Abstract
Physical activity modifies some postprandial responses such as glycemic control, although it is unclear whether this translates into lower postprandial inflammation. Our objective in this study was to determine whether postprandial inflammatory markers are lower in active compared with sedentary middle-aged men. Thirteen active and twelve sedentary middle-aged men consumed a mixed meal on one occasion. Blood was taken via a cannula before and up to 8 h after the meal and with a single-use needle before and 8 h after the meal. Active men had lower fasted IL-6 (0.6 ± 0.2 vs. 1.2 ± 0.3 pg/ml; P = 0.004) and C-reactive protein (1.3 ± 0.3 vs. 2.9 ± 0.6 mg/l; P = 0.04) concentrations than sedentary men. Cannula blood IL-6 concentrations increased by 3.49 pg/ml in the 8 h following the meal (P < 0.001); however, this increase was minimal (0.36 pg/ml) in blood taken via a single-use needle from the contralateral arm (P = 0.013). The sedentary group had larger glucose (P = 0.034), insulin (P = 0.013), and triacylglycerol (P = 0.057) responses to the meal. These results provide further evidence that physical activity is associated with lower inflammatory marker concentrations in a fasted state and a lower postprandial metabolic response to a meal. However, this does not translate into lower postprandial inflammatory markers since the only evidence of postprandial inflammation (a large increase in serum IL-6) was actually due to the cannula used for blood sampling.
Keywords: exercise, cannula, interleukin-6
low-grade chronic inflammation plays a critical role in the etiology of cardiovascular disease and type 2 diabetes (23, 24), and it appears that inflammation plays a key role at different stages of the progression of atherosclerosis (14). The most commonly used marker of chronic inflammation is C-reactive protein (CRP), which is produced by the liver primarily in response to IL-6 concentration (13). There is growing evidence that systemic concentrations of these markers can be useful tools in predicting the risk of cardiovascular disease, possibly to the same degree as more traditional risk factors such as lipid and cholesterol concentrations (23).
Recent evidence from both epidemiological and intervention studies suggests that regular physical activity decreases markers of chronic inflammation (20, 21, 25, 27, 34). Most research has investigated the effect of physical activity on inflammation in the fasted state; however, we spend the majority of a typical day in a postprandial state. Several studies have found an increase in some markers of inflammation, such as IL-6, following the consumption of a meal. This increase seems to be dependent on both the content of the meal (19) and the metabolic characteristics of the subject (2, 3). In theory, a regular and repeated increase in inflammatory mediators in the postprandial period could have implications for cardiovascular risk.
Research has shown that the postprandial response of several metabolic parameters, including lipids and insulin concentrations, are reduced by increased physical activity levels (8, 12). If physical activity has a similar effect on postprandial inflammation, then this may be an additional mechanism to explain the benefits of physical activity on chronic disease. The aim of this study was to determine whether active middle-aged men show a lower postprandial response to a standardized meal than their sedentary counterparts.
SUBJECTS AND METHODS
Subjects.
Thirteen active and 12 sedentary subjects were recruited via local advertisement following ethical approval and after subjects had given written informed consent. Individuals who smoked, were clinically obese (body mass index >35 kg/m2), or were diagnosed with any medical condition or taking regular medication that may have interfered with the results were excluded. Habitual physical activity was determined using combined heart rate and accelerometry (Actiheart, Cambridge Neurotechnology, Cambridge, UK), validated by Thompson et al. (29). The monitor was worn for seven whole consecutive days recording data every minute over this period. Subjects were not aware of the function of the monitor so as to minimize changes in behavior. These data were used to estimate metabolic equivalents (METs) and the amount of time spent in different MET categories for periods of time greater than a given number of minutes (e.g., >6 METs for ≥3 min). Based on these values, only subjects who met physical activity criteria for either the active or sedentary groups were included. Active subjects participated in at least 90 min of structured vigorous (≥6 METS) physical activity per week and 30 min of moderate or greater (≥3 METS) physical activity (in bouts of 10 min or greater) 5 days/wk. Sedentary subjects undertook no structured vigorous physical activity and 30 min of physical activity ≥ 3 METS (in bouts of 10 min or greater) on fewer than 5 days/wk. Subjects had not greatly changed their physical activity patterns over the last 6 mo or more.
Preliminary measures.
Blood pressure, body mass, skinfold thickness (at 4 different sites: biceps, triceps, subscapular, and superiliac) to estimate percent body fat (5), as well as maximal oxygen uptake (V̇o2max) were measured approximately 7–14 days before the main trial. V̇o2max was determined using an incremental incline test on a treadmill (Woodway, ELG 70 Weiss, Germany). The test consisted of 3-min exercise stages with the incline increasing by 3% at the end of each stage, and this was continued until volitional fatigue. Expired gas samples, heart rate, and rating of perceived exertion (RPE) were collected during each stage and during the final minute of exercise.
Experimental design.
Subjects completed one main trial that involved having blood samples taken at baseline and up to 8 h after consuming a test meal. Subjects were asked to complete a weighed food and fluid record 3 days preceding the trial. They were asked to abstain from vigorous physical activity and alcohol for 1 day before the trial. Subjects arrived at the laboratory following an overnight fast. Following the application of topical local anesthetic a cannula was inserted into an antecubital forearm vein. After the subject had rested for 15 min a baseline blood sample was taken via the cannula. In addition, a blood sample was taken using a single-use needle from an antecubital vein from the contralateral arm in 11 of the 25 subjects.
Subjects were given a mixed meal consisting of 200 g of whipping cream (Somerfield) and 200 ml of water mixed with 75 g of porridge (Quaker Oats) and cooked in a microwave (M1736N; Samsung, UK) at 800 W for 4 min, and a glucose drink consisting of 250 ml of water and 75 g glucose powder (Nutrivit) to consume over a 15-min period. The total energy content of the meal was 1,300 kcal with 55% (120 g) carbohydrate, 39% (85 g) fat, and 6% (12 g) protein.
Regular blood samples were taken from the cannula up to 8 h after the meal, every 15 min for the first hour and then every hour for the next 7 h. Before the cannula was removed a second blood sample was taken from the contralateral arm using a single-use needle from the same subjects as at baseline.
Analytical procedures.
Subjects remained in a seated position for 15 min before and during all blood sampling. Blood samples were distributed into 5-ml serum and EDTA plasma tubes (Sarstedt, Leicester, UK). For all cannula samples 2.5 ml of blood was taken and discarded immediately before blood samples were taken. Immediately after and between blood samples isotonic saline was infused into the cannula to maintain patency. Whole blood glucose and lactate were measured using an automated analyzer (YSI 2300 STAT plus, Yellow Springs, OH). Blood cell counts, hemoglobin content, and hematocrit were measured using an automatic hematology system (SF-3000, Sysmex, Milton Keynes, UK). The remaining blood was centrifuged (Heraeus Biofuge Primo R, Kendro Laboratory Products, Bishops Stortford, United Kingdom) at 5,000 rpm (3,500 g) for 10 min at 5°C. Serum and plasma were aliquoted into Eppendorf tubes before being frozen at −80°C. Commercially available enzyme-linked immunosorbent assays (ELISA) were used to measure CRP (Diagnostic Systems Laboratories, Webster, TX), IL-6 (Quantikine, R & D Systems, Abingdon, UK), soluble intercellular adhesion molecule-1 (sICAM-1; R & D Systems), and soluble vascular cell adhesion molecule-1 (sVCAM-1; R & D Systems). Immunoassays were used to measure plasma triacylglycerol (TAG), free fatty acids (FFA) and cholesterol (Cobas, Roche Diagnostics, Burgess Hill, UK) and serum insulin (AutoDELPFIA, Perkin Elmer).
Statistical analysis.
Statistical analysis was performed using SPSS 14.0 for Windows (SPSS, Chicago, IL). Subject characteristics and activity levels are expressed as means ± SD. All other values are expressed as means ± SE. Statistical significance was set at a value of P ≤ 0.05. All values were checked for normality using Kolmogorov-Smirnov and Shapiro-Wilk tests. Any values that were not normally distributed were subsequently transformed. Repeated-measures ANOVA was used to determine differences between active and sedentary groups for all measures. Two-way ANOVA was used to determine differences between cannula and venepuncture blood sampling methods for IL-6 and white blood cells (WBC). When an interaction effect was found, t-tests were performed to determine specific time points that were significantly different; t-tests were also used to determine any differences between the two groups at baseline. Area under the curve (AUC) was calculated for glucose, insulin, and TAG using the trapezium rule. Normality tests were then performed, and if required, values were transformed; t-tests were then used to test for significant differences between the active and sedentary groups.
RESULTS
Anthropometric and physiological measures.
Anthropometric and physiological measures are summarized in Table 1. There were no significant differences in age, height, and systolic and diastolic blood pressure between the two groups. Body mass (P = 0.011), body mass index (BMI; P = 0.032), and percent body fat (P = 0.011) and V̇o2max (P < 0.001) were significantly different between groups (Table 1).
Table 1.
Descriptive characteristics for active and sedentary
| Active | Sedentary | |
|---|---|---|
| Age, yr | 52±6 | 54±4 |
| Height, m | 1.77±0.07 | 1.80±0.07 |
| Body mass, kg | 77.0±7.0* | 87.0±11.0 |
| BMI, kg/m2 | 24.5±1.6* | 27.0±3.5 |
| Body fat, % | 20±3* | 24±4 |
| Blood pressure, mmHg | 130/85±10/7 | 141/94±20/14 |
| V̇o2max, ml·kg−1·min−1 | 49.2±3.8* | 35.1±3.3 |
| Total vigorous PA time, min/wk | 188±89* | 15±7 |
| Total moderate and vigorous PA time, min/wk | 439±226* | 189±76 |
| Total vigorous PA energy expenditure, kcal/wk | 1,789±808* | 134±68 |
| Total moderate and vigorous PA energy expenditure, kcal/wk | 2,753±903* | 889±374 |
Values are means ± SD for active (n = 13) and sedentary groups (n = 12). Vigorous-intensity physical activity (PA) is all activity >6 metabolic equivalents (METS) for 3 min or more. Moderate and vigorous is all activity >3 METs for 10 min or more. BMI, body mass index; V̇o2max, maximal oxygen uptake.
Significantly different between groups (P < 0.05).
Physical activity levels.
Physical activity levels are summarized in Table 1. The active group participated in, on average, 132% more activity above 3 METs (moderate and vigorous physical activity) per week than the sedentary group (P = 0.003). They also participated in, on average, 12.5 times more vigorous physical activity per week than the sedentary group (P < 0.001).
Metabolic parameters.
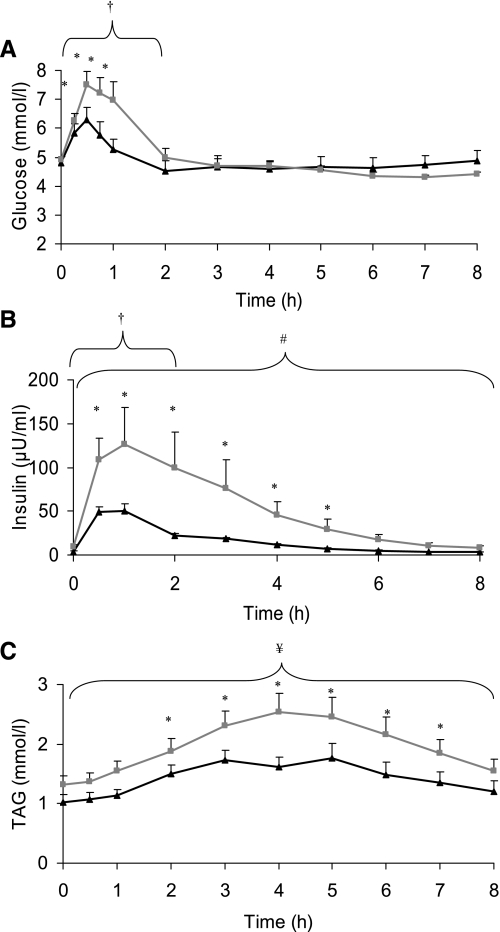
As shown in Fig. 1 blood glucose increased significantly from baseline with concentrations highest at 0.5 h (6.29 ± 0.42 and 7.51 ± 0.46 mmol/l for active and sedentary groups, respectively). The blood glucose AUC for the first 2 h was significantly higher in the sedentary compared with the active group (P = 0.034). Serum insulin also increased significantly following the meal in both groups (P < 0.001) with a mean peak increase at 1 h after the meal of 50 ± 7 and 126 ± 42 μU/ml for active and sedentary groups, respectively. ANOVA showed that there was a group effect for serum insulin, and it was significantly higher in the sedentary group (P = 0.038). The AUC for insulin in the 2 h (P = 0.013) and 8 h (P = 0.003) following the meal was also significantly different between groups. Plasma TAG increased after the meal, peaking at 1.76 ± 0.25 mmol/l (5 h) and 2.54 ± 0.31 mmol/l (4 h) for active and sedentary groups, respectively, and ANOVA showed that there was a group effect with TAG significantly higher in the sedentary group (P = 0.053). There was also a trend for the TAG AUC to be higher in the sedentary group (P = 0.057).
Fig. 1.
Mean blood glucose (A), serum insulin (B), and plasma triacylglycerol (TAG; C) concentrations for active (black triangles; n = 13) and sedentary (gray squares; n = 12) groups at baseline and during the 8 h following consumption of the meal. † 2 h area under curve (AUC) significantly different between groups (P < 0.05). # 8 h AUC significantly different between groups (P < 0.05). ¥ 8 h AUC different between groups (P = 0.057). * Both groups significantly different from baseline (P < 0.05).
Markers of inflammation.
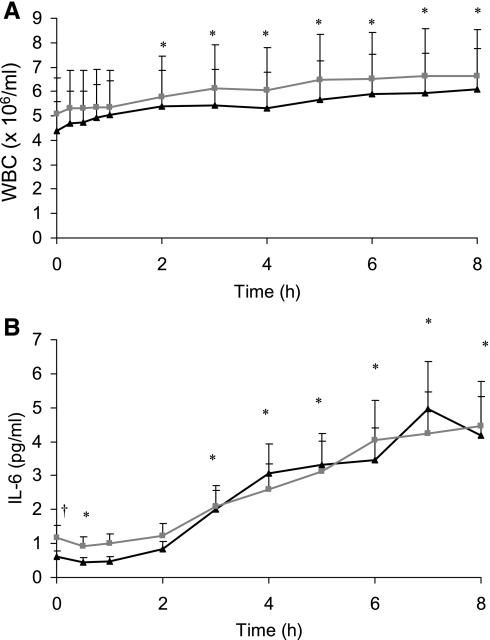
WBC concentration increased over the 8 h following the meal in both groups (P < 0.001; Fig. 2). There was no significant group or interaction effect for leukocyte counts. IL-6 increased in blood taken via the cannula following the meal in both groups (P < 0.001), peaking at a concentration of 5.0 ± 1.3 pg/ml at 7 h for the active group and 4.3 ± 1.3 pg/ml at 8 h for the sedentary group. There was no significant interaction effect for IL-6. However, ANOVA showed that the sedentary group had higher IL-6 concentrations (P = 0.035) with this difference being greatest at baseline (0.61 ± 0.17 vs. 1.18 ± 0.34 pg/ml for active and sedentary groups, respectively; P = 0.004).
Fig. 2.
Mean blood white blood cell (WBC) count (A) and serum IL-6 concentrations (B) for active (black triangles; n = 13) and sedentary (gray squares; n = 12) groups at baseline and during the 8 h following the meal. † Active and sedentary groups significantly different at baseline (P < 0.05). * Both groups significantly different from baseline (P < 0.05).
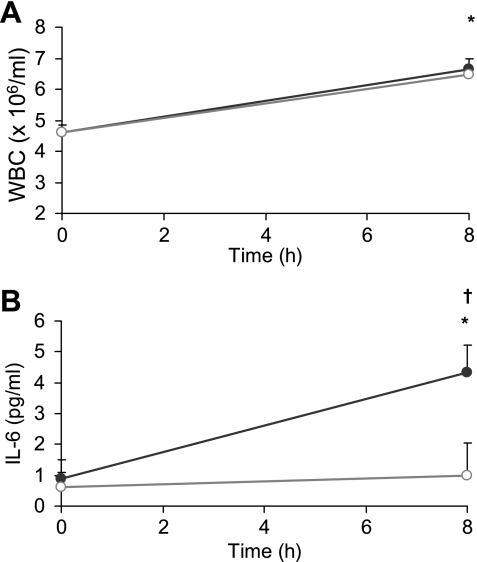
WBC counts in cannula and venous blood were not significantly different (Fig. 3). ANOVA showed a time-method effect (P < 0.001) for IL-6 concentrations measured in blood taken via the two different blood sampling methods (single-use needle venepuncture and cannula). As shown in Fig. 3, IL-6 concentration in blood taken via the cannula increased by 3.49 ± 0.71 pg/ml (P < 0.001), whereas IL-6 taken via venepuncture only increased by 0.36 ± 0.11 pg/ml (P = 0.013) over the 8 h after the meal.
Fig. 3.
Whole blood WBC (A) and serum IL-6 (B) at 0 h and 8 h when taken via the use of single-use needle (open circles; venepuncture) and by use of a cannula (gray circles; n = 11). † Venepuncture and cannula values significantly different (P < 0.05). * Both methods significantly different from baseline (P < 0.05).
CRP did not increase in response to the meal (Table 2). However, ANOVA showed that the mean CRP concentrations for the active group was significantly lower than the sedentary group throughout the trial (P = 0.035). There was a modest 5% increase in sICAM-1 0.5 h following the meal in both groups (Table 2; P = 0.012). There was no significant increase in sVCAM-1 throughout the 8 h following the meal.
Table 2.
Serum CRP, sICAM, and sVCAM concentrations for active and sedentary groups at baseline and during the 8 h following the meal
| CRP, mg/l |
sICAM-1, ng/ml | sVCAM-1, ng/ml | ||||
|---|---|---|---|---|---|---|
| Active | Sedentary | Active | Sedentary | Active | Sedentary | |
| 0 h | 1.3±0.3 | 2.9±0.6* | 210±10 | 227±15 | 727±40 | 648±47 |
| 0.5 h | — | — | 214±11† | 242±18† | 770±49 | 663±45 |
| 1 h | — | — | 212±11 | 237±18 | 756±41 | 627±40 |
| 2 h | 1.3±0.4 | 2.9±0.6* | 218±10 | 248±22 | 735±41 | 661±43 |
| 3 h | — | — | 230±11 | 242±20 | 736±40 | 641±49 |
| 4 h | 1.3±0.4 | 2.8±0.6* | 231±9 | 228±20 | 736±44 | 619±51 |
| 5 h | — | — | 223±9 | 228±16 | 754±47 | 570±37 |
| 6 h | — | — | 221±8 | 226±17 | 721±41 | 605±47 |
| 7 h | — | — | 208±7 | 229±17 | 659±31 | 623±44 |
| 8 h | 1.3±0.4 | 2.7±0.6* | 206±11 | 220±19 | 683±37 | 572±49 |
Values are means ± SE for active (n = 13) and sedentary groups (n = 12). CRP, C-reactive protein; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1.
Sedentary group significantly different from active group (P < 0.05).
Significant time effect from baseline (P < 0.05).
Effect of the blood sampling procedures on IL-6 concentrations.
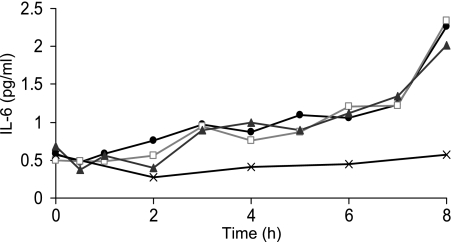
To further investigate the effect of a cannula on postprandial IL-6 concentrations, we examined whether earlier sampling via a single-use needle showed the same differential response to blood taken via a cannula and also whether removing a greater volume of blood from the cannula before analysis made a difference to observed IL-6 concentrations. One 37-yr-old male consumed the test meal on one occasion. Blood was subsequently taken via a cannula at regular intervals and from the contralateral arm via a single-use needle every 2 h. Furthermore, three different blood samples were collected at each time point for cannula samples 1) 2.5 to 5 ml, 2) 5 to 10 ml and 3) 10 to 15 ml. There was an increase in glucose and TAG after the meal, with a peak increase of 1.5 mmol/l and 0.8 mmol/l, respectively. Figure 4 shows the IL-6 response in the blood taken via a single-use needle and samples taken via a cannula after the first 2.5, 5, and 10 ml of blood had been removed. As in our main study, IL-6 in cannula blood increased ∼5-fold after the meal; however, there was no increase in venepuncture IL-6 at any time point. In addition, it is noteworthy that removing up to 10 ml of blood before analysis has very little impact on cannula IL-6 concentration (Fig. 4).
Fig. 4.
Serum IL-6 measured in blood taken via the use of single-use needle (venepuncture; ×) and by use of a cannula after removing 2.5 ml (black circles), 5 ml (open squares), and 10 ml (gray triangles) of blood (n = 1).
DISCUSSION
Regular physical activity has been consistently shown to reduce the glycemic and lipemic response to a meal. We hypothesized that highly active middle-aged men would also have a lower postprandial inflammatory response than sedentary controls. Active subjects showed lower postprandial responses (glucose, insulin, and TAG) and lower fasting markers of inflammation (e.g., IL-6, CRP). However, we found at most only a modest postprandial inflammatory response in either the active or sedentary men. The only inflammatory marker that showed a substantial increase in the time after the meal was IL-6 in blood taken via a cannula. Much of this increase can be attributed to local inflammation in response to the presence of the cannula since at 8 h the IL-6 measured in cannula blood was fivefold higher than that measured in blood taken via a single-use needle from the contralateral arm.
There was a significant increase in glycemic and lipemic parameters after the consumption of the meal in both active and sedentary middle-aged men. As anticipated and shown previously, postprandial insulin, glucose, and TAG were significantly lower in the active group than the sedentary controls (9, 17, 26). Subjects abstained from vigorous exercise the day before the trial. However, since moderate-intensity activity can modify postprandial responses (18), the differences we found in TAG, glucose, and insulin between the two groups could have been due to an acute or chronic effect of training (or a combination of both).
The energy content of the meal was ∼1,300 kcal (85 g fat and 120 g carbohydrate), and the postprandial increase in insulin, glucose, and TAG was of a similar or greater magnitude than shown previously for active or sedentary men (2, 9, 19). Therefore, the meal provided a similar or greater challenge than used in previous studies that have investigated the postprandial response to a single meal.
Despite the large glycemic and lipemic response observed in the present study, there was only evidence of a very modest postprandial inflammatory response, and this was not different between the active and sedentary groups. CRP and sVCAM-1 did not significantly increase after the meal, and soluble ICAM-1 only increased by ∼5% in the first half hour after the meal. Although WBC increased over time, this was of a similar magnitude to the increase found in diurnal variation that has been previously reported (22). On the face of it, it appears that venous IL-6 concentrations in blood taken using a cannula increase markedly in both active and sedentary middle-aged men following the consumption of the high-fat, high-glucose meal. However, recent research has suggested that IL-6 may increase simply in response to the presence of a cannula (11). The results from the present investigation offer support for this finding since IL-6 taken via a single-use needle from the opposite arm 8 h after the meal was only marginally increased above baseline. This suggests that in the present study, the increase in cannula blood IL-6 was mainly the result of an increase in local inflammation in response to the cannula and not an increase in systemic IL-6 in response to the meal. The cannula and its tubing can hold less than 0.5 ml, and since we removed and discarded more than 2.5 ml of blood before each sample, this indicates that the observed increase in IL-6 was from blood drawn from the vein in which the cannula was sited. Furthermore, we also show in a subsequent experiment in one subject that removing up to 10 ml of blood before taking a sample does not overcome the effect of the cannula on serum IL-6 concentrations. This secondary experiment also confirms that IL-6 taken via venepuncture does not increase at any time point after the meal. Collectively, our results suggest that the measurement of IL-6 in cannula blood is not a good measure of the inflammatory response to feeding, and we recommend that investigators pay particular attention to the blood sampling procedures used to examine postprandial changes in IL-6. Because the increase in IL-6 can be discounted, it appears that no inflammatory marker showed a pronounced systemic response to a meal in either active or sedentary subjects.
It is noteworthy that even the very considerable glycemic and lipemic response seen in the inactive subjects did not translate into a postprandial inflammatory response. Although the mechanisms behind the postprandial inflammation reported in other studies has not been determined, it has been suggested that the increase in TAG and/or glucose could result in an increased oxidative stress (1, 3, 19, 33), an increase in endothelial damage (8), or could alter postprandial metabolic processes (3), all resulting in a proinflammatory cascade. In support of these suggestions, research has previously shown that when the glucose, insulin, and TAG response to a meal is reduced through weight loss, this translates into a lower postprandial IL-6 (3) and sICAM (2). Therefore, we anticipated that the very different exercise patterns, body composition, and a large differentiation in glycemic and lipemic responses to a meal would translate into a more marked postprandial inflammatory response in the sedentary subjects. However, we did not find an inflammatory response to this meal, and this suggests that the postprandial glycemic/lipemic and inflammatory response to a meal are not tightly coupled.
Since other research has investigated postprandial inflammation using different meals and blood sampling methods, these results cannot be used to rule out a postprandial inflammatory response to all types of meal. Of the research that has found an increase in postprandial IL-6, most investigators have used a cannula for blood sampling (3, 8, 9, 14, 30, 31). However, Nappo et al. (19) took blood via venepuncture and found an increase in IL-6 after a high-fat meal of sausages, bread, and eggs, but not after a pizza meal. Since these meals had a similar energy content, this suggests that the inflammation was not dependent on the energy load. Interestingly, while neither meal was particularly high in glucose, the fat content was obviously much greater in the high-fat meal (59.2% of energy) compared with the pizza meal (20.6% of energy). However, since the former has a lower fat content (50 g) than the present meal (85 g), this does not explain why there was no evidence of a similar increase in IL-6 after the meal used in the present study. It may be possible that the type of fat or the cooking method could be critically important. One tentative explanation is that postprandial inflammation is the result of an endotoxin that cotransits with dietary nutrients, most likely fats, from the gut after a meal (6). The amount of endotoxemia could be dependent on the nature of the meal (i.e., type of fat, etc.), and this may explain why Nappo et al. (19) found a large IL-6 increase after their sausage and egg breakfast but we found no response to our porridge and cream meal. Further research using a variety of meals, with single-use needle blood sampling, is needed to clarify the effect of food and physical activity on postprandial inflammation.
Many epidemiological and intervention studies have shown increased physical activity to be associated with decreased fasting inflammatory markers such as CRP and IL-6 (10, 16, 21, 34). This is supported by our results that show that fasting CRP and IL-6 were significantly lower in active compared with sedentary individuals. We hypothesized that differences at baseline would translate into even more profound differences in the postprandial period. However, based on the results of the present study alone, there seemed to be no additional prognostic benefit to measuring postprandial inflammatory markers compared with measuring fasted concentrations alone. In fact postprandial IL-6 measured using a cannula actually confounded any differences between the groups. Since increased concentrations of inflammatory markers are associated with increased risk of cardiovascular disease and diabetes, a chronic reduction of fasting inflammatory measures may be one mechanism by which regular physical activity protects against chronic disease. It is not clear how physical activity contributes to the maintenance of lower proinflammatory mediators, although alterations in anti-inflammatory cytokine secretion such as increased IL-10 (27), increased insulin-selective effects on hepatic acute-phase protein secretion (7), and reduced oxidized low-density lipoproteins (28, 32) are all potential mechanisms.
Due to the nature of the inclusion criteria in the present investigation all active subjects were above and all sedentary subjects were below public health physical activity recommendations (4). In addition to much higher physical activity levels, active subjects also had higher fitness (i.e., V̇o2max). It is important to highlight that the active group also had lower total mass, BMI, and proportion of body fat, and therefore it is not possible to determine whether the differences reported in the present study are directly due to differences in physical activity behavior and adaptation (e.g., fitness) or due to differences in body composition as a result of this behavior. Further research is needed to determine whether the benefits of exercise are independent of or dependent on the maintenance of appropriate weight and body composition.
To summarize, in support of previous literature, we found that active middle-aged men had lower concentrations of fasting markers of inflammation than their sedentary counterparts. Interestingly, although active men also exhibited the well-documented reduction in postprandial lipemic and glycemic response to a high-fat, high-glucose meal, this did not translate into a difference in postprandial inflammation. Collectively, these results indicate that the inflammatory response to feeding in the present study was at best only very modest and that the marked change in IL-6 actually appears to be due to the cannula used for blood sampling.
GRANTS
This study was supported by funding from The Biotechnology and Biological Research Council, United Kingdom, and Unilever, United Kingdom.
REFERENCES
- 1.Bae JH, Bassenge E, Kim KB, Kim YN, Kim KS, Lee HJ, Moon KC, Lee MS, Park KY, Schwemmer M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 155: 517–523, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Esposito K, Giugliano D. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 53: 701–710, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Corpeleijn E, Saris WH, Jansen EH, Roekaerts PM, Feskens EJ, Blaak EE. Postprandial interleukin-6 release from skeletal muscle in men with impaired glucose tolerance can be reduced by weight loss. J Clin Endocrinol Metab 90: 5819–5824, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: National Center for Chronic Disease Prevention and Health, 1996.
- 5.Durnin JVGA, Womersle. J. Body fat assessed from total body density and its estimation from skinfold thickness—measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32: 77–97, 1974. [DOI] [PubMed] [Google Scholar]
- 6.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86: 1286–1292, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Festa A, D'Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome—The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102: 42–47, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Gill JM, Caslake MJ, McAllister C, Tsofliou F, Ferrell WR, Packard CJ, Malkova D. Effects of short-term detraining on postprandial metabolism, endothelial function, and inflammation in endurance-trained men: dissociation between changes in triglyceride metabolism and endothelial function. J Clin Endocrinol Metab 88: 4328–35, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Gill JMR, Al-Mamari A, Ferrell WR, Cleland SJ, Packard CJ, Sattar N, Petrie JR, Caslake MJ. Effects of prior moderate exercise on postprandial metabolism and vascular function in lean and centrally obese men. J Am Coll Cardiol 44: 2375–2382, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol 100: 93–99, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmacher T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology 27: 921–931, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hardman AE, Lawrence JEM, Herd SL. Postprandial lipemia in endurance-trained people during a short interruption to training. J Appl Physiol 84: 1895–1901, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 265: 621–626, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellema A, Plat J, Mensink RP. Weight reduction, but not a moderate intake of fish oil, lowers concentrations of inflammatory markers and PAI-1 antigen in obese men during the fasting and postprandial state. Eur J Clin Invest 34: 766–773, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Libby P Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 83: 456S–460S, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol 45: 1563–1569, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Merrill JR, Holly RG, Anderson RL, Rifai N, King ME, Demeersman R. Hyperlipemic response of young trained and untrained men after a high-fat meal. Arteriosclerosis 9: 217–223, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita M, Burns SF, Stensel DJ. Accumulating short bouts of brisk walking reduces postprandial plasma triacylgerol concentrations and resting blood pressure in healthy young men. Am J Clin Nutr 88: 1225–1231, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 39: 1145–1150, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev Med 40: 432–437, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Pitsavos C, Chrysohoou C, Panagiotakos DB, Skoumas J, Zeimbekis A, Kokkinos P, Stefanadis C, Toutouzas PK. Association of leisure-time physical activity on inflammation markers (C-reactive protein, white cell blood count, serum amyloid A, and fibrinogen,) in healthy subjects (from the ATTICA study). Am J Cardiol 91: 368–370, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Pocock SJ, Ashby D, Shaper AG, Walker M, Broughton PM. Diurnal variations in serum biochemical and haematological measurements. J Clin Pathol 42: 172–179, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342: 836–843, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Wilson PWF, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation 109: 2818–2825, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med 163: 1200–1205, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Seals DR, Hagberg JM, Allen WK, Hurley BF, Dalsky GP, Ehsani AA, Holloszy JO. Glucose tolerance in young and older athletes and sedentary men. J Appl Physiol 56: 1521–1525, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281: 1722–1727, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T-Lymphocytes from human atherosclerotic plaques recognize oxidized low-density-lipoprotein. Proc Natl Acad Sci USA 92: 3893–3897, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson D, Batterham AM, Bock S, Robson C, Stokes K. Assessment of low-to-moderate intensity physical activity thermogenesis in young adults using synchronized heart rate and accelerometry with branched-equation modeling. J Nutr 136: 1037–1042, 2006. [DOI] [PubMed] [Google Scholar]
- 30.van Oostrom AJ, Sijmonsma TP, Verseyden C, Jansen EH, de Koning EJ, Rabelink TJ, Castro Cabezas M. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J Lipid Res 44: 576–583, 2003. [DOI] [PubMed] [Google Scholar]
- 31.van Oostrom AJ, Rabelink TJ, Verseyden C, Sijmonsma TP, Plokker HW, De Jaegere PP, Cabezas MC. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis 177: 175–182, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Vasankari TJ, Kujala UM, Vasankari TM, Ahotupa M. Reduced oxidized LDL levels after a 10-month exercise program. Med Sci Sports Exerc 30: 1496–1501, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Wright E, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract 60: 308–314, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab 89: 1739–1746, 2004. [DOI] [PubMed] [Google Scholar]