Abstract
Concomitant smoke inhalation trauma in burn patients is a serious medical problem. Previous investigations in our sheep model revealed that these injuries lead to significant airway hyperemia, enhanced pulmonary fluid extravasation, and severely impaired pulmonary function. However, the pathophysiological mechanisms are still not fully understood. The lung is innervated by sensory nerves containing peptides such as substance P and calcitonin gene-related peptide. Noxious stimuli in the airways can induce a neurogenic inflammatory response, which has previously been implicated in several airway diseases. Calcitonin gene-related peptide is known to be a potent vasodilator. We hypothesized that calcitonin gene-related peptide is also a mediator of the pulmonary reaction to toxic smoke and planned experiments to evaluate its role in this model. We tested the effects of pretreatment with a specific antagonist of the major receptor for calcitonin gene-related peptide (BIBN4096BS; 32 μg/kg, followed by continuous infusion of 6.4 μg·kg−1·h−1) until the animal was killed 48 h after injury in an established ovine model of burn (40% total body surface, third degree) and smoke inhalation (48 breaths, <40°C) injury. In treated animals (n = 7), the injury-related increases in tracheal blood flow and lung lymph flow were significantly attenuated compared with untreated controls (n = 5). Furthermore, the treatment significantly attenuated abnormalities in respiratory gas exchange. The data suggest that calcitonin gene-related peptide contributes to early airway hyperemia, transvascular fluid flux, and respiratory malfunction following ovine burn and smoke inhalation injury. Future studies will be needed to clarify the potential therapeutic benefit for patients with this injury.
Keywords: airway blood flow, neurogenic inflammation, neuropeptides, pulmonary microcirculation, bronchial circulation, sheep
despite improved surgical care, respiratory support, and effective fluid resuscitation management, the mortality rates of patients with severe burn trauma remain high. The presence of concomitant inhalation injury is a major determinant in morbidity and mortality of fire victims (42). It has long been suspected that neuropeptides mediate the initial reaction of the respiratory system to toxic smoke. Stimulation of C-fibers in the airways leads to release of multiple neuropeptides (29, 46). Previous studies from this laboratory indicated that capsaicin pretreatment reduced the severity of the reaction to smoke inhalation injury in sheep (26). The pathophysiological changes in the lung following combined burn and smoke inhalation injury in sheep closely reflect the situation seen in humans with these injuries (12, 18, 19). They are characterized by marked increases in airway blood flow and pulmonary microvascular permeability, leading to greatly enhanced transvascular fluid flow into the bronchopulmonary lymphatic vessels and secretion of mucus by airway glands, which together lead to airway obstruction and, ultimately, result in severe impairment of respiratory gas exchange (7, 12, 18, 19, 43, 47). To gain improved understanding of the pathophysiology involved in the pulmonary reaction to burn and smoke inhalation injury, extensive research has been conducted in the sheep model (11, 12, 19, 38, 43, 47). However, the pathogenetic mechanism of the injury-induced airway hyperemia and transvascular fluid flux is still not fully understood.
The lung is innervated by a dense network of vagal nerve sensory C-fibers, containing peptides such as substance P, neurokinins, and calcitonin gene-related peptide (CGRP) (4, 8). These sensory nerves represent an effective nociceptor system, enabling the lung to react to noxious stimuli in the airways by release of neuropeptides via an axon reflex (22, 46, 52). Increased liberation of neuropeptides may trigger the development of neurogenic inflammatory responses, resulting in airway hyperemia, microvascular hyperpermeability, mucus secretion, and bronchoconstriction (22, 52). Neurogenic inflammation in the airways owing to increased neuropeptide release has previously been implicated in smoke inhalation injury and in several other disease processes (27, 28, 51, 53).
We hypothesized that enhanced CGRP liberation is a major mechanism in the pulmonary reaction to combined burn and smoke inhalation injury. Therefore, the aims of the present study were to test the role of CGRP in the regulation of airway blood flow and transvascular fluid flux in response to burn and smoke inhalation injury in sheep and to evaluate the potential therapeutic benefit of a CGRP antagonist.
METHODS
This study was approved by the Animal Care and Use Committee of the University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health and the American Physiological Society for the care and use of laboratory animals.
Animal model.
Thirteen healthy adult female sheep with a body weight of 31 ± 2 kg were included in this study. Following induction of anesthesia with ketamine (500 mg im, 300 mg iv), endotracheal intubation was performed. Anesthesia was maintained using an isoflurane (1.4–1.8 vol%)-oxygen mixture. The right femoral artery was cannulated with a polyvinylchloride catheter (Intracath, 16 gauge, 24 in., Becton Dickinson Vascular Access; Sandy, UT) for continuous measurement of systemic arterial pressure and intermittent sampling of arterial blood. A thermodilution catheter (model 93A-131-7F, Edwards Critical Care Division; Irvine, CA) was inserted into the right external jugular vein through an introducer sheath (Edwards Lifescience; Irvine, CA) and advanced into the common pulmonary artery. Through the left fifth intercostal space, a Silastic catheter (0.062-in. ID and 0.125-in. OD, Dow Corning; Midland, MI) was positioned in the left atrium for continuous measurement of left arterial pressure. Through the right fifth intercostal space, a Silastic catheter (0.025-in. ID and 0.047-in. OD) was placed into an efferent lymphatic vessel from the caudal mediastinal lymph node for the measurement of lung lymph flow (QL). Ligation of the tail of the caudal mediastinal lymph node and cauterization of the systemic diaphragmatic lymph vessels were performed to remove the systemic lymph contribution (44, 48). After a recovery period of 5–7 days, a baseline measurement was performed in spontaneously breathing sheep. Thereafter, the animals were anesthetized using intravenous ketamine (5 mg/kg). Tracheotomy was performed, and anesthesia was maintained with a halothane (1.1–2.0 vol%)-oxygen mixture. The animals received combined burn and smoke inhalation injury according to an established protocol (12, 43, 44, 50). In brief, sheep received a third-degree burn of 40% of the total body surface area (TBSA) using a Bunsen burner and inhalation injury with 4 × 12 breaths of cotton smoke (<40°C) using a modified bee smoker filled with 40 g of burning cotton toweling. Arterial carboxyhemoglobin concentrations were determined immediately after each set of smoke inhalation. Anesthesia was then discontinued, and the sheep were allowed to awaken. All sheep were mechanically ventilated (Servo Ventilator 900C, Siemens, Elema, Sweden) with a tidal volume of 12–15 ml/kg and a positive end-expiratory pressure of 5 mmHg throughout the study period of 48 h. The respiratory rate was initially set at 20 breaths/min and was further adjusted according to blood gas analysis. All animals were fluid resuscitated with lactated Ringer solution according to the Parkland formula (4 ml·kg−1·%TBSA−1 for the first 24 h, and 2 ml·kg−1·%TBSA−1 for the second 24 h). All animals had free access to dry food but had no oral intake of water.
Measured variables.
Systemic and pulmonary hemodynamic variables were determined using pressure transducers (Baxter-Edwards Critical Care, Irvine, CA) and recorded on a hemodynamic monitor (monitor V24C, Philips Medizin Systeme Böblingen, Böblingen, Germany). Cardiac output was measured in triplicate with the thermodilution technique (Monitor 9530, Baxter-Edwards Critical Care). Blood gases were measured using a blood gas analyzer (Synthesis 15, Instrumentation Laboratories; Lexington, MA). The partial arterial oxygen pressure (PaO2)/inspired oxygen fraction (FiO2) ratio, pulmonary shunt fraction (Qs/Qt), vascular resistances, and pulmonary capillary pressure (PCP) were calculated using standard equations. To exclude acute adverse effects of BIBN4096BS on systemic or pulmonary hemodynamics, acid-base status, and pulmonary gas exchange, we performed a second set of baseline measurements 1 h after the start of drug infusion, immediately before the injury (n = 7 animals). The peak and pause ventilatory pressures were recorded from the indicators on the servoventilator. Lung lymph flow (QL) was measured with graduated test tubes and a stopwatch. Plasma (πP) and lung lymph (πL) colloid oncotic pressures were measured with a colloid osmometer (model 4420, Wescor; Logan, UT). The plasma (CP) and lung lymph (CL) protein concentrations were measured with a refractometer (National Instrument; Baltimore, MD). For estimation of pulmonary microvascular permeability to protein, the lymph-to-plasma protein ratio (CL/CP) was calculated (25). At the end of the 48-h study period, the animals were deeply anesthetized with ketamine (15 mg/kg) and killed by intravenous injection of 60 ml saturated potassium chloride. Immediately after death, the right lung was removed, and a 1-cm thick section was taken from the middle of the lower lobe and inflated with 10% formalin for histological examination. Fixed samples were embedded in paraffin, sectioned into 4-μm pieces, and stained with hematoxylin-eosin. A pathologist who was unaware of the group assignment analyzed the histological changes according to a standardized protocol as described by Cox et al. (7). Twenty-four areas of lung parenchyma in each animal were observed at ×10 objective magnification and graded on a scale of 0–4 (0: absent, appears normal; 1: light; 2: moderate; 3: strong; 4: intense) for congestion, septal edema, alveolar edema, septal inflammation, and hemorrhage. Airway obstruction was evaluated at the same time when the injury score was assigned. For each cross-sectioned airway, a score of 0–100% was made as an estimate of the degree of luminal obstruction. Each airway was classified as a bronchus or a bronchiole (7). Microvascular blood flow of the middle and distal trachea and the right and left main bronchus were measured at baseline, 3 h, and 24 h postinjury by injection of colored microspheres (Interactive Medical Technologies, Los Angeles, CA) as described previously (12, 34).
Treatment groups.
Twelve sheep were randomly allocated to the following study groups: injured, untreated animals (control; n = 5) and injured animals, treated with the selective, nonpeptide CGRP receptor antagonist BIBN4096BS (Boehringer-Ingelheim Pharma KG; Biberach, Germany; n = 7) (20, 36). One hour before the injury, treated animals received an intravenous bolus of 32 μg/kg BIBN4096BS followed by continuous infusion of 6.4 μg·kg−1·h−1 throughout the study, i.e., for 49 h. The drug was dissolved in saline containing a minimum volume of HCl and titrated with NaOH to adjust the pH to neutral (14, 20). In pilot studies in our institution, the dosing regimen of BIBN4096BS was determined by observing its effect on CGRP-induced vasodilation in precontracted small ovine cerebral arteries incubated ex vivo. A concentration of 1 μM was found to increase the dose of CGRP required by more than one order of magnitude. The infusion rate for the present study was chosen to yield and maintain an approximate plasma drug level of 1 μM. The infusion rate was based on published data for the half-life of the drug in humans (20). The animals of the control group received the same amount of the carrier solution.
Statistical analysis.
All values are expressed as means ± SE. After confirming normal distribution (Kolmogorov-Smirnov test), results were compared by analysis of variance with appropriate Student-Newman-Keuls post hoc comparisons to compare differences within and between groups, or by the unpaired t-test. The study was conducted as a two-factor factorial arrangement of treatments with repeated measures on one factor (time) in a completely randomized design. There were thirty treatment combinations. One factor was group and the second factor was the repeated-measures factor, time. Error a, pooled animal variability within groups, was used to compare overall group means, while error b, the pooled animal-by-group interaction within groups, was used to compare the overall time-related means, as well as (since the interaction was highly significant) the group differences at each time period. A value of P < 0.05 was regarded as statistically significant.
RESULTS
At baseline, there was no significant difference in any of the measured variables between groups, as shown in the tables and figures. Infusion of the CGRP receptor antagonist BIBN4096BS in healthy sheep (n = 7) 1 h before the injury did not affect any measured variable of the systemic or pulmonary circulation, acid-base status, or pulmonary gas exchange (Table 1).
Table 1.
Systemic and pulmonary hemodynamic variables, pH, and pulmonary gas exchange of seven healthy sheep at baseline and 1 h after the start of treatment with the CGRP receptor antagonist BIBN4096BS (32 μg/kg, followed by continuous infusion of 6.4 μg·kg−1·h−1)
| Baseline | 1 h After Start of Drug Infusion | |
|---|---|---|
| MAP, mmHg | ||
| BIBN4096BS | 93±2 | 93±2 |
| SVR, dyn·s·cm−5·m2 | ||
| BIBN4096BS | 1,421±42 | 1,424±56 |
| CO, l/min | ||
| BIBN4096BS | 4.9±0.1 | 4.9±0.2 |
| CVP, mmHg | ||
| BIBN4096BS | 6±1 | 6±1 |
| MPAP, mmHg | ||
| BIBN4096BS | 20±1 | 21±1 |
| PVR, dyn·s·cm−5·m2 | ||
| BIBN4096BS | 144±7 | 152±6 |
| pH, −log10[H+] | ||
| BIBN4096BS | 7.49±0.01 | 7.50±0.01 |
| PaO2/FiO2, mmHg | ||
| BIBN4096BS | 506±10 | 503±8 |
| Qs/Qt | ||
| BIBN4096BS | 0.14±0.01 | 0.14±0.01 |
Values are means ± SE. There were no statistically significant differences between time points. CGRP, calcitonin gene-related peptide; MAP, mean arterial pressure; SVR, systemic vascular resistance; CO, cardiac output; CVP, central venous pressure; MPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; PaO2, partial arterial oxygen pressure; FiO2, inspired oxygen fraction; Qs/Qt, pulmonary shunt fraction.
Airway microvascular blood flow.
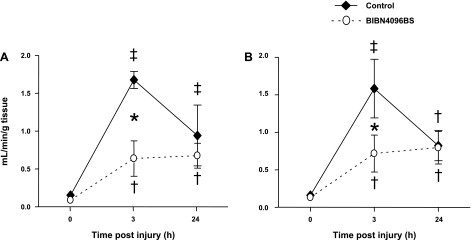
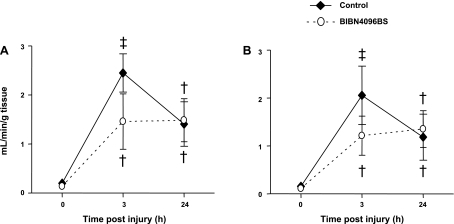
Microvascular blood flow in the trachea and both main bronchi was markedly increased 3 h after the injury in untreated control animals (up to 11-fold in the middle trachea and 12-fold in the right main bronchus). In sheep pretreated with the CGRP receptor antagonist, the elevation in tracheal blood flow was significantly attenuated. Right and left bronchial blood flow also tended to be lower in treated than in control sheep 3 h postinjury, while the drug was still being infused (P = 0.08 and P = 0.1, respectively). At the 24-h time point, no differences between groups could be detected. The time changes in microvascular blood flows are illustrated in Figs. 1 and 2.
Fig. 1.
Impact of the calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS on microvascular blood flow in the middle (A) and distal trachea (B) after burn and smoke inhalation injury. †P < 0.05, ‡P < 0.01 vs. 0 h group. *P < 0.05 between treated and untreated groups.
Fig. 2.
Impact of the CGRP receptor antagonist BIBN4096BS on microvascular blood flow in the right (A) and left main bronchus (B) after burn and smoke inhalation injury. †P < 0.05 vs. 0 h group. ‡P < 0.01 vs. 0 h group.
Microvascular permeability to fluid and protein.
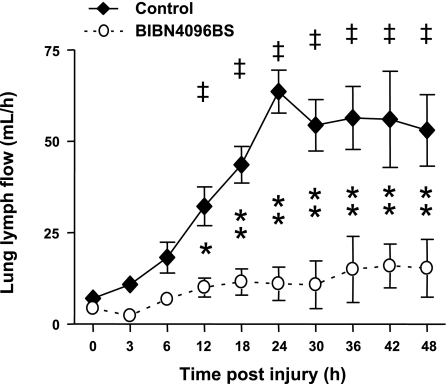
In untreated control animals, pulmonary transvascular fluid flux started to increase immediately after the injury, as evidenced by an elevation in lung lymph flow (QL). QL significantly increased up to nine times the baseline value after 24 h, reaching a plateau for the remainder of the study. In the treatment group, QL was not significantly elevated throughout the study and was significantly lower than in controls from 12 to 48 h (Fig. 3). Following the injury, the plasma protein concentration (CP) and plasma colloid oncotic pressure (πP) decreased significantly toward baseline values in both study groups. However, CP was significantly higher from 24 to 48 h and πP was significantly higher from 36 to 48 h in treated than in control sheep. There were no group differences in lymph-to-plasma protein concentration ratio or lymph-to-plasma colloid oncotic pressure ratio (Table 2).
Fig. 3.
Impact of the CGRP receptor antagonist BIBN4096BS on transvascular fluid flux (lung lymph flow) after burn and smoke inhalation injury. ‡P < 0.01 vs. 0 h group. *P < 0.05, **P < 0.01 between treated and untreated groups.
Table 2.
Time changes in protein concentrations and oncotic pressures of untreated control sheep and sheep treated with the CGRP receptor antagonist BIBN4096BS following burn and smoke inhalation injury
| Time Postinjury, h |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 18 | 24 | 36 | 48 | |
| CP, g/dl | ||||||||
| Control | 4.8±0.1 | 3.8±0.3‡ | 3.8±0.1‡ | 3.5±0.2‡ | 3.0±0.4‡ | 2.8±0.4‡ | 2.8±0.2‡ | 2.9±0.3‡ |
| BIBN4096BS | 4.9±0.2 | 3.9±0.2‡* | 3.6±0.2‡* | 3.6±0.3‡* | 3.6±0.3‡* | 3.7±0.2‡* | 3.6±0.2‡* | 4.0±0.2‡§ |
| CL, g/dl | ||||||||
| Control | 3.0±0.2 | 2.5±0.2† | 2.1±0.2‡ | 2.3±0.2‡ | 2.0±0.2‡ | 1.8±0.2‡ | 1.6±0.2‡ | 1.5±0.2‡ |
| BIBN4096BS | 3.2±0.1 | 3.8±0.1 | 2.2±0.3‡ | 2.6±0.4† | 2.6±0.4† | 2.8±0.4* | 2.6±0.4†* | 2.7±0.5* |
| πP, mmHg | ||||||||
| Control | 20.4±1.2 | 16.1±0.8† | 16.0±0.7† | 15.3±1.3‡ | 12.8±0.9‡ | 12.0±1.1‡ | 10.1±1.0‡ | 11.3±1.0‡ |
| BIBN4096BS | 22.8±0.7 | 16.5±0.7† | 14.9±1.3‡ | 16.8±1.4† | 15.6±1.1‡ | 15.4±1.4‡ | 16.1±1.2‡§ | 17.3±1.4†§ |
| πL, mmHg | ||||||||
| Control | 13.5±0.5 | 11.1±0.9‡ | 9.5±0.7‡ | 10.1±0.7‡ | 9.0±0.9‡ | 8.3±0.8‡ | 7.1±0.7‡ | 6.7±0.5‡ |
| BIBN4096BS | 15.7±1.3 | 10.8±1.7‡ | 8.9±1.2‡ | 10.8±2.0‡ | 11.1±1.6‡ | 10.9±1.4‡ | 10.8±1.6‡* | 11.7±1.9‡* |
| CL/CP | ||||||||
| Control | 0.63±0.03 | 0.67±0.09 | 0.57±0.04 | 0.65±0.04 | 0.69±0.03 | 0.68±0.05 | 0.58±0.05 | 0.51±0.04 |
| BIBN4096BS | 0.69±0.04 | 0.72±0.05 | 0.61±0.04 | 0.68±0.03 | 0.73±0.07 | 0.71±0.04 | 0.71±0.04 | 0.66±0.09 |
| πL/πP | ||||||||
| Control | 0.67±0.02 | 0.69±0.05 | 0.59±0.03 | 0.66±0.02 | 0.70±0.03 | 0.69±0.02 | 0.72±0.09 | 0.60±0.02 |
| BIBN4096BS | 0.69±0.03 | 0.64±0.08 | 0.52±0.04 | 0.60±0.05 | 0.65±0.03 | 0.69±0.03 | 0.63±0.06 | 0.66±0.11 |
| Urine output, ml | ||||||||
| Control | 259±63 | 332±101 | 508±163 | 332±52 | 252±113 | 558±103 | 513±104 | |
| BIBN4096BS | 257±41 | 394±92 | 816±171 | 523±69 | 613±131* | 1,189±126* | 1,116±103* | |
| Net fluid balance, ml | ||||||||
| Control | 515±47 | 933±166 | 1,637±182 | 2,007±165 | 2,274±169 | 2,368±269 | 2,406±421 | |
| BIBN4096BS | 463±58 | 1,105±151 | 1,651±299 | 1,974±353 | 2,228±428 | 2,186±497 | 2,182±579 | |
| Hct, % | ||||||||
| Control | 22±1 | 24±1 | 25±2† | 25±2† | 27±2‡ | 28±3‡ | 29±3‡ | 27±2‡ |
| BIBN4096BS | 24±1 | 25±1 | 23±1 | 23±1 | 23±1 | 23±1* | 23±1* | 22±1* |
| Hb, g/dl | ||||||||
| Control | 7.7±0.5 | 8.7±0.5 | 8.6±0.8 | 8.5±0.8 | 9.3±0.9‡ | 9.5±0.9‡ | 9.8±1.1‡ | 9.0±0.9† |
| BIBN4096BS | 7.9±0.3 | 8.6±0.3 | 7.8±0.3 | 7.7±0.3 | 7.9±0.3 | 7.8±0.3 | 7.9±0.4* | 7.5±0.4 |
Values are means ± SE. CP, plasma protein concentration; CL lung lymph protein concentration; πP, plasma colloid oncotic pressure; πL, lung lymph colloid oncotic pressure; CL/CP, lymph-to-plasma protein ratio; πL/πP, lymph-to-plasma colloid oncotic pressure ratio; Hct, hematocrit; Hb hemoglobin.
P < 0.05,
P < 0.01 between treated and control groups.
P < 0.05,
P < 0.01 vs. 0 h.
Fluid balance, hematocrit, and hemoglobin.
The total urine output was significantly higher in the treatment group (159 ± 22 vs. 84 ± 19 ml/kg; P = 0.03 BIBN4096BS vs. control), but there was no significant difference in total fluid accumulation between groups (72 ± 19 vs. 79 ± 16 ml/kg; P > 0.05 BIBN4096BS vs. control). Despite identical fluid resuscitation (according to the Parkland formula) in both study groups, hematocrit and hemoglobin were significantly elevated toward baseline values only in control sheep, while these variables remained stable in treated sheep. The time changes in net fluid balance, urine output, hemoglobin, and hematocrit are displayed in Table 2.
Pulmonary and systemic hemodynamic variables.
Following the injury, mean pulmonary artery pressure, pulmonary artery occlusion pressure, pulmonary vascular resistance, and calculated pulmonary capillary pressure increased in both groups. No significant differences could be detected between groups. Except for an initial and transient increase in mean arterial pressure, systemic hemodynamic variables (i.e., cardiac output, systemic vascular resistance, and central venous pressure) remained stable in both groups (Table 3).
Table 3.
Time changes in systemic hemodynamic variables of untreated control sheep and sheep treated with the CGRP receptor antagonist BIBN4096BS following burn and smoke inhalation injury
| Time Postinjury, h |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 18 | 24 | 36 | 48 | |
| MAP, mmHg | ||||||||
| Control | 95±3 | 108±6 | 110±5† | 104±5 | 101±4 | 101±6 | 100±7 | 103±6 |
| BIBN4096BS | 95±2 | 107±5 | 116±5† | 108±3† | 106±3 | 102±3 | 100±4 | 101±3 |
| SVR, dyn·s·cm−5·m2 | ||||||||
| Control | 1,374±53 | 1,528±201 | 1,331±114 | 1,585±256 | 1,309±139 | 1,479±172 | 1,475±289 | 1,388±131 |
| BIBN4096BS | 1,421±41 | 1,527±94 | 1,579±101 | 1,599±117 | 1,485±77 | 1,405±97 | 1,427±139 | 1,221±70 |
| CO, l/min | ||||||||
| Control | 5.1±0.1 | 5.0±0.3 | 5.9±0.4 | 5.3±0.7 | 5.8±0.4 | 5.1±0.4 | 5.2±0.5 | 5.3±0.3 |
| BIBN4096BS | 4.9±0.1 | 4.6±0.3 | 5.1±0.3 | 5.1±0.3 | 5.3±0.3 | 5.4±0.3 | 5.2±0.4 | 5.9±0.2 |
| CVP, mmHg | ||||||||
| Control | 7±1 | 8±1 | 9±1 | 7±1 | 9±1 | 10±1 | 10±3 | 10±3 |
| BIBN4096BS | 6±1 | 9±1 | 10±1 | 8±1 | 9±1 | 10±1 | 10±1 | 9±1 |
Values are means ± SE.
P < 0.05 vs. 1 h.
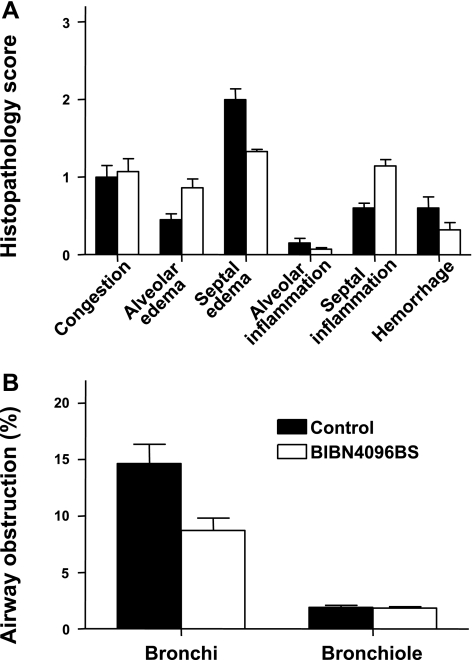
Histopathology scores and airway obstruction.
No statistically significant differences in histopathology scores could be detected between groups. There were also no statistically significant differences in airway obstruction scores between groups, but the mean score for bronchial obstruction was lower in the treatment group (P = 0.12) (Fig. 4B).
Fig. 4.
Impact of the CGRP receptor antagonist BIBN4096BS on histopathology scores (A) and airway obstruction scores (B) after burn and smoke inhalation injury.
Pulmonary function.
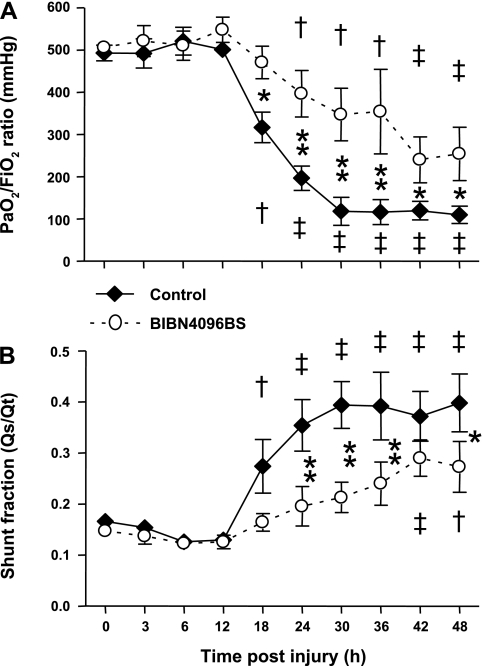
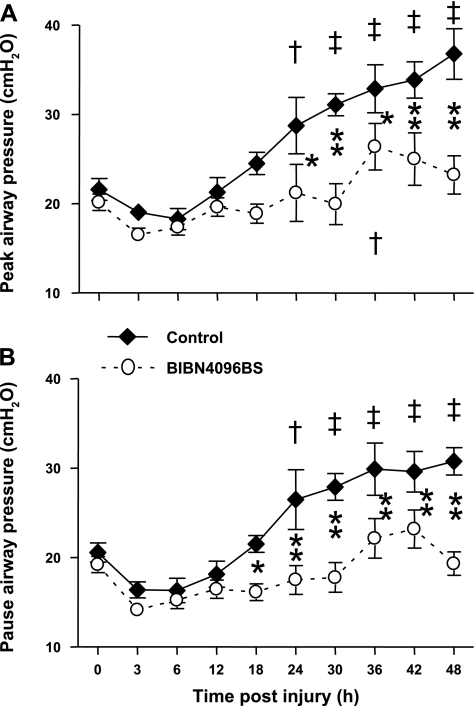
The injury induced a severe impairment in respiratory gas exchange in untreated control animals, as indicated by a decline in PaO2/FiO2 ratio below 200 mmHg and a concomitant increase in pulmonary shunt fraction. In the treatment group, the PaO2/FiO2 ratio remained above 200 mmHg during the entire experimental period and was significantly higher than in the control group from 18 to 48 h. Pulmonary shunt fraction increased significantly less in treated than in control sheep (Fig. 5). The injury was further associated with marked increases in ventilatory pressures in control animals that were significantly attenuated by the treatment (Fig. 6).
Fig. 5.
Impact of the CGRP receptor antagonist BIBN4096BS on partial arterial oxygen pressure (PaO2)/inspired oxygen fraction (FiO2) ratio (A) and pulmonary shunt fraction (B) after burn and smoke inhalation injury. †P < 0.05, ‡P < 0.01 vs. 0 h group. *P < 0.05, **P < 0.01 between treated and untreated groups.
Fig. 6.
Impact of the CGRP receptor antagonist BIBN4096BS on ventilatory pressures [peak airway pressure (A) and pause airway pressure (B)] after burn and smoke inhalation injury. †P < 0.05, ‡P < 0.01 vs. 0 h group. *P < 0.05, **P < 0.01 between treated and untreated groups.
DISCUSSION
The results of the present study suggest that CGRP contributes to early increases in airway blood flow, enhanced pulmonary transvascular fluid flux, and impaired pulmonary gas exchange following combined burn and smoke inhalation injury in sheep. Pretreatment with the specific CGRP receptor antagonist BIBN4096BS partially attenuated these pathophysiological derangements. These findings suggest that CGRP is a major mediator of the early reaction to inhalation of toxic smoke. The fact that inhibition of the effects of CGRP also greatly reduces the decline in PaO2/FiO2 ratio that otherwise occurs around 24 h after injury suggests that the early inflammatory reaction in the large airways is a necessary condition for later development of respiratory malfunction. An alternative explanation of the results would be that CGRP has adverse effects on gas exchange more than 24 h after injury.
The nociceptor nerves of the lung, which sense irritation of the airways, are mostly vagal C-fibers that are processes of neurons that have their cell bodies located in the jugular and nodose ganglia. These neurons contain combinations of substance P, other tachykinins, and CGRP, which function as neurotransmitters in the sensory system. Details of the sensory innervation of the lung have not been well characterized in the sheep to date. Substance P and the tachykinins stimulate neutrophils and mast cell activation and microvascular leakage via NK receptors, while CGRP interacts with separate receptors. Little is known of the effects of simultaneous stimulation of these two groups of receptors on the same cell. Two different isoforms of CGRP are currently known: αCGRP occurs mainly in the lung, whereas βCGRP is prevalent in enteric neurons (37). Within the lung, CGRP is localized in sensory nerves throughout the respiratory tract. Importantly, CGRP-containing nerve fibers can be found adjacent to pulmonary vessels and vascular smooth muscle cells (6, 8, 30). CGRP could further be detected in perikarya of intrapulmonary ganglion cells, epithelial neuroendocrine cells of the airway, and neurons of the parasympathetic plexus within the trachea (13, 22). CGRP mediates its effects via specific receptors, of which two different types have been identified. CGRP1 receptors were found to be expressed in cardiovascular tissues of various organs. Initially, they have been localized in blood vessels of human airways (3, 16, 39). The distribution of CGRP2 receptors is less well defined.
There is good evidence for CGRP-induced vasodilation in pulmonary vessels in different experimental settings (24, 31–33, 35). Via CGRP1 receptors, CGRP mediates its vasodilatory effects by a nitric oxide (NO)- and endothelium-independent mechanism coupled to an increase in intracellular cyclic adenosine monophosphate levels (1, 5). However, there is also evidence for an endothelium-dependent pathway of CGRP-mediated vasodilation in some tissues which involves stimulation of NO production (15). CGRP is degraded by tissue proteases, notably neutral endopeptidase (E.C. 3.4.24.11) (23).
The CGRP receptor antagonist used in this study, BIBN4096BS, has been well characterized as to its specificity and pharmacology in multiple animal models and has undergone trials in humans as a potential therapy for migraine (9, 20, 36). It was found to lack agonist activity and showed no significant affinity to 75 other receptors and enzyme systems (9). The dose of the CGRP receptor antagonist BIBN4096BS chosen for this study was selected to maintain an approximate plasma concentration of one micromolar, which we demonstrated to substantially shift the curve of vasodilation induced by CGRP in ovine cerebral arteries. Cerebral arteries were used for convenience, since they are easy to isolate and had been used in ongoing studies in our institution, and because cerebral blood vessels respond to αCGRP. This was important because the potency of BIBN4096BS varies greatly between species. However, we did not characterize the IC50 for the drug in ovine blood vessels. Tissue concentrations of CGRP were not measured in this study. Although interpretation of these concentrations would need to take into account changes in rate of degradation and potential origin from ganglia as well as sensory nerves, such measurement will be of interest in future studies. It should be noted that BIBN4096BS does not cross the blood-brain barrier to a substantial extent (10), so the effects observed in this study are thought to be most likely due to inhibition of the effects of CGRP peripherally, not in the brain stem. Administration of the drug was not associated with any changes in hemodynamic measurements recorded in the experimental animals immediately before injury, indicating a lack of significant acute hemodynamic effects.
Previous studies in our ovine model of combined burn and smoke inhalation injury demonstrated that this injury is associated with significant increases in airway blood flow and pulmonary transvascular fluid flux (12, 18, 38, 47). However, the exact mechanism of this pathophysiological response is not yet fully understood. By use of this model, multiple mediators have been shown to have significant roles in lung injury following inhalation of toxic smoke, including depletion of antioxidants, NO and related compounds, endothelin, poly(ADP)ribose polymerase, and others. The effects of CGRP appear to be important at the beginning of the reaction of the respiratory system to this injury and may lead indirectly to involvement of multiple additional mediators. In a previous study, we treated groups of sheep with CP96345, an antagonist of the NK1 receptor, which interacts with substance P, at a dose of 1 mg/kg iv every 12 h, using the inactive enantiomer CP96344 as control. Sheep were injured with burn and smoke inhalation using the same protocol as in this study, but no significant effects were found on lung lymph flow or PaO2/FiO2 ratio (17). It is interesting to speculate that CGRP and substance P or other tachykinins might interact, perhaps synergistically (40), in stimulating inflammation in the lung, which would provide a means to modulate the effects of either alone. It is also unclear whether release of CGRP or substance P from neurons in the intrinsic ganglia of the airways, where both are also located, contributes in any way to inflammation in the airways following inhalation of smoke.
The present study provides evidence that CGRP-induced vasodilation is a major contributor to marked airway hyperemia following burn and smoke inhalation injury. Notably, the CGRP-mediated increase in airway blood flow appears to be an early event, because it could only be attenuated 3 h postinjury, while it was not affected 24 h postinjury in BIBN4096BS-treated animals. Of interest, administration of the CGRP receptor antagonist prevented the injury-related increases in regional blood flows more effectively in the middle and distal trachea than in both main bronchi. This finding possibly indicates that CGRP-containing sensory nerves are more abundant in the proximal parts of the respiratory tract, that CGRP receptors are not evenly distributed within the lung with a higher density in the trachea, or that the effects of CGRP receptor binding differ among regions of the airways. Other mechanisms also could contribute to the observed vasodilation in the bronchial circulation. Muscarinic responses to vagal cholinergic stimulation might stimulate some vasodilation. CGRP has been shown recently to affect ganglionic transmission in the airways, and this effect might play a role in our model (21). In addition, we have identified neural nitric oxide synthase in a subset of neurons within airway intrinsic ganglia. It is possible that NO-mediated bronchial vasodilation might occur after inhalation of smoke.
In accordance with previous reports (12, 18, 38, 44, 47), burn and smoke inhalation injury in the present study was associated with a significant increase in pulmonary transvascular fluid flux, and this was effectively prevented by the treatment, suggesting that the increased fluid permeability was mainly mediated by CGRP. Changes in transvascular fluid flux are generally determined by four physical factors (45): 1) the hydrostatic pressure gradient, 2) permeability of the capillary wall, 3) the oncotic pressure gradient, and 4) the perfused surface area of the microvasculature. The present study indicates that the CGRP-mediated increase in pulmonary transvascular fluid flow was mainly due to the latter factor because there were no intergroup differences in 1) parameters of hydrostatic pressure such as mean pulmonary artery pressure and pulmonary capillary pressure, 2) indicators of increased permeability of the pulmonary capillary wall to proteins (lymph-to-plasma protein ratio), or 3) lymph-to-plasma colloid oncotic pressure ratio.
The injury in the present study was further associated with indirect signs of extrapulmonary microvascular hyperpermeability to proteins as indicated by a significant injury-induced decrease in plasma protein concentrations. The hypoproteinemia was unlikely to be a dilutional phenomenon, because hematocrit and hemoglobin significantly increased while fluid was administered. In contrast to the findings in the lung, extrapulmonary microvascular hyperpermeability to fluid and proteins was attenuated by the treatment, suggesting the involvement of CGRP in enhanced fluid and protein loss at other sites in our model, e.g., the cutaneous burn wounds. Fluid extravasation in the burn wound has been described previously in the ovine model (41). It is also possible that renal effects of CGRP could have affected fluid balance in this study (2).
Like humans, sheep subjected to severe smoke inhalation develop significant airway obstruction, which significantly contributes to the progression of respiratory failure following this injury. Smoke-induced airway obstruction results from a combination of bronchoconstriction, bronchial edema, and cast formation. Airway casts typically consist of four components: mucus, fibrin, epithelial cells, and neutrophils. It has previously been described that neurogenic inflammation in the lung is characterized by mucus hypersecretion, protein extravasation and potentiation of bronchial edema formation, neutrophil accumulation, and bronchoconstriction (7, 18). This observation led us to hypothesize that sensory neuropeptides such as CGRP may play a critical role in airway obstruction following inhalation injury. In fact, histologically assessed bronchial obstruction tended to be reduced by pretreatment with the CGRP receptor antagonist in the present study. Additionally, at autopsy, there were apparently less obstructive airway casts in treated sheep, probably resulting in the significantly reduced ventilatory pressures that were observed compared with untreated control animals. The lack of statistical significance in the histological assessment of structural changes partly reflects the biological variability in the experiments and the lack of precision of the histological measures used. The structural changes observed 48 h after injury were likely to be several steps removed from the initial stimulation of CGRP release. Study of morphological differences that may be present in animals treated with a CGRP antagonist during the first few hours after injury will be an interesting aspect of future work. The exact pathophysiological mechanisms of injury-induced airway obstruction and the specific influence of CGRP on this process have not been investigated in this study. Evidence from previous studies with different models and species suggests that CGRP itself is not an effective mediator of bronchoconstriction (6, 30), mucus hypersecretion (49), or protein extravasation (30) in the lung. Furthermore, it needs to be considered that CGRP represents only one of several mediators of neurogenic inflammation. Therefore, it is not surprising that injury-related cast formation and airway obstruction in the present experiment could not be completely prevented by the treatment. Also, it is not clear whether the CGRP that causes tracheal vasodilation arises principally from sensory nerves or from nerves arising in intrinsic airway ganglia. Other nitrergic, muscarinic, or nonadrenergic, noncholinergic (NANC) signals could contribute to the increased systemic blood flow to the airways and lungs in this model.
Nevertheless, administration of the CGRP receptor antagonist resulted in a significantly attenuated deterioration of pulmonary gas exchange in sheep with combined burn and smoke inhalation injury, while no adverse effects on systemic or pulmonary hemodynamic variables were observed. This raises the question whether a treatment strategy based on these findings could be beneficial for patients with this injury. However, some aspects need to be considered in this regard. We only tested the effects of pretreatment with a CGRP receptor antagonist. It remains undetermined whether posttreatment, or treatment of patients with established injury after hospital admission, would be likewise effective. In addition, CGRP is possibly implicated in local airway homeostasis or hypoxia-induced tissue remodeling and protection against hypoxic pulmonary hypertension. These regulatory, protective functions of CGRP could be adversely affected by the suggested treatment in a dose-dependent manner. In a previous study, intravenous bolus administration of 0.1–10 mg BIBN4096BS in healthy volunteers revealed a favorable overall safety profile, but with a trend toward an increased number of (mild) adverse events at the highest dose.
In conclusion, this is the first study demonstrating that CGRP mediates airway hyperemia in the early phase after burn and smoke inhalation injury in sheep. Pretreatment with a specific CGRP receptor antagonist attenuated the injury-related increase in pulmonary transvascular fluid flux and the degree of respiratory malfunction. This finding is significant because it opens a new area for investigation. It appears that after smoke inhalation injury, the initial reactions triggered by CGRP are important in the later development of acute lung injury and ARDS. Thus characterization of the mechanisms of the early inflammatory reaction are relevant in the study of ARDS due to injury by inhalation or aspiration of toxic compounds. Future studies will have to clarify whether posttreatment with a CGRP receptor antagonist is likewise effective. In addition, it needs to be investigated whether pharmacological interruption of the CGRP pathway exerts unfavorable effects on the likely regulatory or protective roles of this neuropeptide.
GRANTS
This study was supported by National Institutes of Health Program Project Grant P01-GM-066312.
Acknowledgments
We thank Collette Jonkam and the entire team of the Investigational Intensive Care Unit at the Univ. of Texas Medical Branch at Galveston for expert technical assistance in conducting this study, and Elbert Whorton for statistical assistance and advice. BIBN4096BS was kindly provided by Henri Doods and Klaus Rudolf, Boehringer-Ingelheim Pharma KG, Biberach, Germany.
REFERENCES
- 1.Aiyar N, Disa J, Siemens IR, Nambi P. Differential effects of guanine nucleotides on [125I]-hCGRP(8-37) binding to porcine lung and human neuroblastoma cell membranes. Neuropeptides 31: 99–103, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Ay I, Tuncer M. Mechanism of CGRP-induced vasodilation in the rat isolated perfused kidney. Pharmacology 71: 209–215, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Brain SD, Cambridge H. Calcitonin gene-related peptide: vasoactive effects and potential therapeutic role. Gen Pharmacol 27: 607–611, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol 147, Suppl 1: S202–S211, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cadieux A, Monast NP, Pomerleau F, Fournier A, Lanoue C. Bronchoprotector properties of calcitonin gene-related peptide in guinea pig and human airways. Effect of pulmonary inflammation. Am J Respir Crit Care Med 159: 235–243, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Cox RA, Burke AS, Kazutaka S, Kazunori M, Katahira J, Traber L, Herndon DN, Schmalstieg F, Traber D, Hawkins H. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol 29: 295–302, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dakhama A, Larsen GL, Gelfand EW. Calcitonin gene-related peptide: role in airway homeostasis. Curr Opin Pharmacol 4: 215–220, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol 129: 420–423, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edvinsson L, Tfelt-Hansen P. The blood-brain barrier in migraine treatment. Cephalalgia 28: 1245–1258, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips G, Parkinson J, Salsbury JR, Biondo N, Schmalstieg F, Burke A, Cox R, Hawkins H, Herndon D, Traber D. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am J Physiol Heart Circ Physiol 285: H2430–H2436, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips GB, Parkinson JF, Cox R, Hawkins H, Herndon D, Traber D. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med 167: 1021–1026, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Fontan JJ, Cortright DN, Krause JE, Velloff CR, Karpitskyi VV, Carver TW Jr, Shapiro SD, Mora BN. Substance P and neurokinin-1 receptor expression by intrinsic airway neurons in the rat. Am J Physiol Lung Cell Mol Physiol 278: L344–L355, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Grant AD, Tam CW, Lazar Z, Shih MK, Brain SD. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS blocks CGRP and adrenomedullin vasoactive responses in the microvasculature. Br J Pharmacol 142: 1091–1098, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray DW, Marshall I. Nitric oxide synthesis inhibitors attenuate calcitonin gene-related peptide endothelium-dependent vasorelaxation in rat aorta. Eur J Pharmacol 212: 37–42, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Hagner S, Haberberger R, Kummer W, Springer J, Fischer A, Bohm S, Goke B, McGregor GP. Immunohistochemical detection of calcitonin gene-related peptide receptor (CGRPR)-1 in the endothelium of human coronary artery and bronchial blood vessels. Neuropeptides 35: 58–64, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins HK, Chandra A, Shimoda K, Enkhabaatar P, Cox RA, Traber DL. Effects of neurokinin receptor blockade on smoke and burn injury in sheep (Abstract). J Burn Care Rehabil 25: S127, 2004. [Google Scholar]
- 18.Herndon DN, Traber DL, Niehaus GD, Linares HA, Traber LD. The pathophysiology of smoke inhalation injury in a sheep model. J Trauma Injury Crit Care 24: 1044–1051, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Herndon DN, Traber LD, Linares HA, Flynn JT, Niehaus G, Kramer G, Traber DL. Etiology of the pulmonary pathophysiology associated with inhalation injury. Resuscitation 14: 43–59, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Iovino M, Feifel U, Yong CL, Wolters JM, Wallenstein G. Safety, tolerability and pharmacokinetics of BIBN 4096 BS, the first selective small molecule calcitonin gene-related peptide receptor antagonist, following single intravenous administration in healthy volunteers. Cephalalgia 24: 645–656, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Kajekar R, Myers AC. Calcitonin gene-related peptide affects synaptic and membrane properties of bronchial parasympathetic neurons. Respir Physiol Neurobiol 160: 28–36, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keith IM The role of endogenous lung neuropeptides in regulation of the pulmonary circulation. Physiol Res 49: 519–537, 2000. [PubMed] [Google Scholar]
- 23.Kramer HH, Schmidt K, Leis S, Schmelz M, Sommer C, Birklein F. Inhibition of neutral endopeptidase (NEP) facilitates neurogenic inflammation. Exp Neurol 195: 179–184, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Laitinen LA, Laitinen A, Salonen RO, Widdicombe JG. Vascular actions of airway neuropeptides. Am Rev Respir Dis 136: S59–S64, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Lange M, Hamahata A, Enkhbaatar P, Esechie A, Connelly R, Nakano Y, Jonkam C, Cox RA, Traber LD, Herndon DN, Traber DL. Assessment of vascular permeability in an ovine model of acute lung injury and pneumonia-induced Pseudomonas aeruginosa sepsis. Crit Care Med 36: 1284–1289, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Lentz CW, Abdi S, Traber LD. The role of sensory neuropeptides in inhalation injury. Proc Am Burn Assoc 10: 27–37, 1992. [Google Scholar]
- 27.Li PC, Chen WC, Chang LC, Lin SC. Substance P acts via the neurokinin receptor 1 to elicit bronchoconstriction, oxidative stress, and upregulated ICAM-1 expression after oil smoke exposure. Am J Physiol Lung Cell Mol Physiol 294: L912–L920, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lin YS, Kou YR. Acute neurogenic airway plasma exudation and edema induced by inhaled wood smoke in guinea pigs: role of tachykinins and hydroxyl radical. Eur J Pharmacol 394: 139–148, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg JM, Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature 302: 251–253, 1983. [DOI] [PubMed] [Google Scholar]
- 30.Martling CR Sensory nerves containing tachykinins and CGRP in the lower airways. Functional implications for bronchoconstriction, vasodilatation and protein extravasation. Acta Physiol Scand Suppl 563: 1–57, 1987. [PubMed] [Google Scholar]
- 31.Martling CR, Saria A, Fischer JA, Hokfelt T, Lundberg JM. Calcitonin gene-related peptide and the lung: neuronal coexistence with substance P, release by capsaicin and vasodilatory effect. Regul Pept 20: 125–139, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Matran R, Alving K, Martling CR, Lacroix JS, Lundberg JM. Effects of neuropeptides and capsaicin on tracheobronchial blood flow of the pig. Acta Physiol Scand 135: 335–342, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Matran R, Alving K, Martling CR, Lacroix JS, Lundberg JM. Vagally mediated vasodilatation by motor and sensory nerves in the tracheal and bronchial circulation of the pig. Acta Physiol Scand 135: 29–37, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Maybauer DM, Maybauer MO, Traber LD, Westphal M, Nakano YY, Enkhbaatar P, Morita N, Herndon DN, Traber DL. Effects of severe smoke inhalation injury and septic shock on global hemodynamics and microvascular blood flow in sheep. Shock 26: 489–495, 2006. [DOI] [PubMed] [Google Scholar]
- 35.McCormack DG, Mak JC, Coupe MO, Barnes PJ. Calcitonin gene-related peptide vasodilation of human pulmonary vessels. J Appl Physiol 67: 1265–1270, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Moreno MJ, Abounader R, Hebert E, Doods H, Hamel E. Efficacy of the non-peptide CGRP receptor antagonist BIBN4096BS in blocking CGRP-induced dilations in human and bovine cerebral arteries: potential implications in acute migraine treatment. Neuropharmacology 42: 568–576, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Mulderry PK, Ghatei MA, Spokes RA, Jones PM, Pierson AM, Hamid QA, Kanse S, Amara SG, Burrin JM, Legon S. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neurosci 25: 195–205, 1988. [DOI] [PubMed] [Google Scholar]
- 38.Murakami K, Traber DL. Pathophysiological basis of smoke inhalation injury. News Physiol Sci 18: 125–129, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 54: 233–246, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Reynier-Rebuffel AM, Mathiau P, Callebert J, Dimitriadou V, Farjaudon N, Kacem K, Launay JM, Seylaz J, Abineau P. Substance P, calcitonin gene-related peptide, and capsaicin release serotonin from cerebrovascular mast cells. Am J Physiol Regul Integr Comp Physiol 267: R1421–R1429, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Sakurai H, Nozaki M, Traber LD, Hawkins HK, Traber DL. Microvascular changes in large flame burn wound in sheep. Burns 28: 3–9, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Shirani KZ, Pruitt BA, Mason AD. The influence of inhalation injury and pneumonia on burn mortality. Ann Surg 205: 82–87, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soejima K, Cox RA, Hawkins HK, Schmalstieg FC, Traber L, Traber D. The role of nitric oxide in inflammation after combined burn and smoke inhalation injury. Am J Respir Crit Care Med 161: A97, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Soejima K, Schmalstieg FC, Sakurai H, Traber LD, Traber DL. Pathophysiological analysis of combined burn and smoke inhalation injuries in sheep. Am J Physiol Lung Cell Mol Physiol 280: L1233–L1241, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Starling EH On the absorption of fluids from the connective tissue spaces. J Physiol 19: 312–326, 1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai CF, Baraniuk JN. Upper airway neurogenic mechanisms. Curr Opin Allergy Clin Immunol 2: 11–19, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Traber DL, Hawkins HK, Enkhbaatar P, Cox RA, Schmalstieg FC, Zwischenberger JB, Traber LD. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther 20: 163–166, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Traber DL, Maybauer MO, Maybauer DM, Westphal M, Traber LD. Inhalational and acute lung injury. Shock 24, Suppl 1: 82–87, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Webber SE, Lim JC, Widdicombe JG. The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br J Pharmacol 102: 79–84, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westphal M, Cox RA, Traber LD, Morita N, Enkhbaatar P, Schmalstieg FC, Hawkins HK, Maybauer DM, Maybauer MO, Murakami K, Burke AS, Westphal-Varghese BB, Rudloff HE, Salsbury JR, Jodoin JM, Lee S, Traber DL. Combined burn and smoke inhalation injury impairs ovine hypoxic pulmonary vasoconstriction. Crit Care Med 34: 1428–1436, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Widdicombe JG The NANC system and airway vasculature. Arch Int Pharmacodyn Ther 303: 83–99, 1990. [PubMed] [Google Scholar]
- 52.Widdicombe JG Overview of neural pathways in allergy and asthma. Pulm Pharmacol Ther 16: 23–30, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Wong SS, Sun NN, Lantz RC, Witten ML. Substance P and neutral endopeptidase in development of acute respiratory distress syndrome following fire smoke inhalation. Am J Physiol Lung Cell Mol Physiol 287: L859–L866, 2004. [DOI] [PubMed] [Google Scholar]