Abstract
We report results from a study designed to explore the utility of artificial gravity (AG) as a countermeasure to bone loss induced by microgravity simulation. After baseline testing, 15 male subjects underwent 21 days of 6° head-down bed rest to simulate the deconditioning associated with spaceflight. Eight of the subjects underwent 1 h of centrifugation (AG; 1 Gz at the heart, 2.5 Gz at the feet) each day for 21 days, whereas seven of the subjects served as untreated controls (Con). Blood and urine were collected before, during, and after bed rest for bone marker determinations. Bone mineral density (BMD) and bone mineral content (BMC) were determined by dual-energy X-ray absorptiometry and peripheral quantitative computerized tomography before and after bed rest. Urinary excretion of bone resorption markers increased during bed rest, but the AG and Con groups did not differ significantly. The same was true for serum C-telopeptide. During bed rest, bone alkaline phosphatase (ALP) and total ALP tended to be lower in the AG group (P = 0.08, P = 0.09). Neither BMC nor BMD changed significantly from the pre-bed rest period in AG or Con groups, and the two groups were not significantly different. However, when AG and Con data were combined, there was a significant (P < 0.05) effect of time for whole body total BMC and total hip and trochanter BMD. These data failed to demonstrate efficacy of this AG prescription to prevent the changes in bone metabolism observed during 3 wk of bed rest.
Keywords: microgravity, countermeasure, bone resorption, bone formation
on earth, bone homeostasis depends a great deal on mechanical loading. The mechanical loading provided by gravity is coupled with bone remodeling, as shown by cellular responses to partial gravity or hypergravity, but the precise mechanism of this coupling is unclear (25, 32, 44, 51). Spaceflight and bed rest studies produce disuse or decreased mechanical loading of muscles, which has a negative effect on bone (18, 21, 30, 36, 42).
On 4- to 6-mo spaceflight missions, it is estimated that the rate of bone mineral loss ranges from 0.5 to 1.5% per month (18, 19, 47) and that over 90% of crew members on these missions lose 5% or more of their bone mineral density (BMD) in at least one site (21). Skylab, Mir, and International Space Station (ISS) data indicate that the degree of change in bone metabolism is related to mission duration (35, 40, 50), but it is unknown whether bone loss will continue with longer exploration missions (29). These data also show that bone mineral is not uniformly lost from all parts of the skeleton or type of bone during spaceflight (21). For example, in the hip, it is estimated that 90% of mineral loss from bone of ISS crew members is from cortical bone (18).
Bone resorption increases in the first 2 wk of spaceflight, as evidenced by increased urinary concentrations of N-telopeptide and pyridinium cross-links (6, 7, 38, 40, 41). Bone formation is unchanged or decreases during spaceflight (7, 40, 41, 47). Calcium absorption in astronauts has been observed to decrease (40, 53) with increased calcium excretion (40, 49, 50, 53). Together, increased resorption and decreased or unchanged bone formation, with decreased calcium absorption and increased calcium excretion, yield an overall negative calcium balance during long-duration spaceflights.
Minimizing the risk of fracture and maximizing the ability of crew members to perform critical tasks when they return to a nominal gravity or reduced gravity environment will be important on long-duration missions, and maintaining bone will aid in both of these actions. To date, bone loss during spaceflight has not been effectively mitigated by countermeasures (21, 42).
In studies of humans and some animals (15, 23, 24, 27, 45), artificial gravity (AG) or hypergravity has been shown to positively affect bone (or related markers), and for this reason, among other benefits, it has been brought to the attention of the National Aeronautics and Space Administration (NASA). Studies in trainee fighter pilots exposed to positive sustained accelerative forces (Gz) over a period of 8–12 mo have shown that positive Gz-induced loading has an osteogenic effect on bone in a site-specific manner. Regions that were maximally strained (head and spine due to helmet and mask assembly) had increased BMD and bone mineral content (BMC) (23, 24). Studies of mature animals show that centrifugation can similarly increase bone density in a site-specific manner (27). Furthermore, Vernikos et al. (45) reported that intermittent exposure to 1 Gz (by standing or walking) during a 4-day head-down tilt bed rest was effective in preventing elevated urinary calcium that typically occurs during bed rest. The optimal AG prescription for bone, including dose, duration, and frequency of centrifugation, is unknown. One goal of the NASA Artificial Gravity Pilot Study was to determine the effectiveness of one AG prescription to mitigate bone loss during bed rest. Our primary hypothesis was that AG would result in a mitigation of the increase in bone resorption associated with bed rest, as indicated by biochemical markers of bone metabolism.
METHODS
Design.
This study consisted of a 21-day period of 6° head-down tilt (HDT) bed rest, with a 12-day period of data collection before bed rest and a 7-day data collection period after bed rest. The bed rest and AG protocols for this study are described in detail in a companion to this article (54). Briefly, during the bed rest phase of the study, subjects were confined to strict 6° HDT bed rest. Subject monitors outside each room ensured round-the-clock compliance. A 6° HDT gurney was used for transport to the centrifuge facility and the shower.
Diets were controlled and were matched for all subjects by relative study day (that is, on any given day, all subjects consumed the same menu). Food quantities for each individual were adjusted to maintain energy intake at a level that would maintain body mass. Details and nutritional aspects of the study have been published elsewhere (58). Blood and urine samples were collected before, during, and after bed rest for analysis of markers of bone metabolism.
Subjects.
A total of 15 healthy male subjects participated in this study. The mean (±SD) age was 27 ± 2 yr for the control (Con) group (n = 7) and 31 ± 3 yr for the artificial gravity (AG) group (n = 8). The Con and AG subjects had an average height of 176 ± 7 and 175 ± 6 cm, respectively. Con subjects weighed 82 ± 8 kg, and AG subjects weighed 81 ± 9 kg.
Treatments.
During the bed rest phase, Con and AG subjects were transported daily on a 6° HDT gurney to the centrifuge facility, where they were transferred to the centrifuge arm (at 6° HDT) and monitored. AG subjects received 1 h of centrifugation, whereas Con subjects did not. Artificial gravity was produced by rotating the subjects on a centrifuge arm with a 3.0-m radius (54). The subjects were allowed to perform antiorthostatic maneuvers (heel raises and shallow knee bends) while spinning but were otherwise passive.
All study protocols were approved by the Johnson Space Center Committee for the Protection of Human Subjects, the University of Texas Medical Branch (UTMB) Institutional Review Board, and the UTMB General Clinical Research Center Advisory Committee. Subjects provided written, informed consent before they were enrolled in the study.
Biological sample collection and processing.
Pre-bed rest blood samples were collected 9 days before bed rest and immediately before bed rest began. Blood was also collected on bed rest days (BR)8, BR15, and BR21 (the last day of bed rest) and again 8 days after bed rest ended (BR+8). Urine was collected throughout the study, but key analyses were performed only on days BR−9, BR−8, BR−4, BR−3, BR−2, and BR−1 before bed rest, on days BR8, BR9, BR15, BR16, BR20, and BR21 during bed rest, and days BR0, BR+1, BR+6, and BR+7 after bed rest. Subjects were fasting for 8 h before each blood draw.
Blood samples were collected into appropriate tubes and processed to yield serum. All single-void samples of urine were collected in individual bottles and stored in coolers until they were processed (within 24 h). Twenty-four-hour urine pools were created, pH was measured, and aliquots were prepared and frozen for analysis. All samples were stored at −80°C until analysis. Fecal samples were collected into individual containers and frozen at −80°C until they were processed. Processing of these samples consisted of homogenization and microwave digestion in nitric acid, as previously reported (39, 40).
Biochemical analyses.
Most analyses were performed using standard commercially available techniques, as described previously (40, 41, 55, 56). Serum, urinary, and fecal calcium concentrations were measured using atomic absorption spectrometry. Ionized calcium was determined using ion-sensitive electrode techniques (i-STAT; Princeton, NJ). Serum intact parathyroid hormone (PTH), receptor activator for nuclear factor-κB ligand (RANKL), and osteoprotegerin (OPG) were measured using immunoradiometric assay (Nichols Institute Diagnostics, San Juan Capistrano, CA, for PTH; Alpco Diagnostics, Salem, NH, for RANKL and OPG). The vitamin D metabolites 25-hydroxycholecalciferol (25-OH vitamin D) and 1,25-dihydroxycholecalciferol [1,25-(OH)2 vitamin D] were also determined using commercially available RIA kits (DiaSorin, Stillwater, MN). Bone alkaline phosphatase (ALP) was measured using ELISA (Quidel, Santa Clara, CA), total ALP was analyzed using a Beckman SYNCHRON CX7 automated clinical chemistry system (Beckman Coulter, Brea, CA), and serum osteocalcin was measured using a commercial RIA kit (Biomedical Technologies, Stoughton, MA). Another bone formation marker, procollagen type I N propeptide (PINP), was analyzed using commercially available RIA kits (Orion Diagnostica, Espoo, Finland).
Bone resorption markers were also determined. In serum, tartrate-resistant acid phosphatase (TRAP-C) was analyzed using commercially available ELISA (SBA Sciences BioCity, Turku, Finland), as was COOH-terminal cross-linking telopeptide of type I collagen (C-telopeptide; CrossLaps ELISA, Nordic Bioscience, Herlev, Denmark). Urinary collagen cross-links were determined: NH2-terminal cross-linking telopeptide of type I collagen (N-telopeptide; Osteomark, Ostex International, Seattle, WA), and pyridinoline and deoxypyridinoline (PYD and DPD, respectively; Pyrilinks, Quidel). Cross-link data were expressed as nanomoles of excretion per day, because we have shown that this reduces within-subject variability (37).
Bone imaging analyses.
Bone density was determined before and after bed rest. All scans were acquired by the same operator to ensure consistency of positioning and were analyzed by Johnson Space Center Bone and Mineral Laboratory personnel.
BMD was measured by dual-energy X-ray absorptiometry (DXA Hologic Discovery W) before bed rest and after 21 days of bed rest. Scans of each volunteer's whole body, pelvis, heel, spine, and proximal femur were acquired in triplicate before and after bed rest. Triplicate measures were averaged before statistical analysis. In accordance with procedures used for analyzing and reporting earlier spaceflight and bed rest data (19, 20, 34), the hip region was manually defined, with the lateral margin placed adjacent to the greater trochanter lateral cortex and the distal border placed relative to the distal margin of the lesser trochanter. Precision of the manual procedure is equivalent to that of the automated procedures available on current DXA analysis software. The highest coefficient of variation (CV) for the DXA procedure in our laboratory is 2.0% for the pelvis and <1.2% for all other regions.
The peripheral quantitative computerized tomography (pQCT) device used was an XCT 3000 (Stratec Medizintechnik). At the time of the first test scan, the length of the subject's tibia was measured. Two scan slices were obtained at 5 and 50% (1 slice at each site) of the tibia length proximal to the distal end plate of the tibia. The slice thickness was 2.4 mm, and the pixel size was 0.4 × 0.4 mm. In addition, three scan slices measured at the insertion of the patellar tendon were obtained. This position was determined by taking a lateral scout scan (starting at the middle of the patella and moving distally) and projecting the intersection of the patellar tendon and tibia. Three adjacent slices were taken at this position, which was referenced to the proximal end plate of the tibia and labeled “proximal 1–3.” To increase the measurement precision, this five-slice sequence was measured in triplicate at each session, and then an average of the three scans was determined. All repeat measurements were referenced to the end plate of the tibia using the same separation measured in the first test. The analysis was performed using the software provided by the manufacturer. The polar strength-strain index (SSI), related to polar moment of inertia, was calculated for each pQCT site and time point. CVs for pQCT at total density ranged (across 2 distal and 3 proximal slices) from 0.4 to 1.6%; for cortical density, from 0.5 to 4.0%; for trabecular density, from 0.7 to 1.5%; and for SSI, from 1.0 to 5.0%.
Statistical analysis.
Statistical analyses were performed on the outcome measures shown in Tables 1–3 (concentrations of the analytes sampled) with the data in their original form (neither transformed nor expressed as percent change). Data from the pre-bed rest days were averaged and used as the pre-bed rest data point. In an exploratory analysis phase, each outcome measure was the dependent variable in a separate repeated-measures ANOVA, with time as the repeated factor and group as a grouping factor. The exploratory phase was designed to flag possible changes in any of the mean outcome measures throughout the study period (before, during, and after bed rest), both overall (“time” main effect) and differentially by treatment group (time × group interaction). After each ANOVA, post hoc Bonferroni t-tests were performed to assess specific differences between pre-bed rest and subsequent time periods, adjusting for multiple comparisons across time; however, no adjustment was made for multiple testing of all outcome measures.
Table 1.
Blood and urinary markers of vitamin D, calcium, and bone metabolism before, during, and after 21 days of bed rest
| Con |
AG |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | BR8/9 | BR15/16 | BR20/21 | BR+0/+1 | BR+6/+7 | Pre | BR8/9 | BR15/16 | BR20/21 | BR+0/+1 | BR+6/+7 | |||||||||||
| 25-OH vit D, nmol/l | 55±16 | 49±15 | 54±14 | 56±17 | 54±15 | 63±17 | 65±17 | 65±24 | 65±15 | 66±14 | ||||||||||||
| 1,25-(OH)2 vit D, pmol/l | 167±63 | 147±74 | 162±93 | 163±89 | 187±96 | 145±57 | 113±34 | 118±36 | 114±40 | 136±39 | ||||||||||||
| PTH,b pg/ml | 50±27 | 41±20 | 35±12c | 39±17c | 39±17c | 50±15 | 46±12 | 40±7c | 41±6c | 41±6c | ||||||||||||
| Ionized calcium, nmol/l | 1.21±0.02 | 1.21±0.02 | 1.24±0.02 | 1.22±0.03 | 1.22±0.03 | 1.22±0.02 | 1.22±0.02 | 1.22±0.03 | 1.22±0.03 | 1.22±0.05 | ||||||||||||
| Serum calcium, mmol/l | 2.299±0.083 | 2.327±0.099 | 2.337±0.092 | 2.277±0.119 | 2.291±0.095 | 2.274±0.141 | 2.273±0.058 | 2.314±0.062 | 2.339±0.092 | 2.285±0.073 | 2.265±0.091 | 2.318±0.076 | ||||||||||
| Urinary calcium, mmol/day | 6.5±1.4 | 5.9±2.5 | 6.7±1.2 | 7.1±1.4 | 7.1±1.2 | 6.1±1.3 | 5.5±2 | 6.3±1.3 | 6.0±2.2 | 6.2±2.1 | 6.1±2.4 | 5.4±1.9 | ||||||||||
| N-telopeptide,b nmol/day | 541±194 | 673±189c | 788±160c | 781±146c | 770±205c | 668±165c | 555±189 | 815±204c | 775±225c | 661±343c | 844±284c | 812±183c | ||||||||||
| Serum C-telopeptide,b mmol/l | 0.9±0.2 | 1.1±0.3c | 1.2±0.2c | 1.2±0.3c | 1.0±0.1 | 0.9±0.2 | 1.0±0.2c | 1.2±0.3c | 1.1±0.2c | 0.9±0.2 | ||||||||||||
| Helical peptide,b mg/day | 772±194 | 802±144c | 926±265c | 1193±347c | 1048±317c | 903±233 | 826±297 | 1075±385c | 1062±411c | 1176±495c | 1082±382c | 953±260 | ||||||||||
| Deoxypyridinoline,b nmol/day | 40±18 | 55±20c | 52±21c | 62±25c | 50±16c | 54±17 | 54±17 | 77±23c | 70±20c | 78±23c | 82±16c | 60±11 | ||||||||||
| Pyridinium cross-links, nmol/day | 223±41 | 285±83 | 260±98 | 319±59 | 308±100 | 248±67 | 259±101 | 285±92 | 299±112 | 321±69 | 285±80 | 268±56 | ||||||||||
| Bone alkaline phosphatase, U/l | 31±5 | 32±8 | 32±6 | 32±4 | 30±5 | 30±7 | 28±7 | 27±6 | 26±5 | 27±5 | ||||||||||||
| Total alkaline phosphatase, U/l | 61±8 | 64±9 | 69±11 | 67±10 | 64±7 | 61±12 | 61±15 | 61±13 | 59±11 | 62±12 | ||||||||||||
| PINP,b ng/ml | 59±15 | 58±16 | 59±13 | 57±12 | 68±15c | 68±15 | 62±12 | 61±16 | 60±10 | 72±20c | ||||||||||||
| Osteocalcin,a ng/ml | 23±4 | 22±5 | 24±5 | 22±4c | 23±4 | 26±4 | 24±4 | 26±5 | 23±4c | 25±5 | ||||||||||||
| Osteoprotegerin,a pmol/l | 4.6±1.3 | 4.3±1.5 | 4.4±1.3 | 4.6±1.5 | 4.7±1.7c | 4.4±1.2 | 4.3±1.1 | 3.9±0.8 | 4.5±1.2 | 5.8±1.9c | ||||||||||||
| TRAP-C,a ng/ml | 3.0±0.7 | 3.3±1.0 | 2.9±0.9 | 3.5±0.9 | 3.7±0.9c | 3.2±0.7 | 3.4±0.9 | 3.5±0.7 | 3.7±0.8 | 3.8±1.1c | ||||||||||||
Values are means ± SD; n = 7 for the control (Con) group and n = 8 for the artificial gravity (AG) group. For urinary variables, the means of individual values from the 2 days at each time point were used to determine the group mean ± SD. 25-OH vit D, 25-hydroxyvitamin D; 1,25-(OH)2 vit D, 1,25-dihydroxyvitamin D; PINP, procollagen type I N propeptide; PTH, parathyroid hormone; TRAP-C, tartrate-resistant acid phosphatase; Pre, pre-bed rest ambulatory period; BR, bed rest days; BR+0/+1 and BR+6/+7, 0 to 1 and 6 to 7 days after bed rest.
P < 0.01;
P < 0.001, significant main effect of time with groups combined.
P < 0.05 vs. Pre (determined by a post hoc Bonferroni t-test in the exploratory ANOVA).
Table 3.
Bone density of the tibia determined by pQCT before and after bed rest
| Con |
AG |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Total density (distal), mg/cm3 | 386±35 | 389±36 | 374±52 | 374±55 | ||
| Cortical region density (distal), mg/cm3 | 492±37 | 498±40 | 493±88 | 493±94 | ||
| Trabecular region density (distal), mg/cm3 | 257±43 | 256±44 | 229±23 | 228±22 | ||
| SSI P (distal), mg/cm3 | 1,864±297 | 1,893±297 | 1,886±561 | 1,887±578 | ||
| Total density (mid), mg/cm3 | 875±58 | 872±59 | 853±64 | 853±68 | ||
| Cortical region density (mid), mg/cm3 | 1,177±23 | 1,170±28 | 1,155±31 | 1,159±37 | ||
| Trabecular region density (mid), mg/cm3 | 505±122 | 509±119 | 482±117 | 480±121 | ||
| SSI P (mid), mg/cm3 | 2,598±249 | 2,605±259 | 2,859±366 | 2,852±385 | ||
| Total density (proximal), mg/cm3 | 311±35 | 312±38 | 308±24 | 308±26 | ||
| Cortical region density (proximal), mg/cm3 | 432±53 | 433±58 | 421±34 | 421±38 | ||
| Trabecular region density (proximal), mg/cm3 | 163±19 | 163±17 | 170±21 | 170±21 | ||
| SSI P (proximal), mg/cm3 | 4,503±937 | 4,556±1079 | 3,985±817 | 3,956±943 | ||
Values are means ± SD; n = 7 for the Con group and n = 8 for the AG group. Proximal means were calculated from the average of 3 slices per session and 3 sessions per time point for each subject; distal and midshaft data were calculated from the average of 3 sessions per time point (1 slice per session) for each subject. pQCT, peripheral quantitative computerized tomography; SSI P, polar strength-strain index.
In a second analysis phase, for the pre-bed rest and in-bed rest periods only, time was treated as a continuous covariate with the aim of identifying possible linear trends, either overall or by group, within the bed rest period for the 17 bone marker variables listed in Table 1. In this analysis phase, P values were adjusted for multiple testing of the bone marker variables, using the free-step-down method of Westfall and Young (48) to control the family-wise type I error rate to 0.05. For BMC and the seven measurements of BMD listed in Table 2, which were made only before and after bed rest, P values from the repeated-measures ANOVA were similarly adjusted for multiple testing. No adjustment was made in analysis of pQCT, because no significant changes were observed for these measurements.
Table 2.
Bone mineral density determined by DXA before and after bed rest
| Con |
AG |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Whole body total BMC,a g | 2,759±269 | 2,728±250 | 2,914±223 | 2,894±213 | ||
| Whole body total BMD, g/cm2 | 1.204±0.059 | 1.200±0.060 | 1.252±0.080 | 1.252±0.080 | ||
| Pelvis BMD, g/cm2 | 1.193±0.096 | 1.180±0.087 | 1.241±0.129 | 1.233±0.136 | ||
| Heel BMD, g/cm2 | 0.711±0.084 | 0.715±0.084 | 0.696±0.063 | 0.695±0.066 | ||
| Spine total BMD, g/cm2 | 1.021±0.064 | 1.015±0.068 | 1.065±0.094 | 1.063±0.095 | ||
| Trochanter BMD,b g/cm2 | 0.779±0.094 | 0.772±0.095 | 0.829±0.129 | 0.823±0.126 | ||
| Femoral neck BMD, g/cm2 | 0.902±0.065 | 0.901±0.067 | 0.950±0.129 | 0.952±0.129 | ||
| Total hip BMD,a g/cm2 | 1.058±0.081 | 1.050±0.081 | 1.099±0.115 | 1.093±0.116 | ||
Values are means ± SD; n = 7 for the Con group and n = 8 for the AG group. BMC, bone mineral content; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; Pre, ambulatory period before bed rest; Post, ambulatory period after bed rest. Individual Pre and Post values are the averages of 3 consecutive scans.
P < 0.05;
P < 0.001, significant effect of time for combined data before adjustment for multiple testing.
Statistical analyses were performed using SigmaStat software version 3.01a (SPSS, Chicago, IL) and Stata statistical software version 10.0 (College Station, TX), and P < 0.05 was the level of significance. Data are means ± SD.
RESULTS
Blood and urinary markers of vitamin D and calcium metabolism and of bone cellular activity are presented by group and time period in Table 1. Neither the exploratory analysis nor the more focused trend analysis showed a change in 25-OH vitamin D or 1,25-(OH)2 vitamin D during bed rest in either group. Similarly, neither blood ionized calcium nor serum calcium changed significantly during bed rest.
Cumulative calcium balance (Fig. 1), determined by subtracting daily fecal and urinary calcium from dietary calcium for each phase of the study, decreased in both groups during bed rest (P < 0.001). Note that because the cumulative balance is “reset” at the beginning of each phase, and the phases have different durations, absolute numbers should not be compared between phases. The data suggest that the subjects tended to be in negative calcium balance even during recovery, but a longer recovery phase would be needed to draw any conclusions about how long it would take to recover to pre-bed rest levels.
Fig. 1.
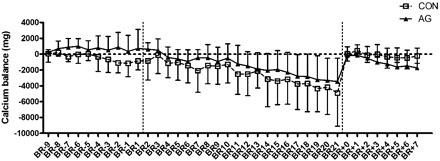
Cumulative calcium balance of control (Con) and artificial gravity (AG) subjects before, during, and after 21 days of bed rest. This was calculated for each individual as the difference between dietary intake of calcium and the combined urinary and fecal excretion of calcium. Cumulative balance was “restarted” with each phase of the study (before, during, and after bed rest; break points are indicated by vertical dotted lines), so that balance calculations are cumulative for days BR−9 through BR1 (before), BR2 through BR21 (during), and BR+0 through BR+7 (after bed rest). Values are means ± SD; n = 6 Con subjects and 8 AG subjects. Dietary intake methodology is described in a companion article (54). There were no differences between the 2 groups, but time had a significant effect. Calcium balance for BR11 through BR21 was significantly more negative than before bed rest (P < 0.001).
Bone resorption was assessed by several markers (Table 1). The exploratory analysis showed that serum intact PTH was significantly lower, but serum C-telopeptide and urinary excretion levels of N-telopeptide, helical peptide, and DPD were significantly greater during bed rest than before bed rest, and the changes did not depend on group (Table 1). All of the increases were corroborated with the trend analysis, even after adjusting for multiple testing of 17 bone markers. Pyridinium cross-link excretion did not change significantly.
With all time points taken into consideration, the exploratory analysis indicated that blood concentrations of bone ALP and total ALP, markers of bone formation, tended to change differently over time in the AG group compared with the Con group (P = 0.08 and P = 0.09, respectively; Table 1). The trend analysis further revealed that concentrations of these markers decreased significantly more in the AG group during bed rest than in the Con group (P = 0.0077 and P = 0.0028 for bone ALP and total ALP, respectively). After adjustment for multiple testing, the group effect on the slope of total ALP during bed rest was still significant (adjusted P = 0.035), but not the effect for bone ALP (adjusted P = 0.087). When the two groups were combined, the serum concentration of PINP, another marker of bone formation, was significantly greater during recovery than before bed rest (P < 0.001), but there was no differential group effect (Table 1). Serum concentrations of osteocalcin showed a significant (P < 0.01) effect of time (lower concentrations on BR20/21 than before bed rest, as shown by the post hoc Bonferroni t-test), but no group effect was found.
Using the exploratory ANOVA, we found that the osteoclastogenesis inhibitory factor OPG was significantly elevated during the recovery phase (BR+7, P < 0.01), and the AG subjects tended to have higher blood concentrations at that time than did the Con subjects (P = 0.054). Concentrations of the OPG ligand RANKL were below the detectable limit of the assay for most subjects; therefore, statistical analyses were not performed. TRAP-C was significantly greater during recovery in both groups than it was before bed rest, indicating that osteoclast activity was higher at that time.
AG had no significant effect on the change over time in whole body total BMC or BMD of the bone regions studied. However, when the two groups were combined, the whole body total BMC and trochanter and total hip BMD were significantly less after bed rest (P = 0.003, 0.014, and 0.0003 respectively; Table 2). After adjustment for multiple testing, the above changes in BMD were still significant (adjusted P < 0.05), but not the whole body total BMC. Bone density of the tibia determined by pQCT was not different after bed rest in any region examined, and it was not different between groups (Table 3).
DISCUSSION
Despite decades of research, it has proven difficult to mitigate weightlessness-induced bone loss. Bone loss as well as other negative physiological effects of weightlessness would be expected to be mitigated by AG. The aim of this project was to determine whether AG would protect multiple physiological systems during unloading; bone, in particular, was thought to be responsive to AG during bed rest.
We hypothesized that AG would protect bone from demineralization during bed rest and that this protection would be evident from changes in markers of bone formation and resorption. The rationale was that the loading produced by AG would stimulate bone formation and decrease bone resorption through a mechanotransduction process by which the mechanical load on bone is transduced into biochemical signals (9, 26). During mechanotransduction, the function of bone cells can change and induce changes in bone structures to adapt to the load.
In the present study, an AG prescription of 1 h/day was used, but it clearly did not protect against the increase in bone resorption that in the past 10 years has become a hallmark of bed rest (3, 14, 21, 30, 34, 36, 46). No evidence was found that bone was protected from demineralization associated with bed rest. Although we did not expect to find any changes in bone density during this relatively short 21-day study, we fully expected that if AG had had a protective effect on bone, we would have observed differences in the biochemical markers of bone resorption and formation.
Several parameters of the AG prescription must be considered to make it optimally effective. Some of these are rate of centrifugation, acceleration, position of the subject on the centrifuge, body mass, timing of exposure, and duration of exposure. The 1 h/day duration of centrifugation (the Gz force) simply may not be long enough to have a protective effect on bone. Vernikos et al. (45) mitigated a bed rest-induced increase in urinary calcium by having subjects walk for 2 or 4 h/day, but standing for 2 or 4 h/day was not enough. Standing for 2 h/day during bed rest was enough to mitigate symptoms of orthostatic intolerance. Minimum requirements for gravity exposure also varied greatly for different physiological systems in an animal study in which rat hindlimbs were unloaded for 28 days (52). The investigators found that the cardiovascular system responded when the rats were exposed to as little as 1 h/day of gravity (1 Gz by standing). They also found that bone was the most resistant system and required up to 4 h/day of standing or 1 h/day of centrifugation (1.5× gravity) to only partially mitigate femur deconditioning (52). No change or a decrease in bone mass was also found after prolonged hypergravity (12, 16, 22). Potential explanations for these disparate results include the age of the animals and the dose of the gravity prescription.
In addition to duration of the force, the Gz force itself that was used in our experiment may not have been great enough to stimulate an effect on bone, or perhaps it could have been enhanced by combining it with exercise. In one 20-day bed rest study in which AG (a maximum of 2 Gz) was combined with ergometric exercise, a protective effect was found on bone as evidenced by a decrease in the urinary excretion of the collagen cross-link DPD (15). This was the only such marker reported, however. Similar effects on other markers might strengthen confidence in this finding.
In another bed rest study, bone loss associated with disuse was effectively mitigated by lower body negative pressure (LBNP) combined with treadmill exercise (36, 56). The LBNP in that study allowed an average ground reaction force (GRF) of 120% of body weight on the treadmill. The GRF provided by the AG prescription in the experiment reported in this study ranged from 112 to 128% of body weight (120 ± 6%). One difference between the force in the AG study and that in the LBNP study is that the force in the AG study was fairly constant over a 1-h period each day. The 120% of body weight average GRF in the LBNP study was intermittent with each step while subjects ran on the treadmill. Several studies suggest that interruption of loading by rest periods amplifies the bone formation response (4, 13, 43). Interestingly, the GRF for AG subjects in this study was positively correlated with their change in heel BMD (r = 0.80, P < 0.01).
Beyond GRF, the other stimulus provided by LBNP is an increase in blood flow and fluid shifting to the lower extremities, with an increase in interstitial fluid pressures. These also have been implicated in bone changes in unloading and spaceflight (5, 8) and may have provided some of the resorption mitigation offered by LBNP combined with treadmill exercise. Centrifugation, as utilized in this study, also results in a fluid shift to the lower body, but at the duration and intensity used, it failed to affect bone metabolism.
Bone ALP of AG subjects decreased during bed rest, and this change was positively correlated with the change in PINP (r = 0.33, P < 0.01). This decrease in bone formation markers of AG subjects during bed rest was not expected, but the mechanism may be related to AG-induced fluid shifts. Several in vitro studies suggest that the flow of interstitial fluid can directly affect bone formation (11, 26). This may explain why, in 6° HDT bed rest and rat hindlimb suspension models, bone mass in the skull and mandible is increased after several weeks (1, 31). Other studies show that osteoblasts are not stimulated very much by mechanotransduction of static loads but that bone formation depends more on strain rate, amplitude, and duration of loading and is threshold driven (11, 17). Perhaps the threshold required to stimulate bone formation was not exceeded by the duration or force of 1 h/day of centrifugation. The fact that the bone formation markers ALP, total ALP, and PINP are not site specific must also be considered. For instance, if bone formation increased to a certain extent in regions that were maximally loaded but decreased to a greater extent in regions that were not loaded, an overall negative change from the pre-bed rest period would result, and we would have seen only the overall negative change.
Urinary calcium did not increase during bed rest as much as we would have expected, although a negative calcium balance during bed rest was observed for both groups. The negative calcium balance resulted from the combination of a decrease in calcium intake (P < 0.05) and an increase in fecal calcium (P < 0.05) during bed rest compared with before bed rest. Urinary calcium excretion, although often considered a hallmark of bed rest, is not always elevated. This can be related to a number of factors, including general variability between subjects. Furthermore, dietary variation between subjects, days, and studies also may contribute to this. Specifically, dietary calcium, sodium, protein (total, or animal vs. vegetable), potassium, and other nutrients can all have an effect on urinary calcium (2, 10, 33, 57).
We know from the data we have presented that 1 h/day of passive centrifugation at 2.5 Gz at the feet is not enough to mitigate bone loss associated with disuse during 21 days of bed rest in men. It is clear that, at some point, bone loss associated with disuse will be mitigated by AG, since it is known that musculoskeletal loading in a 1-G environment protects against such loss. Whether the response of women would differ is also unknown. Further studies with an AG protocol should aim to combine exercise with AG and perhaps include several shorter AG sessions instead of one long session each day.
GRANTS
This work was funded by the NASA Human Research Program and was conducted at the National Institutes of Health-funded (M01 RR 0073) General Clinical Research Center at the University of Texas Medical Branch, Galveston, TX.
Acknowledgments
We thank the Nutritional Biochemistry Laboratory at the Johnson Space Center for sample processing and analysis, the University of Texas Medical Branch for the use of facilities and support of the overall project, the staffs of the Bed Rest and Artificial Gravity Projects at the Johnson Space Center for coordination of this expansive project, and Jane Krauhs for editorial assistance.
REFERENCES
- 1.Arnaud SB, Powell MR, Vernikos-Danellis J, Buchanan P. Bone mineral and body composition after 30 day head down tilt bed rest. J Bone Miner Res 3: S119, 1988 [Google Scholar]
- 2.Arnaud SB, Wolinsky I, Fung P, Vernikos J. Dietary salt and urinary calcium excretion in a human bed rest spaceflight model. Aviat Space Environ Med 71: 1115–1119, 2000 [PubMed] [Google Scholar]
- 3.Baecker N, Tomic A, Mika C, Gotzmann A, Platen P, Gerzer R, Heer M. Bone resorption is induced on the second day of bed rest: results of a controlled crossover trial. J Appl Physiol 95: 977–982, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, Jacobs CR. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech 38: 1909–1917, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield SA, Allen MR, Hogan HA, Delp MD. Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone 31: 149–157, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Caillot-Augusseau A, Lafage-Proust MH, Soler C, Pernod J, Dubois F, Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95). Clin Chem 44: 578–585, 1998 [PubMed] [Google Scholar]
- 7.Caillot-Augusseau A, Vico L, Heer M, Voroviev D, Souberbielle JC, Zitterman A, Alexandre C, Lafage-Proust M-H. Space flight is associated with rapid decreases of undercarboxylated osteocalcin and increases of markers of bone resorption without changes in their circadian variation: observations in two cosmonauts. Clin Chem 46: 1136–1143, 2000 [PubMed] [Google Scholar]
- 8.Colleran PN, Wilkerson MK, Bloomfield SA, Suva LJ, Turner RT, Delp MD. Alterations in skeletal perfusion with simulated microgravity: a possible mechanism for bone remodeling. J Appl Physiol 89: 1046–1054, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344–358, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Fenton TR, Eliasziw M, Lyon AW, Tough SC, Hanley DA. Meta-analysis of the quantity of calcium excretion associated with the net acid excretion of the modern diet under the acid-ash diet hypothesis. Am J Clin Nutr 88: 1159–1166, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Forwood MR, Turner CH. Skeletal adaptations to mechanical usage: results from tibial loading studies in rats. Bone 17: 197S–205S, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Fosse G, Gat H, Holmbakken N, Kvinnsland S. Bone atrophy and hypergravity in mice. Growth 38: 329–342, 1974 [PubMed] [Google Scholar]
- 13.Gross TS, Poliachik SL, Ausk BJ, Sanford DA, Becker BA, Srinivasan S. Why rest stimulates bone formation: a hypothesis based on complex adaptive phenomenon. Exerc Sport Sci Rev 32: 9–13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heer M, Baecker N, Mika C, Boese A, Gerzer R. Immobilization induces a very rapid increase in osteoclast activity. Acta Astronaut 57: 31–36, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Iwase S, Takada H, Watanabe Y, Ishida K, Akima H, Katayama K, Iwase M, Hirayanagi K, Shiozawa T, Hamaoka T, Masuo Y, Custaud MA. Effect of centrifuge-induced artificial gravity and ergometric exercise on cardiovascular deconditioning, myatrophy, and osteoporosis induced by a −6 degrees head-down bedrest. J Gravit Physiol 11: P243–P244, 2004 [PubMed] [Google Scholar]
- 16.Jaekel E, Amtmann E, Oyama J. Effect of chronic centrifugation on bone density of the rat. Anat Embryol (Berl) 151: 223–232, 1977 [DOI] [PubMed] [Google Scholar]
- 17.LaMothe JM, Hamilton NH, Zernicke RF. Strain rate influences periosteal adaptation in mature bone. Med Eng Phys 27: 277–284, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19: 1006–1012, 2004 [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact 1: 157–160, 2000 [PubMed] [Google Scholar]
- 20.LeBlanc AD, Driscol TB, Shackelford LC, Evans HJ, Rianon NJ, Smith SM, Feeback DL, Lai D. Alendronate as an effective countermeasure to disuse induced bone loss. J Musculoskelet Neuronal Interact 2: 335–343, 2002 [PubMed] [Google Scholar]
- 21.LeBlanc AD, Spector ER, Evans HJ, Sibonga JD. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact 7: 33–47, 2007 [PubMed] [Google Scholar]
- 22.Martinez DA, Orth MW, Carr KE, Vanderby R Jr, Vasques M, Grindeland RE, Vailas AC. Cortical bone responses to 2G hypergravity in growing rats. Aviat Space Environ Med 69: A17–A22, 1998 [PubMed] [Google Scholar]
- 23.Naumann FL, Bennell KL, Wark JD. The effects of +Gz force on the bone mineral density of fighter pilots. Aviat Space Environ Med 72: 177–181, 2001 [PubMed] [Google Scholar]
- 24.Naumann FL, Grant MC, Dhaliwal SS. Changes in cervical spine bone mineral density in response to flight training. Aviat Space Environ Med 75: 255–259, 2004 [PubMed] [Google Scholar]
- 25.Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol 19: 91–96, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Owan I, Burr DB, Turner CH, Qiu J, Tu Y, Onyia JE, Duncan RL. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol Cell Physiol 273: C810–C815, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Oyama J. Response and adaptation of beagle dogs to hypergravity. Life Sci Space Res 13: 11–17, 1975 [PubMed] [Google Scholar]
- 29.Parfitt AM. Bone effects of space flight: analysis by quantum concept of bone remodelling. Acta Astronaut 8: 1083–1090, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, and Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol 101: 143–194, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Roer RD, Dillaman RM. Bone growth and calcium balance during simulated weightlessness in the rat. J Appl Physiol 68: 13–20, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Searby ND, Steele CR, Globus RK. Influence of increased mechanical loading by hypergravity on the microtubule cytoskeleton and prostaglandin E2 release in primary osteoblasts. Am J Physiol Cell Physiol 289: C148–C158, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Sellmeyer DE, Schloetter M, Sebastian A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J Clin Endocrinol Metab 87: 2008–2012, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol 97: 119–129, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Smith MC Jr, Rambaut PC, Vogel JM, Whittle MW. Bone mineral measurement-experiment M078. In: Biomedical Results from Skylab (NASA SP-377), edited by Johnston RS and Dietlein LF. Washington, DC: National Aeronautics and Space Administration, 1977, p. 183–190.
- 36.Smith SM, Davis-Street JE, Fesperman JV, Calkins DS, Bawa M, Macias BR, Meyer RS, Hargens AR. Evaluation of treadmill exercise in a lower body negative pressure chamber as a countermeasure for weightlessness-induced bone loss: a bed rest study with identical twins. J Bone Miner Res 18: 2223–2230, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Smith SM, Dillon EL, DeKerlegand DE, Davis-Street JE. Variability of collagen crosslinks: impact of sample collection period. Calcif Tissue Int 74: 336–341, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Smith SM, Nillen JL, LeBlanc A, Lipton A, Demers LM, Lane HW, Leach CS. Collagen cross-link excretion during space flight and bed rest. J Clin Endocrinol Metab 83: 3584–3591, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Wastney ME, Morukov BV, Larina IM, Nyquist LE, Abrams SA, Taran EN, Shih CY, Nillen JL, Davis-Street JE, Rice BL, Lane HW. Calcium metabolism before, during, and after a 3-mo spaceflight: kinetic and biochemical changes. Am J Physiol Regul Integr Comp Physiol 277: R1–R10, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Wastney ME, O'Brien KO, Morukov BV, Larina IM, Abrams SA, Davis-Street JE, Oganov V, Shackelford LC. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the Mir space station. J Bone Miner Res 20: 208–218, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE. Nutritional status assessment of International Space Station crew members. J Nutr 135: 437–443, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Smith SM, Zwart SR, Heer M, Lee SMC, Baecker N, Meuche S, Macias BR, Shackelford LC, Schneider S, Hargens AR. WISE-2005: supine treadmill exercise within lower body negative pressure and flywheel resistive exercise as a countermeasure to bed rest-induced bone loss in women during 60-day simulated microgravity. Bone 42: 572–581, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS. Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol 102: 1945–1952, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI. Mechanical loading modulates glutamate receptor subunit expression in bone. Bone 37: 63–73, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Vernikos J, Ludwig DA, Ertl AC, Wade CE, Keil L, O'Hara D. Effect of standing or walking on physiological changes induced by head down bed rest: implications for spaceflight. Aviat Space Environ Med 67: 1069–1079, 1996 [PubMed] [Google Scholar]
- 46.Watanabe Y, Ohshima H, Mizuno K, Sekiguchi C, Fukunaga M, Kohri K, Rittweger J, Felsenberg D, Matsumoto T, Nakamura T. Intravenous pamidronate prevents femoral bone loss and renal stone formation during 90-day bed rest. J Bone Miner Res 19: 1771–1778, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Weaver CM, LeBlanc A, Smith SM. Calcium and related nutrients in bone metabolism. In: Nutrition in Spaceflight and Weightlessness Models, edited by Lane HW and Schoeller DA. Boca Raton, FL: CRC, 2000, p. 179–201.
- 48.Westfall PH, Young SS. Resampling-Based Multiple Testing. New York: John Wiley & Sons, 1993
- 49.Whedon G, Lutwak L, Rambaut P, Whittle M, Leach C, Reid J, Smith M. Effect of weightlessness on mineral metabolism; metabolic studies on Skylab orbital flights. Calcif Tissue Res 21, Suppl: 423–430, 1976 [PubMed] [Google Scholar]
- 50.Whedon GD, Lutwak L, Rambaut PC, Whittle MW, Smith MC, Reid J, Leach C, Stadler CR, Sanford DD. Mineral and nitrogen metabolic studies, experiment M071. In: Biomedical Results from Skylab (NASA SP-377), edited by Johnston RS and Dietlein LF. Washington, DC: National Aeronautics and Space Administration, 1977, p. 164–174.
- 51.Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem 96: 1049–1060, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Zhang LF, Sun B, Cao XS, Liu C, Yu ZB, Zhang LN, Cheng JH, Wu YH, Wu XY. Effectiveness of intermittent −Gx gravitation in preventing deconditioning due to simulated microgravity. J Appl Physiol 95: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Zittermann A, Heer M, Caillot-Augusso A, Rettberg P, Scheld K, Drummer C, Alexandre C, Horneck G, Vorobiev D. Microgravity inhibits intestinal calcium absorption as shown by a stable strontium test. Eur J Clin Invest 30: 1036–1043, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Zwart SR, Crawford GE, Gillman PL, Kala G, Rodgers AS, Rogers A, Inniss AM, Rice BL, Ericson K, Coburn SP, Bourbeau Y, Hudson E, Booth SL, DeKerlegand DE, Sams CF, Heer MA, Smith SM. Effects of 21 days of bed rest, with or without artificial gravity, on nutritional status of humans. J Appl Physiol (December 12, 2008). 10.1152/japplphysiol.91136.2008 [DOI] [PMC free article] [PubMed]
- 55.Zwart SR, Davis-Street JE, Paddon-Jones D, Ferrando AA, Wolfe RR, Smith SM. Amino acid supplementation alters bone metabolism during simulated weightlessness. J Appl Physiol 99: 134–140, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Zwart SR, Hargens AR, Lee SM, Macias BR, Watenpaugh DE, Tse K, Smith SM. Lower body negative pressure treadmill exercise as a countermeasure for bed rest-induced bone loss in female identical twins. Bone 40: 529–537, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zwart SR, Hargens AR, Smith SM. The ratio of animal protein intake to potassium intake is a predictor of bone resorption in space flight analogues and in ambulatory subjects. Am J Clin Nutr 80: 1058–1065, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Zwart SR, Oliver SM, Fesperman JV, Kala G, Krauhs J, Ericson K, Smith SM. Nutritional status assessment before, during, and after long-duration head-down bed rest. Aviat Space Environ Med 80: A15–A22, 2009 [DOI] [PubMed] [Google Scholar]


