Abstract
The effect of body posture on regional ventilation during bronchoconstriction is unknown. In five subjects with asthma, we measured spirometry, low-frequency (0.15-Hz) lung elastance, and resistance and regional ventilation by intravenous 13NN-saline positron emission tomography before and after nebulized methacholine. The subjects were imaged prone on 1 day and supine on another, but on both days the methacholine was delivered while prone. From the residual 13NN after washout, ventilation defective areas were defined, and their location, volume, ventilation, and fractional gas content relative to the rest of the lung were calculated. Independent of posture, all subjects developed ventilation defective areas. Although ventilation within these areas was similarly reduced in both postures, their volume was smaller in prone than supine (25 vs. 41%, P < 0.05). The geometric center of the ventilation defective areas was gravitationally dependent relative to that of the lung in both postures. Mean lung fractional gas content was greater in the prone position before methacholine and did not increase as much as in the supine position after methacholine. In the prone position at baseline, areas that became ventilation defects had lower gas content than the rest of the lung. In both positions at baseline, there was a gradient of gas content in the vertical direction. In asthma, the size and location of ventilation defects is affected by body position and likely affected by small differences in lung expansion during bronchoconstriction.
Keywords: ventilation-perfusion ratio, pulmonary gas exchange, emission-computed tomography, nitrogen isotopes
imaging studies in animals with normal (24, 32) and acutely injured lungs (27) have shown that the prone position results in a more homogeneous distribution of lung aeration, and pulmonary ventilation (V̇) compared with supine. In humans, some studies have shown a more uniform distribution of V̇ (2, 25) when prone, but other studies have shown no difference in V̇ (3, 11). In asthma, very little is known about the effect of body position on regional V̇. Using an inhalation of hyperpolarized 3He and magnetic resonance imaging, Altes and colleagues (1) noted dorsal (dependent) ventilation defects (Vdefs) in supine asymptomatic asthmatic subjects, which were thought to represent dependent atelectasis. Other studies using nuclear medicine techniques have shown patchy Vdefs, often largely in caudal (dependent) zones of upright subjects (8, 9, 12, 13, 21–23, 26), but none has assessed the effects of changing body position in the location and size of Vdefs.
Previous PET imaging studies of intrapulmonary 13NN-saline kinetics suggested that, during acute bronchoconstriction, Vdefs are the result of severely hypoventilating units clustered in relatively large, contiguous regions (10, 34). Those studies, conducted in supine subjects, showed a tendency for Vdefs to form in dorsal (dependent) lung zones (10). Those results are consistent with a recent theoretical model of bronchoconstriction that includes parallel and serial dynamic interdependence among lung volume, ventilation, and airway size (34, 36). That model predicts that, for a given degree of smooth muscle activation above a critical level, reduction in lung volume should result in the development of Vdefs that increase in size as lung volume is further reduced. Given that the supine position is associated with an average reduction in lung volume of ∼10% compared with prone (20), we theorized that, in bronchoconstricted asthmatic subjects, the formation of Vdefs, under the same level of bronchoconstrictive stimulus, should cover a larger volume of the lung in the supine position compared with that in prone.
METHODS
Subject characteristics.
Five subjects with mild asthma (Table 1) and normal baseline spirometry (Table 2) were studied with protocols and procedures approved by the Human Research Committee of the Massachusetts General Hospital. The subjects were recruited by advertisements posted in the hospital and through general e-mail announcements within the Partners Heathcare System. Subjects were considered eligible if they had been diagnosed with asthma and were over age 18 yr, had not been smoking for the 3 mo before screening, had less than a 10 pack·yr history of smoking in the past, and had not had an upper respiratory infection in the last month before screening. Subjects were questioned to determine whether their asthma met the National Institutes of Health definition for mild to moderate asthma. Subjects were excluded if they were a member of the study staff, had other lung diseases or heart disease, were pregnant, were unresponsive to albuterol, had an absolute contraindication for methacholine (MCh) challenge testing [forced expiratory volume in 1 s (FEV1) < 50% predicted or <1 liter, heart attack or stroke in the last 3 mo, uncontrolled hypertension, or known aortic aneurysm], had been exposed to more than half of the expected radiation dose for the protocol in the past year [375 mrem (milli-Röntgen equivalent in man)], or had taken oral steroids in the past year for their asthma.
Table 1.
Subject characteristics
| Subject | Sex | Age, yr | BMI, kg/m2 | PC20, mg/ml | Medications |
|---|---|---|---|---|---|
| h063 | M | 25 | 25.8 | 0.25 | albuterol |
| h065 | M | 22 | 22.9 | 2.5 | albuterol |
| h068 | F | 27 | 20.5 | 4.0 | fluticasone, salmeterol, loratidine, albuterol |
| h069 | M | 24 | 25.0 | 2.5 | albuterol |
| h073 | M | 28 | 31.0 | 0.25 | albuterol |
| Mean ± SD | 25±2.4 | 25±2.9 | 1.9±1.6 |
BMI, body mass index; PC20, provocative concentration of methacholine causing a 20% fall in forced expiratory volume in 1 s (FEV1); M, male; F, female.
Table 2.
Baseline upright pulmonary function tests before imaging
| Subject | FEV1, liters |
FVC, liters | FEV1/FVC | DlCO | ||||
|---|---|---|---|---|---|---|---|---|
| P | S | P | S | P | S | P | S | |
| h063 | 4.41 (94) | 5.16 (111) | 5.78 (102) | 6.52 (116) | 0.76 | 0.79 | 38.5 (104) | 42.19 (115) |
| h065 | 4.44 (100) | 4.34 (98) | 4.62 (88) | 4.54 (86) | 0.96 | 0.96 | 29.6 (81) | 24.58 (61) |
| h068 | 3.39 (116) | 3.49 (120) | 3.95 (120) | 4.23 (129) | 0.86 | 0.83 | 23.38 (100) | 23.39 (100) |
| h069 | 3.94 (90) | 4.54 (103) | 4.71 (90) | 5.39 (103) | 0.84 | 0.84 | 35.51 (98) | 36.39 (101) |
| h073 | 2.75 (86) | 2.35 (73) | 4.32 (102) | 3.93 (93) | 0.64 | 0.60 | 24.6 (101) | 29.17 (120) |
| Mean | 3.79 (97) | 3.98 (101) | 4.68 (100) | 4.92 (105) | 0.81 | 0.80 | 30.32 (97) | 31.14 (99) |
| SD | 0.72 (12) | 1.09 (18) | 0.69 (13) | 1.05 (17) | 0.12 | 0.13 | 6.62 (9) | 8.01 (23) |
Percent predicted values are in parentheses. FVC, forced vital capacity; DlCO, lung CO diffusing capacity; P, prone day; S, supine day. There were no statistically significant differences comparing prone day and supine day values.
Study protocol.
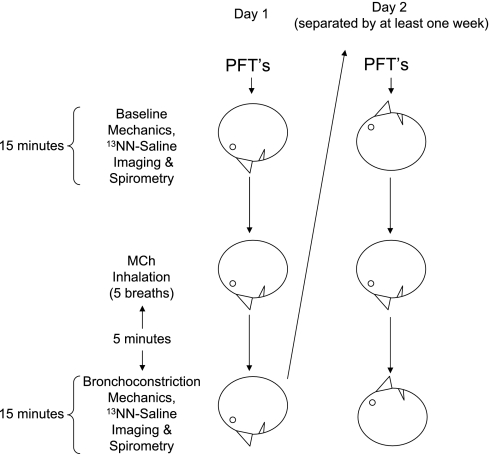
The study protocol (Fig. 1) included imaging sessions on two different days, separated by at least 1 wk. If the subject had not had a standard MCh challenge test within the past year, one was conducted in the seated position at least 1 wk before the first session, and the provocative concentration (Provocholine, Methapharm, Coral Springs, FL) that caused a 20% fall in FEV1 (PC20) was determined. Asthma medications were stopped before MCh challenge testing and imaging, according to MCh challenge guidelines (6). Subjects with a PC20 dose > 8 mg/ml were excluded from the study. On each study day, the subject also had pulmonary function tests in the upright position before any study procedures to verify that the subject was not bronchoconstricted. Subjects were studied in the prone position during the first session and supine on the second, but the inhalation of MCh was always done prone. The subject was positioned to include the largest lung volume within the 10-cm-long field of view of the PET scanner. The imaged lung was estimated to include 75 ± 7% of the whole lung. Lung volume was monitored continuously by impedance plethysmography (SomnoStar PT, SensorMedics, Yorba Linda, CA), and the signal was continuously displayed to the subject on a computer screen. Oscillatory mechanics were measured as previously described (29–31), and the low-frequency (0.15 Hz) resistance and elastance derived. After acquiring the 10-min transmission scan and baseline oscillatory mechanics measurements, the subject was instructed to take two deep breaths. During the exhalation phase of the second breath, the subject was instructed to stop breathing at lung volume equal to the mean lung volume previously estimated from the impedance plethysmographic signal during steady-state breathing. At the start of apnea, a bolus of 13NN-saline (∼30 ml) was injected intravenously (5 ml/s), and dynamic acquisition of emission scans was initiated. After 30–40 s of apnea, the subject was instructed to resume breathing while coached to match his or her previous rate and tidal volume, as displayed on the computer screen. Spirometry was performed in the same position as the preceding emission scan using a hand-held portable spirometer (Satellite Spirometer, Jones Medical Instrument, Oak Brook, IL). While the subject was prone with the head turned to the side and arms outside the scanner resting on armrests, five breaths of MCh were then administered to the subject at his or her previously determined PC20 dose via a DeVilbiss nebulizer and Rosenthal dosimeter (model 646, DeVilbiss Heathcare, Somerset, PA). An identical imaging sequence to that acquired in baseline conditions was repeated starting 5 min after administration of the MCh. On a second day, the subject was positioned supine in the PET scanner so that the imaging field approximated the portion of lung that was imaged on the first session. After baseline oscillatory mechanics, PET imaging, and spirometry, the subject was turned to the prone position for inhalation of five breaths of MCh at PC20 before returning to the supine position. Laser alignment markers from the PET scanner were used to ensure that the same cross section of thorax was imaged at baseline and after bronchoconstriction. Postbronchoconstriction measurements were acquired 5 min later (Fig. 1) and were finished within 20 min to ensure being done between the peak and end of plateau phase of MCh action (4).
Fig. 1.
Protocol schema. Baseline pulmonary function tests (PFTs) were done in the upright position for all subjects, and then spirometry was repeated after imaging in the supine or prone body position. Five different asthmatic subjects were studied on 2 days, separated by at least 1 wk. On the first day, subjects had both imaging and methacholine (MCh) inhalation prone. On the 2nd day, the subjects were imaged supine, but inhaled the MCh prone.
PET imaging.
A PET scanner PC-4096 (Scanditronix AB, Uppsala, Sweden) was used to image 15 contiguous 6.5-mm-thick slices of the thorax. Transmission scans were recorded using a rotating pin source of 68Ge/68Ga. These scans were used to correct the emission scans for energy attenuation caused by body tissues and supporting structures and to demarcate the lung field. To measure regional V̇, dynamic emission scans were acquired following a bolus injection of 13NN in saline solution at the beginning of a 30-s apnea (18, 25, 32). The scanner was programmed to count coincidence data over set periods of time (frames) as follows: 8 frames of 2.5 s, 16 frames of 5 s, and 4 frames of 30 s each, for a total of 28 frames lasting 3 h 40 min. Because of its low solubility in tissues (partition coefficient of water to air = 0.015 at 37°C), upon arrival into the pulmonary capillaries, virtually all 13NN diffuses into the alveolar air space at first pass, where it accumulates for the remainder of apnea. At that point, the subject begins to breath, and the regional ventilation per unit of lung gas volume of perfused alveolar units [specific alveolar ventilation (sV̇a)] was assessed from the 13NN washout rate measured over the following 3 min of breathing, as the tracer was eliminated from a nonatelectatic lung almost exclusively by ventilation (35).
Emission scans were reconstructed by conventional back-projection algorithm and corrected for tissue attenuation and tracer radioactive decay. The lung fields were defined by thresholding the transmission scans and manually removing regions corresponding to main bronchi and large pulmonary vessels. The emission scans were low-pass filtered with edge effect correction to yield an in-plane resolution of 13 mm. Moving average filtering was conducted between contiguous slices in the axial direction to yield equal effective imaging resolution in all axes (13 × 13 × 13 mm).
Data analysis.
Images to display the topographic distribution of tracer retention before and during bronchoconstriction were generated to show the tracer activity remaining in each voxel at the end of the 3-min washout period. Using the tracer retention image taken during bronchoconstriction, a Vdef ROI was defined by selecting a set of voxels containing >20% of the highest tracer concentration on that scan and then manually refining it to only include a contiguous region incorporating a subset of neighboring voxels with elevated residual tracer. The threshold value was a compromise between obtaining a region large enough to reduce the effect of noise and small enough to include only areas of significant tracer retention. Little change in the size of the Vdef regions was seen, with values for thresholding near 20%. Once the Vdef ROI was defined, all voxels outside of this region, but within the lung mask, were considered to be better ventilated areas outside of Vdefs (out). The fraction of Vdef volume was calculated by dividing the number of voxels within the Vdef ROI by the total number of voxels in the imaged lung. Plots comparing upright vs. supine spirometry and baseline spirometry (upright or recumbent) vs. fraction of Vdef volume values were constructed with DeltaGraph 5.6 (Red Rock Software, Salt Lake City, UT), and Pearson correlation coefficients were calculated.
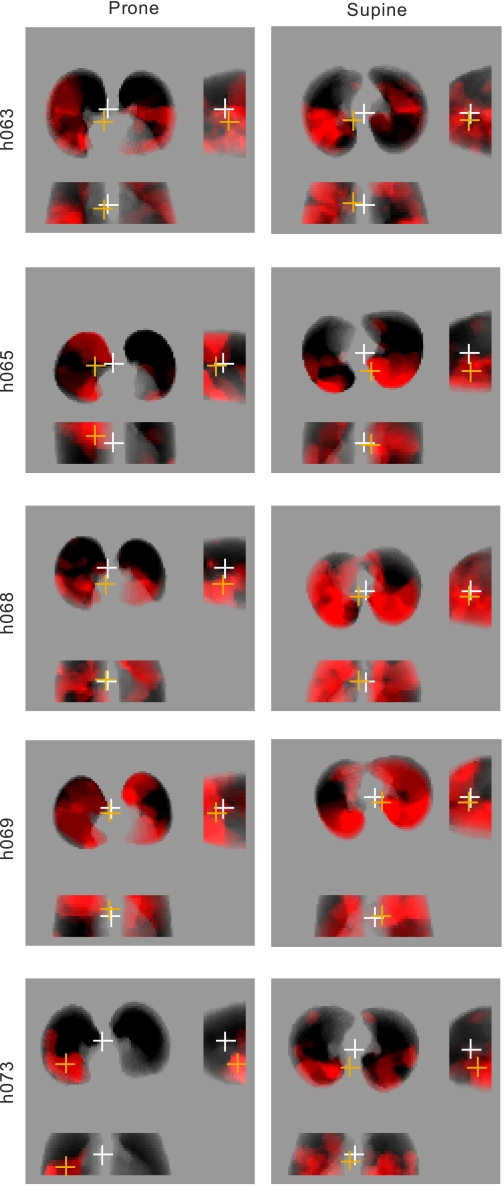
The sV̇a (alveolar ventilation per unit volume) within Vdefs was calculated from the washout kinetics of the average ROI 13NN concentration and expressed as a fraction of the sV̇a of the rest of the imaged lung (V̇Vdefs/out). The heterogeneity of the voxel-by-voxel sV̇a distribution for the imaged lung was characterized by the mean-normalized variance of the tracer washout rate [cov2V̇ = (SD/mean)2]. Regional fractional gas content (Fgas), defined as the volume fraction of a lung region filled with gas, was calculated from the transmission scans (10). Because the transmission scan is acquired during tidal breathing, Fgas is a regional equivalent of the average expansion of the lung. Fgas for the entire imaged lung, the vertical gradient in Fgas, and the relative Fgas of Vdefs regions in relation to that of the rest of the lung (Fgas,Vdef/out) were also calculated. The average location of all Vdef regions within a lung was calculated as the distance between the geometric center of the imaged lung field (GCLung) and that of the Vdefs (GCVdefs) in the left-to-right, dorso-ventral, and caudo-cranial directions (Fig. 2). The deviations between the GCLung and GCVdef were normalized by the corresponding width (right-left), height (ventral-dorsal), and imaged field thickness (cranio-caudal), with the positive sign assigned to the right, ventral, and caudal directions.
Fig. 2.
Analysis of geometric center of the lungs (GCLung) and ventilation defects (GCVdef). Left: projections of Vdefs (in red) for subjects in the prone position; right: same subjects in the supine position. The white cross represents the GCLung, and the orange cross is the GCVdef. There are three views of the lung in each image (clockwise from top left): transverse viewed caudo-cranially, sagittal, and coronal. In these images, Vdefs that deviate toward the bottom of the page result in negative distances between GCLung and GCVdef in the transverse, sagittal, and coronal planes.
Because of the limited sample size of the study, the nonparametric Wilcoxon matched-pairs test was used to assess significance comparing before and after MCh at a level of P < 0.05, and analysis was performed using STATISTICA (StatSoft, Tulsa, OK). Data are expressed as means ± SD. Linear regression was performed on plot of Fgas vs. height using MATLAB (The Mathworks, Natick, MA).
RESULTS
Consistent with our laboratory's previous reports (10, 34), MCh-induced bronchoconstriction generated large and contiguous regions of tracer retention in all subjects. The decrease in FEV1 and forced vital capacity (FVC), and the increase in low-frequency resistance and low-frequency elastance and in the heterogeneity of ventilation (cov2V̇) caused by inhalation of MCh tended to be slightly greater in the supine than in the prone position, but these differences did not reach statistical significance (Table 3). The reduction in FEV1/FVC during bronchoconstriction was greater in the supine position.
Table 3.
Results
| Posture During Imaging | Supine | Prone |
|---|---|---|
| FEV1, %change | −32±18 | −27±13 |
| FVC, %change | −21±19 | −17±18 |
| FEV1/FVC, %change | −14±4.4 | −4.6±11* |
| Rlow, %change | 243±192 | 193±83 |
| Elow, %change | 84±67 | 62±42 |
| covV̇, %change | 70±116 | 59±42 |
| Ventral deviation of Vdef | −0.12±0.07 | 0.14±0.10* |
| Rightward deviation of Vdef | −0.02±0.10 | 0.15±0.18 |
| Basal deviation of Vdef | 0.24±1.19 | 0.08±2.72 |
| V̇Vdef/out (Control) | 1.15±0.24 | 0.96±0.18* |
| V̇Vdef/out (MCh) | 0.56±0.17 | 0.55±0.11 |
| Vertical gradient Fgas, %change | −110±13 | −18±19* |
| Vdef volume/imaged lung volume | 0.41±0.21 | 0.25±0.14* |
Values are means ± SD. Rlow, low-frequency resistance; Elow, low-frequency elastance; covV̇, coefficient of variation of specific ventilation; deviation, fraction of total x-, y-, or z-dimension with positive assigned to ventral, right, and caudal directions; Vdef, ventilation defect; V̇Vdef/out, specific ventilation inside Vdef vs. outside; Fgas, fractional gas content. %Change refers to the change from baseline to post-methacholine.
P < 0.05 compared with supine.
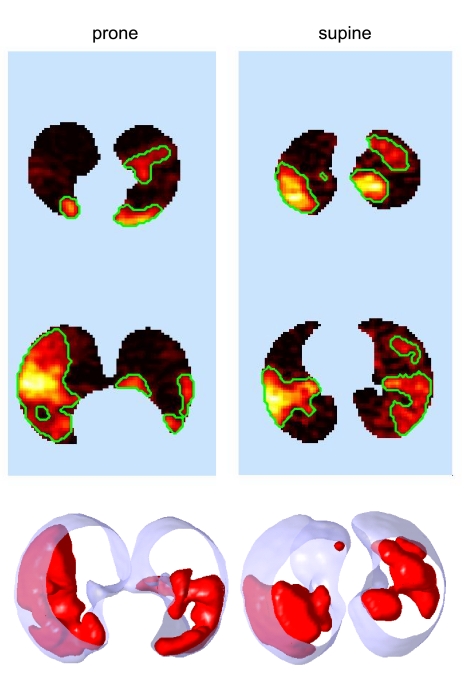
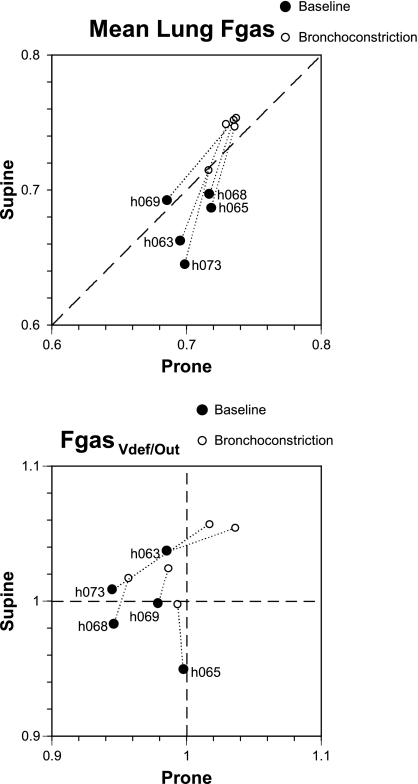
In both body positions, Vdef regions were generally, but not always, located in relatively dependent regions of the lung (Fig. 3). The ventral deviations of the GCVdef had opposite sign, but similar absolute value in the two positions (Table 3). In other words, Vdefs deviated from the GCLung in the gravitational direction by about the same amount in both positions, (i.e., in the supine position they deviated dorsally, and in the prone position ventrally). The average degree of hypoventilation within the Vdefs relative to the rest of the lung after MCh was similar in the two positions (V̇Vdef/out∼ 0.55), but the volume of the lung covered by Vdefs was significantly greater in supine compared with prone (Table 3). Average lung Fgas at baseline was higher in prone compared with supine (P < 0.05) and was elevated after MCh in both positions, but the increase was lower in the prone than in the supine position (0.04 ± 0.02 vs. 0.1 ± 0.02, P < 0.05, Fig. 4, top), as was the percent increase in imaged lung volume (7.0 ± 7.9 and 16 ± 6.1%, P < 0.05). As a result, average lung Fgas after MCh was higher in the supine position. Collectively, these results demonstrate that the prone position was associated with smaller Vdefs, a higher baseline global lung volume, and a lower increase in global lung volume after MCh than the supine position, even though MCh was always inhaled prone.
Fig. 3.
Body posture affects the location and size of Vdefs. Top: two representative slices showing in “hot” color scale the local concentration retained tracer after a washout and the margins (in green) defining the Vdefs. Bottom: three-dimensional rendering of the ventilation defects (in red) and the lung field (transparent blue). Images are from the same subject studied on 2 different days after receiving MCh challenge in the prone posture on both occasions. Note the tendency for Vdefs to be in a dependent location for both postures.
Fig. 4.
Plots of fractional gas content (Fgas) in prone (horizontal axis) vs. supine (vertical axis) before (•) and during bronchoconstriction (○). Top: change in Fgas for the entire imaged lung. Note that, at baseline, Fgas was lower supine than prone (points are below the dashed identity line) for all but one subject. During bronchoconstriction, mean lung Fgas increased for all subjects in both positions, and, for all but one subject, Fgas supine was greater than prone. Bottom: ratio of Fgas inside Vdefs relative to the rest of the lung (Fgas,Vdef/out). Note that, in the prone position, at baseline, all points have values < 1, and thus Vdefs all had lower Fgas than the rest of the lung. Relative Fgas in Vdefs increased during bronchoconstriction in all subjects supine, and in all but one subject prone.
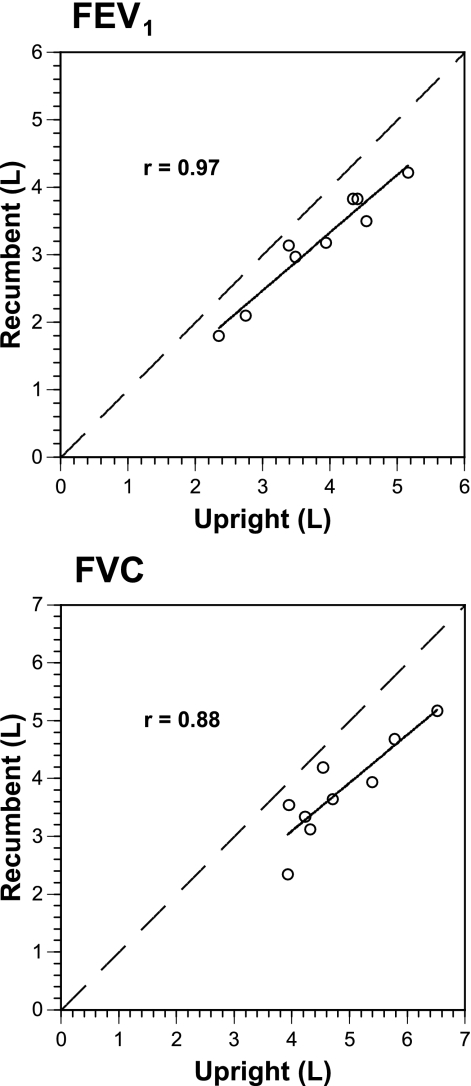
There was excellent correlation between upright FEV1 or FVC and recumbent values (r = 0.97, P < 0.002; r = 0.88, P = 0.002, respectively, Fig. 5), but poor correlation between upright FEV1 or FVC and the size of Vdefs during bronchoconstriction (r = 0.29, P = 0.42; r = 0.16, P = 0.67, respectively). Plotted recumbent values were consistently less (below identity line) than upright for FEV1 (mean 640 ± 240 ml) and FVC (mean 1,050 ± 430 ml, Fig. 5). The change in FEV1 or FVC was not correlated with the change in Vdef volume (r = 0.43, P = 0.25 and r = 0.20, P = 0.60, respectively).
Fig. 5.
Recumbent vs. upright forced expiratory volume in 1 s (FEV1; top) and forced vital capacity (FVC; bottom). Dashed identity line is shown in both panels. Both upright FEV1 and FVC were highly correlated with recumbent values (r = 0.97, P < 0.002; r = 0.88, P = 0.002, respectively), with recumbent values being always less than upright (below identity line). One subject (h065) did not have recumbent spirometry in the prone position.
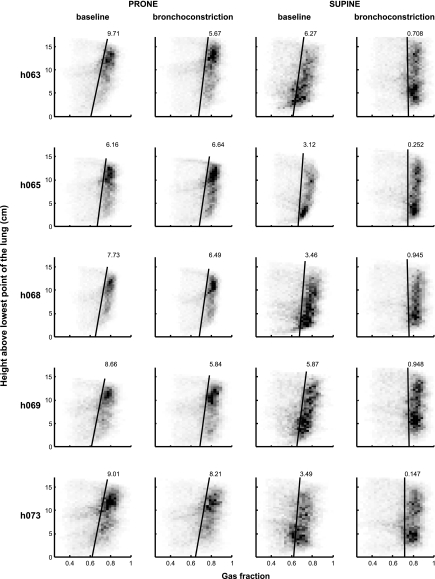
The vertical gradient of Fgas was in the gravitational direction for both positions before MCh (0.0083 ± 0.0014/cm prone and 0.0044 ± 0.0015/cm supine), with gas content increasing in the dependent-to-nondependent direction (Fig. 6). After MCh, the vertical gradient did not change significantly in the prone position (0.0066 ± 0.0010/cm), but did so in the supine position, becoming not different from zero (−0.0005 ± 0.0005/cm).
Fig. 6.
Fgas gradients in the four conditions: prone baseline, prone bronchoconstriction, supine baseline, and supine bronchoconstriction. The gray scale of the squares in the plots corresponds to the number of data points with that value, such that the darker the square, the greater the number of points with that value. The linear regression line (solid black) is shown, and the number at the top of each line is the gradient (×10−3 cm−1) for each plot. Note the larger gradient in the prone position compared with supine at baseline, which changes little during bronchoconstriction. In contrast, the Fgas gradient changes substantially in the supine position during bronchoconstriction compared with baseline.
In the prone position, the Fgas,Vdef/out before MCh was lower than 1 (P < 0.05, Fig. 4, bottom), indicating that, at baseline, areas that were to become Vdefs after MCh were less expanded than those that did not. This systematic behavior was not present in the supine position. However, Fgas,Vdef/out increased after MCh in all subjects supine, and in all but one subject prone became greater than unity (Fig. 4, bottom), indicating that MCh-induced bronchoconstriction was associated with relative overexpansion of Vdef regions with respect to the rest of the lung. In contrast, the relative ventilation of Vdefs with respect to the rest of the lung (V̇Vdef/out) was not systematically different from unity at baseline in either body position.
DISCUSSION
The main result of this study is that, compared with the prone position, the supine position resulted in equally hypoventilated, but larger, ventilation defective regions during bronchoconstriction with MCh. Given that all subjects inhaled MCh in the prone position, these findings suggest that factors other than agonist distribution favored the formation of larger Vdefs in the supine position. One obvious candidate is gravitational effects on regional lung volume, where reduced lung expansion, particularly of dependent areas, decreased lung parenchymal tethering forces on airways, making them smaller and more prone to severe constriction as smooth muscle tone increased.
Before discussing these findings, experimental and methodological limitations of the study should be acknowledged. General experimental limitations of our PET imaging technique have been discussed in previous reports (10, 17, 25, 35). We decided not to randomize the initial body position to minimize the chance of incomplete studies where, having completed a first imaging session, the subject would withdraw before or during the second session. Given the ∼2-h duration of the study and because lying prone in the scanner was less comfortable than lying supine, we reasoned that, if a subject were able to complete the prone session, he or she would be more likely to complete the supine session. This proved correct, and all subjects who completed the first session went on to complete the second one. It could be argued the lack of randomization in the order of body position may have introduced systematic bias in the study. However, the studies were conducted at least 1 wk apart, minimizing carryover effects, and we found no systematic differences in lung function between the two imaging sessions at baseline (Table 2). One could argue that differences in response could be due to changes in baseline lung volume on the 2 imaging days. We could find no relationship between the upright FEV1 or FVC and the resulting Vdef size, despite excellent correlations between upright and recumbent spirometric values (Fig. 5).
What then predisposes a region to become a Vdef? Our laboratory has previously published a lung model of airway constriction that includes both long-range (through redistribution of tidal volume) and short-range (through airway wall stretch by transmural pressure and local parenchymal tethering) feedback mechanisms in an integrative, computational model (34). This model showed that, at low levels of smooth muscle activation, these interactions are self-limiting, and the distribution of ventilation is fairly uniform. However, as smooth muscle activation is higher than a critical level, any small perturbation can trigger a positive feedback that propagates up and down the airway tree, resulting in the formation of large Vdefs. Thus, even minor differences in airway properties (e.g., airway wall thickness, smooth muscle contractile strength, mucus, or reduced parenchymal tethering forces) can be enough to trigger Vdefs in such an airway tree. The model also demonstrated that decreasing end-expiratory lung volume can trigger the formation of Vdefs at lower levels of smooth muscle activation or increase their size, if Vdefs are already present (36). In the present study, we postulate that one of such “small perturbations” could have been the heterogeneity in regional lung volume in the gravitational direction, predisposing Vdef formation in more dependent locations. This is also consistent with the reduced levels of regional lung volume (Fgas,Vdef/out) seen at baseline in the prone position, suggesting that locally reduced lung volume predisposed areas to become Vdefs during bronchoconstriction (Fig. 4). Although, in the supine position at baseline (Fgas,Vdef/out) was not always <1 (i.e., regions that became Vdefs were not necessarily less expanded than the rest of the lung), this does not negate the above hypothesis, because, in this condition, MCh was inhaled by the subjects prone, a position different than that during imaging: the supine Fgas,Vdef/out at baseline did not assess the relative degree of lung expansion that the lung had during exposure to MCh. As a result, Vdefs could have formed before the subject had turned back to the supine position. After reanalyzing the data from 11 subjects previously studied who where imaged and received MCh in the supine position (10), we found that 9 of the 11 subjects had a Fgas,Vdef/out < 1 at baseline, a result consistent with the current findings in the prone position.
In a previous study of MCh-induced bronchoconstricted sheep (33), we reported that the relative ventilation distribution, and thus the deposition of MCh, could have been associated with Vdef formation. Areas that were to become Vdefs had higher ventilation relative to areas outside before administration of the MCh. This is not the case in the present study, since the areas that became Vdefs in the prone position were not statistically more ventilated than the rest of the imaged lung (V̇Vdef/out at baseline = 0.96, Table 1). Although V̇Vdef/out at baseline was greater than unity in the supine position, we do not have ventilation data in the position that the MCh was delivered. From the data in the present study, it appears that the distribution of lung volume, and not the distribution of ventilation, could be the important determinant of Vdefs formation. The extent to which regional lung volume could be related to regional ventilation per unit volume could explain our previous finding in the sheep.
Our study showed, in both body positions, a systematic tendency of Vdefs to occur in dependent regions of the lung despite that MCh was inhaled prone in both cases. This supports the notion that the posture one assumes during bronchial challenge may not be as important as that assumed after exposure in the pattern of the resulting bronchoconstriction (16). However, because regions of Vdefs not only formed in dependent zones (Fig. 3), it suggests that the trigger for Vdefs formation could be multifactorial. One factor that could influence the location of Vdefs is heterogeneous deposition of agonist. We attempted to minimize this factor by inhaling the MCh in the same body position. Despite not controlling other factors that may influence heterogeneous deposition, such as inhalation rate, we still found a systematic dependent location of Vdefs. Gravitational effects on regional lung volume could have also created localized reduction in parenchymal tethering forces on the airways. It is well known that gravitational forces on lung parenchyma and chest wall are responsible for a gradient of lung volume decreasing from nondependent to dependent zones (19, 26). The larger size of the Vdefs observed in supine compared with prone is consistent with this hypothesis, since the gradient in regional lung volume of the prone position was greater than that in the supine position, and the value of Fgas in the most dependent parts of the lung for both positions was nearly the same before MCh (∼0.60–0.65, Fig. 6). The larger overall lung volume prone would tend to increase outward tethering forces on airways and limit the size of Vdefs for a given amount of airway smooth muscle constriction. This is consistent with prior data obtained without imaging, demonstrating that airway hyperresponsiveness is inversely related to lung volume (7). In addition to the larger overall lung volume at baseline in the prone position compared with supine, another possible reason for the smaller size of Vdefs could have been the smaller fraction of the lung in dependent regions due to the shape of the lung. (Note the greater number of voxels in the nondependent zone prone, but not supine, at baseline; Fig. 6.) It should be noted that the Fgas gradients in both the supine and prone positions at baseline measured in these asthmatic subjects were substantially less than those previously observed for normal subjects (25). We speculate that this could be due to a mild gas trapping or reduction in blood volume in dependent zones at baseline in subjects with asthma. Finally, it is also possible that dependent accumulation of secretions could have been responsible for the dependent formation of Vdefs. It is not possible with this protocol to exclude this hypothesis, although it seems unlikely because our subjects did not show evidence of increased mucus production and productive cough in response to MCh-induced bronchoconstriction.
We saw an increase in mean lung Fgas after MCh of ∼10% in the supine position and of ∼4% in the prone position and corresponding increases of imaged lung field of 17% supine and 7% prone. This increase in response to bronchoconstriction in the supine subjects is in the same range (6.6 ± 4.0%, or an approximate increase in lung volume of 20%) of that for supine subjects reported in our laboratory's previous study (10). What is thus notable is that, despite a greater increase in the lung volume in response to MCh of subjects in the supine position, the fraction of lung occupied by Vdefs was still larger in the supine position. Thus, if the increase in mean lung volume in response to MCh was a protective response to an increase in residual volume, the increase in lung volume undergone by the subjects supine was not enough to reduce the size of the Vdefs. In addition to the overall increase in lung volume, we also found a relative increase in regional lung volume of the ventilation defective regions by bronchoconstriction (Fgas,Vdef/out increased in all supine and all but one prone). As our laboratory previously observed (10), this relative increase in Fgas in Vdefs compared with the rest of the lung may be due both to a relative reduction in local blood volume or to dynamic hyperinflation of these regions.
There are several potential clinical implications of these findings. First, patients who are mechanically ventilated with asthma could benefit from the prone position. Second, it is known that the supine position results in greater bronchial hyperresponsiveness compared with the erect position (28), and many patients with asthma often exhibit increased symptoms at night (14). Our data showing reduced lung volume at baseline and larger Vdefs supine compared with prone during bronchoconstriction for the same dose of MCh could be consistent with those findings. Indeed, studies using nocturnal continuous positive airway pressure in asthma have shown improved nocturnal asthma symptoms (5) and quality of life (15).
In conclusion, independent of body position, bronchoconstriction with MCh resulted in ventilation defective areas that tended to be located in gravitationally dependent regions of the lung with the prone position, resulting in smaller Vdefs than those of the supine position. These findings could have important implications for mechanically ventilated patients with asthma or for preventing, or reducing, nighttime asthma symptoms.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-068011 and HL-086717.
Acknowledgments
The authors thank S. A. Barrow and S. B. Weise for technical assistance with image acquisition and processing; Dr. R. J. Callahan and A. Bruce for radiation safety and quality assurance testing of the radioisotope; and Dr. J. A. Correia, W. M. Bucelewicz, and D. F. Lee for preparation of the radioisotope.
REFERENCES
- 1.Altes TA, Powers PL, Knight-Scott J, Rakes G, Platts-Mills TA, de Lange EE, Alford BA, Mugler JP 3rd, Brookeman JR. Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 13: 378–384, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Amis TC, Jones HA, Hughes JM. Effect of posture on inter-regional distribution of pulmonary ventilation in man. Respir Physiol 56: 145–167, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Brudin LH, Rhodes CG, Valind SO, Jones T, Jonson B, Hughes JM. Relationships between regional ventilation and vascular and extravascular volume in supine humans. J Appl Physiol 76: 1195–1204, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Cartier A, Malo JL, Begin P, Sestier M, Martin RR. Time course of the bronchoconstriction induced by inhaled histamine and methacholine. J Appl Physiol 54: 821–826, 1983. [DOI] [PubMed] [Google Scholar]
- 5.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med 99: 529–534, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 161: 309–329, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol 62: 1324–1330, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Engel LA, Landau L, Taussig L, Martin RR, Sybrecht G. Influence of bronchomotor tone on regional ventilation distribution at residual volume. J Appl Physiol 40: 411–416, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Filuk RB, Berezanski DJ, Anthonisen NR. Airway closure with methacholine-induced bronchoconstriction. J Appl Physiol 63: 2223–2230, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Harris RS, Winkler T, Tgavalekos N, Musch G, Melo MF, Schroeder T, Chang Y, Venegas JG. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med 174: 245–253, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneko K, Milic-Emili J, Dolovich MB, Dawson A, Bates DV. Regional distribution of ventilation and perfusion as a function of body position. J Appl Physiol 21: 767–777, 1966. [DOI] [PubMed] [Google Scholar]
- 12.King GG, Eberl S, Salome CM, Meikle SR, Woolcock AJ. Airway closure measured by a technegas bolus and SPECT. Am J Respir Crit Care Med 155: 682–688, 1997. [DOI] [PubMed] [Google Scholar]
- 13.King GG, Eberl S, Salome CM, Young IH, Woolcock AJ. Differences in airway closure between normal and asthmatic subjects measured with single-photon emission computed tomography and technegas. Am J Respir Crit Care Med 158: 1900–1906, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Kraft M, Pak J, Martin RJ, Kaminsky D, Irvin CG. Distal lung dysfunction at night in nocturnal asthma. Am J Respir Crit Care Med 163: 1551–1556, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J 29: 307–311, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Meinero M, Coletta G, Dutto L, Milanese M, Nova G, Sciolla A, Pellegrino R, Brusasco V. Mechanical response to methacholine and deep inspiration in supine men. J Appl Physiol 102: 269–275, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Melo MF, Harris RS, Layfield JD, Venegas JG. Topographic basis of bimodal ventilation-perfusion distributions during bronchoconstriction in sheep. Am J Respir Crit Care Med 171: 714–721, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Mijailovich SM, Treppo S, Venegas JG. Effects of lung motion and tracer kinetics corrections on PET imaging of pulmonary function. J Appl Physiol 82: 1154–1162, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Millar AB, Denison DM. Vertical gradients of lung density in healthy supine men. Thorax 44: 485–490, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno F, Lyons HA. Effect of body posture on lung volumes. J Appl Physiol 16: 27–29, 1961. [DOI] [PubMed] [Google Scholar]
- 21.Munkner T, Bundgaard A. Regional V/Q changes in asthmatics after antigen inhalation. Eur J Respir Dis Suppl 143: 44–47, 1986. [PubMed] [Google Scholar]
- 22.Munkner T, Bundgaard A. Regional V/Q changes in asthmatics after exercise. Eur J Respir Dis Suppl 143: 62–66, 1986. [PubMed] [Google Scholar]
- 23.Munkner T, Bundgaard A. Regional V/Q changes in asthmatics after histamine inhalation. Eur J Respir Dis Suppl 143: 22–27, 1986. [PubMed] [Google Scholar]
- 24.Mure M, Domino KB, Lindahl SG, Hlastala MP, Altemeier WA, Glenny RW. Regional ventilation-perfusion distribution is more uniform in the prone position. J Appl Physiol 88: 1076–1083, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Musch G, Layfield JD, Harris RS, Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol 93: 1841–1851, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Orphanidou D, Hughes JM, Myers MJ, Al-Suhali AR, Henderson B. Tomography of regional ventilation and perfusion using krypton 81m in normal subjects and asthmatic patients. Thorax 41: 542–551, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter T, Bellani G, Scott Harris R, Vidal Melo MF, Winkler T, Venegas JG, Musch G. Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med 172: 480–487, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shardonofsky FR, Martin JG, Eidelman DH. Effect of body posture on concentration-response curves to inhaled methacholine. Am Rev Respir Dis 145: 750–755, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Tgavalekos NT, Musch G, Harris RS, Vidal Melo MF, Winkler T, Schroeder T, Callahan R, Lutchen KR, Venegas JG. Relationship between airway narrowing, patchy ventilation and lung mechanics in asthmatics. Eur Respir J 29: 1174–1181, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Tgavalekos NT, Tawhai M, Harris RS, Musch G, Vidal-Melo M, Venegas JG, Lutchen KR. Identifying airways responsible for heterogeneous ventilation and mechanical dysfunction in asthma: an image functional modeling approach. J Appl Physiol 99: 2388–2397, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Tgavalekos NT, Venegas JG, Suki B, Lutchen KR. Relation between structure, function, and imaging in a three-dimensional model of the lung. Ann Biomed Eng 31: 363–373, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Treppo S, Mijailovich SM, Venegas JG. Contributions of pulmonary perfusion and ventilation to heterogeneity in V̇a/Q̇ measured by PET. J Appl Physiol 82: 1163–1176, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Venegas JG, Schroeder T, Harris S, Winkler RT, Melo MF. The distribution of ventilation during bronchoconstriction is patchy and bimodal: a PET imaging study. Respir Physiol Neurobiol 148: 57–64, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 434: 777–782, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Vidal Melo MF, Layfield D, Harris RS, O'Neill K, Musch G, Richter T, Winkler T, Fischman AJ, Venegas JG. Quantification of regional ventilation-perfusion ratios with PET. J Nucl Med 44: 1982–1991, 2003. [PubMed] [Google Scholar]
- 36.Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol 103: 655–663, 2007. [DOI] [PubMed] [Google Scholar]