Abstract
A fluorescently-labeled, persulfated molecular umbrella (1) has been synthesized from cholic acid, lysine, spermine and Coumarin 343 and found capable of entering live HeLa cells. The distribution of 1 throughout the cytoplasm and the nucleus was diffuse and punctate, respectively. This finding, together with its ability to cross liposomal membranes by passive diffusion, suggests that passive diffusion plays a significant role in the ability of 1 to enter cells. The fact that 1 is concentrated at the nucleus raises the possibility that molecular umbrellas of this type could be used for the nuclear targeting of drugs.
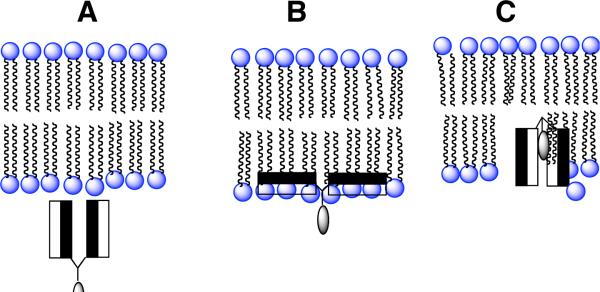
Molecular umbrellas are a unique class of conjugates that are composed of two or more facial amphiphiles that are covalently bound to a central scaffold. Such molecules have been found to possess the ability of crossing liposomal membranes by passive diffusion.1-4 Our working hypothesis has been that the amphiphilic “wings” of a molecular umbrella first bind to the surface of the liposomal membrane, that translocation proceeds through a shielded conformation in which the hydrophilic faces of the molecule point toward one another as it passes through the hydrocarbon interior, and that the umbrella is then released from the adjacent monolayer into the adjoining aqueous phase. In Scheme 1 we illustrate our current model for umbrella transport of a hydrophilic agent across a lipid bilayer. Here, the shaded and unshaded rectangles represent hydrophobic and hydrophilic faces of the facially amphiphilic units, respectively, and the lightly shaded oval represents a covalently attached, hydrophilic agent. Thus, the umbrella first approaches the membrane in a fully exposed conformation (structure A). Hydrophobic interactions with the membrane interior then leads to an adsorbed state in which the hydrophilic faces are in contact with the polar head group region and the hydrophobic faces are in intimate contact with the hydrocarbon region of the lipid bilayer (structure B). Subsquent absorption into the interior of the membrane, being driven by hydrophobic forces, then affords structure C. Translocation to the adjoining leaflet, 180° rotation and reversal of steps B and A (not shown) then releases the conjugate from the other side of the membrane. In essence, we have postulated that the molecular umbrella masks its own hydrophilicity as well as that of an attached polar agent as it crosses the hydrocarbon interior of the bilayer.
Scheme 1.
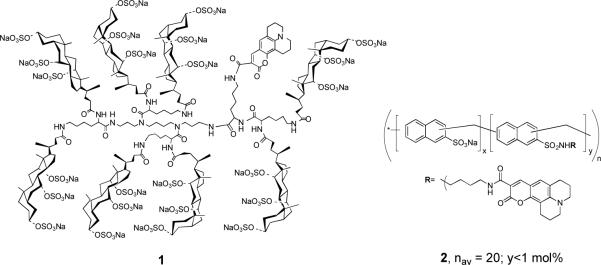
In the work that is reported herein, we sought to answer a long-standing question that bears directly on the potential of molecular umbrellas as drug carriers. Specifically, can a molecular umbrella that is capable of crossing liposomal membranes also cross cellular membranes? To address this question, we designed a fluorescently-labeled molecular umbrella (1) and investigated its interaction with live HeLa cells (Chart 1). We also compared the transport properties of 1 with a non-umbrella analog that is similar in size and charge, but which is devoid of facial amphiphilicity (i.e., 2). Previously, we have shown that a non-fluorescently-labeled form of 1 escapes the aqueous interior of liposomes leaving behind a much smaller, hydrophilic peptide (i.e., glutathione).4 This selective release established that the much larger molecular umbrella crosses lipid bilayers without damaging them.
Chart 1.
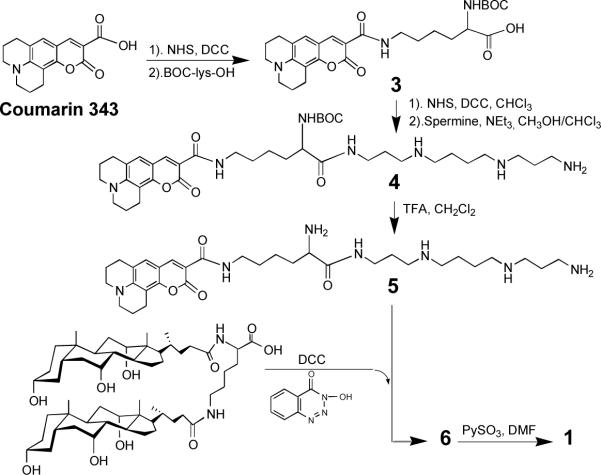
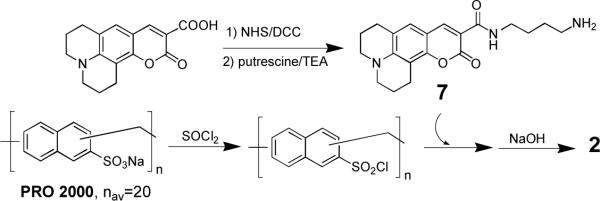
In Scheme 2 we outline the method that was used to prepare 1. Thus, activation of the carboxyl group of Coumarin 343, followed by condensation with α-t-BOC-lysine afforded 3. Subsequent formation of the corresponding N-hydroxylsuccinate ester, and acylation of one of the terminal amino groups of spermine produced 4. Finally, deprotection to give 5, total acylation with lysine dicholamide [activated with 3-hydroxy-1,2,3-benzotriazin-4(3H)-one] to give 6, and persulfation with pyridine sulfur trioxide afforded 1 (6683 Da). Scheme 3 shows the approach that was used to prepare 2 (5 ± 1 kDa). Thus, activation of PRO 2000 (a gift from Indevus Pharmaceuticals, Lexington, MA) with sulfonyl chloride, followed by condensation with less than 1 mol% of a monoconjugate made from Courmarin 343 and putrescine (i.e., 7), and subsequent hydrolysis afforded 2.
Scheme 2.
Scheme 3.
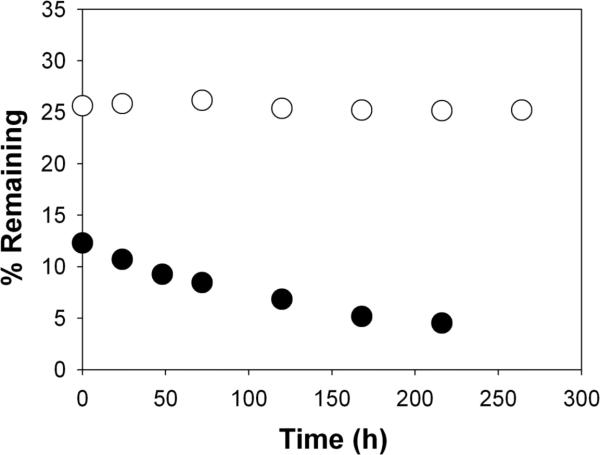
Before examining the ability of 1 and 2 to enter HeLa cells, we first compared their ability to cross liposomal membranes by passive diffusion. Using experimental methods similar to those previously described, liposomes (200 nm, extrusion) were prepared from 40 mg of a mixture of 1-palmitoyl-2-oleyol-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG) [POPC/POPG, 95/5, mol/mol] and 2 mL of phosphate buffered saline [10 mM phosphate, 150 mM NaCl, pH 7.4, PBS] that was 0.5 mM in 1 or 2.4 After dialysis for 52 h at 23°C, the percentage of liposome-entrapped 1 or 2 was monitored as a function of time (Figure 1). In contrast to 1, which exhibited a first-order release, liposome-entrapped 2 showed no detectable release.
Figure 1.
Plot of percentage of liposome-entrapped 1 (●) and 2 (○) remaining as a function of dialysis time at 23°C, after an initial dialysis of 52 h. Experimental methods used were similar to those previously described.4 Here, release of 1 was monitored by removing aliquots of the liposomal dispersion during dialysis, destroying the liposomes with an ethanolic solution of sodium dodecyl sulfate, and analyzing for residual Cascade Blue (460 nm). The half-life for 1 and 2 diffusing across the dialysis membrane was 3.5 and 7 h, respectively.
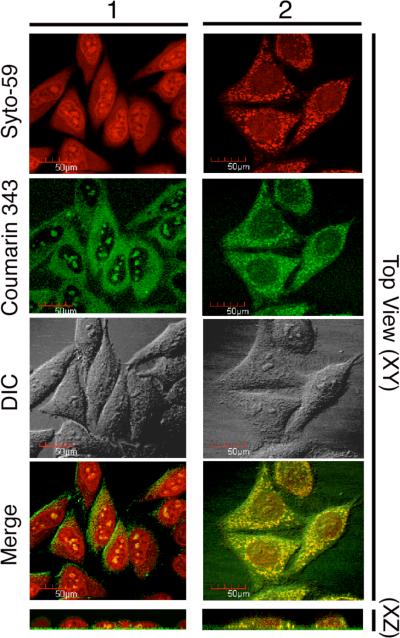
To determine whether 1 can enter live HeLa cells, confocal microscopy was used to section optically through the cellular volume to ascertain that the fluorescent label was dispersed within the cell and not bound to its outer surface. Cells were also labeled with Syto-59 to mark their nucleus and their cytoplasm. After a 90-min incubation period with 4 μM of 1, the molecular umbrella was detected inside the cells (Figure 2). Similar micrographs were observed after 10 min. That 1 had been internalized within the cells was confirmed from profile views that were obtained by computer-based rotation of the image stacks (Figure 2, bottom). Here, the red fluorescence of Syto-59 marks each cell, the fluorescence of Coumarin 343 is shown in green, and the overall cell is revealed by the differential interference contrast (DIC) micrographs. Both the Coumarin 343 and merged micrographs in Figure 2 show a distribution of 1 that is diffuse in the cytoplasm and punctate in the nucleus. The former is generally considered to be an indication of cellular entry via passive diffusion.5,6 In addition, the molecular umbrella that was found in the nucleus was bound in spots that colocalize with nucleoli within the nucleus. At present, the nature of this binding is unclear, although we believe that the high density the sulfate groups within each facial amhiphile is likely to play a key role. It should be noted that no cellular damage of any sort was ever observed in any of these experiments. Specifically, the cells appeared healthy throughout these experiments, as indicated by the fact that they did not retract and that they maintained attachments to the substrate. It is also noteworthy that incubation with 1 for 30 min showed negligible toxicity (<5% cell death) as judged by propidium iodide exclusion measurements. Related studies that have been carried out with CaSki cells, which were fixed with 4% paraformaldehyde, have also shown nuclear localization after 15 min of incubation with 1.7
Figure 2.
Confocal micrographs of HeLa cells incubated with 4 μM of 1 or 2 for 90 min. Living cells were treated with Syto-59 (red in the merged image to mark the nuclei and cytoplasm) and Coumarin 343-labeled 1 or 2 (green in merged images). Single image planes from the centers of the cells are shown. Syto-59 (red), Coumarin 343 (green) and DIC are shown in the X-Y dimension (Top View) and in the X-Z dimension (Profile, bottom). NOTE: The addition of 2 (but not 1) to HeLa cells led to a redistribution of Syto-59.
In sharp contrast to 1, which was consistently taken up by the HeLa cells in more than 20 independent experiments, the internalization of 2 was variable. In most cases no cellular uptake was observed! When cellular uptake was found, 2 entered all of the cells; that is, its uptake was either “all or none”. Another peculiar feature of 2 was that it induced a redistribution of Syto-59 within the HeLa cells. Examples of cellular uptake of 2, in those few cases where internalization was observed, are shown in Figure 2. In contrast to 1, the distribution of 2 within the cytoplasm and the nucleus was punctate and diffuse, respectively. The former implies that 2 enters HeLa cells by a fundamentally different pathway, most probably via endocytosis. Because of the poor reproducibility of cellular internalization by 2, and because it led to a peculiar redistribution of Syto-59, this non-umbrella analog was not investigated further.
The fact that 1 is capable of crossing liposomal membranes, together with its diffuse distribution within the cytoplasm, suggests that passive diffusion plays an important role in its cellular entry. The reason for the slow rate at which 1 is released from liposomes relative to the rapid internalization by cells is not presently clear. Since cell membranes are rich in proteins, one explanation for this difference would be that lipid/protein interfaces provide a pathway for the molecular umbrellas to “slip across” the membrane, possibly via some type of protein-induced “flip-flop” mechanism. Alternatively, rapid cellular entry of 1 could be related to a higher intrinsic affinity of 1 to cellular membranes. In addition, we cannot rule out the possibility that other mechanisms may be contributing to the cellular entry of 1. In preliminary studies, treatment of HeLa cells with NaN3 and 2-deoxyglucose (to deplete ATP production) only modestly inhibited the cellular uptake of 1 (see Supporting Information). Control experiments that were carried out under similar conditions with fluorescently-labeled transferrin, however, showed a very substantial inhibition of endocytotic uptake. This finding is fully consistent with passive diffusion of 1 across lipid bilayers. Although no cellular uptake of 1 was found at 4°C, which is consistent with an endocytotic pathway for cellular entry, we believe that the ATP-depletion experiment is a more reliable indicator of probable mechanism of cellular entry. Specifically, we believe that the lowering of the temperature to 4°C is a more severe perturbation of cellular conditions than ATP-depletion, since it is likely to result in a major reorganization of the two-dimensional structure of the plasma membrane.
The ability of this highly anionic molecular umbrella (1) to enter live cells is in sharp contrast to many cationic cellular delivery systems that have been reported in the literature; e.g., arginine-rich, cell-penetrating peptides.8 This anionic character may well be responsible for the ability of 1 to concentrate at the nucleus of cells. Efforts currently in progress are aimed at defining the relationships that exist between the structure and composition of molecular umbrellas and their ability to diffuse across hydrophobic barriers, enter cells, and concentrate at the nucleus of cells. The results of these studies will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
We are grateful to the National Institues of Health (PHS Grants GM51814 to SLR and GM58025 to LC) for support of this research and to Prof. Susan Fueshko Perry (Lehigh) for valuable discussions.are grateful to the National Science Foundation (CHE-0345248) for support of this research.
Footnotes
Department of Biological Sciences
Supporting Information Available: Experimental procedures. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.a Janout V, Lanier M, Regen SL. Molecular umbrellas. J. Am. Chem. Soc. 1996;118:1573–1574. [Google Scholar]; b Janout V, Lanier M, Regen SL. Design and synthesis of molecular umbrellas. J. Am. Chem. Soc. 1997;119:640–647. [Google Scholar]
- 2.Janout V, DiGiorgio C, Regen SL. Molecular umbrella-assisted transport of a hydrophilic peptide across a phospholipid membrane. J. Am. Chem. Soc. 2000;122:2671–2672. [Google Scholar]; b Janout V, Jing B, Regen SL. Molecular umbrella-assisted transport of an oligonucleotide across cholesterol-rich phospholipid bilayers. J. Am. Chem. Soc. 2005;127:15862–15870. doi: 10.1021/ja053930x. [DOI] [PubMed] [Google Scholar]
- 3.Janout V, Staina IV, Bandyopadhyay P, Regen SL. Evidence for an umbrella mechanism of bilayer transport. J. Am. Chem. Soc. 2001;123:9926–9927. doi: 10.1021/ja016265a. [DOI] [PubMed] [Google Scholar]
- 4.Jing B, Janout V, Herold BC, Klotman ME, Heald T, Regen SL. Persulfated molecular umbrellas as anti-HIV and anti-HSV agents. J. Am. Chem. Soc. 2004;126:15930–15931. doi: 10.1021/ja044400o. [DOI] [PubMed] [Google Scholar]
- 5.Farrera-Sinfreu J, Giralt E, Castel S, Albericio F, Royo M. Cell-penetrating cis-gamma-amino-L-proline-derived peptides. J. Am. Chem. Soc. 2005;127:9459–9468. doi: 10.1021/ja051648k. [DOI] [PubMed] [Google Scholar]
- 6.Fillon A, Anderson JP, Chmielewski J. Cell-penetrating agents based on a polyproline helix scaffold. J. Am. Chem. Soc. 2005;127:11798–11803. doi: 10.1021/ja052377g. [DOI] [PubMed] [Google Scholar]
- 7.Madan RP, Mesquita PMM, Cheshenko N, Jing B, Shende V, Guzman E, Heald T, Keller MJ, Regen SL, Shattock RJ, Herold BC. Molecular umbrellas: a novel class of candidate topical microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. J. of Virology. 2007;81:7636–7646. doi: 10.1128/JVI.02851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yandek LE, Pokorny A, Floren A, Knoelke K, Langel U, Almeida PF. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys. J. 2007;92:2434–2444. doi: 10.1529/biophysj.106.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.