37 of the readily available aldehyde 52 in the presence of (R)-BINOL to afford the homoallylic alcohol 53 in 84% yield and >94% ee (Scheme 6). O-Methylation of the secondary alcohol 53 followed by ozonolysis and oxidation of the resulting aldehyde gave the corresponding carboxylic acid 54. Coupling of 54 with L-serine methyl ester hydrochloride in the presence of isobutyl chloroformate (IBCF) and N-methylmorpholine (NMM) followed by DAST-mediated cyclodehydration and DBU/BrCCl3 oxidation30 of the resulting oxazoline yielded oxazole 55. Debenzylation and subsequent oxidation of the primary alcohol 56 provided the desired aldehyde 57. A three-carbon chain extension of aldehyde 57 via Wittig olefination with TMS-protected propargyl triphenylphosphonium bromide proceeded with poor olefin selectivity (E/Z = 2.5: 1.0) at C-8 and a moderate 49% yield. The inseparable alkene isomers were desilyated to provide the oxazole fragment 43 which was subsequently coupled to vinyl iodide 42 via a Sonogashira cross-coupling reaction to provide dienyne 41 in 58% yield followed by chromatographic separation of alkene isomers.
Scheme 6.
Hoffmann's 1st generation synthesis of a masked triene monomer of disorazole C1. Reagents and conditions: (a) (R)-BINOL, Ti(Oi-Pr)4, 4Å MS, allyltributylstannane, CH2Cl2, −20 °C, 84%; (b) NaH, MeI, THF, 94%; (c) O3/O2, CH2Cl2, −78 °C, then PPh3, −78 °C to rt, 86%; (d) NaClO2, KH2PO4, H2O2, CH3CN/MeOH/H2O, 10 °C, 98%; (e) L-SerOMe·HCl, IBCF, NMM, THF, −25 °C to rt, 71%; (f) DAST, K2CO3, CH2Cl2, −78 °C; (g) DBU, BrCCl3, CH2Cl2, 0 °C to rt, 79% (over 2 steps); (h) H2, Pd/C, EtOH, 97%; (i) SO3·Pyr, Et3N, CH2Cl2/DMSO, 0 °C, 75%; (j) Br−Ph3P+CH2C≡CTMS, n-BuLi, THF, −78 °C to 0 °C, 49%; (k) K2CO3, MeOH, 77%; (l) 42, PdCl2(PPh3)2, CuI, Et3N, CH3CN, −20 °C to rt, 58%.
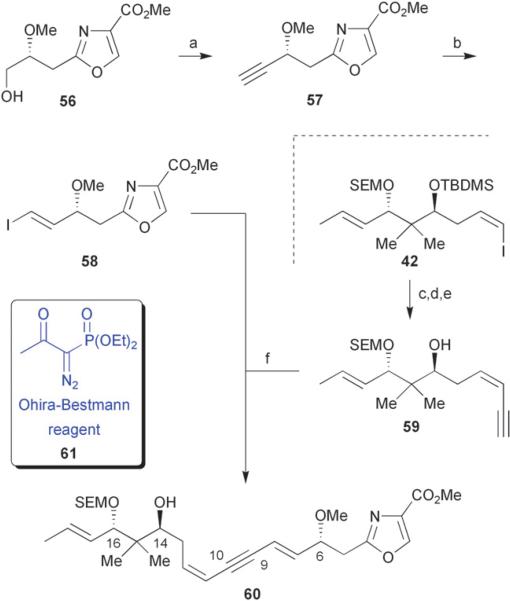
As a result of the problems associated with poor selectivity during the instalment of the (E)-alkene at C-7/C-8, Hoffmann devised a second generation approach that involved a double Sonogashira cross-coupling reaction to selectively construct carbon–carbon bonds at C-8/C-9 and C-10/C-11.38 Oxidation of the primary alcohol 56 followed by immediate treatment of the resulting unstable aldehyde with the Ohira–Bestmann reagent 61 provided terminal alkyne 57 in 75% yield over 2 steps (Scheme 7). Alkyne 57 smoothly underwent hydrostannylation/iododestannylation to selectively afford the (E)-configured vinyl iodide 58 in 88% yield.
Scheme 7.
Hoffmann's 2nd generation synthesis of a masked triene monomer of disorazole C1. Reagents and conditions: (a) (ClCO)2, Et3N, DMSO/CH2Cl2, −78 °C to −40 °C, then 56, K2CO3, MeOH, 0 °C to rt, 75% (over 2 steps); (b) n-Bu3SnH, Pd(PPh3)4, THF, then I2, 88%; (c) TMS-acetylene, PdCl2(PPh3)2, CuI, Et3N, CH3CN, 99%; (d) TBAF (1.1 equiv), THF, 0 °C to rt, 81%; (e) TBAF (10.0 equiv), THF, 95%; (f) PdCl2(PPh3)2, CuI, Et3N, DMF, 89%.
The enyne 59 coupling partner was easily obtained via a three-step sequence involving a Sonogashira cross-coupling with the previously synthesized vinyl iodide 42 and TMS-acetylene, followed by selective removal of the TMS and TBDMS protecting groups. With the newly prepared fragments in hand, vinyl iodide 58 and enyne 59 were coupled via a second Sonogashira reaction to afford the fully resolved dienyne 60 in 89% yield (Scheme 7). The authors found that removal of the TBDMS protecting group at C-14 was required early on, as late stage cleavage resulted in either no reaction or decomposition.38
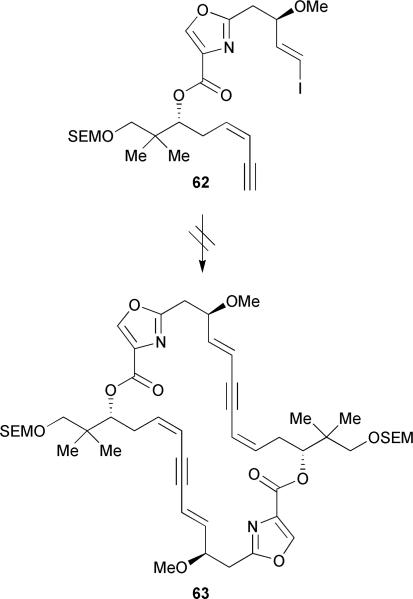
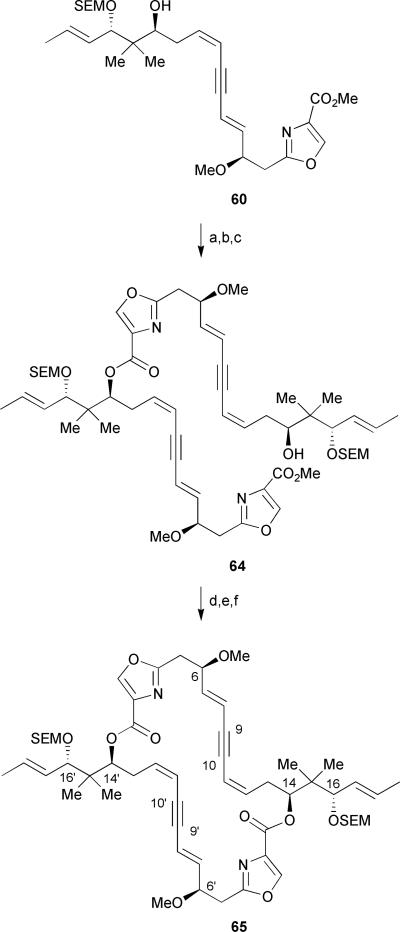
With dienyne 60 in hand, a direct dimerization of the carboxylic acid derived from 60 was examined as part of the endgame strategy but was unsuccessful under a variety of conditions (e.g. DPTC/DMAP, Yamaguchi, and 2-chloro-1-methylpyridinium iodide/DMAP).38 Interestingly, formation of a 15-membered lactone resulting from monomer cyclization33 was not observed, thereby emphasizing the utility of the alkyne placement within the masked triene subunit. A double Sonogashira approach was also briefly explored on a model system to effect the dimerization but met with failure (Scheme 8). In order to circumvent the difficulties encountered during the dimerization strategy, a lengthier, stepwise approach was pursued. Silyl protection of the free secondary alcohol of dienyne 60 with TESOTf followed by hydrolysis of the methyl ester and subsequent Yamaguchi esterification provided the desired ester in a moderate 69% yield (Scheme 9). The authors found that it was important to activate the free carboxylic acid portionwise in order to avoid decomposition of the acylating agent prior to the reaction with alcohol 60. Subsequent cleavage of the silyl ether at C-14 proceeded in 87% yield followed by selective hydrolysis of the methyl ester in the presence of the internal ester linkage to furnish the corresponding acid quantitatively. Finally, Yamaguchi macrolactonization provided the O-silyl protected tetradehydrodisorazole C1 65 in a low 31% yield. Curiously, the authors did not report any attempts to remove the protecting groups of macrodiolide 65 to complete a formal total synthesis of disorazole C1.39
Scheme 8.
Hoffmann's alternative Sonogashira cyclodimerization attempt. Reagents and conditions: PdCl2(PPh3)2, CuI, Et3N, DMF, or slow addition of 62 to PdCl2(PPh3)2, CuI, Et3N, THF.
Scheme 9.
Hoffmann's synthesis of tetradehydrodisorazole C1. Reagents and conditions: (a) TESOTf, 2,6-lutidine, DMAP, CH2Cl2, −40 °C to −20 °C, 85%; (b) 1N LiOH, H2O/THF, quant.; (c) 2,4,6-trichlorobenzoyl chloride, Et3N, toluene, then 60, DMAP, toluene, 40 °C, 69%; (d) TBAF, AcOH, H2O/THF, 87%; (e) Ba(OH)2, H2O/MeOH/THF, quant.; (f) 2,4,6-trichlorobenzoyl chloride, Et3N, toluene, 40 °C, 31%.
4.3 Wipf's total synthesis of (−)-disorazole C1
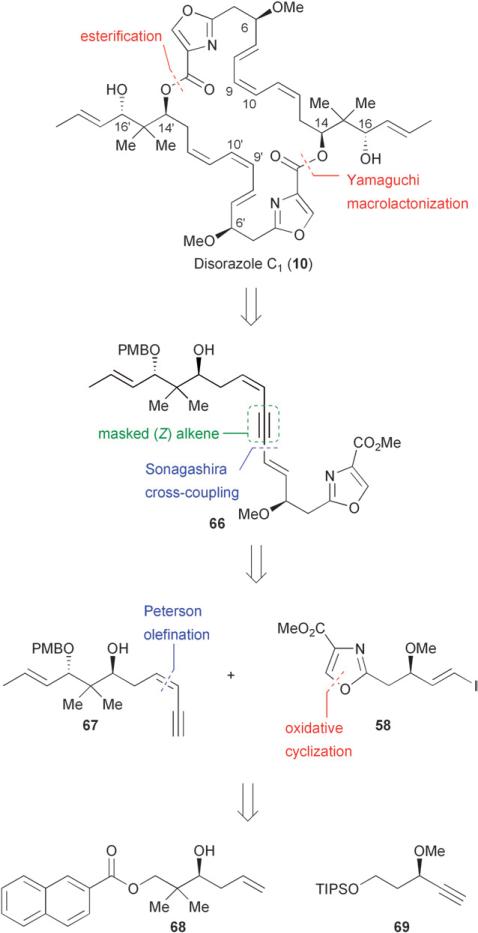
Although the constitution of the disorazoles has been known since 19941 and relative and absolute configurations for disorazole A1 were assigned in 2000,35 it was not until 2004 that a total synthesis of a member of the disorazole class was reported by Wipf and Graham.39 Previous work by Meyers29,33 and Hoffmann36,40,41 revealed important synthetic parameters, including the instability of the triene backbone and the significance of the position of the alkyne which served both to mask the (Z)-olefin(s) of the triene unit as well as facilitate the macrolactonization. Wipf and Graham were able to capitalize on these important findings and devise a synthetic approach that utilized a Sonogashira coupling to construct the carbon–carbon bonds at C-8/C-9 of the dienyne monomer 66 (Fig. 8). Easily under-appreciated but most significant for the success of this synthesis was the selection of a p-methoxybenzyl protecting group for the hydroxyl function at C-16. Due to the sensitive nature of the late stage intermediates, the oxidative but relatively neutral conditions used for PMB removal were superior to basic, fluoride, or acidic conditions. Key fragments 67 and 58 would be derived from known alcohol 6842 and alkyne 69, respectively.
Fig. 8.
Wipf's retrosynthetic analysis of (−)-disorazole C1 (10).
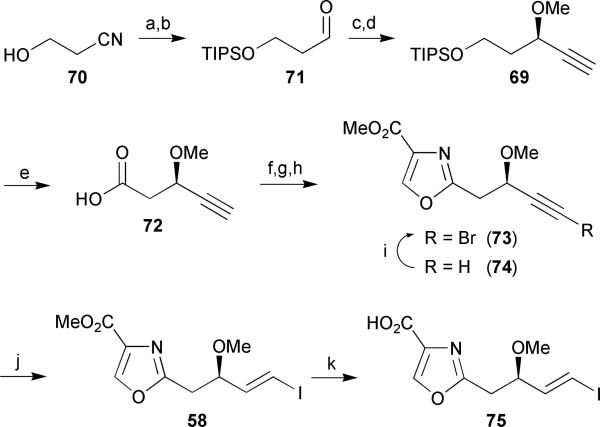
The synthesis of oxazole fragment 58 commenced with the O-silyl protection of hydroxy nitrile 70 followed by partial reduction of the nitrile to afford aldehyde 71 in 78% over two steps (Scheme 10). Alkyne addition to aldehyde 71 was performed under Pu's conditions43 to provide the propargyl alcohol in 66% yield and 92% ee. Other asymmetric alkyne transfer conditions were tried but provided inferior results, in agreement with the findings of Marshall.44 O-Methylation of the secondary alcohol under phase-transfer conditions proceeded with concomitant removal of the TMS group to provide the terminal alkyne 69 in 95% yield. Cleavage of the silyl ether followed by oxidation of the resulting primary alcohol under conditions developed by Merck45 afforded carboxylic acid 72 in excellent yield. Hydroxy amide formation followed by DAST-mediated cyclodehydration and subsequent BrCCl3/DBU oxidation30 yielded a mixture of bromoalkyne 73 and terminal alkyne 74. Both compounds were converted to the (E)-vinyl iodide 58 through a palladium-catalyzed hydrostannylation/iododestannylation sequence, with the bromoalkyne showing greater selectivity. Accordingly, terminal alkyne 74 was further converted to the bromoalkyne 73 in the presence of NBS and AgNO3 in acetone in a moderate 54% yield. Finally, hydrolysis of the methyl ester completed the synthesis of oxazole fragment 75.
Scheme 10.
Wipf's oxazole segment synthesis. Reagents and conditions: (a) TIPSCl, imidazole, DMF; (b) DIBAL-H, CH2Cl2, −10 °C, 78% (over 2 steps); (c) TMS-acetylene, Et2Zn, toluene, Δ, then (S)-BINOL, Ti(Oi-Pr)4, 71, rt, 66%; (d) dimethyl sulphate, n-Bu4NHSO4, NaOH, toluene/H2O, 0 °C to rt, 95%; (e) HF, CH3CN, then NaOCl, NaClO2, TEMPO, CH3CN, phosphate buffer (pH 6.7), 45 °C, 99%; (f) SerOMe·HCl, EDC, HOBt, NMM, CH2Cl2, 0 °C to rt, 55%; (g) DAST, CH2Cl2, then K2CO3, −78 °C to rt; (h) DBU, BrCCl3, CH2Cl2, 0 °C to 4 °C; (i) NBS, AgNO3, acetone, 54%; (j) n-Bu3SnH, PdCl2(PPh3), THF, −78 °C to rt, then I2, 0 °C, 92%; (k) 1N LiOH, H2O/THF, 97%.
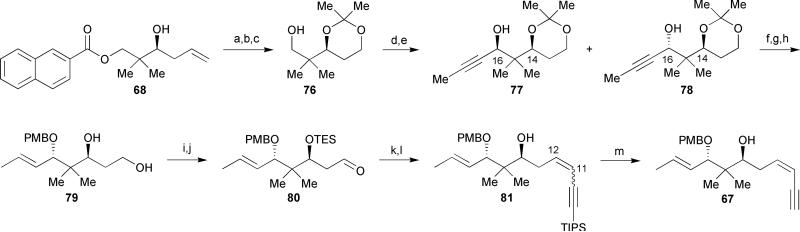
The key enyne 67 was obtained from the readily available homoallylic alcohol 6842 in 13 overall steps (Scheme 11). Ozonolysis of alkene 68 followed by reductive work-up with NaBH4 afforded the corresponding alcohol. 1,3-Diol protection followed by hydrolysis of the naphthyl ester provided acetonide 76. Swern oxidation of the primary alcohol proceeded smoothly, but, unfortunately the subsequent alkyne addition using 1-lithiopropyne proved problematic, resulting in formation of a 1.1: 1.0 (syn: anti) ratio of separable diastereomers at C-16. Hoffmann also reported difficulties in the selective formation of the neopentyl anti-1,3-diol.36,41,46 Attempts at improving the diastereoselectivity for the generation of this diol segment have thus far proved unsuccessful. Nevertheless, the anti-1,3-diol 78 was carried forward to provide diol 79 via alkyne reduction using Red-Al in degassed THF followed by PMB protection of the resulting allylic alcohol and subsequent acetonide cleavage. Protection of the free hydroxyl groups with TESOTf and selective Swern oxidation of the primary silyl ether gave aldehyde 80 in 75% yield over two steps. With aldehyde 80 in hand, Wipf utilized a Peterson olefination in the presence of Corey's lithiated 1,3-bis(triisopropylsilylpropyne)47 to install the (Z)-alkene at C-11/C-12 to afford, upon selective cleavage of the TES ether, enyne 81 as a separable 8: 1 (Z/E) mixture in 62% overall yield over two steps. Completion of enyne fragment 67 was successfully accomplished in 94% yield via a TIPS-deprotection of enyne 81.
Scheme 11.
Wipf's enyne segment synthesis. Reagents and conditions: (a) O3/O2, Sudan III, MeOH/CH2Cl2, −78 °C, then NaBH4, −78 °C to rt, 88%; (b) 2,2-dimethoxypropane, PPTS, THF, 0 °C to rt, 97%; (c) 1N LiOH, THF/MeOH, 0 °C to rt, 82%; (d) oxalyl chloride, DMSO, Et3N, −78 °C; (e) propyne, n-BuLi, THF, −78 °C to 0 °C; (f) Red-Al, THF (degassed), Δ, 83%; (g) PMBBr, Et3N, KHMDS, THF, −78 °C to rt; (h) AcOH/THF/H2O (4:1:1), 60 °C, 84% (over 2 steps); (i) TESOTf, 2,6-lutidine, CH2Cl2, 0 °C; (j) oxalyl chloride, DMSO, Et3N, CH2Cl2, −78 °C, 75% (over 2 steps); (k) 1,3-bis(TIPS) propyne, n-BuLi, THF, −78 °C; (l) chloroacetic acid, MeOH/CH2Cl2; (m) TBAF, THF, 0 °C to rt, 94%.
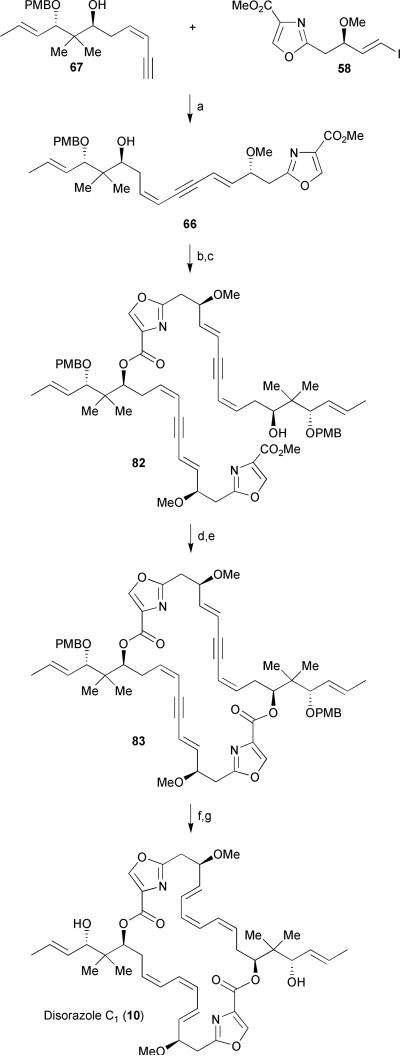
The two coupling components, oxazole 58 and enyne 67, were subjected to Sonogashira cross-coupling conditions to afford dienyne 66 in an excellent 94% yield (Scheme 12). A stepwise formation of (bis)dienyne 82 was achieved by DCC-mediated coupling of dienyne 66 and carboxylic acid 75 followed by another Sonogashira coupling of the resulting vinyl iodide to enyne 67. Selective hydrolysis of the methyl ester in the presence of the internal ester linkage using LiOH followed by Yamaguchi macrolactonization led to the advanced intermediate tetradehydrodisorazole C1 83. A direct dimerization of the carboxylic acid derived from hydrolysis of dienyne 66 under high-dilution (0.6 mM) Yamaguchi esterification/macrolactonization conditions to afford bisdienyne 83 in 59% yield was also reported by Wipf.26 After extensive optimizations, oxidative cleavage of both PMB ethers of 83 in the presence of DDQ under buffered conditions was found to generate the free bisallylic alcohol in a satisfactory 61% yield. Finally, unmasking of the labile triene unit was realized through a carefully monitored partial reduction of bisalkyne 83 in the presence of Lindlar's catalyst and excess quinoline to complete the first total synthesis of (−)-disorazole in 20 linear steps from known alcohol 68 and in 1.5% overall yield. Synthetic (−)-disorazole C1 was compared to the analytical data reported for the original sample1 and was found to be identical in all regards (1H NMR, 13C NMR, HRMS, [α]D). As of late 2008, Wipf's synthesis of (−)-disorazole C1 remains the only report of a completed synthesis of a member of the disorazole family, fourteen years after their initial discovery.
Scheme 12.
Wipf's end game strategy for disorazole C1. Reagents and conditions: (a) PdCl2(PPh3), CuI, Et3N, CH3CN, −20 °C to rt, 94%; (b) 75, DCC, DMAP, CH2Cl2, 0 °C to rt, 80%; (c) 67, PdCl2(PPh3)2, CuI, Et3N, CH3CN, −20 °C to rt, 94%; (d) 1N LiOH, H2O/THF, 98%; (e) 2,4,6-trichlorobenzoyl chloride, Et3N, THF, then DMAP, toluene, 79%; (f) DDQ, phosphate buffer (pH 6.7), CH2Cl2, 61%; (g) H2, Lindlar's catalyst, quinoline, EtOAc, 57%.
4.4 Hoffmann's synthesis of a masked northern hemisphere of disorazoles A1 and D1
Disorazole A1 constitutes the largest component of the original 29 macrodiolides isolated from the fermentation broth of Sorangium cellulosum.1 Although disorazole A1 was found to be too cytotoxic for direct application in anticancer therapy (IC50 = 3 pg/mL, cell line L929, mouse fibroblasts, vide supra),12 the mode of action of the disorazoles as well as their structure–activity relationships (SARs) remain an interesting topic for chemists and biologists. The structure of disorazole A1 is particularly challenging among members of the disorazole family due to the presence of the electrophilic vinyl-dienyl epoxide at C-9/C-10. The synthetic approach of Hoffmann and co-workers involved an esterification/lactonization of southern hemisphere 41 and northern hemisphere 84 (Fig. 9).40 The synthesis of dienyne fragment 41 was described earlier by Hoffmann (Scheme 6, vide supra).36,38 Vinyl epoxide 84 was derived from phosphonium salt 86 and oxazole 85 via a Wittig olefination. The labile (Z)-alkenes at C-9′/C-10′ and C-5/C-6 were again masked as alkynes.
Fig. 9.
Hoffmann's retrosynthetic analysis of disorazole A1 (1).
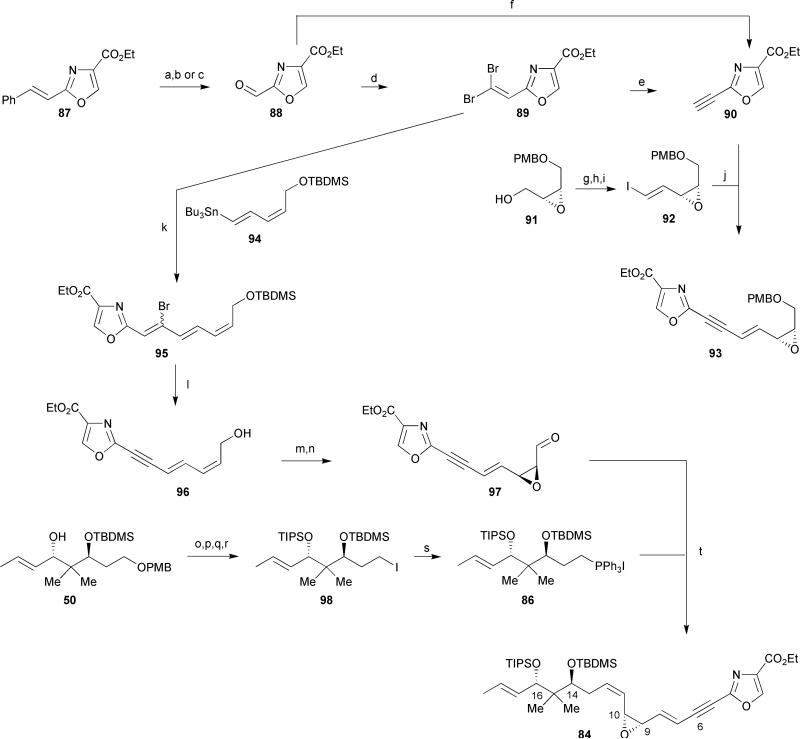
Hoffmann's synthesis of northern fragment 84 commenced with a Sharpless dihydroxylation of the known oxazole 87,48 followed by oxidative cleavage of the resulting glycol in the presence of Pb(OAc)4 to provide aldehyde 88 in 87% overall yield (Scheme 13). Alternatively, aldehyde 88 was obtained in 68% yield by a one step protocol using NaIO4 on silica gel in the presence of catalytic RuCl3 hydrate. Corey-Fuchs homologation of aldehyde 88 provided alkyne 90 in a disappointing 21% yield. Through brief optimizations, Hoffmann et al. found that treatment of aldehyde 88 with Ohira–Bestmann reagent afforded the desired alkyne 90 in a moderately improved yield of 50%. Model studies under a variety of reaction conditions (e.g. variations of catalyst, base, solvent, concentration, order of addition, and temperature) revealed that a direct Sonogashira coupling of alkyne 90 to vinyl epoxide test substrate 92 yielded at best 15% of enyne 93. The authors realized that the sensitive epoxide at C-9/C-10 required a late stage instalment, thereby suggesting a slightly revised synthetic strategy.
Scheme 13.
Hoffmann's synthesis of a masked northern half of disorazole A1. Reagents and conditions: (a) K3[Fe(CN)6], K2CO3, OsO4, (DHQD)2PHAL, t-BuOH/H2O, 96%; (b) Pb(OAc)4, K2CO3, benzene, 0 °C, 80%; (c) NaIO4, silica gel, RuCl3·xH2O, CH2Cl2, 68%; (d) CBr4, PPh3, CH2Cl2, 70%; (e) n-BuLi, THF, −78 °C, 30%; (f) Ohira–Bestmann reagent 61 (Scheme 7, vide supra), K2CO3, EtOH, 0 °C to rt, 50%; (g) SO3·Pyr, Et3N, CH2Cl2/DMSO, 0 °C to rt, 95%; (h) PPh3, CHI3, t-BuOK, THF, 75%; (i) MeLi, THF, −100 °C, 75%; (j) PdCl2(PPh3), CuI, Et3N, DMF, 15%; (k) 94, tris(2-furyl)phosphine, Pd2dba3, toluene, 100 °C, 86%; (l) TBAF, THF, 0 °C, 87%; (m) D-(−)-diethyl tartrate, Ti(Oi-Pr)4, t-BuOOH, 4Å MS, CH2Cl2, −18 °C, 84%; (n) PhI(OAc)2, TEMPO, CH2Cl2, 81%; (o) TIPSOTf, 2,6-lutidine, CH2Cl2, 99%; (p) DDQ, CH2Cl2/H2O, 0 °C, 99%; (q) MsCl, Et3N, DMAP, THF, 0 °C, 99%; (r) NaI, NaHCO3, acetone, Δ, 91%; (s) PPh3, Hünig's base, sealed flask, 85 °C; (t) 86, LiHMDS, THF, −78 °C to rt, then 97, HMPA, −78 °C to rt, 43% (from 98).
As a modified approach, Sonogashira cross-coupling of dibromide 89 to readily available vinyl stannane 9440 provided bromotriene 95 in 86% yield. A single step debromination and silyl ether cleavage was accomplished in the presence of excess TBAF to yield allylic alcohol 96 in 87%. Sharpless asymmetric epoxidation of dienyne 96 followed by oxidation of the primary alcohol afforded the desired vinyl epoxy aldehyde 97 in 86% enantiomeric excess. Wittig olefination of aldehyde 97 in the presence of phosphonium salt 86, prepared in a straightforward manner from the previously synthesized allylic alcohol 50 in 5 steps (Scheme 13),36 completed the synthesis of the key fragment 84. As a side note, the TIPS ester at C-16 of alkyliodide 98 was utilized in place of the corresponding MOM and SEM ethers, as described previously in Hoffmann's approach towards disorazole C1 (vide supra), to circumvent stability issues encountered during the halogenation step providing iodide 98.
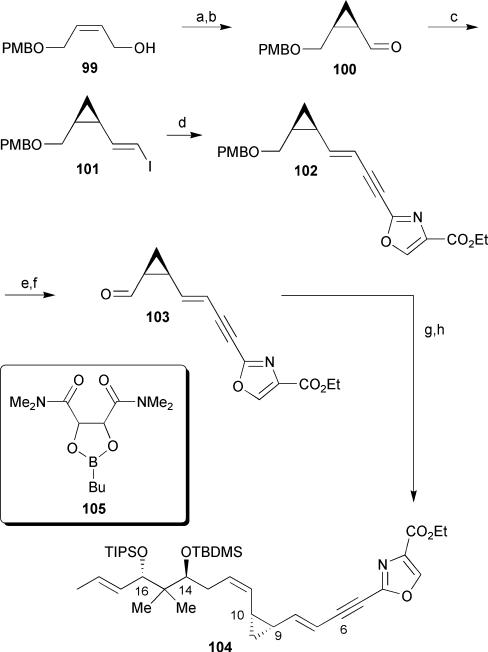
In addition to the completion of the masked northern hemisphere 84 of disorazole A1, Hoffmann successfully synthesized a more stable analog by replacing the vinyl epoxide at C-9/C-10 of disorazole A1 with a cyclopropane unit (Scheme 14).41,49 Asymmetric Charette cyclopropanation of mono-PMB protected cis-butene diol 99 followed by Dess–Martin oxidation afforded aldehyde 100. Subsequent Takai olefination of aldehyde 100 provided vinyl iodide 101 in a moderate 49% yield. Sonogashira cross-coupling of the vinyl iodide with previously prepared alkyne 90 followed by DDQ cleavage of the PMB ether and subsequent Dess–Martin oxidation of the resulting primary alcohol yielded the advanced oxazole 103. Finally, completion of the masked cyclopropane analog was achieved by a Wittig olefination between aldehyde 103 and the previously prepared alkyl iodide 98 to provide 104 in a 40% yield and ≥5: 1 Z/E selectivity. Unfortunately, no biological data on this fragment or any corresponding macrocyclic C-9/C-10 cyclopropane-containing analogs are available.
Scheme 14.
Hoffmann's synthesis of a masked cyclopropane analog of the northern hemisphere of disorazole A1. Reagents and conditions: (a) Et2Zn, CH2I2, dioxaborolane 105, CH2Cl2, 0 °C, 81%; (b) Dess–Martin periodinane, NaHCO3, CH2Cl2, 88%; (c) CrCl2, CHI3, THF, 0 °C, 49%; (d) 90, PdCl2(PPh3)2, CuI, Et3N, DMF, 49%; (e) DDQ, CH2Cl2/H2O; (f) Dess–Martin periodinane, NaHCO3, 72% (over 2 steps); (g) 98, PPh3, Hünig's base, 90 °C, then 103, LiHMDS, HMPA, THF, −78 °C to rt, 40%.
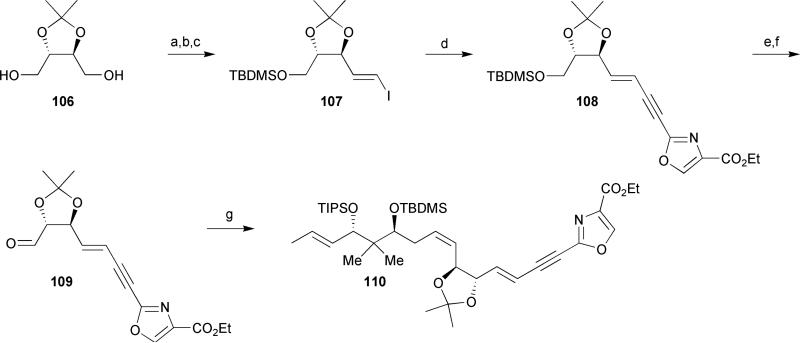
As an extension of Hoffmann's approach towards the synthesis of a more stable analog of disorazole A1, the vinyl epoxide at C9/C10 was substituted with an acetonide protected 1,2-diol unit, effectively providing a masked northern fragment of disorazole D1 (6) (Scheme 15).41 Although the absolute stereochemistry of the 1,2-diol segment of disorazole D1 has yet to be unambiguously assigned, Hoffmann et al. began their synthesis with the C2-symmetric diol 106 derived from tartaric acid utilizing a protocol reported by Seebach and Hunger-bühler.50 Mono-protection of diol 106 followed by Parikh–Doering oxidation and Takai olefination provided vinyl iodide 107. Sonogashira cross-coupling with the previously reported alkyne 90 (Scheme 13, vide supra) afforded enyne 108 in 86% yield. Completion of the masked analog 110 was achieved by an initial silyl ether cleavage followed by Dess–Martin oxidation and subsequent Wittig olefination of the resulting aldehyde with phosphonium iodide 86 (Z/E = 10: 1).
Scheme 15.
Hoffmann's synthesis of a masked northern half of disorazole D1. Reagents and conditions: (a) NaH, TBDMSCl, THF, 85%; (b) SO3·Pyr, Et3N, DMSO/CH2Cl2, 0 °C, 96%; (c) CrCl2, CHI3, THF, 72%; (d) 90, PdCl2(PPh3)2, CuI, Et3N, DMF, 86%; (e) TBAF, THF, 0 °C, 99%; (f) Dess–Martin periodinane, pyridine, CH2Cl2, 0 °C, 75%; (g) 86, LiHMDS, HMPA, THF, −78 °C to rt, 32%.
5 Conclusions
The disorazoles have proven themselves as structurally intriguing, synthetically challenging natural products with impressive in vitro anti-cancer activities. The exact binding site of the disorazoles remains uncertain although several biological studies suggest that they bind at or near the vinca domain. The identification of the biosynthetic gene cluster of the disorazoles has opened the possibility for genetic engineering to provide substantial quantities of these metabolites in an expedient manner. Both their complex architecture and labile functionalities present organic chemists with formidable tasks, and only a single total synthesis has been completed in the past fourteen years. Although industries have shifted their focus to small organic molecules of non-natural origin as a primary source for therapeutic drugs, it is without a doubt that the vast amount of information available in the field of natural products will continue to provide an intellectual playground for biologists and synthetic chemists alike. It is eminently feasible that a disorazole or a synthetic compound inspired by the disorazoles will emerge as a therapeutic agent in the not too distant future.51
6 Acknowledgments
The authors thank the National Cancer Institute for grant support (CA78039). We also gratefully acknowledge the contributions and stimulating discussions of our collaborators Thomas Graham, Andreas Vogt, Rachel Sikorski, Alexander Ducruet, Fenfeng Yu, Marni Brisson, Carolyn Kitchens, Bethany Petrik, Brianne Raccor, Raghavan Balachandran, Jane Stout, Claire Walczak, Celeste Reese, William Saunders, Billy Day, and, especially, John Lazo.
Biography
 Chad Hopkins obtained his B.Sc. (2001) in Chemistry from Mississippi State University. He then moved to the University of Kansas to pursue his Ph.D. under the supervision of Professor Helena Malinakova, where his research was focused on the development of novel palladium-catalyzed multi-component coupling and cascade processes for the synthesis of homoallylic alcohols, amines, δ-lactones, and unnatural amino acids. After completion of his Ph.D. in 2007 he joined the group of Professor Peter Wipf at the University of Pittsburgh as a postdoctoral research associate and is currently working on the synthesis of analogs of the natural product disorazole C1.
Chad Hopkins obtained his B.Sc. (2001) in Chemistry from Mississippi State University. He then moved to the University of Kansas to pursue his Ph.D. under the supervision of Professor Helena Malinakova, where his research was focused on the development of novel palladium-catalyzed multi-component coupling and cascade processes for the synthesis of homoallylic alcohols, amines, δ-lactones, and unnatural amino acids. After completion of his Ph.D. in 2007 he joined the group of Professor Peter Wipf at the University of Pittsburgh as a postdoctoral research associate and is currently working on the synthesis of analogs of the natural product disorazole C1.
 Peter Wipf received his Dipl. Chem. and Ph.D. in Chemistry in 1984 and 1987, respectively, from the University of Zürich under the direction of Prof. Heinz Heimgartner. From 1988−1990, he was a Swiss National Science Postdoctoral Fellow in the laboratory of Prof. Robert E. Ireland at the University of Virginia. In 1990, he joined the faculty at the University of Pittsburgh, and since 2004 he has been a Distinguished University Professor of Chemistry. At the center of his research program is natural product synthesis, the study of chemical reactivity and the use of synthesis to augment the chemical toolbox and develop new therapeutic strategies.
Peter Wipf received his Dipl. Chem. and Ph.D. in Chemistry in 1984 and 1987, respectively, from the University of Zürich under the direction of Prof. Heinz Heimgartner. From 1988−1990, he was a Swiss National Science Postdoctoral Fellow in the laboratory of Prof. Robert E. Ireland at the University of Virginia. In 1990, he joined the faculty at the University of Pittsburgh, and since 2004 he has been a Distinguished University Professor of Chemistry. At the center of his research program is natural product synthesis, the study of chemical reactivity and the use of synthesis to augment the chemical toolbox and develop new therapeutic strategies.
7 References
- 1.Jansen R, Irschik H, Reichenbach H, Wray V, Höfle G. Liebigs Ann. Chem. 1994:759–773. [Google Scholar]
- 2.Gerth K, Pradella S, Perlova O, Beyer S, Müller R. J. Biotechnol. 2003;106:233–253. doi: 10.1016/j.jbiotec.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Pronzato P. Drugs. 2008;68:139–146. doi: 10.2165/00003495-200868020-00001. [DOI] [PubMed] [Google Scholar]; Trivedi M, Budihardjo I, Loureiro K, Reid TR, Ma JD. Future Oncol. 2008;4:483–500. doi: 10.2217/14796694.4.4.483. [DOI] [PubMed] [Google Scholar]; Reichenbach H, Höfle G. Drugs in R&D. 2008;9:1–10. doi: 10.2165/00126839-200809010-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Altaha R, Fojo T, Reed E, Abraham J. Curr. Pharm. Des. 2002;8:1707–1712. doi: 10.2174/1381612023394043. [DOI] [PubMed] [Google Scholar]
- 4.Jansen R, Washausen P, Kunze B, Reichenbach H, Höfle G. Eur. J. Org. Chem. 1999:1085–1089. [Google Scholar]; Jansen R, Kunze B, Reichenbach H, Höfle G. Liebigs Ann. 1996:285–290. [Google Scholar]; Kunze B, Jansen R, Sasse F, Höfle G, Reichenbach H. J. Antibiot. 1995;48:1262–1266. doi: 10.7164/antibiotics.48.1262. [DOI] [PubMed] [Google Scholar]
- 5.Jansen R, Kunze B, Reichenbach H, Höfle G. Eur. J. Org. Chem. 2000:913–919. [Google Scholar]; Kunze B, Jansen R, Sasse F, Höfle G, Reichenbach H. J. Antibiot. 1998;51:1075–1080. doi: 10.7164/antibiotics.51.1075. [DOI] [PubMed] [Google Scholar]
- 6.Sasse F, Steinmetz H, Höfle G, Reichenbach H. J. Antibiot. 1995;48:21–25. doi: 10.7164/antibiotics.48.21. [DOI] [PubMed] [Google Scholar]
- 7.Horstmann N, Menche D. Chem. Commun. 2008:5173–5175. doi: 10.1039/b810405k. [DOI] [PubMed] [Google Scholar]; Sasse F, Steinmetz H, Höfle G, Reichenbach H. J. Antibiot. 1993;46:741–748. doi: 10.7164/antibiotics.46.741. [DOI] [PubMed] [Google Scholar]
- 8.Sasse F, Steinmetz H, Heil J, Höfle G, Reichenbach H. J. Antibiot. 2000;53:879–885. doi: 10.7164/antibiotics.53.879. [DOI] [PubMed] [Google Scholar]
- 9.Reichenbach H. J. Ind. Microbiol. Biotech. 2001;27:149–156. doi: 10.1038/sj.jim.7000025. [DOI] [PubMed] [Google Scholar]
- 10.Kopp M, Irschik H, Pradella S, Müller R. ChemBioChem. 2005;6:1277–1286. doi: 10.1002/cbic.200400459. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho R, Reid R, Viswanathan N, Gramajo H, Julien B. Gene. 2005;359:91–98. doi: 10.1016/j.gene.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Irschik H, Jansen R, Gerth K, Höfle G, Reichenbach H. J. Antibiot. 1995;48:31–35. doi: 10.7164/antibiotics.48.31. [DOI] [PubMed] [Google Scholar]
- 13.Hearn BR, Arslanian RL, Fu H, Liu F, Gramajo H, Myles DC. J. Nat. Prod. 2006;69:148–150. doi: 10.1021/np050367n. [DOI] [PubMed] [Google Scholar]
- 14.Elnakady YA, Sasse F, Lünsdorf H, Reichenbach H. Biochem. Pharmacol. 2004;67:927–935. doi: 10.1016/j.bcp.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 15.For a review on the function of tubulin and role of antitubulin drugs, see: Pellegrini F, Budman DR. Cancer Invest. 2005;23:264–273. doi: 10.1081/cnv-200055970.
- 16.Cruz-Monserrate Z, Mullaney JT, Harran PG, Pettit GR, Hamel E. Eur. J. Biochem. 2003;270:3822–3828. doi: 10.1046/j.1432-1033.2003.03776.x. [DOI] [PubMed] [Google Scholar]
- 17.Khalil MW, Sasse F, Lünsdorf H, Elnakady YA, Reichenbach H. ChemBioChem. 2006;7:678–683. doi: 10.1002/cbic.200500421. [DOI] [PubMed] [Google Scholar]
- 18.Wipf P, Reeves JT, Day BW. Curr. Pharm. Des. 2004;10:1417–1437. doi: 10.2174/1381612043384853. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 19.Schiff PB, Fant J, Horowitz SB. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 20.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 21.ter Haar E, Kowalski RJ, Hamel E, Lin CM, Longley RE, Gunasekera SP, Rosenkranz HS, Day BW. Biochemistry. 1996;35:243–250. doi: 10.1021/bi9515127. [DOI] [PubMed] [Google Scholar]
- 22.Madiraju C, Edler MC, Hamel E, Raccor BS, Van Balachandran R, Zhu G, Guiliano KA, Vogt A, Shin Y, Fournier J-H, Fukui Y, Brueckner AM, Curran DP, Day BW. Biochemistry. 2005;44:15053–15063. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]
- 23.Mooberry SL, Tien G, Hernandez AH, Plubrukarn A, Davidson BS. Cancer Res. 1999;59:653–660. [PubMed] [Google Scholar]
- 24.Gallagher BM., Jr. Curr. Med. Chem. 2007;14:2959–2967. doi: 10.2174/092986707782794014. [DOI] [PubMed] [Google Scholar]; Altmann K-H, Gertsch J. Nat. Prod. Rep. 2007;24:327–357. doi: 10.1039/b515619j. [DOI] [PubMed] [Google Scholar]
- 25.Ross DD. Leukemia. 2000;14:467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]; Hsia T-C, Lin C-C, Wang J-J, Ho S-T, Kao A. Lung. 2002;180:173–179. doi: 10.1007/s004080000091. [DOI] [PubMed] [Google Scholar]; Lehne G. Curr. Drug Targets. 2000;1:85–99. doi: 10.2174/1389450003349443. [DOI] [PubMed] [Google Scholar]; Dumontet C, Sikic BI. J. Clin. Oncol. 1999;17:1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]; Gottesman MM, Fojo T, Bates SE. Nat. Rev. Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 26.Wipf P, Graham TH, Xiao J. Pure Appl. Chem. 2007;79:753–761. [Google Scholar]
- 27.Wipf P, Graham TH, Vogt A, Silkorski RP, Ducruet AP, Lazo JS. Chem. Biol. Drug Des. 2006;67:66–73. doi: 10.1111/j.1747-0285.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 28.Tierno MB, Kitchens CA, Petrik B, Graham TH, Wipf P, Xu F, Saunders W, Raccor BS, Balachandran R, Day BW, Stout JR, Walczak CE, Ducruet AP, Reese CE, Lazo JS. J. Pharmacol. Exp. Ther. 2009;328:715–722. doi: 10.1124/jpet.108.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillier MC, Park DH, Price AT, Ng R, Meyers AI. Tetrahedron Lett. 2000;41:2821–2824. [Google Scholar]
- 30.Phillips AJ, Uto Y, Wipf P, Reno MJ, Williams DR. Org. Lett. 2000;2:1165–1168. doi: 10.1021/ol005777b. [DOI] [PubMed] [Google Scholar]
- 31.Stork G, Zhao K. Tetrahedron Lett. 1989;30:2173–2174. [Google Scholar]
- 32.Kiyooka S, Kaneko Y, Komura M, Matsuo H, Nakano M. J. Org. Chem. 1991;56:2276–2278. [Google Scholar]
- 33.Hillier MC, Price AT, Meyers AI. J. Org. Chem. 2001;66:6037–6045. doi: 10.1021/jo010249e. [DOI] [PubMed] [Google Scholar]
- 34.Kira M, Iwamoto T. In: Chemistry of Organic Silicon Compounds. Rappoport. Apeloig ZY, editor. Vol. 3. John Wiley & Sons Ltd.; Chichester: 2001. pp. 853–948. [Google Scholar]; Hillier MC, Meyers AI. Tetrahedron Lett. 2001;42:5145–5147. [Google Scholar]
- 35.Höfle G. GBF Annual Report. 19992000.
- 36.Hartung IV, Niess B, Haustedt LO, Hoffmann HMR. Org. Lett. 2002;4:3239–3242. doi: 10.1021/ol026468j. [DOI] [PubMed] [Google Scholar]
- 37.Keck GE, Krishnamurthy D. Org. Synth. 1998;75:12–18. [Google Scholar]
- 38.Niess B, Hartung IV, Haustedt LO, Hoffmann HMR. Eur. J. Org. Chem. 2006:1132–1143. [Google Scholar]
- 39.Wipf P, Graham TH. J. Am. Chem. Soc. 2004;126:15346–15347. doi: 10.1021/ja0443068. [DOI] [PubMed] [Google Scholar]
- 40.Hartung IV, Eggert U, Haustedt LO, Niess B, Schäfer PM, Hoffmann HMR. Synthesis. 2003:1844–1850. [Google Scholar]
- 41.Haustedt LO, Panicker SB, Kleinert M, Hartung IV, Eggert U, Niess B, Hoffmann HMR. Tetrahedron. 2003;59:6967–6977. [Google Scholar]
- 42.Bode JW, Gauthier DR, Carreira EM. Chem. Commun. 2001:2560–2561. [Google Scholar]
- 43.Gao G, Moore D, Xie R-G, Pu L. Org. Lett. 2002;4:4143–4146. doi: 10.1021/ol026921r. [DOI] [PubMed] [Google Scholar]
- 44.Marshall JA, Bourbeau MP. Org. Lett. 2003;5:3197–3199. doi: 10.1021/ol034918h. [DOI] [PubMed] [Google Scholar]
- 45.Zhao M, Li J, Mano E, Song Z, Tschaen DM, Grabowski EJJ, Reider PJ. J. Org. Chem. 1999;64:2564–2566. [Google Scholar]
- 46.Vakalopoulos A, Smits R, Hoffmann HMR. Eur. J. Org. Chem. 2002:1538–1545. [Google Scholar]
- 47.Corey EJ, Rucker C. Tetrahedron Lett. 1982;23:719–722. [Google Scholar]
- 48.Panek JS, Beresis RT. J. Org. Chem. 1996;61:6496–6497. doi: 10.1021/jo960532r. [DOI] [PubMed] [Google Scholar]
- 49.For examples involving the substitution of an epoxide with a cyclopropane unit in the epothilone and radicicol series, see: Kuzniewski CN, Gertsch J, Wartmann M, Altmann K-H. Org. Lett. 2008;10:1183–1186. doi: 10.1021/ol800089x.;Nicolaou KC, Sasmal PK, Rassias G, Reddy MV, Altmann K-H, Wartmann M, O'Brate A, Giannakakou P. Angew. Chem., Int. Ed. 2003;42:3515–3520. doi: 10.1002/anie.200351819.;Nicolaou KC, Ritzén A, Namoto K, Buey RM, Díaz JF, Andreu JM, Wartmann M, Altmann K-H, O'Brate A, Paraskevi G. Tetrahedron. 2002;58:6413–6432.;Nicolaou KC, Namoto K, Li J, Ritzén A, Ulven T, Shoji M, Zaharevitz D, Gussio R, Sackett DL, Ward RD, Hensler A, Fojo T, Giannakakou P. ChemBioChem. 2001;2:69–75. doi: 10.1002/1439-7633(20010105)2:1<69::AID-CBIC69>3.0.CO;2-8.;Yang Z-Q, Geng X, Solit D, Pratilas CA, Rosen N, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:7881–7889. doi: 10.1021/ja0484348.;Yamamoto K, Garbaccio RM, Stachel SJ, Solit DB, Chiosis G, Rosen N, Danishefsky SJ. Angew. Chem., Int. Ed. 2003;42:1280–1284. doi: 10.1002/anie.200390329.
- 50.Hungerbühler E, Seebach D. Helv. Chim. Acta. 1981;64:687–702. [Google Scholar]
- 51.Newman DJ, Cragg GM. J. Nat. Prod. 2004;67:1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]