Abstract
The goal of this project was to examine the effects of artificial gravity (AG) on skeletal muscle strength and key anabolic/catabolic markers known to regulate muscle mass. Two groups of subjects were selected for study: 1) a 21 day-bed rest (BR) group (n = 7) and 2) an AG group (n = 8), which was subjected to 21 days of 6° head-down tilt bed rest plus daily 1-h exposures to AG (2.5 G at the feet). Centrifugation was produced using a short-arm centrifuge with the foot plate ∼220 cm from the center of rotation. The torque-velocity relationships of the knee extensors and plantar flexors of the ankle were determined pre- and posttreatment. Muscle biopsy samples obtained from the vastus lateralis and soleus muscles were used for a series of gene expression analyses (mRNA abundance) of key factors implicated in the anabolic vs. catabolic state of the muscle. Post/pre torque-velocity determinations revealed greater decrements in knee extensor performance in the BR vs. AG group (P < 0.04). The plantar flexors of the AG subjects actually demonstrated a net gain in the torque-velocity relationship, whereas in the BR group, the responses declined (AG vs. BR, P < 0.001). Muscle fiber cross-sectional area decreased by ∼20% in the BR group, whereas no losses were evident in the AG group. RT-PCR analyses of muscle biopsy specimens demonstrated that markers of growth and cytoskeletal integrity were higher in the AG group, whereas catabolic markers were elevated in the BR group. Importantly, these patterns were seen in both muscles. We conclude that paradigms of AG have the potential to maintain the functional, biochemical, and structural homeostasis of skeletal muscle in the face of chronic unloading.
Keywords: gene expression, muscle biopsy, messenger ribonucleic acid, anabolic/catabolic markers
human space exploration during the past four decades has unequivocally demonstrated that gravity plays a critical role in determining the function of various physiological systems. In microgravity, the lack of gravitational forces acting on the body produces a physiological state that is characterized by substantial losses of muscle mass and function (1), bone mass (27, 28, 33), and cardiovascular function (12, 15, 36) as evidenced by losses in orthostatic tolerance (14, 19, 30, 40). With respect to skeletal muscle, the loss of muscle mass can be both rapid and extensive (16, 29, 37). For instance, Edgerton et al. (16) observed that 5–11 days of microgravity produced significant reductions (16–36%) in muscle fiber cross-sectional area in the vastus lateralis muscle. Long-duration spaceflight (1) and bed rest (1) studies have demonstrated that losses in the mass of the triceps surae muscle group can approach 25%. An important consequence of the muscle atrophy that occurs in microgravity is a commensurate loss in muscle function, which, in some instances, can approximate a 40% deficit (1).
The significance of muscle atrophy and loss of muscle strength in a microgravity environment extends well beyond skeletal muscle itself. Skeletal muscles play a unique role in maintaining whole body homeostasis. As the organ system of movement, the activity of skeletal muscle plays a central role in dictating the loading conditions imposed on the skeletal system, and, in so doing, is critical for maintaining/regulating bone mass. In addition, the energetic requirements of skeletal muscle dictate the demands placed on the cardiovascular system, thereby influencing key elements such as heart mass, stroke volume, and capillary density (6).
It also should be noted that the effects of microgravity on other physiological systems occur independently of skeletal muscle activity. For instance, the loss of orthostatic tolerance is a well-known consequence of even short-duration exposures to microgravity (11, 14, 19, 30, 40), and exercise (using both treadmills and cycle ergometers) has been shown to have little protective effect in maintaining orthostatic tolerance (13, 17, 18, 20, 21, 30–32).
Given these considerations, the question arises as to whether a single integrated microgravity countermeasure can be developed to effectively prevent/mitigate the breadth of physiological adaptations noted above. In this context, a short-arm centrifuge represents a potentially unique approach because it can generate “artificial gravity” (AG), which can be used in a variety of ways to impose a spectrum of forces on various physiological systems. For instance, short-arm centrifuges can be used to produce hypergravity loading conditions (38, 39) that might effectively protect both skeletal muscle and bone. Short-arm centrifuges also provide the opportunity to impose orthostatic challenges on the cardiovascular system, and it may be possible to develop AG paradigms that couple the countermeasure needs of skeletal muscle, bone, and the cardiovascular system, thereby preventing the physiological deconditioning that normally occurs in microgravity.
Given this background, the current study represents an initial step in developing a long-term research program dedicated to exploring the use of AG as a countermeasure to microgravity. This involved building a unique research short-arm centrifuge, assembling a multidisciplinary research team, and performing a demonstration project whereby subjects underwent 21 days of bed rest with or without daily 1-h exposures to AG. With respect to skeletal muscle, we tested two primary hypotheses: 1) AG is effective in maintaining/blunting the loss of muscle function that normally occurs as a result of bed rest; and 2) AG is effective in blunting the induction of a catabolic state as reflected by molecular markers of growth and atrophy.
MATERIALS AND METHODS
Subjects and centrifugation.
Male subjects (29 ± 3 yr, 81 ± 9 kg, 176 ± 7 cm) were recruited to participate in this study and were subjected to either 21 days of 6° head-down tilt bed rest (BR group; n = 7) or 21 days of bed rest plus daily exposures to artificial gravity (AG group; n = 8). Those subjects in the AG group were subjected to daily 1-h bouts of 2.5 Gz (as measured at the feet) and were instructed to perform ad libitum shallow knee bends and heel raisers so that the muscles could act as pumps (i.e., muscle pumps), minimizing the pooling of blood in the legs. Centrifugation was performed using a short-radius centrifuge whose foot support was ∼220 cm from the center of rotation. The dimensions of a short-arm centrifuge represent an important advantage because 1) they are compatible with the dimensions of the Shuttle Transport System and the International Space Station and 2) they obviate the need for alternative strategies such as spinning the space craft to achieve similar Gz values. The results presented in this study are from a broad multisystem study that also examined the responses of the cardiovascular, vestibular, skeletal, and immune systems. Given the pilot nature of this study, 2.5 Gz was selected because it had the potential to impose high loading conditions on the musculoskeletal system and a strong orthostatic challenge on the cardiovascular system without producing loss of consciousness. Based on the positive findings of this study, future investigations are planned to attempt to optimize loading conditions to preserve muscle mass and function. All subjects were recruited and screened through the Johnson Space Center (JSC) Human Test Subject Facility. All subjects were required to provide written consent to participate in this study. The protocol was approved by the JSC Committee for the Protection of Human Subjects, the UTMB Institutional Review Board, and the General Clinical Research Center (GCRC) Advisory Committee.
Isokinetic testing of muscle strength.
In this study, muscle strength was assessed by making measurements of torque-velocity relationships for the knee flexors/extensors and ankle plantar/dorsiflexors by using a Cybex dynamometer. The subjects were seated on a chair with a slightly reclined backrest with the left thigh resting on and securely strapped to the seat. The subjects were allowed to become accustomed to the function of the test apparatus 1 wk before the first test session. During each test session, the subjects performed three to five trials at each test speed with ∼10 s of rest between efforts. Subjects were encouraged to make maximal efforts throughout the range of motion (−1.75 to 0 rad; where 0 rad = horizontal plane). The test speeds used for the knee extensors were 0, 0.87, 1.74, 2.61, 3.49, 4.36, and 5.24 rad/s. For the plantar flexors, we used angular velocities of 0, 0.52, 1.05, 1.57, 2.09, 2.62, 3.14, 3.66, and 4.19 rad/s. The best effort at each speed was used for data analyses. Calibration of the dynamometer was checked before each test session. To more accurately describe the torque-velocity relationships of the knee extensors, we employed a correction for the gravitational moment of the limb-lever system.
Measurements of muscle fiber cross-sectional area.
Muscle biopsy samples were taken from the vastus lateralis and soleus muscles. Biopsies of the soleus muscle were obtained by having the subjects lie on their side with the foot in a plantar flexed position (to provide better separation from the gastrocnemius muscle). Muscle samples were mounted on cork and quickly frozen in isopentane cooled with liquid nitrogen. Subsequently, muscle samples were serially sectioned in a cryostat (−20°C) at 10-μm thickness and placed on glass slides. The tissue sections were allowed to air dry and subsequently stained using hematoxylin and eosin. Sections were examined using a Leica DMLS light/fluorescent microscope, and images were digitally captured for analyses of muscle cross-sectional area (n = 50 fibers per muscle sample) using NIH Image J software (Scion).
Total RNA isolation.
Total RNA was extracted from preweighed frozen muscle samples using the TRI reagent (Molecular Research Center, Cincinnati, OH) according to the company's protocol, which is based on the method described by Chomczynski and Sacchi (10). Extracted RNA was precipitated from the aqueous phase with isopropanol and, after being washed with ethanol, was dried and suspended in a known volume of nuclease-free water. The RNA concentration was determined by optical density at 260 nm (using the conversion factor of 40 μg/ml per unit of optical density of 260 nm). The muscle total RNA concentration was calculated based on total RNA yield and the weight of the analyzed sample. The RNA samples were stored frozen at −80°C for subsequent analyses for specific gene expression.
RNA slot blotting.
Slot blotting was utilized to determine total myosin heavy chain (MHC) mRNA expression as described previously (22). One microgram of total RNA was denatured and applied onto a positively charged nylon membrane (GeneScreen plus; NEN) using a slot blot apparatus (Schleicher and Schuell). After ultraviolet fixation, the membrane was hybridized with a common antisense MHC mRNA probe used to determine the total MHC mRNA expression. The MHC probe is complementary to the coding region 500 nucleotides upstream from the stop codon of type I MHC mRNA. This region is 100% identical in all the MHC isoforms, and the obtained signal corresponds to the total population of MHC mRNA expressed in human muscle. After the MHC signal was obtained, the membrane was washed off via boiling in 1% SDS, and the membrane was reprobed to determine 18S ribosomal RNA. An antisense 18S ribosomal RNA probe in which the signal is directly proportional to the amount of total RNA was used to normalize for possible variability in the amount of loaded RNA per slot. Probes were 5′ end-labeled with 32P using -ATP and T4 polynucleotide kinase. Hybridization and washing procedures were carried out as described previously (23). Hybridization signals were detected and analyzed using a Phosphorimager and Image Quant analysis software (Molecular Dynamics). For each sample, the MHC mRNA signal was normalized to the corresponding 18S signal. The slot blot hybridization signal for these probes was strongly correlated with the amount of loaded total RNA ranging from 0.25 to 2 μg per slot. The sequence of oligonucleotides probes used for hybridization is reported in Table 1. For each sample, the total MHC mRNA was normalized to the muscle weight by using the corresponding muscle RNA concentration as the normalizing factor.
Table 1.
Oligonucleotides sequence for the probes used in slot blotting and primers for PCR for specific mRNA analyses
| Target mRNA | Sequence 5′→3′ | PCR Product Size, bp | GenBank Accession No. |
|---|---|---|---|
| All MHC isoforms | TGGTGTCCTGCTCCTTCTT | Used in slot blot | NM_000257 |
| 18S ribosomal RNA | GTGCAGCCCCGGACATCTAAG | Used in slot blot | NR_003286 |
| α-Skeletal actin | Fwd: GCGGGCATTCACGAGACCAC | 214 | NM_001100 |
| Rev: CGCCGATCCACACCGAGTATT | |||
| Myostatin | Fwd: CTACAACGGAAACAATCATTACCA | 243 | NM_005259 |
| Rev: GTTTCAGAGATCGGATTCCAGTAT | |||
| Atrogin | Fwd: AAGGGCAGCTGGATTGGAAGAAGATG | 198 | NM_058229 |
| Rev: TGAACAAGTTGATAAAGTCCTGGGGTGAAA | |||
| Type I MHC | Fwd: GGTGCGGGAGCTGGAGAATG | 404 | NM_000257 |
| Rev: GGGGCTTTGCTGGCACCTC | |||
| Type IIa MHC | Fwd: GGGTACGGGAGCTGGAAGGAGAGG | 426 | NM_017534 |
| Rev: TTACAGAGGGAAATGACCAAAGATG | |||
| Type IIx MHC | Fwd: CAGGACACCAGCGCCCATCT | 524 | NM_005963 |
| Rev: TTTCTTTGGTCACCTTTCAGCAGTT |
MHC, myosin heavy chain; Fwd, forward primer; Rev, reverse primer. Note that PCR primers are on exons spanning introns; this way, the PCR signal is from cDNA amplification, since any genomic DNA will not amplify under the applied PCR conditions. These primers were purchased from Operon Biotechnology (Huntsville, AL).
Reverse transcription-polymerase chain reaction.
An end-point RT-PCR method was applied to study the expression of specific mRNAs. The sequences for the primers used for the specific target mRNAs are shown in Table 1. One microgram of total RNA was reverse transcribed into cDNA for each muscle sample using the SuperScript II RT from Invitrogen (Life Technologies, Carlsbad, CA) and a mix of oligo(dT) (100 ng/reaction) and random primers (200 ng/reaction) in a 20-μl total reaction volume at 45°C for 50 min, according to the provided protocol. At the end of the RT reaction, the tubes were heated at 90°C for 5 min to stop the reaction and were then stored at −80°C until used in the PCR reactions for specific mRNA analyses. For each specific target mRNA, the RT and PCR reactions were carried out under identical conditions by using the same reagents premixed for all of the samples that were compared in the study. To validate the consistency of the procedures, at least one representative from each group was included in each RT-PCR run, and each sample was run in duplicate for the PCR reaction. For each primer set, PCR conditions (cDNA dilutions, MgCl2 concentration, and annealing temperature) were set to optimal conditions and normalized so that the target mRNA product yields were in the linear range of the semi logarithm plot when the yield was expressed as a function of the number of PCR cycles, and, for a given condition, target mRNA PCR yields were tightly correlated to input cDNA (5). As an additional consideration, non-reverse transcribed RNA was used in the PCR reaction at the same dilution as used in the cDNA and gave no signals, ensuring that the PCR signals were from cDNA products and not genomic DNA. PCR signal representing a specific mRNA expression was normalized to tissue weight by using total RNA concentration to generate the calculation. Reporting RNA expression per unit muscle weight is preferred, since the total RNA concentration may vary in muscle tissue in response to training or other activity paradigms, whereas the traditional internal controls such as GAPDH and large ribosomal proteins were shown not to vary in muscle subjected to different activity paradigms (24).
Statistical analyses.
For the torque-velocity relationship, the post-bed rest value was expressed as a percentage of the pre-bed rest value. These data were then analyzed using a two-way ANOVA (group and velocity as main factors). The RNA and PCR data (pre vs. post) were analyzed using a paired t-test. Each data point is reported as a mean ± SE. Statistical significance was defined as P ≤ 0.05, and all analyses were performed using Systat 10.2.01 (Systat Software, San Jose, CA).
RESULTS
Torque-velocity relationships.
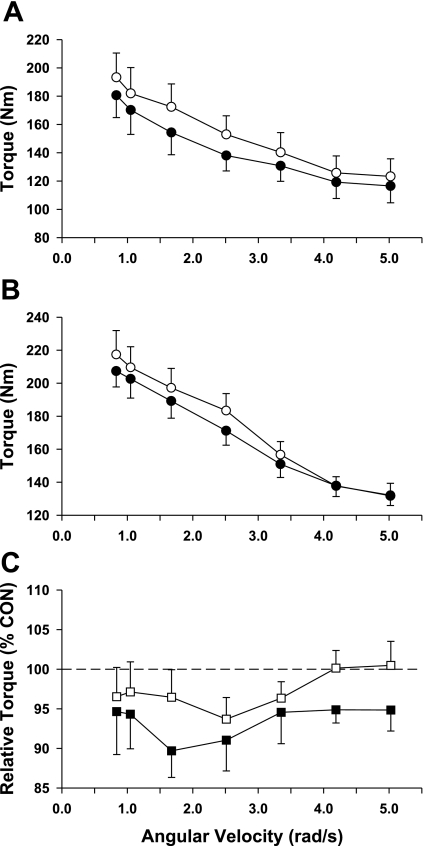
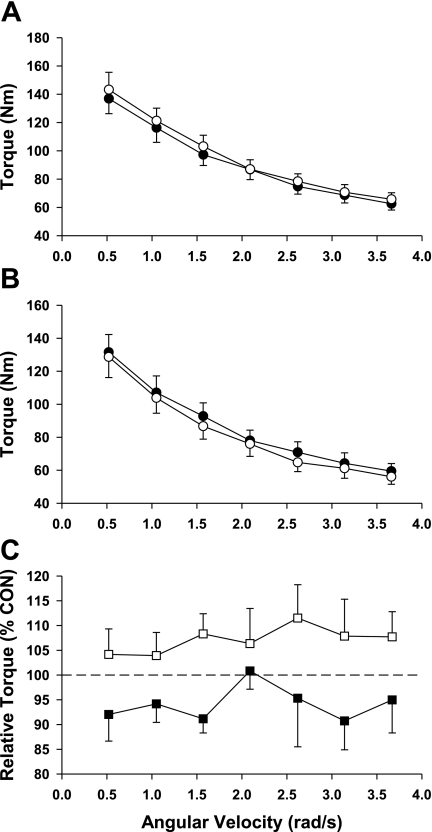
The torque-velocity relationships for the knee extensors and plantar flexors are shown in Figs. 1 and 2. As shown in Fig. 1, the knee extensors for both the BR and AG groups lost strength as reflected by the lower post-bed rest torque values (see Fig. 1C). Interestingly, this loss in strength was greatest in the BR group as evidenced by a significant group effect (P = 0.039; F ratio = 4.4). As shown in Fig. 2, 21 days of bed rest produced a loss in the control group plantar flexor strength that averaged ∼7% across the spectrum of test speeds. In contrast, AG was effective in producing an increase in muscle strength (mean = 7%; Fig. 2C) at each of the test speeds, and comparisons between the plantar flexors of the control and AG groups resulted in a significant group effect (P <0.001; F ratio = 15.2).
Fig. 1.
Knee extensor torque before (Pre; ○) and after (Post; •) bed rest (BR). Data for the BR and artificial gravity (AG) groups are shown in A and B, respectively. Relative changes in knee extensor torque for the BR (▪) and AG groups (□) are shown in C; relative changes in torque reflect the Post values expressed as a percentage of the Pre value (control, Con). Each data point represents a mean ± SE. Analysis using a 2-way ANOVA demonstrated that there was a significant group effect.
Fig. 2.
Plantar flexor torque Pre (○) and Post (•) BR. Data for the BR and AG groups are shown in A and B, respectively. Relative changes in torque for the BR (▪) and AG groups (□) are shown in C; relative changes in torque reflect the Post values expressed as a percentage of the Pre value (Con). Each data point represents a mean ± SE. Analysis using a 2-way ANOVA demonstrated that there was a significant group effect.
Muscle fiber cross-sectional area.
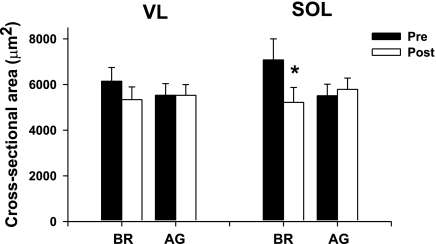
As shown in Fig. 3, the cross-sectional area of the vastus lateralis was unaffected by both BR and AG. In contrast, the muscle fiber cross-sectional area of the BR soleus muscle was reduced by ∼20%. Importantly, it should be noted that AG was very effective in maintaining the muscle fiber cross-sectional area of the soleus muscle (Fig. 3; P < 0.05).
Fig. 3.
Effects of BR and AG on muscle fiber cross-sectional area. Note that although 21 days of BR did not affect the muscle fiber cross-sectional area of the vastus lateralis (VL), there was a significant loss in that of the soleus (SOL) muscle. Importantly, AG was effective in preventing any loss in muscle fiber cross-sectional area of the SOL muscle. Each value is a mean ± SE. *P ≤ 0.05.
Molecular markers of growth and atrophy.
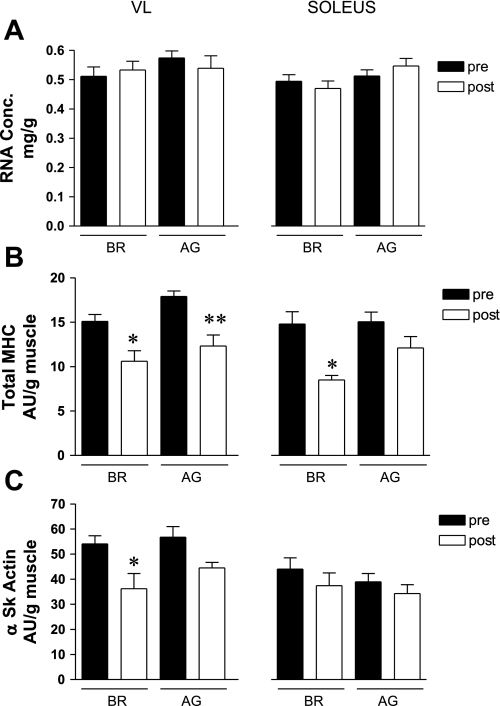
In the current study, we used total RNA concentration as a marker of the translational capacity. As shown in Fig. 4A, neither bed rest nor the AG countermeasure significantly affected total RNA concentration. The transcriptional capacity of key cytoskeletal proteins was assessed by examining changes in the mRNA levels of actin and total MHC. Total MHC mRNA levels in the vastus lateralis were markedly reduced (∼20–35%) in both the BR and AG groups (Fig. 4B). There also was a large decrease (∼40%) in the total MHC mRNA level in the BR soleus muscle, whereas AG appeared to effectively prevent such changes. As shown in Fig. 4C, the actin mRNA level was significantly decreased in the vastus lateralis of the BR group. Neither intervention affected the actin mRNA levels in the soleus muscle.
Fig. 4.
Effects of BR and AG on key markers of transcriptional and translational capacity. Note that neither BR or AG affected the total RNA concentration (Conc), as shown in A. Total myosin heavy chain (MHC) mRNA levels were significantly reduced by BR, and AG was unable to blunt or prevent these changes in the SOL muscle (B). Total α-skeletal actin (α Sk actin) mRNA levels were unaffected by either intervention (C). AU, arbitrary unit. Each value is a mean ± SE. *P ≤ 0.05; **P ≤ 0.01.
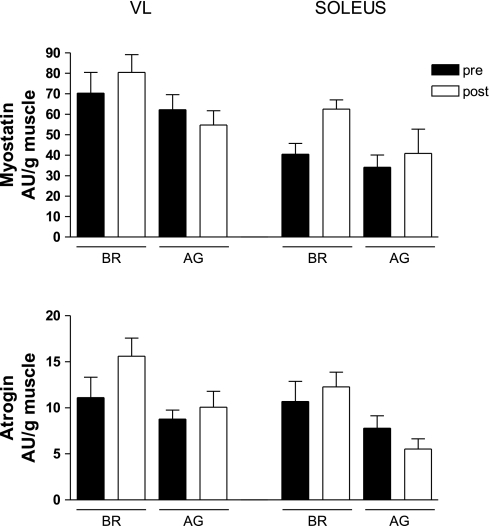
Myostatin and atrogin are genes that exert a negative influence over muscle mass, and on this basis, it might be proposed that 1) the catabolic state created by microgravity would lead to an elevation in their mRNA levels, and 2) the loading imposed on the muscle by AG would prevent such changes in myostatin and atrogin mRNA levels. The trends shown in Fig. 5 appear to be consistent with this perspective; however, none of the changes were statistically significant.
Fig. 5.
Effects of BR and AG on key catabolic markers. As shown in A, 21 days of BR produced an elevation in myostatin mRNA levels in the SOL muscle, and this effect was blunted by the AG countermeasure. A similar trend also was observed for atrogin mRNA levels (B). Each value is a mean ± SE.
Myosin mRNA isoform phenotypes.
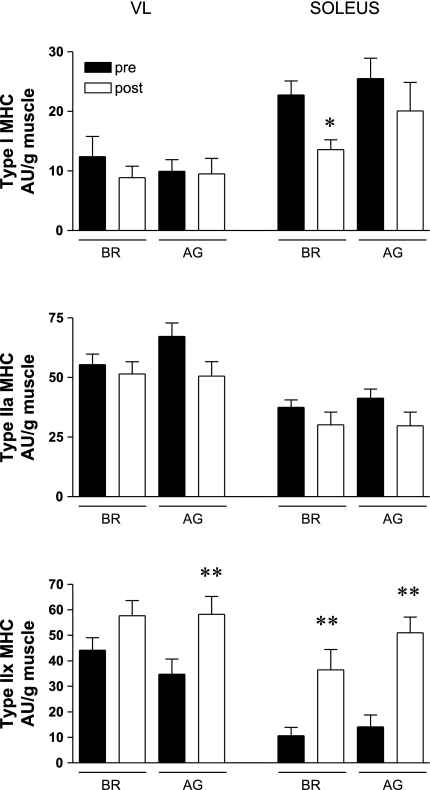
The MHC mRNA isoform profile for vastus lateralis and soleus muscles in the control and AG groups are shown in Fig. 6. Note that neither BR nor AG had a significant effect on the slow type I MHC mRNA levels in the vastus lateralis, whereas there was a large reduction in the soleus muscle of the BR group (Fig. 6A). In contrast, AG appears to have been effective in minimizing the loss of the slow type I MHC mRNA in the soleus muscle of the AG group (Fig. 6A). The levels of the fast type IIA MHC mRNA were unchanged in the vastus lateralis and soleus muscles of the BR and AG groups (Fig. 6B). Bed rest produced significant increases in the fast type IIX MHC mRNA levels in both the vastus lateralis and soleus muscles (Fig. 6C). Similar increases also were observed in the AG group. Collectively, these findings suggest that BR produced a faster MHC mRNA phenotype and that this was partially blunted by the AG countermeasure (see slow type I MHC mRNA levels in the soleus muscle).
Fig. 6.
Effects of BR and AG on MHC mRNA isoform profile in the VL and SOL muscles. As shown in A, BR produced a significant reduction in the slow type I MHC mRNA isoform in the SOL muscle, and AG was partially effective in preventing a loss in this key MHC mRNA pool. Neither BR nor AG affected the mRNA levels of the fast type IIA MHC isoform (B). BR produced a significant elevation in the fast type IIX MHC mRNA levels in both the VL and SOL muscles (C). Importantly, it should be noted that AG was unable to blunt this effect. Each value is a mean ± SE. *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
Many physiological systems are influenced by the loading conditions imposed by gravity. With respect to a microgravity environment, the types of adaptations that occur result in structural and functional alterations that reduce overall physiological capacity, and these are perhaps best illustrated by overt losses in muscle mass, strength, and orthostatic tolerance. Currently, optimal countermeasures for preventing these adaptations have not been developed, and this gives rise to the possibility that periodic or continuous exposure to AG may be an effective means for preventing these adaptations. In this context, the findings of the current study are unique in several regards. First, they demonstrate that human subjects can undergo daily exposures to a rotating environment without developing significant degrees of motion sickness. Second, we observed that AG was partially effective in maintaining the functional properties of the knee extensors and plantar flexors as evidenced by torque-velocity measurements. Third, periodic exposure to AG appears to be beneficial with respect to maintaining muscle mass as evidenced by muscle fiber cross-sectional area. Finally, we observed a trend suggesting that AG may be effective in minimizing the development of a catabolic (i.e., atrophy) profile.
Burton (8) commented that “the human centrifuge has been used for over 50 years as an enormously important research tool for delving into the mysteries of the acceleration environment. It is time that we develop an applied use for this valuable tool and let it support the human in space. But, as in any developmental process, applied uses will have to be determined, so we had better get started!” This statement nicely summarizes the genesis for the current set of projects. During the past 5–10 years, several human-powered short-arm centrifuge designs (29, 39, 53) have been described in the literature. However, few studies have examined the effectiveness of short-arm centrifuges as countermeasures to microgravity, and in this regard, the current collection of studies is unique. From a conceptual perspective, the use of AG as a countermeasure to microgravity is attractive because it produces inertial forces that can be used to mimic those normally produced by gravity. In a sense then, short-arm centrifuges can functionally be used to add back gravity while in a microgravity environment. Given the lack of research in this area, the optimal AG countermeasure prescription is unknown, and as a result, even the most rudimentary issues remain unresolved. As just one example, it is unclear whether astronauts must be continuously exposed to AG or whether short periods of AG are just as effective.
AG can be used to mitigate functional losses induced by microgravity.
In this regard, the findings of this study are quite important because they demonstrate that even short exposures to AG may be effective in mitigating the effects of microgravity. For instance, we observed that daily exposures to AG partially reversed the loss in knee extensor muscle strength due to bed rest. With respect to the plantar flexors, AG actually increased the muscle strength as reflected by increases throughout the torque-velocity relationship. Importantly, the success of this low-intensity AG countermeasure program raises the intriguing possibility that more intense loading regimes produced by higher Gz may be capable of further mitigating the effects of microgravity. Yang et al. (38) recently demonstrated that subjects could perform squats under hypergravity conditions that produce foot forces equivalent to those produced while performing 10-repetition-maximum squats in a normal 1-Gz environment (i.e., foot forces >3 times body weight). Hence, it is clear that AG can be used to produce very high loading conditions on skeletal muscle.
The concept that AG may be used as an effective countermeasure to microgravity is further supported by the work of Akima et al. (2), who demonstrated that cycle ergometry under hypergravity conditions was partially effective in preventing losses in strength and muscle volume. These investigators observed that 20 days of bed rest produced a 23% loss in isometric strength, whereas bed rest subjects who performed cycle ergometry under hypergravity conditions lost only 7% of their isometric strength. Correspondingly, there was a 9% loss in muscle volume in the bed rest subjects, whereas AG countermeasure subjects lost only 1% of their muscle volume. Of importance, it should be noted that AG countermeasure program employed by Akima et al. (2) required the knee extensors to produce relatively low forces (i.e., with respect to their maximal isometric strength), and this raises the possibility that high levels of AG may not be necessary to maintain muscle strength.
In developing AG countermeasure paradigms to prevent muscle atrophy and loss of muscle function, it will be important to determine whether the response to AG is muscle specific and dependent on muscle fiber type. These are important considerations given the findings of other countermeasure studies. For instance, Alkner and Tesch (3) observed that a flywheel resistance training program was effective in maintaining knee extensor muscle function and volume during 29 (3) and 90 days (4) of bed rest. Importantly, in both studies, the effects were not as robust for the plantar flexors of the ankle. In light of these observations, it is interesting to note that the AG countermeasure used in the current study appeared to be more effective (at the functional level) for maintaining the function of the plantar flexors. This might be due to the fact that the plantar flexors were loaded (on a relative basis) to a greater extent than the knee extensors. Since both muscle groups worked against the same load and the plantar flexors have a smaller cross-sectional area, they must have worked at a higher percentage of their maximal isometric tension, perhaps producing a more effective countermeasure to bed rest.
AG can prevent loss in muscle fiber cross-sectional area and induction of catabolic state.
The protective effects of AG on the plantar flexors were manifested not only at the functional level but also at the cellular and molecular levels, as well. For instance, 21 days of BR produced a 20% reduction in muscle fiber cross-sectional area in the soleus muscle, whereas it was unchanged in the AG group. Consistent with these observations, we observed a trend in the soleus muscle whereby the mRNA levels of myostatin (P = 0.09) and atrogin (P = 0.12) were elevated to a greater extent in the BR group. The elevation of both of these factors in the BR group is consistent with the induction of a catabolic state. Myostatin is thought to be a negative regulator of muscle mass, and although its exact role in adult animals has not been clarified, reports indicate that its elevation may negatively influence protein synthesis (25). In addition, various types of muscle atrophy (25) appear to be associated with elevations in myostatin. With respect to atrogin, a number of studies have shown that models of disuse result in an elevation of its mRNA levels (26, 34, 35). Studies with genetically modified mice have demonstrated that knockout of this gene blunts the atrophy that normally results from denervation (7). Collectively, the ability of AG to maintain muscle fiber cross-sectional area and prevent elevations in myostatin and atrogin mRNA levels is an important corollary to the functional data.
Can artificial gravity prevent slow-to-fast transitions in MHC phenotype?
Given this background, it is somewhat surprising to note that AG was not more effective in preventing 1) losses in total MHC mRNA levels and 2) slow-to-fast isoform MHC mRNA isoform transitions. Bed rest produced significant reductions (∼35%) in the total MHC mRNA levels in both the vastus lateralis and soleus muscles. This is an interesting observation given that the actin mRNA levels were unaffected in either muscle, and this observation perhaps suggests that the transcriptional regulation of myosin is much more sensitive to loading state than is actin. Mechanical unloading of skeletal muscle (especially in slow muscles like the soleus) typically produces a slow-to-fast transition in MHC isoform expression at both the protein and mRNA levels (9). Consistent with this classic hallmark of unloading, we observed that bed rest produced a large increase in the fast type IIX MHC mRNA level that was greatest in the soleus muscle. Interestingly, AG was unable to mitigate this slow-to-fast transition in either the vastus lateralis or soleus muscle. We suspect that this reflects the minimal amount of resistance loading placed on these muscle groups.
Key limitations.
It is somewhat surprising that 21 days of bed rest did not produce a more robust loss of muscle strength. This may reflect an overall low level of fitness in these subjects. The mean maximal oxygen uptake was ∼36 ml O2·kg−1·min−1 in the BR subjects, and this is certainly at the lower limit of the current pool of astronauts. Hence, the response of subjects with a greater fitness level (i.e., similar to that of astronauts) may have been more robust than the subjects who participated in this study. As indicated in materials and methods, subjects were allowed to perform shallow knee bends and heel raisers. Given the “pilot project” nature of this study, no attempt was made to control the number of repetitions or the loading conditions imposed on the target muscles. It is clear that these represent important variables that need to be controlled in future studies, and in so doing, it should be possible to determine the true extent to which AG can be used as a countermeasure to microgravity (i.e., preventing the loss of muscle mass and function and shifts in key contractile protein isoforms).
Summary.
There are three key findings of this study. First, AG proved effective in maintaining muscle function relative to the BR group. This is very encouraging given that it is unlikely that the approach used in the current study represents an optimal AG countermeasure paradigm. In this regard, it seems reasonable to suggest that AG paradigms that employ greater loading conditions may prove to be even more effective. Second, AG was effective in preventing losses in muscle fiber cross-sectional area in the plantar flexors. Finally, it is important to note that AG was not able to protect total MHC mRNA levels and the slow phenotype of the soleus muscle. Although these effects were not manifested at either the functional or the morphological levels, this later finding suggests that the AG paradigm employed in the current study did not universally protect all cellular/molecular systems. Certainly, this provides an important basis for pursuing additional studies that attempt to optimize the AG countermeasure prescription, especially as it relates to longer durations of microgravity as might occur during exploration class missions.
GRANTS
This study was supported by National Aeronautics and Space Administration Human Research Program Grant NASA-NNJ-068HC6IG (to K. M. Baldwin) and was conducted at the National Institutes of Health-funded (M01 RR0073) GCRC at UTMB, Galveston, TX.
REFERENCES
- 1.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol 95: 2185–2201, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Akima H, Katayama K, Sato K, Ishida K, Masuda K, Takada H, Watanabe Y, Iwase S. Intensive cycle training with artificial gravity maintains muscle size during bed rest. Aviat Space Environ Med 76: 923–929, 2005. [PubMed] [Google Scholar]
- 3.Alkner BA, Tesch PA. Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand 181: 345–357, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Alkner BA, Tesch PA. Knee extensor and plantar flexor muscle size and function following 90 days of bed rest with or without resistance exercise. Eur J Appl Physiol 93: 294–305, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol 94: 2255–2262, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol 45: 169–189, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Burton RR The role of artificial gravity in the exploration of space. Acta Astronaut 33: 217–220, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Caiozzo VJ Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle Nerve 26: 740–768, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Clement G, Pavy-Le Traon A. Centrifugation as a countermeasure during actual and simulated microgravity: a review. Eur J Appl Physiol 92: 235–248, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Convertino VA Consequences of cardiovascular adaptation to spaceflight: implications for the use of pharmacological countermeasures. Gravit Space Biol Bull 18: 59–69, 2005. [PubMed] [Google Scholar]
- 13.Convertino VA Endurance exercise training: conditions of enhanced hemodynamic responses and tolerance to LBNP. Med Sci Sports Exerc 25: 705–712, 1993. [PubMed] [Google Scholar]
- 14.Convertino VA Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Convertino VA Mechanisms of microgravity induced orthostatic intolerance: implications for effective countermeasures. J Gravit Physiol 9: 1–13, 2002. [PubMed] [Google Scholar]
- 16.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR, Day MK, Greenisen M. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol 78: 1733–1739, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Franke WD, Mills KK, Lee K, Hernandez JP. Training mode does not affect orthostatic tolerance in chronically exercising subjects. Eur J Appl Physiol 89: 263–270, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Frey MA Considerations in prescribing preflight aerobic exercise for astronauts. Aviat Space Environ Med 58: 1014–1023, 1987. [PubMed] [Google Scholar]
- 19.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 289: R109–R116, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Greenleaf JE Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc 29: 207–215, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Greenleaf JE, Dunn ER, Nesvig C, Keil LC, Harrison MH, Geelen G, Kravik SE. Effect of longitudinal physical training and water immersion on orthostatic tolerance in men. Aviat Space Environ Med 59: 152–159, 1988. [PubMed] [Google Scholar]
- 22.Haddad F, Baldwin KM, Tesch PA. Pretranslational markers of contractile protein expression in human skeletal muscle: effect of limb unloading plus resistance exercise. J Appl Physiol 98: 46–52, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Haddad F, Herrick RE, Adams GR, Baldwin KM. Myosin heavy chain expression in rodent skeletal muscle: effects of exposure to zero gravity. J Appl Physiol 75: 2471–2477, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol 102: 573–581, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287: C834–C843, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289: E969–E980, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19: 1006–1012, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Leblanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7: 33–47, 2007. [PubMed] [Google Scholar]
- 29.McCarthy JP, Bamman MM, Yelle JM, LeBlanc AD, Rowe RM, Greenisen MC, Lee SM, Spector ER, Fortney SM. Resistance exercise training and the orthostatic response. Eur J Appl Physiol Occup Physiol 76: 32–40, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Moore AD, Lee SM, Charles JB, Greenisen MC, Schneider SM. Maximal exercise as a countermeasure to orthostatic intolerance after spaceflight. Med Sci Sports Exerc 33: 75–80, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Pawelczyk JA, Kenney WL, Kenney P. Cardiovascular responses to head-up tilt after an endurance exercise program. Aviat Space Environ Med 59: 107–112, 1988. [PubMed] [Google Scholar]
- 32.Raven PB, Smith ML, Hudson DL, Graitzer HM. The effects of exercise training on factors affecting orthostatic tolerance. Physiologist 30: S147–S150, 1987. [PubMed] [Google Scholar]
- 33.Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, Felsenberg D. Muscle atrophy and bone loss after 90 days' bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone 36: 1019–1029, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Rourke BC, Yokoyama Y, Milsom WK, Caiozzo VJ. Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Physiol Biochem Zool 77: 582–593, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21: 140–155, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Sides MB, Vernikos J, Convertino VA, Stepanek J, Tripp LD, Draeger J, Hargens AR, Kourtidou-Papadeli C, Pavy-LeTraon A, Russomano T, Wong JY, Buccello RR, Lee PH, Nangalia V, Saary MJ. The Bellagio Report. Cardiovascular risks of spaceflight: implications for the future of space travel. Aviat Space Environ Med 76: 877–895, 2005. [PubMed] [Google Scholar]
- 37.Tesch PA, Berg HE, Bring D, Evans HJ, LeBlanc AD. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur J Appl Physiol 93: 463–468, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Baker M, Graf S, Larson J, Caiozzo VJ. Hypergravity resistance exercise: the use of artificial gravity as potential countermeasure to microgravity. J Appl Physiol 103: 1879–1887, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Kaplan A, Pierre M, Adams G, Cavanagh P, Takahashi C, Kreitenberg A, Hicks J, Keyak J, Caiozzo V. Space cycle: a human-powered centrifuge that can be used for hypergravity resistance training. Aviat Space Environ Med 78: 2–9, 2007. [PubMed] [Google Scholar]
- 40.Yates BJ, Kerman IA. Post-spaceflight orthostatic intolerance: possible relationship to microgravity-induced plasticity in the vestibular system. Brain Res Rev 28: 73–82, 1998. [DOI] [PubMed] [Google Scholar]