Abstract
Recent studies show that brief exercise alters circulating neutrophil and peripheral blood mononuclear cell (PBMC) gene expression, ranging from cell growth to both pro-and anti-inflammatory processes. These initial observations were made solely in males, but whether PBMC gene expression is altered by exercise in females is not known. Ten early-pubertal girls (8–11 yr old) and 10 late-pubertal girls (15–17 yr old) performed ten 2-min bouts of cycle ergometry (∼90% peak heart rate) interspersed with 1-min rest intervals. Blood was obtained at rest and after exercise, and microarrays were performed in each individual subject. RNA was hybridized to Affymetrix U133+2.0 Arrays. Exercise induced significant changes in PBMC gene expression in early (1,320 genes)- and late (877 genes)-pubertal girls. The expression of 622 genes changed similarly in both groups. Exercise influenced a variety of established gene pathways (EASE < 0.04) in both older (6 pathways) and younger girls (11 pathways). Five pathways were the same in both groups and were functionally related to inflammation, stress, and apoptosis, such as natural killer cell-mediated cytotoxicity, antigen processing and presentation, B cell receptor signaling, and apoptosis. In summary, brief exercise alters PBMC gene expression in early- and late-pubertal girls. The pattern of change involves diverse genetic pathways, consistent with a global danger-type response, perhaps readying PBMCs for a range of physiological functions from inflammation to tissue repair that would be useful following a bout of physical activity.
Keywords: leukocytes, puberty, gender, microarrays, immune system
in this study we examined for the first time the effects of exercise on peripheral blood mononuclear cell (PBMC) gene expression in early- and late-pubertal girls. There are mounting data that leukocytes are not only involved in eradicating pathogens but also play a role in wound repair, muscle growth, other key developmental processes, and childhood diseases like asthma and arthritis (12, 13, 27, 31). Moreover, brief exercise can trigger bronchoconstriction and anaphylaxis, both serious conditions in which the inflammatory response appears to be dysregulated (7). Our knowledge of the acute effects of exercise on leukocyte gene expression in humans is still rudimentary.
In both adults and children, a leukocytosis occurs following brief, heavy exercise (29). In addition, we now know from recent studies performed in this and other laboratories that there are remarkable changes in the gene expression profile pattern of the circulating leukocytes rapidly accompanying exercise (2, 4, 9, 25). The impact of exercise on the immune response is increasingly seen as one of the fundamental mechanisms through which levels of physical activity modulate health, growth, and disease risk in both children and adults.
We hypothesized, first, that exercise would stimulate gene expression in PBMCs in girls; and second, that the genomic response would include alterations in pro- and anti-inflammatory cytokines, stress factors, and growth mediators. The analysis of individual gene responses through techniques like microarray is becoming a powerful tool in understanding mechanisms that control the physiological response of immune cells to a variety of perturbations, but the enormity of the data generated by such analyses can be perplexing, and, at the same time, it is increasingly recognized that the functional, physiological significance of changes in gene expression may be better understood by examining the coordinated modulation of groups of genes acting in discrete pathways. Since very little in general is yet known about individual gene expression of leukocytes in response to exercise, and even less about specific gene profiles in particular, one major objective of this study was to analyze PBMC gene responses to exercise in terms of pathways.
In general, there are fewer studies focused on immune responses to perturbations like exercise in females compared with males. However, it is well recognized that both gender and puberty can influence immune function (11, 17, 28), and there is a small but growing body of literature suggesting that leukocyte gene expression may also be influenced by these factors (14). The primary focus of the present study was to continue to test the hypothesis that exercise alters circulating leukocyte gene expression in children as well as in adults. We also began to explore whether puberty altered leukocyte gene expression in response to exercise by recruiting females specifically in either the early or late pubertal stages of childhood and adolescence.
MATERIALS AND METHODS
Subjects
Twenty healthy females participated in this study (Table 1). Ten early-pubertal participants (age range 8–11 yr old) and 10 late-pubertal participants (age range 15–17 yr old) comprised the study sample. We used a validated self-administered questionnaire that has been widely used to assess pubertal status (23, 26). Using this tool, only girls who were at Tanner 1 were included in the early-pubertal group and those at Tanner 5 were included in the late-pubertal group. In the late-pubertal girls, we made no attempt to perform exercise selectively in either the luteal or follicular phase of the menstrual cycle. Individuals participating in competitive sports and with a history of any chronic medical conditions or use of any medications were excluded from participation. The Institutional Review Board at the University of California, Irvine approved the study, and written informed assent and consent was obtained from all participants and their parents upon enrollment.
Table 1.
Anthropometric and physiological characteristics of the 20 subjects
| Early-Pubertal Girls (n = 10) | Late-Pubertal Girls (n = 10) | |
|---|---|---|
| Age, yr | 10.0±0.3 | 16.1±0.4* |
| Height, cm | 137.3±1.9 | 161.6±1.4* |
| Weight, kg | 30.6±1.8 | 56.9±2.2* |
| %Fat | 24.3±1.9 | 27.6±1.7 |
| Peak V̇o2, ml·min−1·kg−1 | 35.6±1.4 | 33.2±2.2 |
Values are means ± SE. Peak V̇o2, peak oxygen uptake.
Significant difference between early- and late-pubertal girls (P < =0.0002).
Anthropometric Measurements
Standard calibrated scales and stadiometers were used to determine height and body mass. Dual-energy X-ray absorptiometry (DXA) was used to measure body fat, expressed as a percentage.
Measurement of Fitness
Each subject performed a ramptype progressive cycle ergometer using the SensorMedics metabolic system (Ergoline 800S, Yorba Linda, CA). Subjects were vigorously encouraged during the high-intensity phases of the exercise protocol. Gas exchange was measured breath-by-breath, and the anaerobic (lactate) threshold and peak V̇o2 were calculated using standard methods (6).
Exercise Protocol
At least 48 h but not exceeding 7 days following the completion of the ramp test, each subject performed exercise consisting of ten 2-min bouts of constant-work-rate cycle ergometry, with a 1-min rest interval between each bout of exercise. The work rate was individualized for each girl and was calculated to be equivalent to the work rate corresponding roughly to 50% of the work rate between the anaerobic threshold and the peak oxygen uptake (as determined noninvasively from the ramptype test). This resulted in a relative work rate that was equivalent between study subjects. We have used this protocol in the past, first to more closely mimic the “stop-start” nature of spontaneous physical activity (1), and second to ensure that the exercise input was standardized to physiological indicators of each subject's exercise capacity (32).
Blood Sampling and Analysis
An indwelling catheter was inserted into the antecubital vein. A baseline sample was taken 30 min after the placement of the catheter and before the onset of exercise. We waited 30 min to ensure that measurable physiological parameters of stress (e.g., heart rate and blood pressure) were at baseline levels. Subjects then completed the ten 2-min bouts of constant work rate, and additional blood samples were obtained immediately after exercise (total of 40 samples). The plasma was separated and stored at −80°C and thawed only once for analysis. Complete blood counts (CBC) for white blood cell analysis were obtained by standard methods from the clinical hematology laboratory at University of California, Irvine.
PBMC Separation
PBMCs were isolated using OptiPrep density gradient medium (Sigma). Standard and consistent practices were employed in an effort to minimize any potential changes in mRNA expression levels due to manipulation of PBMCs. The duration from blood draw to stabilization of RNA never exceeded 60 min.
RNA Extraction
Total RNA was extracted using TRIzol (Gibco BRL Life Technologies, Rockville, MD) reagent and purified using the RNeasy Midi columns method (Qiagen, Valencia, CA). RNA pellets were resuspended in diethyl pyrocarbonate-treated water. RNA integrity was assessed (before beginning target processing) by running out a small amount of each sample (typically 25–250 ng/well) onto a RNA Lab-On-A-Chip (Caliper Technologies, Mountain View, CA) that was evaluated on an Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA).
Preparation of Labeled cRNA
The detailed protocol for preparation and microarray processing was performed as recommended by the manufacturer and is available in the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). Briefly, 4 μg total RNA was used as a template for double-stranded cDNA synthesis. Single-stranded then double-stranded cDNA was synthesized from the poly(A) spike-in controls and mRNA present in the isolated total RNA using the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) and a T7-oligo(dT) primer (Integrated DNA Technologies, Coralville, IA) that contains a T7 RNA polymerase promoter site added to its 3′-end. A portion of the resulting double-stranded cDNA was used as a template to generate biotin-tagged cRNA from an in vitro transcription reaction (IVT), using the Affymetrix GeneChip IVT Labeling Kit.
Hybridization to Microarray
A total of 15 μg of the resulting biotin-tagged cRNA was fragmented to an average strand length of 100 bases (range 35–200 bases) following prescribed protocols (Affymetrix GeneChip Expression Analysis Technical Manual). Subsequently, 10 μg of this fragmented target cRNA was hybridized at 45°C with rotation for 16 h (Affymetrix GeneChip Hybridization Oven 640) to probe sets present on an Affymetrix U133+2 arrays. The GeneChip arrays were washed and then stained (SAPE, streptavidin-phycoerythrin) on an Affymetrix Fluidics Station 450, followed by scanning on a GeneChip Scanner 3000. Microarrays were performed in each individual subject, not in pooled samples.
Real-time PCR (RT-PCR).
For confirmation of microarray gene expression findings, RT-PCR was carried out on six genes selected from the natural killer cell-mediated cytotoxicity pathway (FASLG, GZMB, PRF1, CASP3, CD247, and KLRD1). This pathway was significantly altered by exercise in both the early- and late-pubertal girls. One microgram of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Reagents kit (Applied Biosystems) according to the manufacturer's instructions, using random primers in a 20-μl reaction. The RT-PCR analysis was performed with the ABI PRISM 7000 Sequence Detection System (Applied Biosystems) by using TaqMan Universal PCR Master Mix and Assays-on-Demand Gene Expression probes (Applied Biosystems) (FASLG: assay ID, Hs00181226_g1; GZMB: assay ID, Hs00188051_m1; PRF1: assay ID, Hs00169473_m1; CASP3: assay ID, Hs00263337_m1; CD247: assay ID, Hs00609515_m1; KLRD1: assay ID, Hs00233844_m1). Actin beta was used as an endogenous control.
Data Analysis
Microarray analysis.
The results were quantified and analyzed using GCOS 1.4 software (Affymetrix) using default values (Scaling Target Signal Intensity = 500). The microarray data were analyzed using ArrayAssist version 5.2.2 (StrataGene). We normalized the data using GC-RMA. Only probe sets that reached a signal value ≥20 in at least one array and a present call by MAS5 criteria in at least 30 arrays were selected for further analysis. Overall, 24,089 of 54,675 probe sets represented on the array met these criteria. The microarray CEL files and GC-RMA normalized data have been deposited in the GEO database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14642). We further applied BRB-ArrayTools software version 3.6.0 (http//linus.nci.nih.gov/brb/tool.htm) to determine significantly changed probe sets from before to after exercise for early-pubertal and late-pubertal girls separately. Traditional Student's paired t-test was first applied to each probe set, and then significantly changed probe sets were identified with permutation tests (30). With 95% confidence, the final list of significantly changed probe sets in each group has less than a 5% false discovery rate (FDR). The change of gene expression from preexercise to postexercise was additionally compared between early- and late-pubertal girls using a two-sample t-test and FDR adjustment.
Gene annotation.
The final list of significantly changed probe sets was then additionally analyzed using the functional annotation tools provided by DAVID, the Database for Annotation, Visualization and Integrated Discovery (http://david.abcc.ncifcrf.gov), to classify the genes into pathways using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Only pathways with Expression Analysis Systematic Explorer (EASE) score < 0.044 are presented in this analysis. EASE score is a modified Fisher exact P value in the DAVID system used for gene-enrichment analysis. EASE score P value = 0 represents perfect enrichment. P value ≤ 0.05 is considered as gene enrichment in a specific annotation category (http://david.abcc.ncifcrf.gov/helps/functional_annotation.html#summary).
Comparison of Microarray Results to RT-PCR
We used a paired t-test to determine the effect of exercise on the six genes selected in the 10 early-pubertal and 10 late-pubertal girls.
Physiological Data
The physiological data are presented as means and SE. The two-sided paired t-test was applied for testing changes from before to after the exercise within each group, and the two-sample t-test was applied for examining group difference. All analyses were done using SAS9 (Cary, NC), and the significance level was set at 0.05.
RESULTS
Anthropometric and Physiological Characteristics
The anthropometric and physiological characteristics of the 20 participants appear in Table 1. The subjects were of normal fitness (early-pubertal girls: 93.5 ± 3.7% of the subjects' predicted V̇o2max; late-pubertal girls: 98.8 ± 6.7% of the subjects' predicted V̇o2max) (5).
Exercise Intensity and Lactic Acid Levels
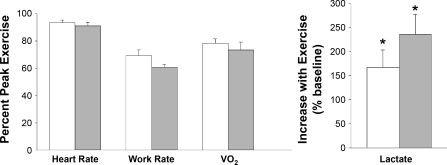
Relative exercise intensity as determined by heart rate, work rate, and V̇o2 was similar between the two groups (Fig. 1). In the older girls, lactate increased from 1.94 ± 0.61 to 6.27 ± 1.98 mmol/l, P < 0.0001 (+235.8 ± 74.6%). Lactate also increased significantly in the early-pubertal girls [from 2.14 ± 0.67 mmol/l to 5.40 ± 1.70 mmol/l, P = 0.0008 (+166.8 ± 52.8%)].
Fig. 1.
Assessment of exercise intensity during the ten 2-min bouts used to gage the peripheral blood mononuclear cell (PBMC) genomic response in early (white bars)- and late (gray bars)-pubertal girls. The values represent the means ± SE of heart rate, work rate, and oxygen uptake (V̇o2) expressed as percentage of the individual participant's peak values obtained in an earlier session from the progressive exercise protocol. As can be seen, the relative exercise intensity was virtually the same in the 2 groups. Lactate increased in both groups (*P < 0.05)
PBMC Response to Exercise
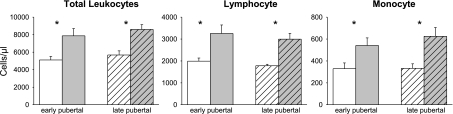
As shown in Fig. 2, the number of total WBCs, lymphocytes, and monocytes was found to be significantly elevated at peak exercise in both early-pubertal girls [WBC increases 2,760 ± 466 (51%, P = 0.0002), lymphocytes 1,262 ± 280 (60%, P = 0.0015), and monocytes 212 ± 52 (92%, P = 0.0026)] and late-pubertal girls [WBC 2,800 ± 414 (52%, P = 0.0001), lymphocytes 1,174 ± 321 (72%, P = 0.0064), monocytes 287 ± 86 (94%, P = 0.012)]. There was not significant difference in the number of cells increased in late- compared with early-pubertal girls.
Fig. 2.
Effect of exercise on PBMCs in the early and late pubertal girls. Data represent the means ± SE before (white bars) and immediately after exercise (gray bars) for all leukocytes, lymphocytes, and monocytes. Hatched bars represent the late-pubertal girls. *Significant (P < 0.05) increase from before to after exercise.
Effect of Exercise on Gene Expression
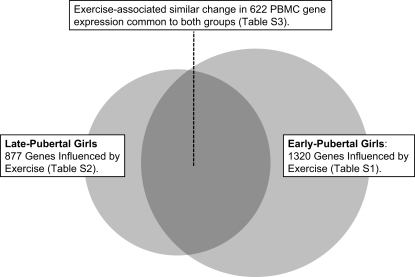
Exercise induced significant changes in PBMC gene expression in both the early- and late-pubertal girls. Exercise altered 877 genes (611 up, 266 down) (FDR < 0.05) in late-pubertal girls and 1,320 (829 up, 491 down) in the early-pubertal girls. Detailed lists of genes that were altered by exercise in early- and late-pubertal girls are shown in Supplemental Tables S1 and S2, respectively, which are available with the online version of this article. Figure 3 schematically compares the magnitude of the PBMC gene change in the two groups and shows the 622 genes that were significantly affected in a similar direction (420 up, 202 down) in both groups (Supplemental Table S3).
Fig. 3.
Comparison of the effect of exercise on PBMC genes in early- and late-pubertal girls, showing the relative magnitude of the effect (circles) and the size of the overlap (shaded area). There were 622 PBMC genes that were significantly altered by exercise in both groups. Tables S1–S3 are available with the online version of this article.
We characterized genes affected by exercise in each group using gene pathway analysis using KEGG. We found 11 pathways in the early-pubertal girls and six pathways in the late-pubertal girls that were significantly affected by exercise (EASE score < 0.044) (Table 2). Five pathways were the same in both groups. A complete detailed list of the individual genes in the five common pathways is shown in Table 3. Many of the pathways identified were involved in innate or early immune responses.
Table 2.
Gene pathways as classified by KEGG that were significantly changed by exercise in early (11 pathways)- and late (6 pathways)-pubertal girls
| Pathway | No. of Genes |
P Value (EASE Score) | ||
|---|---|---|---|---|
| EG | LG | EG | LG | |
| Natural killer cell-mediated cytotoxicity | 22 | 18 | 2.8×10−4 | 1.8×10−5 |
| Antigen processing and presentation | 16 | 13 | 4.6×10−4 | 9.2×10−5 |
| B cell receptor signaling pathway | 14 | 10 | 5.7×10−4 | 1.3×10−3 |
| Type I diabetes mellitus | 9 | 7 | 5.6×10−3 | 4.9×10−3 |
| Hematopoietic cell lineage | 14 | 7.0×10−3 | NS | |
| Apoptosis | 12 | 9 | 3.7×10−2 | 2.2×10−2 |
| Adherens junction | 11 | 3.7×10−2 | NS | |
| Jak-STAT signaling pathway | 18 | 4.4×10−2 | NS | |
| Focal adhesion | 22 | 2.8×10−4 | NS | |
| Ubiquitin-mediated proteolysis | 16 | 4.6×10−4 | NS | |
| Glycan structures- biosynthesis 1 | 15 | 5.7×10−4 | NS | |
| Keratan sulfate biosynthesis | 9 | NS | 2.6×10−2 | |
EG, early-pubertal girls; LG, late-pubertal girls; KEGG, Kyoto Encyclopedia of Genes and Genomes; EASE, Expression Analysis Systematic Explorer; NS, not significant.
Table 3.
Five pathways that were altered by exercise in both early- and late-pubertal girls, classified by KEGG
| Pathway/Gene Name | Gene Symbol | Fold Change* |
|
|---|---|---|---|
| EG | LG | ||
| Antigen processing and presentation (EG, 16 genes; LG, 13 genes) | |||
| Class ii, major histocompatibility complex, transactivator | CIITA | 0.71 | 0.77 |
| Major histocompatibility complex, class ii, dm alpha | HLA-DMA | 0.83 | |
| Major histocompatibility complex, class ii, do alpha | HLA-DOA | 0.71 | 0.70 |
| Major histocompatibility complex, class ii, do beta | HLA-DOB | 0.67 | 0.63 |
| Major histocompatibility complex, class ii, dp alpha 1 | HLA-DPA1 | 0.80 | |
| Heat shock 70-kDa protein 1a | HSPA1B | 2.90 | 3.37 |
| Heat shock 70-kDa protein 8 | HSPA8 | 1.15 | 1.14 |
| Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 3 | KIR2DL3 | 2.78 | 3.33 |
| Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 4 | KIR2DL4 | 2.34 | 2.43 |
| Killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail, 1 | KIR3DL1 | 2.91 | 2.98 |
| Killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail, 2 | KIR3DL2 | 3.04 | 3.27 |
| Killer cell lectin-like receptor subfamily c, member 3 | KLRC3 | 1.49 | 1.62 |
| Killer cell lectin-like receptor subfamily d, member 1 | KLRD1 | 2.08 | 1.85 |
| Killer cell lectin-like receptor subfamily c, member 4 | KLRK1 | 1.27 | |
| Transporter 1, ATP-binding cassette, sub-family b (mdr/tap) | TAP1 | 1.18 | |
| Natural killer cell mediated cytotoxicity (EG, 22 genes; LG, 18 genes) | |||
| vav 2 oncogene | VAV2 | 0.84 | |
| Hematopoietic cell signal transducer | HCST | 1.16 | |
| Interferon (alpha, beta and omega) receptor 1 | IFNAR1 | 1.21 | |
| fyn oncogene related to src, fgr, yes | FYN | 1.24 | |
| Caspase 3, apoptosis-related cysteine peptidase | CASP3 | 1.26 | 1.28 |
| zeta-chain (tcr) associated protein kinase 70 kDa | ZAP70 | 1.26 | |
| Killer cell lectin-like receptor subfamily c, member 4 | KLRK1 | 1.27 | |
| Phosphoinositide-3-kinase, catalytic, gamma polypeptide | PIK3CG | 1.37 | 1.33 |
| cd244 natural killer cell receptor 2b4 | CD244 | 1.37 | |
| cd3z antigen, zeta polypeptide (tit3 complex) | CD247 | 1.46 | 1.60 |
| sh2 domain protein 1a, Duncan's disease (lymphoproliferative syndrome) | SH2D1A | 1.46 | 1.45 |
| Killer cell lectin-like receptor subfamily c, member 3 | KLRC3 | 1.49 | 1.62 |
| Fc fragment of igg, low affinity iiib, receptor (cd16b) | FCGR3B | 1.91 | |
| Killer cell lectin-like receptor subfamily d, member 1 | KLRD1 | 2.08 | 1.85 |
| Perforin 1 (pore forming protein) | PRF1 | 2.24 | 2.08 |
| Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 4 | KIR2DL4 | 2.34 | 2.43 |
| Granzyme b (granzyme 2, cytotoxic t-lymphocyte-associated serine esterase 1) | GZMB | 2.39 | 2.25 |
| Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 3 | KIR2DL3 | 2.78 | 3.33 |
| Killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail, 1 | KIR3DL1 | 2.91 | 2.98 |
| Killer cell immunoglobulin-like receptor, three domains, long cytoplasmic tail, 2 | KIR3DL2 | 3.04 | 3.27 |
| Sh2 domain containing 1b | SH2D1B | 3.10 | 2.72 |
| fas ligand (tnf superfamily, member 6) | FASLG | 4.36 | 4.46 |
| Mitogen-activated protein kinase 1 | MAPK1 | 1.30 | |
| Nuclear factor of activated t-cells, cytoplasmic, calcineurin-dependent 2 | NFATC2 | 1.33 | |
| Son of sevenless homolog 1 (Drosophila) | SOS1 | 1.42 | |
| B cell receptor signaling pathway (EG, 14 genes; LG, 10 genes) | |||
| b-cell cll/lymphoma 10 | BCL10 | 0.54 | 0.62 |
| cd19 antigen | CD19 | 0.58 | 0.58 |
| cd22 antigen | CD22 | 0.58 | 0.58 |
| b-cell linker | BLNK | 0.66 | 0.70 |
| cd72 antigen | CD72 | 0.66 | 0.70 |
| cd79a antigen (immunoglobulin-associated alpha) | CD79A | 0.66 | 0.66 |
| Nuclear factor of kappa light polypeptide gene enhancer in b-cells inhibitor, alpha | NFKBIA | 0.68 | 0.70 |
| ras guanyl releasing protein 3 (calcium and dag-regulated) | RASGRP3 | 0.71 | 0.64 |
| vav 2 oncogene | VAV2 | 0.84 | |
| Bruton agammaglobulinemia tyrosine kinase | BTK | 0.85 | |
| v-akt murine thymoma viral oncogene homolog 3 (protein kinase b, gamma) | AKT3 | 1.24 | |
| v-fos fbj murine osteosarcoma viral oncogene homolog | FOS | 1.36 | |
| Phosphoinositide-3-kinase, catalytic, gamma polypeptide | PIK3CG | 1.37 | 1.33 |
| Nuclear factor of activated t-cells, cytoplasmic, calcineurin-dependent 2 | NFATC2 | 1.33 | |
| Type I diabetes mellitus (EG, 9 genes; LG, 7 genes) | |||
| Major histocompatibility complex, class ii, do beta | HLA-DOB | 0.67 | 0.63 |
| Major histocompatibility complex, class ii, do alpha | HLA-DOA | 0.71 | 0.70 |
| Major histocompatibility complex, class ii, dp alpha 1 | HLA-DPA1 | 0.80 | |
| Major histocompatibility complex, class ii, dm alpha | HLA-DMA | 0.83 | |
| Major histocompatibility complex, class ii, dq beta 1 | HLA-DQB1 | 0.83 | |
| Islet cell autoantigen 1, 69 kDa | GLCCI1 | 1.20 | 1.27 |
| Perforin 1 (pore forming protein) | PRF1 | 2.24 | 2.08 |
| Granzyme b (granzyme 2, cytotoxic t-lymphocyte-associated serine esterase 1) | GZMB | 2.39 | 2.25 |
| fas ligand (tnf superfamily, member 6) | FASLG | 4.36 | 4.46 |
| Major histocompatibility complex, class i, f | HLA-F | 1.25 | |
| Apoptosis (EG, 12 genes; LG, 9 genes) | |||
| Nuclear factor of kappa light polypeptide gene enhancer in b-cells inhibitor, alpha | NFKBIA | 0.68 | 0.70 |
| Protein kinase, camp-dependent, regulatory, type ii, beta | PRKAR2B | 0.76 | |
| Baculoviral iap repeat-containing 2 | BIRC2 | 1.12 | |
| Receptor (tnfrsf)-interacting serine-threonine kinase 1 | RIPK1 | 1.16 | 1.22 |
| v-akt murine thymoma viral oncogene homolog 3 (protein kinase b, gamma) | AKT3 | 1.24 | |
| Caspase 3, apoptosis-related cysteine peptidase | CASP3 | 1.26 | 1.28 |
| Caspase 10, apoptosis-related cysteine peptidase | CASP10 | 1.33 | 1.32 |
| Tumor necrosis factor receptor superfamily, member 1a | TNFRSF1A | 1.35 | |
| Phosphoinositide-3-kinase, catalytic, gamma polypeptide | PIK3CG | 1.37 | 1.33 |
| Baculoviral iap repeat-containing 4 | BIRC4 | 1.40 | |
| fas (tnfrsf6)-associated via death domain | FADD | 1.49 | 1.42 |
| fas ligand (tnf superfamily, member 6) | FASLG | 4.36 | 4.46 |
| casp8 and fadd-like apoptosis regulator | CFLAR | 1.30 | |
| Interleukin-1 receptor-associated kinase 2 | IRAK2 | 1.38 | |
EG, early-pubertal girls; LG, late-pubertal girls.
Fold change is defined as the geometric mean of expression levels of After/Before. A fold change of 0.7 indicates the expression level after exercise is about 70% of the expression level before the exercise. A fold change of 1.6 indicates the expression level after the exercise is about 160% of the expression level before the exercise.
Comparison between Early- and Late-Pubertal Females
There were 255 genes affected by exercise in the late-pubertal girls that were not affected in the early-pubertal girls, and conversely, there were 698 genes affected by exercise in the early pubertal girls that were not affected in the late (Fig. 3). However, when we applied the statistical analysis using two-sample t-test described above, we found no significant differences (10% FDR) in individual PBMC gene expression in response to exercise between early- and late-pubertal girls.
RT-PCR Corroboration of Specific Genes
The six genes selected for RT-PCR all were found to be significantly upregulated by microarray analysis. Similarly, analysis of RT-PCR in all 20 participants (early- and late-pubertal girls) revealed significant changes in these same genes (P < 0.004, by paired t-test). Similar to our previous studies (4, 24, 25), the RT-PCR changes parallel those found by microarray analysis.
DISCUSSION
We found that a relatively brief bout of exercise, designed to mimic more natural patterns of physical activity in children, induced a remarkable change in PBMC gene expression in healthy females. These observations were made using a stringent statistical analysis of the microarray data to limit the possibility of false-positive results. In the early-pubertal girls the relatively brief but heavy exercise protocol altered 1,320 genes, whereas in the late-pubertal girls the expression of 877 genes was changed. The expression of 622 genes changed similarly in both groups (Fig. 3, Supplemental Table S3). We also found that exercise influenced a variety of established gene pathways (EASE < 0.044) in both older (6 pathways) and younger girls (11 pathways, Table 2). The five pathways that were the same in both groups and their individual genes are shown in Table 3. The majority of the significant pathways was related to inflammation, stress, and apoptosis, consistent with previous work from this and other laboratories on gene expression following exercise in circulating leukocytes in humans.
Two alternative mechanisms might explain the robust effect of exercise on PBMC gene pathways: the first, a direct effect of exercise on gene expression within the population of circulating PBMCs; and the second, an indirect effect, the mobilization into the circulation of PBMCs that were expressing genes differently in their marginal pools (e.g., lung, lymphatics) or because of different maturational status of the mobilized PBMCs compared with those PBMCs already in the circulating blood. In human studies, it would be unfeasible to sample gene expression of marginal pools of PBMCs. Nonetheless, our data do permit us to draw some inferences concerning possible mechanisms.
Consider first, for example, the gene with the highest increase in expression—fas ligand (FSALG). The 4.4-fold increase that we observed for this gene could occur, however, only if marginal PBMCs that entered the circulation during exercise (N.B., we observed about a 65% increase) were expressing FASLG at levels ∼10-fold greater than the circulating PBMCs. The decrease in expression for another gene, fusion (FUS), which had a fold change of 0.4, is possibly even more definitive. If, in the extreme case, the marginal PBMCs had no detectable expression of this gene, the lowest possible postexercise fold change would be 0.6. Thus it is reasonable to speculate from the current data that exercise has at least some direct effect on gene expression even in the circulating population of PBMCs.
Particularly intriguing was our finding of what appeared to be activation of the natural killer (NK) cell-mediated cytotoxicity pathway. When upregulated, the genes in this pathway enable circulating leukocytes to identify, attach to, and ultimately kill cells perceived as foreign or dangerous (NK cells express an array of activating cell surface receptors that can trigger cytolytic programs, as well as cytokine or chemokine secretion). Although this particular pathway was so named because of work done in NK cells, many of the same genes are expressed in cytotoxic lymphocytes as well (16). Consequently, changes in gene expression that we identified did not, most likely, result from alterations of gene expression solely in the relatively small number of NK cells but also in CD8+ lymphocytes as well.
In the early-pubertal girls, 22 genes in the NK cell-mediated cytotoxicity pathway were altered following exercise (21 had higher expression), and in the late-pubertal girls 18 genes in this pathway had higher expression after exercise (Table 3). Many of the genes that had higher expression after exercise involved cell receptors (10 receptors in the early-pubertal girls and 7 receptors in the late-pubertal girls) (Table 3). NK surface receptors can trigger cytolytic programs, as well as cytokine or chemokine secretion associated with inducing apoptosis in target cells. Indeed, there is evidence suggesting that exercise does induce physiological activation of innate immune cells like NK cells (18, 19), providing a possible link between the exercise-induced changes in gene expression and functional outcomes.
The NK cell-mediated cytotoxic pathway includes processes in which potential target cells must be recognized as “foreign”; consequently, it is not surprising that the antigen processing and presentation pathway, too, was altered by exercise (early-pubertal girls, 16 genes; late-pubertal girls, 13 genes, Table 3). In the early-pubertal girl group, 10 genes within this pathway had higher expression after exercise—all of them within the class I major histocompatibility complex (MHC I) pathway that inhibits the cytotoxic activity of NK cells. Similarly, six genes in the antigen processing and presentation pathway within the class II major histocompatibility complex had lower expression after exercise consistent with a muting of cytotoxic activity in CD4T cells. We speculate that the seeming paradox that activation by exercise of genes that attenuate the killing function of NK and cytotoxic t-cells occurs at the same time the numbers of these cells increase in the circulation is another manifestation of the ability of exercise to simultaneously stimulate both pro- and anti-inflammatory leukocyte function. The regulation of leukocyte function in response to exercise might parallel that of circulating cytokines, as noted earlier by Ostrowski et al. (22): “… cytokine inhibitors and anti-inflammatory cytokines restrict the magnitude and duration of the inflammatory response to exercise.”
In the present study, heat shock 70-kDa protein 1a (HSPA1B), one of the genes in the antigen processing and presentation pathway, had higher expression immediately after exercise in both early (2.90-fold change)- and late-pubertal girls (3.37-fold change). These data corroborate the growing body of evidence implicating the heat shock protein family of chaperone genes and proteins in the cellular response to exercise. Most studies have focused on muscle tissue in which exercise is now known to elicit a robust Hsp gene and protein response (20). Intriguingly, there are also data from this and other laboratories pointing toward an Hsp gene and protein response in circulating leukocytes following exercise as well (4, 8). For example, in our own earlier studies in PBMCs in young men and in early- and late-pubertal boys (25), we also found that members of the HSP gene regulatory family were altered by exercise. HSPs are increasingly seen as early responders in the signaling that occurs in the systemic immunological response to “danger” (21). Hsp70, one of the HSPs found in the circulation, is emerging as pluripotent cytokine and mediator (3). The consistent increase in Hsp70 gene expression in PBMCs in early- and late-pubertal girls suggests that its role is likely to be in modulating activation of leukoctyes early in the danger or stress response paradigm. The functional consequences of this exercise-altered gene expression in Hsp 70 have yet to be elucidated.
We found a general suppression of the B-cell receptor signaling pathway. Circulating B-cells are activated through stimulus of their receptors (by interaction with APCs) and then differentiate into either circulating memory B-cells or immunoglobulin producing plasmacytes. The impact of exercise on B-cell immunoglobulin production is not fully elucidated; however, there are several studies suggesting that even moderate exercise (e.g., ∼35% peak V̇o2) leads to suppression of immunoglobulin production (10). Our finding, that exercise attenuated the B-cell receptor signaling pathway, is consistent with these previous observations, and again, suggests the possibility of regulation of leukocyte function at the gene expression level following exercise.
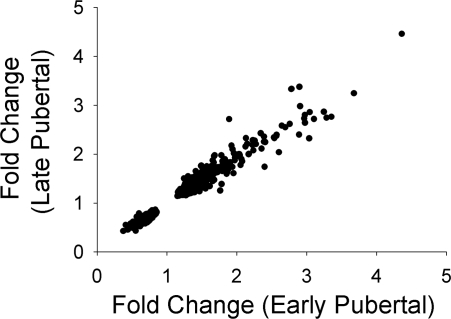
We attempted to examine possible differences and similarities in the gene expression response to exercise between the early- and late-pubertal girls. One remarkable finding was the consistency of the fold change in the group of genes that were significantly influenced by exercise in both groups (Fig. 4). Clearly, there is a well-controlled pattern of gene expression in leukocytes in response to exercise that is manifest in both early- and late-pubertal girls.
Fig. 4.
Comparison of fold change in 622 PBMC genes that were significantly affected by exercise in both the early- and late-pubertal girls. Data points represent the mean fold change for each of the exercise-sensitive genes in both groups. The fold change in the 2 groups was highly correlated (r = 0.98).
In examining Fig. 3, we wondered whether within the set of genes that did not overlap (i.e., genes whose expression significantly changed in one group following exercise but not the other) there were examples of genes that differed significantly between the early-and late-pubertal girls. Applying rigorous statistical modeling, however, revealed no genes that achieved statistically significant differences despite the fact that there were 698 genes affected by exercise in the early-pubertal girls only, and 255 genes affected by exercise in the late-pubertal girls only. It is possible that given the large number of genes examined in the microarray, and the relatively small sample size, we inadvertently limited our ability to discover moderate maturational differences in gene expression evoked by exercise. Other techniques, such as the use of RT-PCR on targeted gene candidates, may prove to be better adept at finding smaller but consequential differences related to maturation.
We also compared the results in females with those obtained in recently published early- and late-pubertal boys (24). A number of hypotheses suggesting sex differences do emerge from the data. Indeed, there were many more genes that changed similarly in the late-pubertal boys and girls (453 genes in common) than in the early-pubertal subjects (80 genes). These data are included in Data Supplements 4 and 5, available with the online version of this article. In the early-pubertal subjects, the younger boys had far fewer genes influenced by exercise (109 genes) than did any of the other groups (i.e., early- and late-pubertal girls: 1,320 and 877, respectively; late-pubertal boys: 1,246). These initial observations raise intriguing questions regarding the role of sex hormones, maturation of physiological responses to exercise, and sex-associated changes in body composition on the PBMC response to exercise that await further investigation.
Exercise is a complex and profound physiological perturbation in which, not surprisingly, the sudden insult to cellular homeostasis leads to a systemic “danger-type” response (15), one reflecting the increased vulnerability of an organism required to flee a predator or pursue prey. Our data indicate that in early- and late-pubertal girls brief exercise is associated not only with increased numbers of immune cells in the circulation but also may serve as a “wake-up” call in which key gene pathways are activated preparing PBMCs for fighting infection, wound repair, and, at the same time, setting the stage for apoptosis should these functions not be needed and the activated cells be eliminated. We could not, in these experiments using minimally invasive procedures in healthy children, determine whether the changes in gene expression resulted from direct effects of exercise on PBMCs or, alternatively, from a shift of PBMC type cells in marginal pools that were expressing genes differently than those in the circulation. By whatever mechanism, however, exercise-associated changes in gene expression in the circulating pool of PBMCs was substantial. The extent to which changes in gene expression in PBMCs are accompanied by functional or physiological changes in these cells, and either ameliorate, or, if abnormal, stimulate disease, has yet to be determined.
GRANTS
This work was supported in part by National Institutes of Health Grants RO1-HL-080947, P01-HD-048721 and the University of California-Irvine Satellite Grant GCRC-MO1-RR00827.
Supplementary Material
REFERENCES
- 1.Bailey RC, Olson J, Pepper SL, Barstow TJ, Porszsasz J, Cooper DM. The level and tempo of children's physical activities: an observational study. Med Sci Sports Exerc 27: 1033–1041, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Buttner P, Mosig S, Lechtermann A, Funke H, Mooren FC. Exercise affects the gene expression profiles of human white blood cells. J Appl Physiol 102: 26–36, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Calderwood SK, Mambula SS, Gray PJ Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci 1113: 28–39, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung SP, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol 97: 1461–1469, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol 56: 628–634, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol 56: 628–634, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DM, Radom-Aizik S, Schwindt CD, Zaldivar F. Dangerous exercise—lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol 103: 700–709, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Fehrenbach E, Niess AM, Veith R, Dickhuth HH, Northoff H. Changes of HSP72-expression in leukocytes are associated with adaptation to exercise under conditions of high environmental temperature. J Leukoc Biol 69: 747–754, 2001. [PubMed] [Google Scholar]
- 9.Fehrenbach E Multifarious microarray-based gene expression patterns in response to exercise. J Appl Physiol 102: 7–8, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Gleeson M Immune function in sport and exercise. J Appl Physiol 103: 693–699, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Gourdy P, Araujo LM, Zhu R, Garmy-Susini B, Diem S, Laurell H, Leite-de-Moraes M, Dy M, Arnal JF, Bayard F, Herbelin A. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood 105: 2415–2420, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12: 1065–1074, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis JN, Petty HR, Tang Y, Frank MB, Tessier PA, Dozmorov I, Jiang K, Kindzelski A, Chen Y, Cadwell C, Turner M, Szodoray P, McGhee JL, Centola M. Evidence for chronic, peripheral activation of neutrophils in polyarticular juvenile rheumatoid arthritis. Arthritis Res Ther 8: R154, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamason R, Zhao P, Rawat R, Davis A, Hall JC, Chae JJ, Agarwal R, Cohen P, Rosen A, Hoffman EP, Nagaraju K. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunol 7: 2, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matzinger P Friendly and dangerous signals: is the tissue in control? Nat Immunol 8: 11–13, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Mori S, Jewett A, Murakami-Mori K, Cavalcanti M, Bonavida B. The participation of the Fas-mediated cytotoxic pathway by natural killer cells is tumor-cell-dependent. Cancer Immunol Immunother 44: 282–290, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC. Sexual dimorphism in innate immunity. Arthritis Rheum 46: 250–258, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Moyna NM, Acker GR, Fulton JR, Weber K, Goss FL, Robertson RJ, Tollerud DJ, Rabin BS. Lymphocyte function and cytokine production during incremental exercise in active and sedentary males and females. Int J Sports Med 17: 585–591, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Nieman DC, Miller AR, Henson DA, Warren BJ, Gusewitch G, Johnson RL, Davis JM, Butterworth DE, Nehlsen-Cannarella SL. Effects of high- vs. moderate-intensity exercise on natural killer cell activity. Med Sci Sports Exerc 25: 1126–1134, 1993. [PubMed] [Google Scholar]
- 20.Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab 33: 1050–1065, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol 197: 1–8, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515: 287–291, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen AC, Crockett L, Richards M, Boxer A. Self-report measure of pubertal status-reliability, vailidity, and initial norms. J Youth Adolescence 17: 117–133, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Radom-Aizik S, Zaldivar F Jr, Leu SY, Cooper DM. Brief bout of exercise alters gene expression in peripheral blood mononuclear cells of early- and late-pubertal males. Pediatr Res 65: 447–452, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radom-Aizik S, Zaldivar F Jr, Leu SY, Galassetti P, Cooper DM. Effects of 30 min of aerobic exercise on gene expression in human neutrophils. J Appl Physiol 104: 236–243, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Robertson EB, Skinner ML, Love MM, Elder GH, Conger RD, Dubas JS, Petersen AC. The Pubertal Development Scale: a rural and suburban comparison. J Early Adolescence 12: 174–186, 1992. [Google Scholar]
- 27.Sabroe I, Whyte MK. Toll-like receptor (TLR)-based networks regulate neutrophilic inflammation in respiratory disease. Biochem Soc Trans 35: 1492–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Schneider CP, Schwacha MG, Chaudry IH. Influence of gender and age on T-cell responses in a murine model of trauma-hemorrhage: differences between circulating and tissue-fixed cells. J Appl Physiol 100: 826–833, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Schwindt CD, Zaldivar F, Wilson L, Leu SY, Wang-Rodriguez J, Mills PJ, Cooper DM. Do circulating leucocytes and lymphocyte subtypes increase in response to brief exercise in children with and without asthma? Br J Sports Med 41: 34–40, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon RM, Korn EL, McShane LM, Radmacher MD, Wright GW, Zhao Class Prediction Y. In: Design and Analysis of DNA Microarray Investigations. New York: Springer, 2003, p. 65–94.
- 31.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol Online 578: 327–336, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tirakitsoontorn P, Nussbaum E, Moser C, Hill M, Cooper DM. Fitness, acute exercise, and anabolic and catabolic mediators in cystic fibrosis. Am J Respir Crit Care Med 164: 1432–1437, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.