Abstract
Rodent models of chronic intermittent hypoxia (IH) are commonly used to investigate the pathophysiological sequelae that result from hypoxic exposure in patients experiencing obstructive sleep apnea (OSA). Despite the widespread use of IH models, little attention has been paid to carefully defining the degree of oxyhemoglobin desaturation that occurs during each hypoxic period. Therefore, we developed a rapid blood sampling technique to determine the arterial blood gas changes that occur in conscious unrestrained mice during a single IH event and hypothesized that the arterial Po2 (PaO2) at the nadir level of the inspired oxygen profile causes oxyhemoglobin saturation to fall to between 80% and 90%. Mice were exposed to 120–180 cycles of IH at a rate of 60 cycles/h, and arterial blood samples were withdrawn (<3 s) at baseline and at 10-s time intervals over the course of a single IH cycle. The IH regimen caused a decline in the fraction of inspired oxygen from room air levels to a transient nadir of 6.0 ± 0.2% over the 30-s hypoxic period. The PaO2 and arterial oxyhemoglobin saturation reached a nadir of 47 ± 2 mmHg and 85 ± 2% at 30 s, respectively. Arterial Pco2 decreased to a nadir of 26 ± 2 mmHg at 30 s, associated with a rise in arterial pH to 7.46 ± 0.2. We conclude that the magnitude of oxyhemoglobin desaturation that is induced in our murine model of IH is consistent with the degree of hypoxic stress that occurs in moderate to severe clinical OSA.
Keywords: arterial oxyhemoglobin saturation, hypercapnia, obstructive sleep apnea, pH, inspired oxygen, mouse, oxyhemoglobin desaturation, arterial partial pressure of oxygen
obstructive sleep apnea (OSA) is characterized by recurrent collapse of the upper airway during sleep, leading to repeated periods of intermittent hypoxia (IH). OSA and the resulting IH have been associated with an increased risk of hypertension (15, 18), insulin resistance (22), cardiovascular disease (7, 14), and cerebrovascular disease (34). Rodent models of IH have been commonly used to simulate the hypoxic stress in OSA and can produce cardiovascular (6), metabolic (8, 13), and neurocognitive (23) dysfunction. However, results have sometimes been equivocal, as in blood pressure studies in rats, where systemic hypertension may (6) or may not (11) occur in response to IH exposure. Disparities in outcomes in rodent models of IH have often been ascribed to differences in the severity of the hypoxic stress, which may vary considerably between studies.
Multiple factors determine the severity of hypoxic stress that occurs with IH exposure. For each single hypoxic exposure, these include the duration of the hypoxic event, the nadir of inspired oxygen reached, the rate of hypoxia development, the length of time the inspired oxygen is maintained at the nadir, and the subsequent rate of reoxygenation. For repeated episodes of IH, factors include the number of hypoxic events per hour, the number and timing of hypoxic episodes within each 24-h period, and the number of days or weeks of total exposure time. It is not surprising given these multiple parameters that almost every laboratory that models IH has their own distinct exposure paradigm. Indeed, the question can be asked as to how relevant are specific exposure paradigms of IH as a model of the hypoxic stress experience by OSA patients?
The fundamental component of any IH paradigm is the hypoxic profile that defines each single IH event. In most IH paradigms, an increasing hypoxic stimulus is used to model the decline in oxyhemoglobin desaturation that commonly occurs in OSA. The impact of the hypoxic profile on arterial blood gas status is an important factor in determining the hypoxic stress an animal experiences and the clinical relevance of the stimulus. Despite the very large number of studies that have used IH paradigms in rodents, no previous study has attempted to assess the changes in arterial blood gas status that accompany a single hypoxic profile in conscious freely behaving animals.
We (31) have previously measured arterial blood gas levels in conscious mice during steady-state hypoxic exposures of 1–4 min and observed severe hypoxemia (27.3 ± 3.5 mmHg) when inspired oxygen was reduced to 5%. The purpose of the present study was to measure the arterial blood gas changes that occur in conscious mice during the course of a 30-s IH event in which the inspired oxygen is decreased from room air levels to a transient nadir of 5–6% before rapid reoxygenation. We hypothesized that during a single IH event, the arterial Po2 (PaO2) at the nadir level of inspired oxygen is not as severe as occurs in steady-state exposure to 5% inspired oxygen in mice and simulates the hypoxic stress that occurs with moderate to severe OSA in patients.
MATERIALS AND METHODS
Animals and surgical preparation.
Animal handling and experimentation were in accordance with approved Institutional Animal Care and Use Committee protocols at the University of Pittsburgh. Male C57BL/6J mice aged 10–12 wk (n = 9) were kept on a 12:12-h light-dark cycle beginning at 8 AM with free access to food and water. Femoral arterial catheters were chronically implanted as previously described (1). In brief, for catheterization, mice were anesthetized with inhaled 2% isoflurane. Micro-renathane catheters (MRE-025, Braintree Scientific, Braintree, MA) were prepared by heating, pulling, cutting to the appropriate diameter, shaping into a J-form in hot oil, and sterilizing (ethylene oxide). Catheters were inserted in the left femoral artery, tied in place, stabilized with superglue (Henkel, Rocky Hill, CT), tunneled subcutaneously to the upper back by threading through a blunt needle, taped to a wire attached to posterior cervical muscles for stiffness (792500, A-M-Systems, Sequim, WA), and connected to a 360° swivel designed for mice (375/D/22QM, Instech, Plymouth Meeting, PA). In contrast to previous studies (1, 35), we used a longer arterial catheter (38 in.) so that the deadspace outside the animal could contain ∼80 μl of blood (see below for details of blood sampling). Patency of the catheters was maintained by continuously flushing 7 μl/h saline containing 20 U/ml heparin (Baxter, Deerfield, IL) using a syringe pump with a multisyringe adaptor (R99-EM, Razel Scientific Instruments, St. Albans, VT). Catheters were monitored for patency daily and kept unclogged by manual flushes using a 1-ml syringe with a 26-gauge needle when necessary. Animals were allowed 72 h to recover from surgery and were required to have a pH in room air of 7.38 or above to begin the protocol.
IH.
A gas control delivery system was designed to regulate the flow of nitrogen and room air into a customized cage housing individual tethered mice during the experimental period, as previously described (21). The cage was pyramidal in shape with a base and height of 7 in. The mouse was housed on grated metal flooring that was 1.5 in. above the bottom of the cage, allowing a mixing area for gases that entered the cage from all four sides below the mouse. The gas exhausted passively up past the mouse and through a 1-in. hole at the apex of the pyramid, which served as the outlet for the catheters to connect to the fluid swivel. The inspired gas was monitored from outlet ports from three sides of the cage positioned at the level of the nose of the mouse. A series of programmable solenoids and flow regulators altered the inspired oxygen over a defined and repeatable profile by switching between room air and nitrogen at flow rates of 5–7 l/min. During each period of IH, the inspired oxygen was reduced from 20.9% to ∼5–6.0% over a 30-s period and rapidly reoxygenated (supplemented by 5 s of 100% oxygen at ∼1 l/min) to room air levels in the succeeding 30-s period (i.e., 60 cycles/h). This profile matched that used in our previous study (20), in which larger rectangular customized cages were used to expose up to four untethered mice at a time to IH.
On experimental days, mice were exposed to 2–3 h (i.e., 120–180 cycles) of IH before arterial blood gases were withdrawn and the fraction of inspired oxygen (FiO2) was detected at nose level using a Vacumed fast response oxygen analyzer (Vacumed, Ventura, CA).
Blood gas withdrawal and analysis.
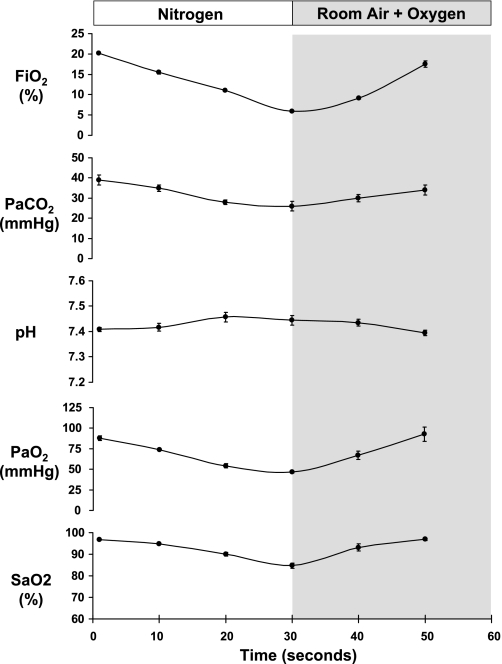
Arterial blood samples were drawn at baseline and at 10-s time points during a single IH cycle (Fig. 1). The catheter was of sufficient length so that the 60-μl sample volume could be pulled quickly into the catheter deadspace outside the animal in <3 s. The withdrawal syringe was disconnected, and the sample transferred to a capillary tube for immediate analysis with a Rapidlab 348 (Bayer) blood gas analyzer (an automated, compact analyzer used in clinical and laboratory settings that requires a minimum sample size of 60 μl). The pH, Pco2, and Po2 were directly measured, and the arterial oxyhemoglobin saturation (SaO2) was calculated assuming a standard oxyhemoglobin dissociation curve. No more than two blood samples were taken from a mouse on a single day. In the total of nine mice studied, a minimum of two and maximum of seven arterial blood samples were taken per mouse depending on the duration of catheter patency.
Fig. 1.
Mean ± SE changes in the fraction of inspired oxygen (FiO2; %), arterial Pco2 (PaCO2), arterial pH, arterial Po2 (PaO2), and arterial oxyhemoglobin saturation (SaO2) at 10-s intervals throughout a 30-s period of hypoxia and 30-s period of reoxygenation. Numbers of blood gas samples and numbers of mice at each time point were as follows: room air, 16 samples and 8 mice; 10 s, 6 samples and 3 mice; 20 s, 8 samples and 6 mice; 30 s, 7 samples and 3 mice; 40 s, 6 samples and 4 mice; and 50 s, 6 samples and 4 mice. SEs were calculated using the number of mice.
RESULTS
All animals recovered from surgery and exhibited normal blood gas levels [PaO2 = 88 ± 3 mmHg, arterial pH = 7.41 ± 0.01, arterial Pco2 (PaCO2) = 39 ± 3 mmHg, and SaO2 = 96 ± 1%] under baseline room air conditions (Fig. 1). The IH regimen caused an essentially linear decline in FiO2 from room air levels to 6.0 ± 0.2% over the 30-s hypoxic period. The PaO2 and SaO2 mirrored the time-related profile of FiO2, reaching nadirs of 47 ± 2 mmHg and 85 ± 2% at 30 s, respectively. The PaCO2 also decreased over the course of the hypoxic event, consistent with a compensatory hyperventilation, and reached a nadir of 26 ± 2 mmHg at 30 s. Concomitant with the fall in PaCO2, there was a corresponding rise in arterial pH.
DISCUSSION
Despite the widespread use of rodent IH models to study the pathological sequelae of OSA, the profile of arterial blood gas disturbances that occur within a single IH episode has not been carefully characterized. The data for our specific regimen of IH show that in the C57BL/6J mouse, the PaO2 falls continuously over a 30-s period to a nadir slightly below 50 mmHg, which corresponds to an arterial oxyhemoglobin saturation of ∼85%. The strength of our study was that the data were collected in a sequential, time-dependent manner in awake, chronically instrumented, and unhandled mice, and the data exhibited high reproducibility, as evidenced by the small SEs. The femoral artery was catheterized, in preference to the common carotid artery, to preserve the blood supply to both carotid chemoreceptors and allow development of the full ventilatory response to hypoxia. In the discussion below, we address the significance of these findings and relate them to the broader field of IH paradigms and the associated limitations and strengths of the model.
Severity of a single IH event.
The term “intermittent hypoxia” has been applied to many aspects of physiology, ischemic preconditioning, exercise training, altitude acclimation, and medicine. The concept of IH applied to exercise training can involve nighttime periods of hypoxic exposure (e.g., sleeping in a hypoxic tent) that last for several hours. In contrast, the IH that is used to simulate the hypoxic stress that occurs in OSA is commonly in a range from 12 to 90 s/hypoxic event (6, 11). The choice of the hypoxic exposure time, coupled with the time profile of oxygenation and reoxygenation, is the defining feature for any regimen of IH.
In our model, we chose a 30-s hypoxic exposure time for two reasons. First, a 30-s period of complete airway obstruction in an OSA patient represents a significant degree of hypoxic stress, with SaO2 likely to fall into the 80–90% range or below (4). Second, we (31) have previously developed a murine model of sleep-induced hypoxia in which 100% nitrogen is infused into a customized chamber housing a mouse instrumented for polysomnography. In this model, the infusion of nitrogen during sleep causes a linear decline in the level of inspired oxygen experienced by the mouse until arousal occurs and the nitrogen infusion is instantly switched back to room air. We observed that infusion of nitrogen into the customized chambers during sleep resulted in spontaneous arousals occurring on average after 25–30 s of hypoxic exposure at a resulting nadir of inspired oxygen of ∼10–13%. However, we also observed that in >10% of the sleep-induced hypoxic events, the FiO2 decreased to below 7% before spontaneous arousal occurred. Based on these observations, and the desire to develop a model of IH that resembled moderate to severe clinical OSA, we designed a protocol of nonsleep-induced hypoxia that incorporated 30-s periods of a ramp hypoxic exposure that reduced FiO2 to ∼5–6% (20). We subsequently validated in a later study (21) that this specific IH stimulus causes a transient arousal (assuming the animal is asleep at stimulus onset; see further discussion below) that is precisely timed to the nadir of inspired oxygen at 5–6%. Thus, a physiological rationale exists for the 30-s IH profile that we developed in mice to produce a severe, but clinically relevant, model of OSA.
Despite a physiological basis for our profile of FiO2 during IH exposure, we had no evidence that the degree of oxyhemoglobin desaturation that occurs in our mouse model is comparable with that experienced by a patient with moderate to severe OSA. Indeed, we (31) have shown previously that 60–90 s of exposure to 5% oxygen reduced PaO2 from 87 ± 5 to 27 ± 3 mmHg in conscious spontaneously breathing C57BL/6J mice. If a comparable degree of arterial hypoxemia occurred in our IH profile when FiO2 is reduced to 6% transiently, the model would represent a degree of OSA so severe that the clinical relevance would be questionable. However, we now show in the present study that the transient nadir of 6% reduced PaO2 from a room air value of 88 ± 3 mmHg to only 47 ± 2 mmHg. These data demonstrate that the resulting calculated nadir SaO2 of 85 ± 2% is within a range representing moderate to severe clinical OSA.
Applying the hypoxic profile to the overall IH regimen.
Once the specific FiO2 profile for a single IH event is determined, it is necessary to define the remaining parameters for the overall IH regimen. For our protocol, we used an exposure rate of 60 events/h since this was equivalent to the rate of induced hypoxic episodes that occurred per hour of sleep in our murine model of sleep-induced hypoxia described above. In other IH studies, the rate of exposure has varied from a high of 120 events/h (6) to a low of 15 events/h (17). The majority of these studies used a daily exposure period of 8–12 h, coinciding with the light or sleeping cycle of rodents, with the shorter exposures sometimes the result of systems that are not fully automated and require the presence of personnel. The chronicity of the IH regimen is entirely dependent on the study goal, with some experiments being as short as 1 day (e.g., the development of insulin resistance) (8) or as long as 3 mo (e.g., the development of atherosclerosis) (26). Consequently, the paradigm of IH can vary markedly between studies.
Technical considerations in IH rodent models.
There are several technical considerations that could be standardized between studies to enable data derived from rodent models of IH to be compared. First, the cyclical FiO2 profile that the animal experiences is most accurately determined by placing a sensor at the level of the nose. If regular animal cages are placed inside a larger chamber from which the FiO2 is either monitored or controlled, then the change in FiO2 that the individual animals experience within their chamber during each hypoxic cycle may be overestimated. Second, animals that are transferred between a home cage and a stimulus cage each day may experience psychological stress in addition to the hypoxic stress. Animals exposed to hypoxia could potentially develop a preconditioning or fear response to placement in the stimulus chambers, producing a physiological response that may be independent of the effects of IH. Ideally, animals should be continually housed in their home cages throughout the entire IH protocol with a second group of animals exposed to a control stimulus of intermittent air that produces the same rate of gas flows through the cage but without the presence of nitrogen. Finally, when animals are first exposed to IH, the nadir of hypoxia should be ramped down slowly over ∼1–2 h to allow them to adapt to the hypoxic stress, particularly if the nadir FiO2 is in the 5% range; immediate exposure to a 5% nadir FiO2 can sometimes induce panic, seizures, and very occasionally death. If a consensus could be reached on the standardization of these technical considerations, as well as the IH regimen itself (see below), outcome responses could be more easily compared between studies.
Limitations of IH models.
There are several limitations and strengths of rodent IH models that are worthy of discussion. The major limitation of the IH model is that it does not produce airway obstruction in a manner developed in larger animals, such as dogs (10, 16). Consequently, the IH stimulus does not produce the intrathoracic pressure swings or hypercapnia that occurs with airway obstruction, both of which can have important cardiovascular effects (28, 29). However, there is evidence that the addition of an intermittent hypercapnic component to the IH stimulus does not exacerbate the hypertension that occurs in a rat model of IH (12). It is possible, as demonstrated for sympathetic nerve activity, that any interactive effects of intermittent hypercapnia and IH are most apparent in the presence of large intrathoracic pressure changes during obstructed inspiratory efforts (30). Also, the arousals and sleep fragmentation that characterize OSA are often not present in rodent IH models, as occurs when the hypoxic stimulus is delivered while the animal is awake. However, we have demonstrated for our specific IH regimen in mice that if the animal is asleep when the IH stimulus is delivered, there is invariably an arousal response that is tightly coupled in time to the nadir in FiO2 (21). In contrast, during the dark period, when animals are maintained continuously in room air, the animals experience approximately 5 h of sleep in the absence of any hypoxic exposure (21). Another significant limitation of rodent models of IH, as alluded to in the Introduction, is that the majority of laboratories have their own unique IH paradigms, making it difficult to compare results between studies. Also, the IH model is now commonly used in mice, and it is possible that mice and rats may have different susceptibilities to specific levels of nadir hypoxia exposure, resulting in disparate outcomes. It is conceivable that the development of a pseudosteady-state period at the nadir FiO2 may produce much greater falls in arterial oxyhemoglobin saturation than we report in the present study using a transient nadir. The former may be closer to a model of sustained hypopneas, as are common in children with OSA, compared with the transient nadir modeled in the persent study, which is more typical of frank obstructive apneas.
Despite the many limitations of the IH rodent model, there is one overriding strength. Multiple studies have demonstrated that IH can produce pathophysiological outcomes that are known to occur in OSA patients from clinical studies. These include hypertension (6), elevated sympathetic nerve activity (2, 5), impaired vascular responsiveness (19, 32) and cardiac function (3), insulin resistance (8, 20), hyperlipidemia (13), liver injury and hepatitis (25, 27), atherosclerosis (26), learning and memory deficits (23, 24), chronic sleep disturbances (33), and alterations in respiratory control (17). However, it is important to note that IH exposure does not always lead to pathological changes and may, at least initially, induce a form of compensatory response. For example, 3 wk of exposure to IH in dogs was found to reduce the CO2 reserve (the difference in the pressure of end-tidal CO2 between eupnea and the apneic threshold) and, contrary to the authors' hypothesis, act to stabilize breathing and reduce the propensity for apnea (9). Thus, IH is a powerful stimulus that has a wide range of physiological effects and often induces pathology typical of the sequelae of OSA.
Another advantage of the rodent IH model over more sophisticated models of sleep-induced airway obstruction or sleep-induced hypoxia is the relative technical simplicity of the approach. Large numbers of animals can be exposed to IH simultaneously and for extended periods of time, since no instrumentation is required. It is likely that IH rodent models will continue to provide new insights into the pathophysiological outcomes of OSA and the potential mechanisms that account for these changes.
Summary.
Rodent models of IH are commonly used to study pathology related to OSA, but paradigms vary between laboratories, and little is known about the blood gas disturbances produced by the transient episodes of hypoxia. We now show that the fall in PaO2 and the associated oxyghemoglobin desaturation that occurs with a linear 30-s reduction in FiO2 from room air to 6% oxygen produces a nadir SaO2 of ∼85%. The degree of oxyhemoglobin desaturation that our model induces at a rate of 60 events/h is consistent with the magnitude of hypoxic stress that occurs in moderate to severe clinical OSA.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-077785 and HL-063767.
REFERENCES
- 1.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce β-cell replication. Diabetes 56: 1792–1801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83: 95–101, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher EC, Costarangos C, Miller T. The rate of fall of arterial oxyhemoglobin saturation in obstructive sleep apnea. Chest 96: 717–722, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20: 612–619, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 352: 1206–1214, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katayama K, Smith CA, Henderson KS, Dempsey JA. Chronic intermittent hypoxia increases the CO2 reserve in sleeping dogs. J Appl Physiol 103: 1942–1949, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Kimoff RJ, Makino H, Horner RL, Kozar LF, Lue F, Slutsky AS, Phillipson EA. Canine model of obstructive sleep apnea: model description and preliminary application. J Appl Physiol 76: 1810–1817, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Kraiczi H, Magga J, Sun XY, Ruskoaho H, Zhao X, Hedner J. Hypoxic pressor response, cardiac size, and natriuretic peptides are modified by long-term intermittent hypoxia. J Appl Physiol 87: 2025–2031, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829–1836, 2000. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell CP, King ED, Schwartz AR, Smith PL, Robotham JL. A dog model to investigate the relationship between obstructive sleep apnoea and blood pressure regulation. J Sleep Res 4: 89–92, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 286: H388–H393, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160: 521–530, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med 167: 1548–1553, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G871–G877, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology 45: 1007–1013, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Schneider H, Schaub CD, Andreoni KA, Schwartz AR, Smith PL, Robotham JL, O'Donnell CP. Systemic and pulmonary hemodynamic responses to normal and obstructed breathing during sleep. J Appl Physiol 83: 1671–1680, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Schneider H, Schaub CD, Chen CA, Andreoni KA, Schwartz AR, Smith PL, Robotham JL, O'Donnell CP. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. J Appl Physiol 88: 1093–1102, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia–implications for sleep apnea. Clin Exp Hypertens A 10, Suppl 1: 413–422, 1988. [DOI] [PubMed] [Google Scholar]
- 31.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol 91: 2758–2766, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol 90: 2007–2013, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 353: 2034–2041, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Yokoe T, Alonso LC, Romano LC, Rosa TC, O'Doherty RM, Garcia-Ocana A, Minoguchi K, O'Donnell CP. Intermittent hypoxia reverses the diurnal glucose rhythm and causes pancreatic beta-cell replication in mice. J Physiol 586: 899–911, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]