Abstract
Serum retinol-binding protein (RBP4) is secreted by liver and adipocytes and is implicated in systemic insulin resistance in rodents and humans. RBP4 normally binds to the larger transthyretin (TTR) homotetramer, forming a protein complex that reduces renal clearance of RBP4. To determine whether alterations in RBP4-TTR binding contribute to elevated plasma RBP4 levels in insulin-resistant states, we investigated RBP4-TTR interactions in leptin-deficient ob/ob mice and high-fat-fed obese mice (HFD). Gel filtration chromatography of plasma showed that 88–94% of RBP4 is contained within the RBP4-TTR complex in ob/ob and lean mice. Coimmunoprecipitation with an RBP4 antibody brought down stoichiometrically equal amounts of TTR and RBP4, indicating that TTR was not more saturated with RBP4 in ob/ob mice than in controls. However, plasma TTR levels were elevated approximately fourfold in ob/ob mice vs. controls. RBP4 injected intravenously in lean mice cleared rapidly, whereas the t1/2 for disappearance was approximately twofold longer in ob/ob plasma. Urinary fractional excretion of RBP4 was reduced in ob/ob mice, consistent with increased retention. In HFD mice, plasma TTR levels and clearance of injected RBP4 were similar to chow-fed controls. Hepatic TTR mRNA levels were elevated approximately twofold in ob/ob but not in HFD mice. Since elevated circulating RBP4 causes insulin resistance and glucose intolerance in mice, these findings suggest that increased TTR or alterations in RBP4-TTR binding may contribute to insulin resistance by stabilizing RBP4 at higher steady-state concentrations in circulation. Lowering TTR levels or interfering with RBP4-TTR binding may enhance insulin sensitivity in obesity and type 2 diabetes.
Keywords: obesity, adipose, high-fat diet
Resistance to insulin action is a major risk factor for type 2 diabetes, cardiovascular disease, and early mortality (16, 39). Multiple factors secreted by adipocytes contribute to regulation of systemic insulin sensitivity, fuel metabolism, energy balance, cardiovascular function, and immune function (27, 41). Serum retinol-binding protein (RBP4) is secreted from liver and adipocytes. Previously, its only known function was to deliver retinol (vitamin A) to tissues (46). We recently discovered that RBP4 is elevated in serum in insulin-resistant rodents and humans (23, 59). Serum levels correlate highly with the magnitude of insulin resistance and with many other features of the “metabolic syndrome” (2, 9, 20, 23, 28, 32, 49, 50, 56, 59), a constellation of insulin resistance and cardiovascular risk factors. Experimentally elevating serum RBP4 levels in mice causes insulin resistance, whereas lowering serum RBP4 in normal mice or in mice on a high-fat diet (HFD) enhances insulin sensitivity (59). A few studies have not found a correlation between insulin resistance and serum RBP4 (6, 7, 45, 55, 57), which may be due to methodological problems (22). Recent human genetic studies link single nucleotide polymorphisms in the RBP4 gene to altered insulin secretion and insulin sensitivity (15) and type 2 diabetes (36), raising the possibility that RBP4 might be involved in the pathogenesis of diabetes in some humans. In this study, we aimed to determine whether altered clearance of RBP4 could contribute to its elevation in serum in insulin-resistant states.
Although the major site of RBP4 synthesis and secretion is the hepatocyte, other organs and tissues express RBP4, including adipose tissue (46). RBP4 mRNA and protein are upregulated in adipose tissue in some insulin-resistant states (30, 50, 59). RBP4 is a compact, globular, 21-kDa protein that is filtered freely through the renal glomerular membrane. However, RBP4 normally binds to the larger (56 kDa) transthyretin (TTR) homotetramer to form a protein complex that resists glomerular filtration and reduces renal clearance of RBP4. TTR is also the major thyroid-binding protein in mice. Interestingly, TTR-deficient mice [TTR-knockout (KO)] have normal thyroid function due to enhanced production of triiodothyronine in tissues (37) but have markedly reduced plasma retinol and RBP4 levels (~5% of wild-type levels) (17). Moreover, compounds that interfere with RBP4 binding to TTR, such as certain synthetic retinoids, profoundly reduce serum RBP4 levels (3, 18). Thus, formation of an RBP4-TTR complex in serum is critical for maintaining RBP4 levels. Conditions that increase RBP4-TTR binding affinity in serum could be key determinants of serum RBP4 levels in vivo. Interestingly, circulating TTR levels are elevated in some obese, insulin-resistant people in conjunction with increased serum RBP4 (30).
In vitro studies in the presence of saturating concentrations of RBP4 have suggested more than one binding site for RBP4 on TTR (35). However, under normal conditions in vivo, RBP4 and TTR are thought to exist as a 1:1 molar complex due to the limiting concentration of RBP4 compared with TTR (46). Studies (21) have reported a three- to fivefold excess of TTR over RBP4 in human and rodent serum. Therefore, one potential mechanism contributing to elevated serum RBP4 levels in insulin-resistant states could be altered stoichiometry of RBP4-TTR binding.
For many years, it was thought that circulating levels of retinol-RBP4 remained very constant, changing only in response to extremes in nutritional intake of vitamin A, protein, calories, and zinc or to hormonal factors, stress, or some disease states (46). Although some mechanisms responsible for maintaining and regulating RBP4 levels in the circulation have been characterized, the processes involved in maintaining abnormally elevated RBP4 levels in insulin-resistant states have not been investigated, partly because chronic elevation of RBP4 has only recently been described (23, 59). Here we investigate whether alterations in RBP4-TTR binding could contribute to the elevated serum RBP4 levels that are characteristic of obesity, type 2 diabetes, and the “metabolic syndrome.”
METHODS
Mice and diets
Female ob/ob mice and lean littermate controls (either +/+ or ob/+) were obtained from Jackson Laboratories, and female FVB mice were obtained from Charles River Laboratories. Mice with a targeted disruption of the RBP4 gene (RBP4-KO) were generously provided by Drs. Max Gottesman and William Blaner, (Columbia University, New York, NY). RBP4-KO mice were of mixed C57BL/6J × 129/Sv background. All mice were fed Formulab chow diet 5008 (4.5% of calories from fat). After an acclimation period of 1 wk, female FVB mice were randomly assigned into chow or HFD groups. HFD mice were fed a diet high in fat [55% of calories derived from corn oil (18%) and lard (37%); Harlan Teklad 93075] for 16 wk. Mice were housed four per cage in a temperature-controlled room and were maintained on a 14:10-h light-dark cycle. Mice had ad libitum access to both food and water.
Purification and analysis of recombinant RBP
cDNA encoding human RBP4 (hRBP4) or mouse RBP4 (mRBP4) lacking the NH2-terminal 17-amino acid signal sequence was expressed in E. coli and purified as described previously (59). Briefly, an isopropylthiogalactoside-inducible expression system yielded bacterial inclusion bodies containing >50% pure recombinant hRBP4. The inclusion bodies were solubilized in 5 M guanidine-hydrochloride denaturing buffer, and RBP4 was refolded in the presence of retinol and then subjected to anion exchange chromatography.
Purified hRBP4 bound retinol efficiently, based on approximately equal UV absorbance ratios at wavelengths of 280 (detecting purified hRBP4 protein) and 330 nm (detecting retinol). The quality of refolding of purified recombinant hRBP4 was further assessed by measuring its interaction with a column matrix composed of 1 mg of human transthyretin (Sigma) cross-linked to 1 ml of NHS-Sepharose (GE Healthcare). As expected, >90% of the purified hRBP4 was retained on the TTR affinity column under physiological salt and pH conditions, and the retained RBP4 was quantitatively eluted under high-pH/low-salt conditions. Endotoxin was measured by limulus amoebocyte assay (Cambrex/Biowhittaker) to be <0.01 endotoxin U/ml for both the RBP4 and vehicle control solutions after endotoxin removal, which is less than the ambient endotoxin levels of reverse-osmosis, double-deionized water (Millipore). Purified RBP4 protein was dialyzed in a buffer containing 10 mM HEPES and 100 mM NaCl, stored frozen at stock concentrations of 7–8 mg/ml, and protected from exposure to light.
Gel filtration chromatography for separation of RBP4-TTR complexes
Plasma (0.1 ml) or recombinant protein was loaded onto a Superdex 200 Tricorn 10/300 GL column connected to an ÄKTA purifier 10 system (GE Healthcare). Protein complexes were separated by passing phosphate-buffered saline at 0.5 ml/min for 90 min at room temperature. Eluting proteins were detected by absorbance at 280 nm and collected in 1.8-ml fractions for analysis by SDS-PAGE. Resolution of separation was increased by collecting smaller (0.3 ml) fractions for some analyses.
Western blotting for plasma RBP4 and TTR
Plasma was diluted 30 times in 1× SDS-PAGE sample buffer, and proteins were separated by 15% SDS-PAGE and transferred to nitrocellulose. Mouse and human RBP4 proteins were detected using an anti-human RBP4 polyclonal antibody (no. A0040; Dako). This antibody also recognizes mouse RBP4, but with approximately three times lower affinity (data not shown). TTR was detected with an anti-rat TTR polyclonal antibody generously provided by Dr. William Blaner (Columbia University). Quantification was perfomed with GeneGnome chemiluminescence imaging system and GeneTools software (Syngene, MD). One microliter of plasma was used to detect RBP4, and only 0.3 µl of plasma was used for the more abundant TTR. Gel filtration fractions (8 µl of each fraction) were analyzed similarly.
Immunoprecipitation of plasma RBP4-TTR complex
Mouse plasma was subjected to immunoprecipitation using anti-human RBP4 followed by SDS-PAGE. Western blot for RBP4 and TTR was then performed, and levels of these proteins were quantified as described in Western blotting for plasma RBP4 and TTR. Plasma from RBP4-KO mice was used to control for nonspecific background.
RBP4 pharmacokinetics
Mice were injected with recombinant hRBP4 (100 µg) via tail vein, and 20 µl of blood was collected at intervals (15 min and 1, 2, 4, and 24 h). Mice had ad libitum access to both food and water. Plasma (0.3 µl) was subjected to SDS-PAGE and Western blotting. RBP4 concentrations were quantified as described in Western blotting for plasma RPB4 and TTR complex, and two-parameter exponential curves were plotted with 1- to 24-h time points for each mouse using SigmaPlot (Systat Software). The two-parameter exponential equation CT = a × exp(− b × x) was used to calculate t1/2, where CT is the plasma concentration at time x.
Fractional excretion of RBP4
The entire volume of urine spontaneously produced over 6- or 8-h intervals from individual mice was collected at room temperature. Plasma was collected during the urine collection period. Urine RBP4 was measured using an ELISA for mouse or rat RBP4 (Adipogen). Creatinine was measured in urine and plasma using a colorimetric assay (Bio Assay Systems) according to the manufacturer’s instructions. Creatinine clearance was calculated as follows: (urine creatinine ÷ plasma creatinine) × (urine volume/min). Fractional excretion of RBP4 was calculated as follows: (urine RBP4 × plasma creatinine) ÷ (plasma RBP4 × urine creatinine).
mRNA preparation and quantitative PCR
Total RNA was extracted from liver of fed mice using Tri Reagent (Molecular Research Center). Mouse TTR mRNA was quantified using the Taqman gene expression assay Mm00443267_m1 (Applied Biosystems). 18S mRNA was also measured and used to normalize TTR mRNA values.
Measurement of serum insulin and glucose levels
Serum insulin levels were determined by enzyme-linked immunosorbent assay (Crystal Chem). Serum glucose levels were determined by glucose oxidase assay.
Statistical analyses
All data are expressed as means ± SE. Significance is set at P < 0.05.
RESULTS
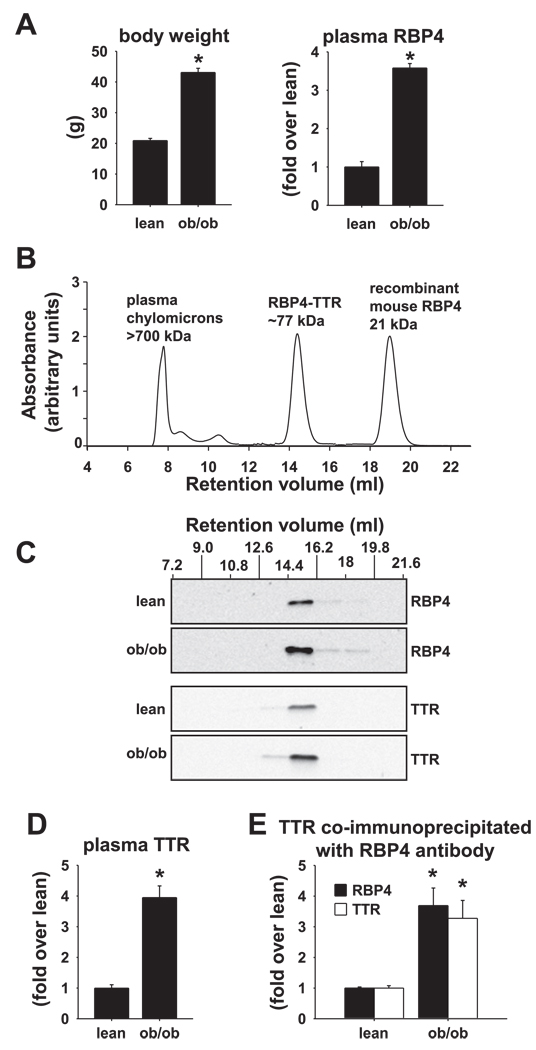
We investigated RBP4-TTR interactions in two obese, insulin-resistant models: leptin-deficient ob/ob mice, because they exhibit the highest levels of RBP4 among insulin-resistant mouse models that we have studied (59), and mice with obesity due to HFD feeding, because of the relevance to dietary obesity in humans. ob/ob mice (females, age 6 wk) exhibit approximately twofold increased body weight (Fig. 1A, left) and approximately fourfold increased serum RBP4 levels compared with their lean littermate controls (Fig. 1A, right). Plasma insulin and glucose measured in the ad libitum-fed state were elevated in ob/ob mice as expected, indicating insulin resistance and impaired glucose homeostasis (insulin: lean 1.6 ± 0.3 ng/ml, ob/ob 144 ± 18 ng/ml, P < 0.01; glucose: lean 225 ± 12 mg/dl, ob/ob 334 ± 36 mg/dl, P < 0.01). We used size exclusion (gel filtration) chromatography to analyze the RBP4-TTR complex. The gel filtration column was calibrated by determining individual retention volumes for plasma chylomicrons, purified RBP4-TTR (precomplexed), and purified RBP4 alone (Fig. 1B). Bovine serum albumin (66 kDa) was tested as a separate calibrator and found to elute just after the RBP4-TTR complex (77 kDa), consistent with its relative molecular weight (not shown).
Fig. 1.
Retinol-binding protein 4 (RBP4) is elevated in plasma of ob/ob mice and cofractionates with an equimolar amount of transthyretin (TTR) at a column volume consistent with the size of the RBP4-TTR complex. A: body weight and plasma RBP4 levels determined by Western blotting in lean and ob/ob mice in the fed state at 6 wk of age. Data are means ± SE of 4 mice/genotype. *P < 0.001. B: elution profile of chylomicrons in mouse plasma, purified and precomplexed mouse RBP4-TTR, and purified recombinant mouse RBP4 alone. Purified proteins were detected in column eluents by monitoring absorbance at 280 nm. Plasma chylomicrons were detected by monitoring retinyl ester absorbance at 330 nm. C: Western blotting of RBP4 and TTR in plasma pooled from 2 lean and 2 ob/ob mice separated by gel filtration chromatography (column eluent fractions 7.2–21.6 ml). These data are representative of 3 experiments on a total of 6 mice/genotype. D: TTR levels in plasma from lean and ob/ob mice (determined by Western blotting). The same mice were used as in A. Data are means ± SE of 4 mice/genotype. *P < 0.001. E: relative levels of RBP4 and TTR present in the RBP4-TTR complex coimmunoprecipitated from plasma of ob/ob (n = 5) or lean littermates (n = 3) using anti-RBP4 antibody and detected by Western blotting with anti-RBP4 antibody or anti-TTR antibody. *P < 0.03 vs. lean.
Lean and ob/ob plasma samples were subjected to gel filtration chromatography, and column fractions were analyzed by Western blotting (Fig. 1C). The majority of immunoreactive RBP4 and TTR eluted together as a peak at the same retention volume as the purified RBP4-TTR complex calibrator (Fig. 1, B and C). A small amount of immunoreactive RBP4 (6% of total RBP4 in ob/ob, 12% in lean) eluted after this major peak in two subsequent fractions (fractions 16.2–18 and 18–19.8 ml; Fig. 1C). These fractions represent non-TTR-bound RBP4, since there was no associated TTR immunoreactivity, and could represent unknown forms of low-molecular-weight RBP4 aggregates. Therefore, despite an approximately fourfold elevation of plasma RBP4 in ob/ob mice, very little “free” RBP4 is detected in plasma, and the majority appears to be contained in a complex consistent in molecular size with the RBP4-TTR complex.
A very small fraction of TTR has been detected as a component of plasma chylomicrons in the fasted condition or after lipid intake (48). In the present study, immunoreactive RBP4 or TTR did not coelute with chylomicrons in the plasma of ob/ob or lean control mice; plasma was obtained in the fed state, when chylomicrons would be relatively high (retention volume 7.2–9 ml; Fig. 1, B and C).
TTR plays a critical role in stabilizing RBP4 in circulation (17), and since the majority of RBP4 is associated with TTR in ob/ob mice, we sought to determine 1) whether the binding affinity and/or capacity of TTR for RBP4 is altered in ob/ob mouse plasma and 2) whether clearance of circulating RBP4 is reduced in ob/ob mice. Western blotting of ob/ob plasma revealed that TTR concentrations are elevated approximately fourfold in plasma of ob/ob vs. lean mice (Fig. 1D). To determine whether TTR, which is normally present in molar excess to RBP4, binds more than one molecule of RBP4 in plasma of ob/ob mice, we analyzed the relative stoichiometries of RBP4 and TTR in RBP4 immunoprecipitates by Western blotting. All of the RBP4 present in plasma was immunoprecipitated as determined by Western blotting of the plasma supernatant after immunoprecipitation (not shown). The stoichiometry of RBP4 and TTR coimmunoprecipitation was the same in ob/ob and lean mice (Fig. 1E), suggesting no change in the stoichiometry of RBP4 and TTR binding in ob/ob mice.
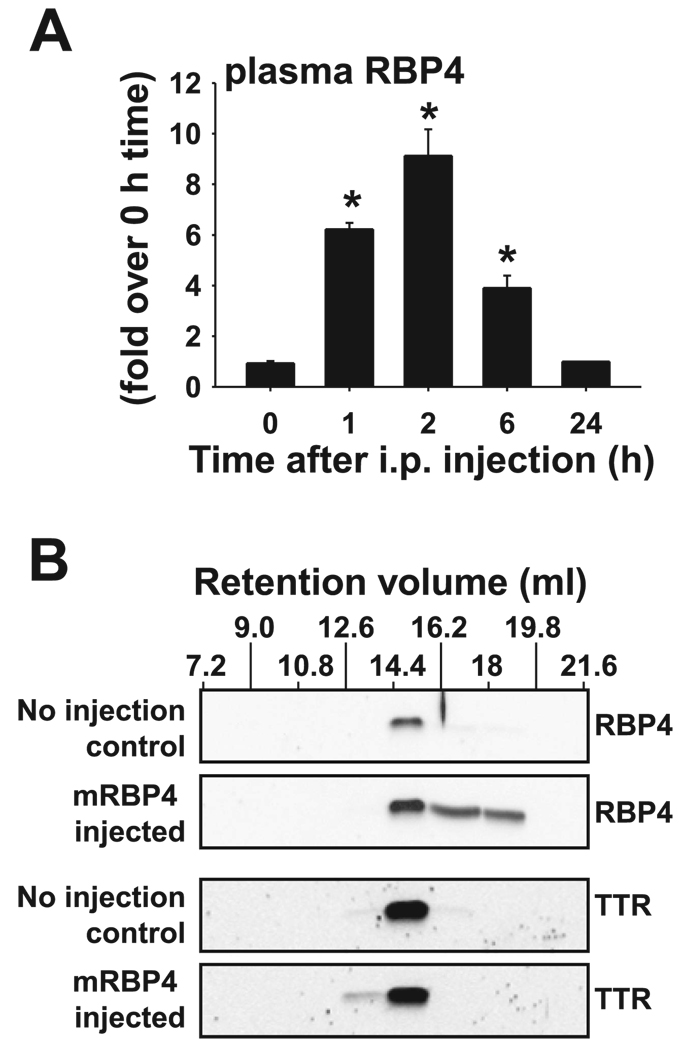
We previously reported that intraperitoneal (ip) injection of purified recombinant RBP4 causes elevation of plasma RBP4 to levels that are comparable with those observed in ob/ob mice (59) and in some insulin-resistant human subjects (23). However, the levels decline relatively rapidly after peak levels are achieved. Since purified recombinant RBP4 exhibits normal binding to TTR in vitro (59), we hypothesized that binding capacity of TTR for RBP4 may be exceeded in the setting of an acute elevation of circulating RBP4. To test this, we analyzed the RBP4-TTR complex following ip injection of purified RBP4 in normal FVB mice. Plasma RBP4 concentrations reached a peak at ~2 h after ip injection (Fig. 2A) and declined relatively rapidly to a normal baseline level within 24 h. Gel filtration chromatography of plasma obtained at the peak concentration of RBP4 (2 h after injection) revealed that 60% of RBP4 coeluted with TTR at the same retention volume (fraction 14.4 – 16.2 ml; Fig. 2B) observed for the purified RBP4-TTR complex and for the endogenous RBP4-TTR complex in ob/ob and lean mouse plasma (Fig. 1C). The remaining 40% of injected RBP4 eluted at higher retention volumes, consistent with the presence of “free” RBP4 protein or unknown forms of RBP4 aggregate, as seen in Fig. 1C. Furthermore, RBP4-TTR stoichiometry did not differ between RBP4-injected mice and noninjected control mice at the time of peak RBP4 concentration (data not shown).
Fig. 2.
Injected RBP4 binds to endogenous TTR in the circulation but is cleared within 24 h. A: normal FVB mice were injected with 400 µg of recombinant mouse RBP4 intraperitoneally (ip) in the ad libitum-fed state and killed after 0, 1, 2, 6, and 24 h. Mice were euthanized, cardiac bleeds were performed, and plasma RBP4 was measured by Western blotting; n = 5 at each time point. *P < 0.001 vs. levels at times 0 and 24 h. B: plasma from mice killed 2 h after injection with mouse RBP4 (mRBP4 injected) and from control littermates that were not injected (no injection control) was subjected to gel filtration chromatography. Fractions 7.2–21.6 ml were subjected to SDS-PAGE and Western blotting for RBP4 and TTR. These data are representative of 2 experiments on a total of 8 mice/genotype.
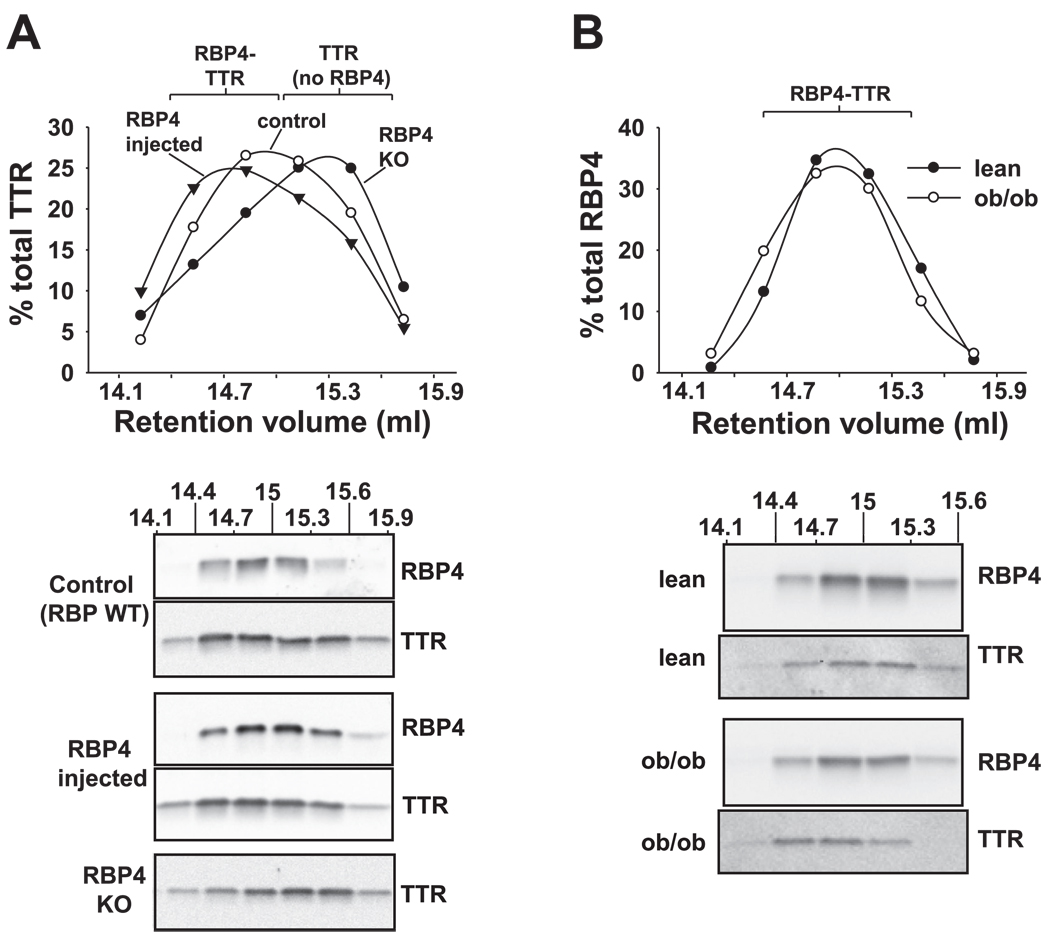
We further characterized RBP4-TTR binding by Western blotting smaller volume chromatographic fractions in the region of interest, resulting in a higher resolution analysis (Fig. 3). To test the sensitivity of the system to changes in plasma RBP4 concentrations, we first performed the analysis on plasma from RBP4-injected and RBP4-KO mice. Increased RBP4-TTR binding in serum of RBP4-injected mice and loss of RBP4-TTR binding in RBP4-KO mice could be detected as a shift in TTR size distribution to lower and higher retention volumes, respectively (Fig. 3A). RBP4 in plasma of ob/ob mice displayed a size distribution very similar to that observed in lean control mice (Fig. 3B, graph and first and third gels) and in normal wild-type control mice (Fig. 3A), suggesting no change in the stoichiometry of RBP4-TTR binding in ob/ob plasma. However, the distribution of TTR in plasma of ob/ob mice was shifted slightly toward lower retention volumes compared with lean mice, suggesting that a fourfold increase in TTR in ob/ob mice may lead to an increase in the binding of TTR to other small-molecular-weight circulating factors. Together these findings indicate that the total circulating pool of TTR in plasma provides an increased binding capacity for RBP4.
Fig. 3.
Analysis of size distribution of the RBP4-TTR complex by high-resolution gel filtration chromatography. A, top: effects of saturating RBP4 concentrations or absence of RBP4 on the size distribution (eluent fractions 14.1–15.9 ml) of TTR in plasma of normal control mice (○), plasma from normal mice in which RBP4 binding sites on TTR have been saturated by injection of purified RBP4 (▼), and plasma of RBP4-knockout (KO) mice in which no RBP4 is bound to TTR (●). A, bottom: Western blotting of column fractions (14.1–15.9 ml) from mouse plasma with antibodies to RBP4 and/or TTR, corresponding to the size distribution graph above. WT, wild type. Data are representative of 3 mice/genotype. B, top: size distribution of RBP4 in plasma of ob/ob mice (○) or lean littermate control mice (●). The graph shows quantification of bands from Western blotting fractions 14.1–15.6, which contain the RBP4-TTR complex (Fig. 1C). B, bottom: representative Western blots. The volume of ob/ob plasma fractionated was adjusted to approximate the quantity of RBP4 present in lean control plasma. Similar results were obtained in 3 mice of each genotype.
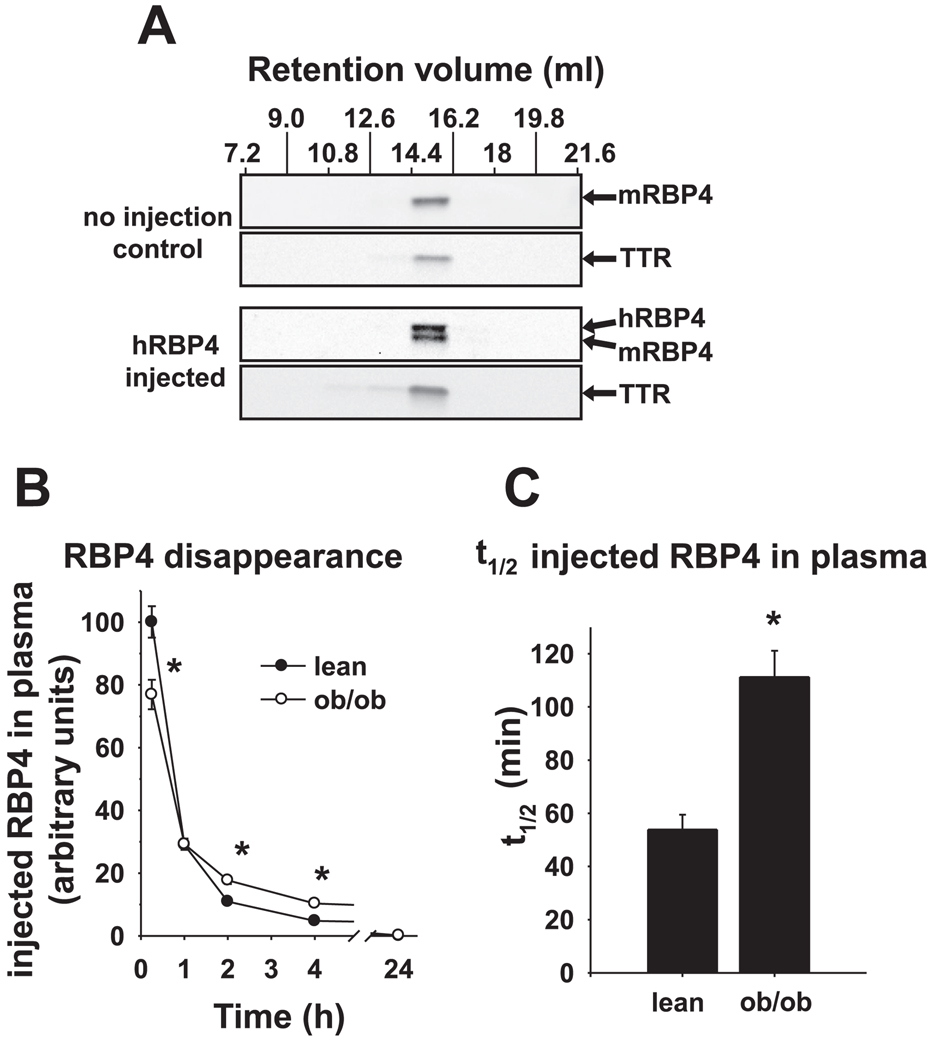
To determine whether clearance of circulating RBP4 is altered in ob/ob mice, we injected purified hRBP4 intravenously and monitored its disappearance from serum. We used hRBP4 for these studies because it can be distinguished from endogenous mouse RBP4 due to its slower mobility on SDS-PAGE, and in separate studies we found that its clearance is similar to that of injected mouse RBP4 (not shown). Moreover, hRBP4-transgenic mice exhibit an approximately threefold elevation of RBP4 in plasma, indicating that endogenous mouse TTR can interact with and stabilize hRBP4 (38). We tested the ability of injected hRBP4 to interact with endogenous mouse TTR in plasma of normal mice by performing gel filtration analysis of plasma. After injection of purified hRBP4, we found that all of the hRBP4 coeluted with endogenous mouse RBP4 and TTR in the expected fraction for the RBP4-TTR complex (Fig. 4A).
Fig. 4.
Altered pharmacokinetics of plasma RBP4 in ob/ob mice. A: plasma from a normal FVB mouse 3 h after injection with recombinant human RBP4 (hRBP4) and a control mouse that was not injected was subjected to gel filtration chromatography. Fractions 7.2–21.6 ml (low-resolution separation) were subjected to SDS-PAGE and Western blotting for RBP4 and TTR. These data are representative of 3 experiments. B: lean and ob/ob mice were injected with hRBP4 (100 µg) via tail vein, and then hRBP4 levels in plasma were measured at 15 min and 1, 2, 4, and 24 h. C: 2-parameter exponential curve fits of hRBP4 levels in plasma of individual mice at 1, 2, 4, and 24 h were used to calculate t1/2 of disappearance from plasma after intravenous injection, using a 1-compartment model. For B, and C, values are means ± SE. *P ≤ 0.02 vs. lean; n = 16/group.
In both lean and ob/ob mice, injection of hRBP4 produced elevated levels of plasma RBP4. RBP4 concentrations measured 15 min after intravenous injection were ~25% greater in lean mice than in ob/ob mice (Fig. 4B), which may reflect greater dilution of the bolus in the expanded blood volume of ob/ob mice (61). Injected hRBP4 disappeared rapidly from plasma during the first hour after injection in both lean and ob/ob mice (Fig. 4C). We used a one-compartment kinetic model to determine the rate of disappearance of hRBP4 starting at 1 h postinjection, since plasma levels were matched for both models at that time point. The t1/2 for disappearance of RBP4 from ob/ob mouse serum was more than twofold greater than in lean mice (ob/ob 111.2 ± 9.9 vs. lean control 53.9 ± 5.6 min, P < 0.001; Fig. 4C). Therefore, ob/ob mice exhibited decreased clearance of RBP4 from plasma, consistent with the observation of enhanced RBP4-binding capacity of TTR in ob/ob plasma. Further supporting this observation, we found that ob/ob mice exhibited ~70% reduced fractional excretion of RBP4 compared with lean littermates [0.040 ± 0.010 (lean) vs. 0.012 ± 0.001 (ob/ob), P < 0.05], calculated on the basis of steady-state RBP4 and creatinine concentrations in plasma and urine. The reduced RBP4 fractional excretion was not due to impaired renal function since creatinine clearance was similar in both groups.
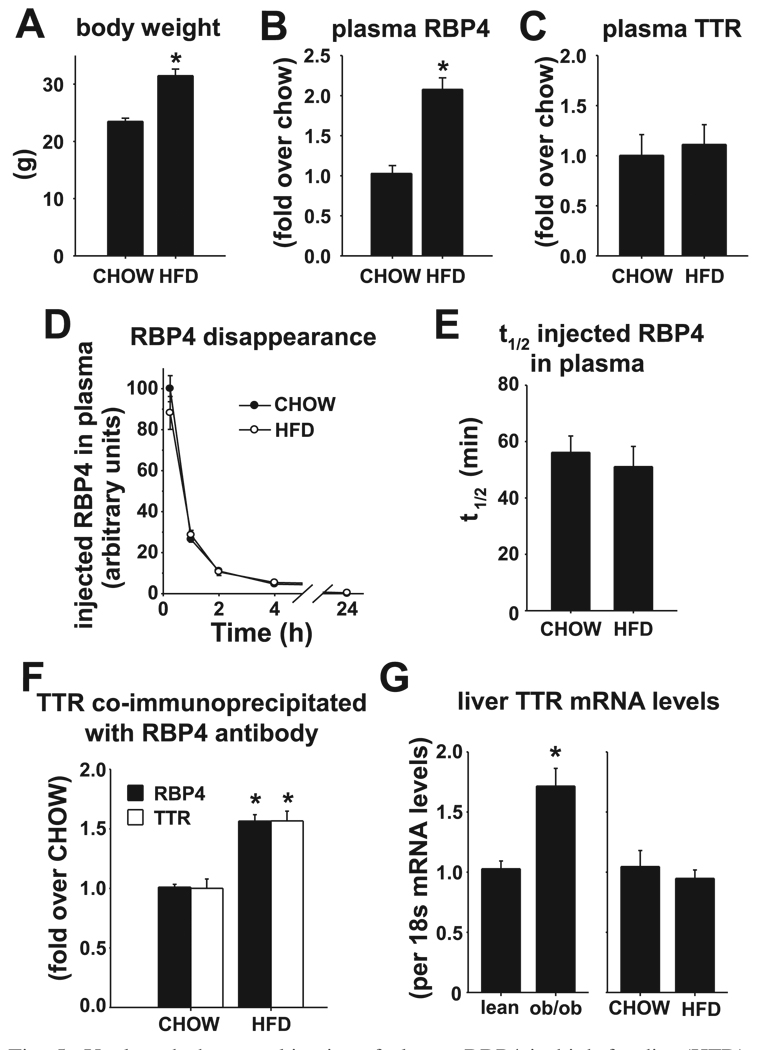
To determine whether enhanced RBP4-binding capacity of TTR and decreased RBP4 clearance are features of other insulin-resistant states, we studied FVB mice with obesity and insulin resistance due to HFD feeding (55% fat calories). Baseline RBP4 concentrations in lean chow-fed FVB control mice were similar to those in lean control littermates of ob/ob mice (not shown). HFD mice are less obese and exhibit less severe insulin resistance and glucose intolerance than ob/ob mice. Sixteen weeks of HFD feeding resulted in 34% increased body weight (Fig. 5A), threefold elevated plasma insulin (CHOW 1.0 ± 0.1 ng/ml, HFD 3.3 ± 0.9 ng/ml, P < 0.003), modestly elevated plasma glucose (CHOW 128.9 ± 6.0 mg/dl, HFD 165 ± 8.7 mg/dl, P < 0.003) in the ad libitum-fed state, and a twofold elevation of endogenous plasma RBP4 levels (Fig. 5B) relative to lean chow-fed control mice. In contrast to ob/ob mice, plasma TTR levels were not elevated in HFD-fed mice compared with lean chow-fed control mice (Fig. 5C). Similar results were obtained in HFD-induced obese mice on the C57BL/6J × 129/Sv mixed background (not shown).
Fig. 5.
Unaltered pharmacokinetics of plasma RBP4 in high-fat diet (HFD)-induced obese mice with elevated plasma RBP4 levels. A: body weight. B: plasma RBP4. C: plasma TTR as determined by Western blotting in HFD-induced obese and chow-fed (CHOW) mice after 16 wk of diet. D: CHOW and HFD mice were injected with hRBP4 (100 µg) via tail vein, and then hRBP4 levels in plasma were measured at 15 min and 1, 2, 4, and 24 h. E: 2-parameter exponential curve fits of hRBP4 levels in plasma of individual mice at 1, 2, 4, and 24 h were used to calculate t1/2 of disappearance from plasma after intravenous injection. F: relative levels of RBP4 and TTR present in the RBP4-TTR complex coimmunoprecipitated from plasma of CHOW or HFD mice using anti-RBP4 antibody and detected by Western blotting with anti-RBP4 antibody or anti-TTR antibody. G: RNA was extracted from liver of lean, ob/ob (left), CHOW, and HFD mice (right), and TTR and 18S mRNA were measured by RT-PCR. TTR mRNA is expressed relative to 18S mRNA. All values are means ± SE. For A, B, and F: *P <0.001 vs. CHOW; n = 7 CHOW and 8–9 HFD. For G: *P < 0.05 vs. lean; n = 6–8 in each group.
Thus, elevated plasma TTR concentrations do not play a role in stabilizing the twofold elevated levels of plasma RBP4 in mice that are insulin-resistant due to HFD. However, this observation does not rule out the possibility that RBP4-TTR binding affinity could be increased in mice fed HFD, causing reduced dissociation of the RBP4-TTR complex and decreased clearance of free RBP4. To test this, we measured clearance of injected hRBP4 in HFD mice using the same method employed for ob/ob mice. At 15 min after intravenous injection, hRBP4 reached the same peak concentrations in plasma of HFD- and chow-fed mice (Fig. 5D), and the t1/2 of clearance of hRBP4 was identical for both groups (56 ± 6 min for CHOW vs. 51 ± 7 min for HFD; Fig. 5, D and E) and similar to the t1/2 observed in lean mice (Fig. 4C). Furthermore, RBP4-TTR stoichiometry did not differ between mice fed chow and mice fed HFD (Fig. 5F).
To determine whether differences in plasma TTR levels in these models reflect altered expression in liver, which could result in increased secretion, we measured TTR mRNA in liver, the primary tissue source of circulating TTR. TTR mRNA was increased in liver of ob/ob mice but not in mice on HFD relative to their respective lean controls (Fig. 5G), consistent with the elevated circulating TTR levels in ob/ob but not in HFD mice. In contrast, both ob/ob and HFD-fed mice exhibit reduced RBP4 mRNA in liver relative to lean controls (data not shown). RBP4 mRNA per gram of adipose tissue is not increased in adipose tissue of ob/ob or HFD-fed mice relative to lean controls, unlike in obese humans (30, 50, 59). However, in both mouse models, RBP4 mRNA expressed per fat pad is increased due to the expanded fat mass (not shown). Therefore, increased fat mass in these models may contribute, at least in part, to elevated serum RBP4 concentrations.
Together these data indicate that there are multiple mechanisms for elevation of plasma RBP4 in insulin-resistant states. Increased levels of circulating TTR may contribute to increased plasma RBP4-binding capacity and altered RBP4 clearance in some but not all states of insulin resistance. Since elevation of circulating RBP4 causes insulin resistance and glucose intolerance in mice, these findings further suggest that increased TTR or alterations in RBP4-TTR binding may contribute to the development or worsening of insulin resistance by stabilizing RBP4 at higher steady-state concentrations in circulation.
DISCUSSION
RBP4 is elevated in many studies of insulin-resistant mice and human subjects (2, 9, 20, 23, 28, 32, 49, 50, 56, 59). Lack of elevation in a few studies may be due to methodological problems in measuring serum RBP4 levels (22). Although RBP4 is expressed primarily in liver under normal conditions, adipose tissue may be an important secondary source of RBP4 in insulin-resistant states (53, 59). RBP4 concentrations may be elevated up to fivefold above the normal range in some insulinresistant human subjects and from 4- to > 10-fold elevated in ob/ob mice (Ref. 59 and the present study). Extreme elevations in circulating RBP4 may reflect altered production and/or altered clearance of RBP4 from circulation. Here we report that impaired clearance of RBP4 from circulation may contribute to the very high concentrations of RBP4 observed in ob/ob mice but not to the more modest approximately twofold elevation observed in mice that are obese from HFD. In addition, we found increased RBP4-binding capacity in plasma that appears to be secondary to a fourfold increase in TTR concentrations observed in ob/ob mice but not in mice fed HFD. Since TTR stabilizes RBP4 in serum (17), elevated TTR concentrations may play a role in maintaining the very high RBP4 concentrations observed in ob/ob mice. The important role of TTR in determining circulating RBP4 levels is also evident in prior observations that TTR-KO mice have extremely low circulating RBP4 levels (~5% of those observed in wild-type mice) due to more rapid renal clearance of RBP4 from circulation (54). Since serum TTR levels are elevated in some insulinresistant humans (30), these findings may reveal at least one mechanism for the elevation of RBP4 in some obese and type 2 diabetic humans.
The potential importance of high TTR levels in insulin-resistant states is further highlighted by the fact that TTR and RBP4 were elevated in serum in human subjects with lipid profiles that were associated with increased cardiovascular risk (62). The authors of that study concluded that TTR, as well as RBP4, is a marker of dyslipidemia, “overnutrition,” and possibly the metabolic syndrome in humans. Whether TTR could have metabolic effects that contribute to insulin resistance independent of RBP4 is not known, although studies (34, 43) suggest TTR exerts effects on triacylglycerol synthesis and glucose transport through acylation-stimulating protein. TTR has also been shown (40) to affect insulin secretion.
Our findings suggest that delayed RBP4 clearance in some obese models such as ob/ob mice may be due, at least in part, to elevated TTR levels. However, the elevated serum RBP4 levels in HFD mice are not associated with elevated serum TTR. Since TTR circulates in a three- to fivefold molar excess over RBP4, only ~20% of serum TTR is bound to RBP4 normally. Thus, the RBP4 elevation in this model probably reflects occupancy of an increased number of TTR molecules by RBP4. The mechanism by which TTR molecules act to retain an increased number of RBP4 molecules could involve structural modifications of TTR that affect TTR-binding affinity for RBP4 and thereby influence RBP4 clearance in insulin-resistant models. The only known modulators of RBP4-TTR affinity are synthetic retinoids that cause a conformational change upon binding RBP4 and reduce the affinity of RBP4 for TTR (3, 18) and changes in the primary sequence of TTR due to genetic mutation. More than 80 variants of TTR have been identified (10, 42), many due to their role in forming or preventing amyloidogenic intermediates and amyloid fibrils in familial amyloidosis. One of these variants (Ile84Ser mutation) has a dramatically reduced affinity for RBP4 (4), whereas an individual with the compound mutation Arg104His/Val30Met was found (51) to have high serum TTR and RBP4 levels. No information regarding insulin-glucose homeostasis was reported in either case. In addition to genetic variability, several posttranslationally modified forms of TTR have been identified (33, 47, 52, 63) in serum of healthy humans and in subjects with familial amyloidosis. Future studies are needed to assess whether genetic or posttranslational variants of TTR contribute to elevation of RBP4 and insulin resistance in human subjects.
Since ob/ob mice and HFD-fed mice were different strains in this study, it remains possible that differences in genetic background might explain some differences in RBP4 clearance and serum TTR concentrations observed in the two models. However, we found that the same HFD feeding protocol caused a similar magnitude of RBP4 elevation with unchanged TTR concentrations in mixed C57Bl/6 × 129/Sv strain mice (not shown). Further work is necessary to determine whether certain strains may be more or less susceptible to impaired clearance of RBP4 in insulin-resistant states.
Since ob/ob mice exhibit multiple abnormalities related to their leptin-deficient, insulin-resistant, and severely obese state (5, 8, 11, 29, 31), the question arises whether the decreased RBP4 clearance could reflect generally diminished renal function compared with the lean controls. This is highly unlikely since chronic renal dysfunction without anuria/oliguria in insulin-resistant states, including type 2 diabetes in humans, is generally associated (1, 19, 25, 26, 44, 60) with increased urinary RBP4 excretion. Therefore, renal dysfunction associated with early stages of diabetic nephropathy is not likely to result in impaired clearance of RBP4. In fact, we found that creatinine clearance was normal in ob/ob mice, but the fractional urinary excretion of RBP4 was markedly reduced. Thus, the retention of RBP4 in plasma does not result from advanced renal dysfunction and is likely to be due, at least in part, to elevated TTR.
It is not yet known whether the increased hepatic expression of TTR and elevated serum TTR concentrations observed in ob/ob mice result primarily from leptin deficiency or secondarily from the state of severe obesity and insulin resistance in this model. However, we found that short-term leptin therapy (over the course of 24 h) does not affect serum RBP4 or TTR levels in ob/ob mice compared with saline injected controls (data not shown). We did not examine the effect of longer-term leptin replacement therapy in ob/ob mice due to the rapid effects on food intake and body weight, which would confound interpretation of the results (24). Studies of transcriptional control of TTR expression (12–14, 58) have identified DNA binding sites for several liver-enriched transcription factors that could be involved in the induction of TTR expression in ob/ob mice.
In addition to providing new insight into potential mechanisms for elevated RBP4 in the setting of some insulin-resistant states, our findings further emphasize the utility of targeting the RBP4-TTR complex as a pharmacological strategy for treating insulin resistance and type 2 diabetes. Treatment of obese HFD-fed mice with fenretinide, a drug that lowers RBP4 by reducing its affinity for TTR, thereby increasing its renal excretion, improves insulin sensitivity and glucose intolerance (59). Even in the setting of elevated TTR concentrations, fenretinide or other drugs that reduce RBP4-TTR binding affinity would be predicted to lower serum RBP4 levels and improve insulin-glucose homeostasis. Therefore, insulin-resistant human subjects with or without elevated TTR levels may benefit from lowering serum TTR levels or from targeted pharmacological disruption of the RBP4-TTR complex.
ACKNOWLEDGMENTS
We acknowledge Takeda Pharmaceutical Company for recombinant mRBP4 andW. Blaner, M. Gottesman, and L. Quadro for RBP4-KO mice. We also thank L. Zemany, K. Catalano, O. Peroni, E. Eckersdorf, and P. Aryal for their expert technical assistance.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-43051 (to B. B. Kahn) and K08-DK-69624 (to T. E. Graham) and by Takeda Pharmaceutical (to B. B. Kahn). N. Mody was supported by fellowships from the American Heart Association and American Diabetes Association-European Association for the Study of Diabetes.
REFERENCES
- 1.Abahusain MA, Wright J, Dickerson JW, de Vol EB. Retinol, alphatocopherol and carotenoids in diabetes. Eur J Clin Nutr. 1999;53:630–635. doi: 10.1038/sj.ejcn.1600825. [DOI] [PubMed] [Google Scholar]
- 2.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 3.Berni R, Formelli F. In vitro interaction of fenretinide with plasma retinol-binding protein and its functional consequences. FEBS Lett. 1992;308:43–45. doi: 10.1016/0014-5793(92)81046-o. [DOI] [PubMed] [Google Scholar]
- 4.Berni R, Malpeli G, Folli C, Murrell JR, Liepnieks JJ, Benson MD. The Ile-84→Ser amino acid substitution in transthyretin interferes with the interaction with plasma retinol-binding protein. J Biol Chem. 1994;269:23395–23398. [PubMed] [Google Scholar]
- 5.Briley LP, Szczech LA. Leptin and renal disease. Semin Dial. 2006;19:54–59. doi: 10.1111/j.1525-139X.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 6.Broch M, Vendrell J, Ricart W, Richart C, Fernández-Real JM. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007;30:1802–1806. doi: 10.2337/dc06-2034. [DOI] [PubMed] [Google Scholar]
- 7.Cabré A, Lázaro I, Girona J, Manzanares JM, Marimón F, Plana N, Heras M, Masana L. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 2007;195:e150–e158. doi: 10.1016/j.atherosclerosis.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 8.Cervero A, Dominguez F, Horcajadas JA, Quiñonero A, Pellicer A, Simón C. The role of the leptin in reproduction. Curr Opin Obstet Gynecol. 2006;18:297–303. doi: 10.1097/01.gco.0000193004.35287.89. [DOI] [PubMed] [Google Scholar]
- 9.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 10.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 11.Correia ML, Haynes WG. Leptin, obesity and cardiovascular disease. Curr Opin Nephrol Hypertens. 2004;13:215–223. doi: 10.1097/00041552-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Costa RH, Grayson DR. Site-directed mutagenesis of hepatocyte nuclear factor (HNF) binding sites in the mouse transthyretin (TTR) promoter reveal synergistic interactions with its enhancer region. Nucleic Acids Res. 1991;19:4139–4145. doi: 10.1093/nar/19.15.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa RH, Grayson DR, Darnell JE., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989;9:1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa RH, Lai E, Darnell JE., Jr Transcriptional control of the mouse prealbumin (transthyretin) gene: both promoter sequences and a distinct enhancer are cell specific. Mol Cell Biol. 1986;6:4697–4708. doi: 10.1128/mcb.6.12.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig RL, Chu WS, Elbein SC. Retinol binding protein 4 as a candidate gene for type 2 diabetes and prediabetic intermediate traits. Mol Genet Metab. 2007;90:338–344. doi: 10.1016/j.ymgme.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 17.Episkopou V, Maeda S, Nishiguchi S, Shimada K, Gaitanaris GA, Gottesman ME, Robertson EJ. Disruption of the transthyretin gene results in mice with depressed levels of plasma retinol and thyroid hormone. Proc Natl Acad Sci USA. 1993;90:2375–2379. doi: 10.1073/pnas.90.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Formelli F, Carsana R, Costa A, Buranelli F, Campa T, Dossena G, Magni A, Pizzichetta M. Plasma retinol level reduction by the synthetic retinoid fenretinide: a one year follow-up study of breast cancer patients. Cancer Res. 1989;49:6149–6152. [PubMed] [Google Scholar]
- 19.Galanti LM, Jamart J, Dell’omo J, Donckier J. Comparison of urinary excretion of albumin, alpha 1-microglobulin and retinol-binding protein in diabetic patients. Diabetes Metab. 1996;22:324–330. [PubMed] [Google Scholar]
- 20.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886–1890. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 21.Goodman D. Plasma retinol-binding protein. In: Sporn M, Roberts A, Goodman D, editors. The Retinoids: Biology, Chemistry, and Medicine. Orlando, FL: Academic; 1984. pp. 41–86. [Google Scholar]
- 22.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 2007;50:814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]
- 23.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 24.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 25.Holm J, Nielsen NV, Hemmingsen L. Retinopathy in type II diabetes mellitus associated with above-normal urinary excretion of RBP. Kidney Int Suppl. 1994;47:S105–S108. [PubMed] [Google Scholar]
- 26.Hong CY, Chia KS, Ling SL. Urinary protein excretion in Type 2 diabetes with complications. J Diabetes Complications. 2000;14:259–265. doi: 10.1016/s1056-8727(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 28.Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K. Association of serum-retinol binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3224–3229. doi: 10.1210/jc.2007-0209. [DOI] [PubMed] [Google Scholar]
- 29.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, Bluher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 32.Lee DC, Lee JW, Im JA. Association of serum retinol binding protein 4 and insulin resistance in apparently healthy adolescents. Metabolism. 2007;56:327–331. doi: 10.1016/j.metabol.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Lim A, Prokaeva T, McComb ME, Connors LH, Skinner M, Costello CE. Identification of S-sulfonation and S-thiolation of a novel transthyretin Phe33Cys variant from a patient diagnosed with familial transthyretin amyloidosis. Protein Sci. 2003;12:1775–1785. doi: 10.1110/ps.0349703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maslowska M, Wang HW, Cianflone K. Novel roles for acylation stimulating protein/C3adesArg: a review of recent in vitro and in vivo evidence. Vitam Horm. 2005;70:309–332. doi: 10.1016/S0083-6729(05)70010-8. [DOI] [PubMed] [Google Scholar]
- 35.Monaco HL, Rizzi M, Coda A. Structure of a complex of two plasma proteins: transthyretin and retinol-binding protein. Science. 1995;268:1039–1041. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- 36.Munkhtulga L, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, Kumada M, Erdenebulgan B, Zolzaya K, Lkhagvasuren T, Iwamoto S. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum Genet. 2007;120:879–888. doi: 10.1007/s00439-006-0264-4. [DOI] [PubMed] [Google Scholar]
- 37.Palha JA, Episkopou V, Maeda S, Shimada K, Gottesman ME, Saraiva MJ. Thyroid hormone metabolism in a transthyretin-null mouse strain. J Biol Chem. 1994;269:33135–33139. [PubMed] [Google Scholar]
- 38.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–223. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 40.Refai E, Dekki N, Yang SN, Imreh G, Cabrera O, Yu L, Yang G, Norgren S, Rossner SM, Inverardi L, Ricordi C, Olivecrona G, Andersson M, Jornvall H, Berggren PO, Juntti-Berggren L. Transthyretin constitutes a functional component in pancreatic beta-cell stimulus-secretion coupling. Proc Natl Acad Sci USA. 2005;102:17020–17025. doi: 10.1073/pnas.0503219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraiva MJ. Transthyretin mutations in hyperthyroxinemia and amyloid diseases. Hum Mutat. 2001;17:493–503. doi: 10.1002/humu.1132. [DOI] [PubMed] [Google Scholar]
- 43.Scantlebury T, Maslowska M, Cianflone K. Chylomicron-specific enhancement of acylation stimulating protein and precursor protein C3 production in differentiated human adipocytes. J Biol Chem. 1998;273:20903–20909. doi: 10.1074/jbc.273.33.20903. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu H, Negishi M, Shimomura Y, Mori M. Changes in urinary retinol binding protein excretion and other indices of renal tubular damage in patients with non-insulin dependent diabetes. Diabetes Res Clin Pract. 1992;18:207–210. doi: 10.1016/0168-8227(92)90147-j. [DOI] [PubMed] [Google Scholar]
- 45.Silha JV, Nyomba BL, Leslie WD, Murphy LJ. Ethnicity, insulin resistance, and inflammatory adipokines in women at high and low risk for vascular disease. Diabetes Care. 2007;30:286–291. doi: 10.2337/dc06-1073. [DOI] [PubMed] [Google Scholar]
- 46.Soprano D, Blaner W. Plasma retinol-binding protein. In: Sporn M, Roberts A, Goodman D, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven; 1994. pp. 257–282. [Google Scholar]
- 47.Suhr OB, Ando Y, Ohlsson PI, Olofsson A, Andersson K, Lundgren E, Ando M, Holmgren G. Investigation into thiol conjugation of transthyretin in hereditary transthyretin amyloidosis. Eur J Clin Invest. 1998;28:687–692. doi: 10.1046/j.1365-2362.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Ando Y, Haraoka K, Katsuragi S, Yamashita T, Yamashita S, Okajima M, Terazaki H, Okabe H. Role of VLDL/chylomicron in amyloid formation in familial amyloidotic polyneuropathy. Biochem Biophys Res Commun. 2003;311:344–350. doi: 10.1016/j.bbrc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–2719. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]
- 50.Tan BK, Chen J, Lehnert H, Kennedy R, Randeva HS. Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: effects of gonadal and adrenal steroids. J Clin Endocrinol Metab. 2007;92:2764–2772. doi: 10.1210/jc.2007-0091. [DOI] [PubMed] [Google Scholar]
- 51.Terazaki H, Ando Y, Misumi S, Nakamura M, Ando E, Matsunaga N, Shoji S, Okuyama M, Ideta H, Nakagawa K, Ishizaki T, Ando M, Saraiva MJ. A novel compound heterozygote (FAP ATTR Arg104His/ATTR Val30Met) with high serum transthyretin (TTR) and retinol binding protein (RBP) levels. Biochem Biophys Res Commun. 1999;264:365–370. doi: 10.1006/bbrc.1999.1514. [DOI] [PubMed] [Google Scholar]
- 52.Terazaki H, Ando Y, Suhr O, Ohlsson PI, Obayashi K, Yamashita T, Yoshimatsu S, Suga M, Uchino M, Ando M. Post-translational modification of transthyretin in plasma. Biochem Biophys Res Commun. 1998;249:26–30. doi: 10.1006/bbrc.1998.9097. [DOI] [PubMed] [Google Scholar]
- 53.Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem. 1992;267:1805–1810. [PubMed] [Google Scholar]
- 54.van Bennekum AM, Wei S, Gamble MV, Vogel S, Piantedosi R, Gottesman M, Episkopou V, Blaner WS. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem. 2001;276:1107–1113. doi: 10.1074/jbc.M008091200. [DOI] [PubMed] [Google Scholar]
- 55.Vitkova M, Klimcakova E, Kovacikova M, Valle C, Moro C, Polak J, Hanacek J, Capel F, Viguerie N, Richterova B, Bajzova M, Hejnova J, Stich V, Langin D. Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol-binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet-induced changes in insulin sensitivity. J Clin Endocrinol Metab. 2007;92:2330–2335. doi: 10.1210/jc.2006-2668. [DOI] [PubMed] [Google Scholar]
- 56.Weiping L, Qingfeng C, Shikun M, Xiurong L, Hua Q, Xiaoshu B, Suhua Z, Qifu L. Elevated serum RBP4 is associated with insulin resistance in women with polycystic ovary syndrome. Endocrine. 2006;30:283–287. doi: 10.1007/s12020-006-0006-3. [DOI] [PubMed] [Google Scholar]
- 57.Yagmur E, Weiskirchen R, Gressner AM, Trautwein C, Tacke F. Insulin resistance in liver cirrhosis is not associated with circulating retinol-binding protein 4. Diabetes Care. 2007;30:1168–1172. doi: 10.2337/dc06-2323. [DOI] [PubMed] [Google Scholar]
- 58.Yan C, Costa RH, Darnell JE, Jr, Chen JD, Van Dyke TA. Distinct positive and negative elements control the limited hepatocyte and choroids plexus expression of transthyretin in transgenic mice. EMBO J. 1990;9:869–878. doi: 10.1002/j.1460-2075.1990.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 60.Yaqoob M, McClelland P, Patrick AW, Stevenson A, Mason H, White MC, Bell GM. Evidence of oxidant injury and tubular damage in early diabetic nephropathy. QJM. 1994;87:601–607. [PubMed] [Google Scholar]
- 61.Yen TT, Stienmetz J, Simpson PJ. Blood volume of obese (ob-ob) and diabetic (db-db) mice. Proc Soc Exp Biol Med. 1970;133:307–308. [PubMed] [Google Scholar]
- 62.Yoshida A, Matsutani Y, Fukuchi Y, Saito K, Naito M. Analysis of the factors contributing to serum retinol binding protein and transthyretin levels in Japanese adults. J Atheroscler Thromb. 2006;13:209–215. doi: 10.5551/jat.13.209. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q, Kelly JW. Cys-10 mixed disulfide modifications exacerbate transthyretin familial variant amyloidogenicity: a likely explanation for variable clinical expression of amyloidosis and the lack of pathology in C10S/V30M transgenic mice? Biochemistry. 2005;44:9079–9085. doi: 10.1021/bi050378f. [DOI] [PubMed] [Google Scholar]