Abstract
We examined the effects of endurance training on parameters of glucose flux during rest and exercise in postmenopausal women. Ten sedentary, but healthy women (55 ± 1 yr) completed 12 wk of endurance exercise training on a cycle ergometer [5 days/wk, 1 h/day, 65% peak oxygen consumption (V̇o2peak)]. Flux rates were determined by primed continuous infusion of [6,6-2H]glucose (D2-glucose) during 90 min of rest and 60 min of cycle ergometer exercise during one pretraining exercise trial [65% V̇o2peak (PRE)] and two posttraining exercise trials [the power output that elicited 65% pretraining V̇o2peak (ABT) and 65% posttraining V̇o2peak (RLT)]. Training increased V̇o2peak by 16.3 ± 3.9% (P < 0.05). Epinephrine and glucagon were lower during ABT and lactate was lower during ABT and RLT (P < 0.05), but the apparent insulin response was unchanged. Whole body glucose rate of appearance decreased posttraining during exercise at a given power output (4.58 ± 0.39 mg·kg−1·min−1 during ABT compared with 5.21 ± 0.48 mg·kg−1·min−1 PRE, P < 0.05), but not at the same relative workload (5.85 ± 0.36 mg·kg−1·min−1). Training resulted in a 35% increase in glucose MCR during exercise at the same relative intensity (7.16 ± 0.42 ml·kg−1·min−1 during RLT compared with 5.28 ± 0.42 ml·kg−1·min−1 PRE, P < 0.05). Changes in parameters of glucose kinetics during exercise were accomplished without changes in dietary composition, body weight, or body composition. We conclude that despite changes in the hormonal milieu that occur at menopause, endurance training results in a similar magnitude in training-induced alterations of glucose flux as seen previously in younger women.
Keywords: menopause, glucose kinetics, body composition, exertion, exercise training
declining levels of endogenous estrogen (estradiol, E2) and progesterone (P4) at menopause have diverse consequences including effects on glucose metabolism, energy substrate partitioning, and exercise capacity. Although the effects of menopause on glucose flux during exercise have been little studied, data are available on younger subjects with variable levels of ovarian hormones. Ruby et al. (35) and Carter et al. (7) found that glucose flux decreased during moderate intensity exercise in amenorrheic women (35) and men (7) after 17β-estradiol administration (E2). Furthermore, D'eon et al. (14) reported that glucose flux in eumenorrheic women tended to be lower under conditions of high estrogen and progesterone and estrogen alone compared with baseline (14). As well, studies on younger women (8, 21) and men (3, 8, 10, 11, 20, 28, 30) have demonstrated that endurance training decreases glucose flux during a given exercise task. In aggregate, previous studies lead to the idea that the withdrawal of ovarian hormones at menopause may lead to an increase in glucose flux during physical activity relative to that during exercise in the premenopausal time of life.
To date studies that have examined the effects of exercise and endurance training on glucose flux in older individuals have grouped men and women together in the analyses (36, 37). For example, Sial et al. (36) measured glucose flux rates during 60 min of moderate intensity cycle ergometer exercise and found that the glucose Ra in men and women aged 73 ± 2 yr was higher than in younger individuals (age 26 ± 2 yr) exercising at the same absolute intensity. However, when subjects exercised at the same relative intensity (50% of V̇o2max), glucose Ra was higher in the younger subjects (36). In addition to describing the effects of exercise on glucose flux in the aged, to understand the effects of training on glucose metabolism Sial et al. (37) studied elderly subjects (age 74 ± 2 yr) before and after 16 wk of endurance training and found that training decreased glucose Ra at the same absolute intensity. However, a comparison of similar relative intensities was not reported. Given that lean body mass and aerobic capacity decrease with age (18) a comparison of similar relative intensities may be more informative when comparing different age groups. As well, because data obtained on elderly men and women were pooled in analysis, it is unclear whether the same effects of exercise and endurance training would apply to women in early postmenopausal years.
To contribute to the literature on the effects of exercise and endurance training on parameters of glucose flux, we sought to determine the effects of 12 wk of moderate intensity endurance training on parameters of glucose flux in sedentary, but otherwise healthy postmenopausal women. On the basis of previously observed effects of 17β-estradiol and progesterone on glucose flux, we predicted that the decline in ovarian endocrine function after menopause might elevate glucose flux in women relative to premenopause. Because exercise intensity is the key determinant of glucose flux (5, 21, 39) we predicted that as in younger individuals (3, 8, 20, 21, 33) exercise training would decrease glucose flux at the same absolute, but not relative exercise intensity. Additionally we suspected that because of nascent insulin resistance following menopause, the magnitude of training-induced alterations in glucose flux in postmenopausal women might be more pronounced than seen previously in premenopausal women.
METHODS
Subjects.
Ten healthy, nonsmoking, weight-stable postmenopausal women (55 ± 0.61 yr) were recruited from the University of California campus and the surrounding community by posted notices and internet advertisements. The women were considered to be postmenopausal if they reported not menstruating for at least a year, and their plasma follicle-stimulating hormone (FSH) levels were >30 mIU/ml. Subjects were considered sedentary if they participated in <2 h of regular strenuous activity per week for the previous year and if they had peak oxygen consumption (V̇o2peak) values between 15 and 35 ml·kg−1·min−1 as determined by a leg cycle ergometer stress test. The women were admitted into the study if they met the following criteria: 1) were diet and weight stable for ≥6 mo, 2) did not have osteoporosis, 3) had not taken estrogen ≥6 mo or blood thinners such as aspirin ≥3 mo before the study, 4) had not had a hysterectomy, 5) had normal lung function (forced expiratory volume in 1 s of 70% or more), and 6) were disease and injury free as determined by a health history questionnaire and physical examination. Subjects were excluded if they had the metabolic syndrome, which is defined by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP/ATP III) (32). The University of California Committee for the Protection of Human Subjects approved the study protocol (CPHS 2005-10-29) and subjects provided written informed consent.
Experimental design.
Subjects underwent a total of three isotope infusion trials over the course of the study: one pretraining trial and two posttraining trials. The trials consisted of a 90-min rest period followed by 60 min of continuous pedaling on a cycle ergometer. This task was selected as it is consistent in duration with Institute of Medicine (IOM) physical activity recommendations (6), and because from practical experience it was the most strenuous exercise task that sedentary participants were capable of doing. The first trial was performed at 65% of V̇o2peak (PRE). One of the posttraining trials was performed at the same absolute workload (65% of pretraining V̇o2peak, ABT) while the other posttraining trial was performed at the same relative workload (65% of new V̇o2peak, RLT). The two posttraining trials were performed 2 wk apart at weeks 10 and 12, respectively, of the training period. The order of the two posttraining trials was randomized and training continued between the two trials.
Screening tests.
Anthropometric and ergonometric data have been reported (42), but are reiterated for convenience to readers. Briefly, body composition was measured by DEXA; waist circumference was determined at the smallest circumference between the xiphoid process and the anterior iliac crest; hip circumference was measured as the largest circumference around the buttocks, and a 12-h fasting blood sample was taken from each of the prospective subjects to obtain baseline measurements of plasma glucose, FSH, TG, HDL, and total cholesterol. FSH levels were used to confirm postmenopausal status, while levels of TG and HDL were used in conjunction with other parameters to assess whether the subjects had metabolic syndrome. Three-day diet records (2 weekdays and 1 weekend day) were recorded and analyzed before and after the 12-wk endurance training program to monitor subjects' caloric intake and macronutrient composition and to ensure that the subjects had maintained the same dietary habits throughout the course of the study. Subjects were instructed not to alter their dietary habits or discretionary physical activity level over the course of the study, were weighed before every training session and trial, and told to increase their energy intake to compensate for the increase in energy expenditure to maintain weight stability.
V̇o2peak tests were conducted under medical supervision as per ACSM guidelines (1). Subjects were considered to have reached their V̇o2peak when the following criteria were met: 1) leveling off of oxygen consumption (V̇o2) with increasing workload, 2) RER values greater than 1.1, and 3) a heart rate within 10% of their age-predicted maximum.
Tracer protocol.
Subjects were instructed not to exercise on the day before isotope trials and to only eat the standardized diet that was provided to them (2,051 ± 58 total calories: 24% fat, 58% carbohydrate, and 18% protein). This standardized diet was based on IOM predictive equations for total energy expenditure (TEE) assuming a physical activity coefficient (PA) of 1.14 or physical activity level (PAL) of 1.5, low active (6, 26). The PA is used in the prediction equations for total energy expenditure, while the PAL represents a measure of TEE in relation to basal energy expenditure (BEE) (PAL=TEE/BEE) (6). Subjects were instructed not to drink caffeine-containing beverages 24 h prior to the trials. Subjects reported to the laboratory 9-h fasted on the morning of the trial and catheters were then placed into a hand vein to obtain “arterialized” blood samples using the “heated hand vein” technique for measurements of fasting blood glucose and background blood glucose enrichment. A previous study in our laboratory (24) showed that measurement of blood glucose isotopic enrichment was the same whether it was taken from the radial artery or using the “heated hand vein” technique.
After collection of the background blood and breath measurements, subjects were given a standardized breakfast (560 kcal; 60% carbohydrate, 26% fat, and 14% protein) to consume in the laboratory. Due to a work conflict, one subject completed her isotope trials in the afternoon, arriving at the laboratory at noon to begin each trial. However, this subject consumed the same standardized pretrial meal as the other subjects. A different standardized breakfast (565 kcal; 61% carbohydrate, 27% fat, 12% protein) was provided to this subject to eat on the morning of the trials. We choose to study subjects under postprandial conditions to mimic the normal free-living conditions and report data on postmenopausal women with stable exercise blood glucose levels and normal preexercise liver glycogen stores.
Ninety minutes after the subjects finished consuming the standardized breakfast, a venous catheter was placed in the contralateral arm and a 15 ml priming bolus of [6,6-2H2]glucose (D2-glucose) was administered at a dose that was 125 times the resting infusion rate. [6,6-2H2]glucose was continuously infused (Baxter Travenol 6300 infusion pump) for the 90-min rest period while the subjects rested supine or semi-supine. The [6,6-2H2]glucose cocktail was at a concentration of 8 mg/ml and the resting infusion minute rate was 0.025 mg·kg−1·min−1. Infusion rates were increased during exercise to 0.052 mg·kg−1·min−1 during the posttraining trial performed at 65% pretraining V̇o2peak (ABT), 0.059 mg·kg−1·min−1 during 65% pretraining (PRE), and 0.078 mg·kg−1·min−1 during 65% posttraining V̇o2peak (RLT). These infusion rates were chosen based on experience to maintain stable isotopic enrichment values for the different exercise workloads to perform the calculations for glucose kinetics.
Isotope tracers were obtained from Cambridge Isotope Laboratories (Woburn, MA), diluted in 0.9% sterile saline, tested for sterility and pyrogenicity (University of California, San Francisco, School of Pharmacy), and passed through a 0.2-μm Millipore filter (Nalgene, Rochester, NY) on the day of each experimental trial.
Respiratory gas exchange measurements and blood samples were collected during the trial at 0, 60, 75, and 90 min of rest and during 15, 30, 45, and 60 min of exercise. Heart rate and blood pressure measurements were recorded throughout rest and exercise at the same frequency as the blood and breath sampling.
Blood sampling and analyses.
After background sampling and subsequent to the start of the tracer infusion, blood samples were taken at 60, 75, and 90 min of rest and at 15, 30, 45, and 60 min of exercise. Blood samples for analysis of glucose, lactate, and glucose enrichment were immediately deproteinized with 8% perchloric acid, mixed thoroughly, and then stored on ice. Blood for analysis of insulin and cortisol were collected in tubes containing aprotinin. Blood samples for analysis of glucagon and growth hormone (GH) were collected in glass tubes containing EDTA and aprotinin while those for determination of catecholamines were stored in tubes containing glutathione and EGTA to prevent oxidation. Samples for blood analysis, except for insulin, were immediately stored on ice and then centrifuged at 3,000 g for 10 min. Samples for serum insulin analysis were allowed to clot at room temperature before centrifugation. Perchlorate supernatants for glucose and lactate analyses were stored at −20°C while the other samples were stored at −80°C until further analysis. All the samples from a given subject were analyzed at the same time in duplicate to reduce variability.
I125-radioimunoassays were performed to measure the concentrations of serum insulin (Linco Research, St. Charles, MO) and plasma glucagon (Diagnostic Products, Los Angeles, CA) and cortisol (Coat-A-Count Kits, Diagnostic Products). Plasma growth hormone concentrations were determined by ELISA (Bio Quant, San Diego, CA). The sensitivities of the assays were 2 μU/ml for insulin, 13 pg/ml (3.7 pmol/l) for glucagon, 0.2 μg/dl (5.5 nmol/l) for cortisol, and 0.2 ng/ml for growth hormone. Epinephrine and norepinephrine concentrations were determined by high performance liquid chromatography with electrochemical detection. Hematocrit was measured at each of the time points using a circular microcapillary tube reader (no. 2201, International) and verified to be stable so as not to compromise the metabolite and hormone concentration measurements. Subjects drank tap water ad libitum during each trial to maintain hydration status.
Isotopic enrichment analyses.
Glucose concentrations and isotopic enrichments (IE) were measured using gas chromatography-mass spectrometry (GC model 6890 series and MS model 5973N, Agilent Technologies) of the pentaacetate derivative using an Agilent DB-17 GC column and [U-13C]glucose as the internal standard as used previously (23). Chemical ionization was performed using methane gas, and selected ion monitoring was used to monitor the ions mass-to-charge ratios of 331, 333, and 337. The selected ion abundances were compared against the external standard curve to calculate IE, and normalized to the internal standard curve to determine the glucose concentration.
Calculations.
Glucose rates of appearance (Ra), disappearance (Rd), and metabolic clearance rate (MCR) were calculated assuming a single compartment model and using the equations defined by Steele (38) and modified for use with stable isotopes (41).
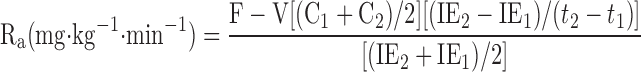 |
(1) |
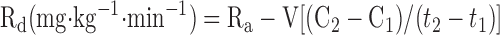 |
(2) |
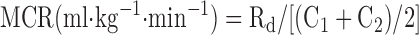 |
(3) |
where F represents the isotopic infusion rate; V is the estimated volume distribution of glucose (100 ml/kg); C1 and C2 are the concentrations at sampling times t1 and t2, respectively; and IE1 and IE2 are the glucose isotopic enrichments of D2-glucose at sampling times t1 and t2, respectively. The background blood samples were used to correct the isotopic enrichment values.
Training protocol.
The training protocol on a leg cycle ergometer has been reported elsewhere (42), but is repeated here for convenience to the reader. The duration of the exercise training sessions was gradually increased from 30 to 60 min during the first 4 wk. The number of supervised training sessions was increased gradually from 3 days/wk during the first 3 wk to 5 days/wk during weeks 5–12. By week 5, subjects were exercising for 60 min at 65% V̇o2peak 5 days/wk; this intensity and duration was continued throughout the course of the intervention. Interval training was added during the last 4 wk, during which subjects performed four 1-min bouts at a power output that elicited 100% V̇o2peak. Training was supervised by student personal trainers who used Polar heart rate monitors and data from the intermediate (5 wk) V̇o2peak test to monitor and standardize the relative exercise intensity in training. Subjects were weighed before each training session and asked to increase their energy intake to maintain the same body weight. All subjects complied with the exercise training protocol and remained in the study for the entire duration.
Statistical analyses.
Data are presented as group means ± SE. For evaluation of significance of responses to exercise and training on parameters of metabolism values for the last 15 min of rest (75 and 90 min) and the last 30 min of exercise (30, 45, and 60 min) were averaged to give representative values. Significance of differences among the metabolite concentrations, glucose kinetics, and hormone concentrations were determined using one-way ANOVA with repeated measures while glucose concentration and isotopic enrichment measurements over time were analyzed using a two-way ANOVA with repeated measures. Significance of differences among the mean values in physical characteristics of the subjects were analyzed with paired Student's t-tests. Post hoc comparisons were made with Fischer's protected least significant difference test. Statistical significance was defined as an α ≤ 0.05.
RESULTS
Subjects.
The physical characteristics and the metabolic profiles of the subjects before and after training have been reported in detail elsewhere (42), but some results are reiterated here for convenience of readers.
Subjects were weight stable and body composition did not change as the result of training. Maximal power output achieved during V̇o2max testing increased by 25.3 ± 3.4% and V̇o2peak increased by 16.3 ± 3.9% as a result of training (P < 0.05; 42). Because of the training effect, the ABT posttraining trial was conducted at 55% of the posttraining V̇o2peak. Resting heart rate values were significantly lower after training. Pulmonary minute ventilation, heart rate, diastolic blood pressure, and mean arterial pressure were all reduced during exercise at ABT (P < 0.05), but not RLT (42). There were no changes in the total energy intake (1,845 ± 124 and 1,823 ± 98 kcal/day before and after training, respectively), percentage of energy intake as carbohydrate (48 ± 2.7 and 49 ± 2.7%, respectively), percentage of energy intake as fat (40 ± 2.2 and 42 ± 3.7%, respectively), and percentage of energy intake as protein (18 ± 1.1 and 18 ± 1.0%, respectively) as a result of the exercise intervention.
Blood glucose concentration.
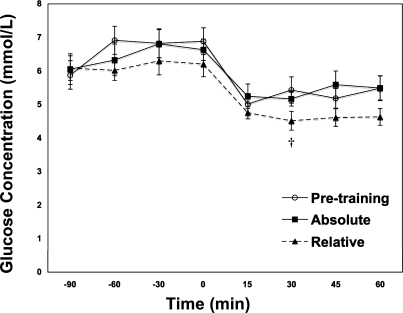
There was no significant difference between the resting 3-h postprandial plasma glucose concentrations before and after training (Fig. 1). Before training blood glucose concentrations decreased 22% in the transition from rest to exercise (P < 0.05). After training, during ABT blood glucose concentration decreased 16% in the rest to exercise transition and 30% during RLT compared with preexercise rest. After training, blood glucose concentrations during the RLT trial were 16% lower than PRE and 17% lower than ABT (P < 0.05).
Fig. 1.
Blood glucose concentrations (mM) over time in middle-aged (55 ± 0.6 yr) postmenopausal women at rest and during exercise before and after 12 wk of endurance training. Exercise commenced at 0 time. Values are means ± SE. †Significantly different from pretraining 65% (PRE) at P < 0.05.
Blood lactate concentration.
There was no significant difference between the resting 3-h postprandial plasma lactate concentrations before and after training (Fig. 2). Lactate concentrations significantly increased from rest to exercise during PRE and during RLT (P < 0.05), but not during ABT. Compared with PRE, lactate concentrations decreased 62% during ABT and 45% during RLT although power output during RLT was 35% greater than during PRE (P < 0.05; Fig. 2).
Fig. 2.
Plasma lactate concentration (mM) at rest and during exercise before and after 12 wk of endurance training. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. *Significantly different from resting conditions at P < 0.05. †Significantly different from pretraining 65% (PRE) at P < 0.05. §Significantly different from posttraining (ABT) at P < 0.05.
Hormone concentrations.
In all trials the plasma insulin concentration and insulin-to-glucagon ratio were significantly higher at rest compared with exercise, but there were no significant differences between trials (Table 1). Glucagon concentration was significantly higher during exercise than at rest for PRE and RLT, but not ABT. Glucagon concentration was 15% lower during ABT compared with PRE (P < 0.05). Growth hormone concentration was significantly higher during exercise during all trials, but there was no significant difference between the trials. There were no significant differences in cortisol concentrations between rest and exercise or between any of the trials.
Table 1.
Hormonal and metabolic responses of subjects following similar exercise and training protocols
| Variable | Rest |
Exercise | |||
|---|---|---|---|---|---|
| Pretraining | Posttraining | PRE | ABT | RLT | |
| Insulin, μU/ml | 33.0±5.12 | 26.8±4.40 | 7.10±0.78* | 6.23±0.71* | 7.30±0.96* |
| Glucagon, pg/ml | 74.9±7.81 | 80.0±10.3 | 105±14.12* | 89.3±14.5† | 106±14.2* |
| I/G | 11.9±2.91 | 11.9±3.61 | 2.06±0.54* | 2.46±0.99* | 2.05±0.54* |
| Cortisol, μg/dl | 9.42±1.06 | 11.9±0.73 | 14.08±2.40 | 10.5±1.78 | 13.15±2.07 |
| Growth hormone, ng/ml | 1.48±0.18 | 2.34±0.52 | 8.92±2.22* | 6.88±1.76* | 7.99±1.76* |
| Epinephrine, pg/ml | 35.1±4.87 | 54.2±15.7 | 189±27.8* | 126±18.0*† | 130±25.5* |
| Norepinephrine, pg/ml | 320±47.8 | 326±67.5 | 876±109* | 743±111* | 979±160* |
Values are means ± SE. I/G, insulin-to-glucose ratio; PRE, pretraining exercise trial (65% V̇o2peak); ABT, posttraining exercise trials (the power output that elicited 65% pretraining V̇o2peak); RLT, 65% posttraining V̇o2peak.
Significantly different from resting conditions at P < 0.05.
Significantly different from PRE at P < 0.05.
§Significantly different from ABT at P < 0.05.
Epinephrine concentrations were significantly higher during exercise compared with rest during all three trials (Table 1). After training, epinephrine concentrations during ABT were 33% lower than PRE (P < 0.05), but there was no significant difference between PRE and RLT. Norepinephrine concentrations were significantly higher during exercise during all trials compared with rest, but there was no significant difference between the trials.
Blood glucose kinetics.
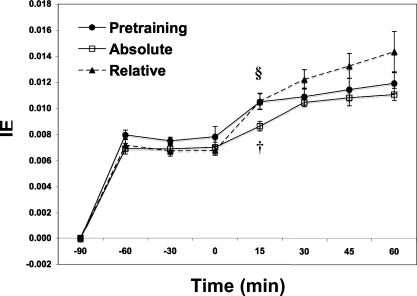
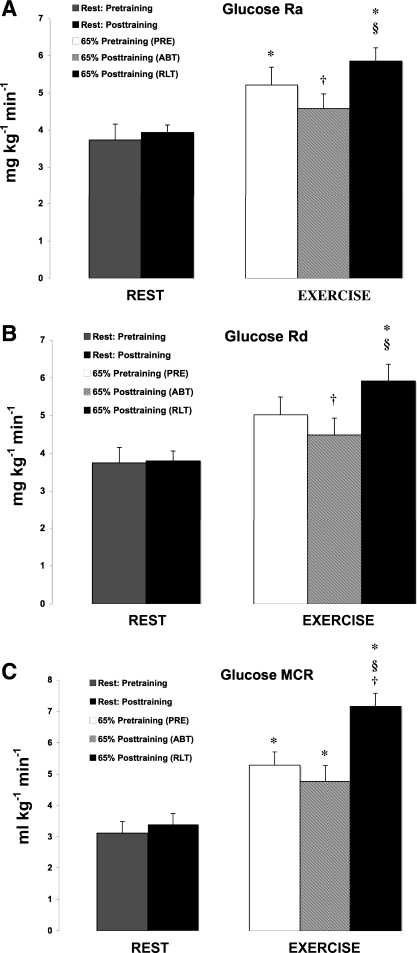
Glucose isotopic enrichments achieved steady levels during rest and exercise under all conditions studied (Fig. 3). Glucose Ra increased from rest to exercise during PRE and RLT (P < 0.05; Fig. 4A). Glucose Ra during ABT was 12% lower than PRE and 22% lower than RLT (P < 0.05). However, there was no difference in glucose Ra between PRE and RLT, the exercise power output during RLT (74.9 ± 4.07 W) being 35% higher than that during PRE (55.5 ± 5.90 W).
Fig. 3.
Isotopic enrichments (IE) of [6,6-2H2]glucose (D2-glucose) over time for the 3 isotope trials. Values are means ± SE. PRE, 65% pretraining trial; ABT, same absolute workload as PRE; RLT, same relative workload (65% of posttraining V̇o2peak). †Significantly different from pretraining 65% (PRE) at P < 0.05. §Significantly different from posttraining (ABT) at P < 0.05.
Fig. 4.
A: effect of 12 wk of endurance training on plasma glucose rate of appearance (Ra) in postmenopausal women. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. *Significantly different from resting conditions at P < 0.05. †Significantly different from PRE at P < 0.05. §Significantly different from ABT at P < 0.05. B: effect of 12 wk of endurance training on plasma glucose rate of disappearance (Rd) in postmenopausal women. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. *Significantly different from resting conditions at P < 0.05. †Significantly different from PRE at P < 0.05. §Significantly different from ABT at P < 0.05. C: effect of 12 wk of endurance training on plasma glucose metabolic clearance rate (MCR) in postmenopausal women. Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. *Significantly different from resting conditions at P < 0.05. †Significantly different from PRE at P < 0.05. §Significantly different from ABT at P < 0.05.
The response of glucose Rd tracked glucose Ra with the exception that glucose Rd during PRE was not different from resting conditions (P > 0.05; Fig. 4B). Glucose Rd during ABT was 11% lower than PRE and 24% lower than RLT. Similar to the trend of glucose Ra, there was no difference in glucose Rd between PRE and RLT.
Glucose MCR significantly increased from rest to exercise during all the trials (P < 0.05; Fig. 4C). After training glucose MCR during RLT was 35% higher than PRE and 50% higher than ABT (P < 0.05).
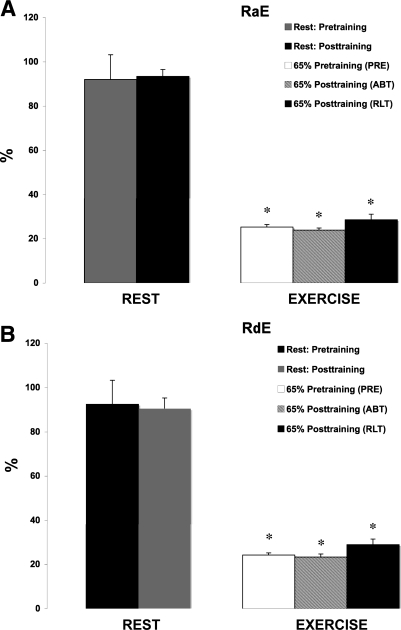
Because the energy expenditure during RLT was 16.7% greater than during ABT (6.1 ± 0.34 vs. 5.3 ± 0.24 kcal/min) we expressed the values of glucose Ra and Rd as a percentage of the total energy expenditure (RaE and RdE, respectively). There was a decrease in RaE and RdE between rest and exercise for all trials (P < 0.05), but there was no significance between the exercise trials (Fig. 5).
Fig. 5.
A: effect of 12 wk of endurance training on Ra as a function of the total energy expenditure (RaE). Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. *Significantly different from resting conditions at P < 0.05. †Significantly different from PRE at P < 0.05. §Significantly different from ABT at P < 0.05. B: effect of 12 wk of endurance training on Rd as a function of the total energy expenditure (RdE). Values are means ± SE of the last 15 min of rest and the last 30 min of exercise. *Significantly different from resting conditions at P < 0.05. †Significantly different from PRE at P < 0.05. §Significantly different from ABT at P < 0.05.
DISCUSSION
In previous studies on young women treated similarly to the postmenopausal women enrolled in the current investigation, endurance training decreased blood glucose flux by an average of 15% during continuous exercise at a given submaximal moderate intensity exercise power output (21). Now we report that endurance training without weight loss or changes in body composition simultaneously decreased blood glucose appearance and disappearance rates by 12% and increased clearance rates by 35% during exercise in postmenopausal women after 12 wk of supervised endurance training. The finding of a significant adaptation in glucose flux was accompanied by an increase in V̇o2peak and a decrease in lactate concentration at the given absolute and relative intensities. In aggregate, these findings indicate that postmenopausal women are capable of similar training-induced adaptations as seen in younger populations.
Our study that employed a longitudinal design is unique in that postmenopausal women remained diet and weight stable over the course of study. Because caloric restriction and weight loss can independently affect metabolism, we successfully isolated the effects of endurance training on glucose metabolism and other parameters of metabolic and cardiovascular fitness. However, with caution data in the present report can be contrasted with those obtained in studies using a mixed sex study design including older postmenopausal women (37) as well as results of studies in which the investigators altered diet or allowed subjects to lose body weight (27).
Glucose flux during rest.
Glucose flux in resting subjects was relatively higher than those seen in some previous studies under similar experimental conditions (2.81 mg·kg−1·min−1 in younger women vs. 3.73 mg·kg−1·min−1 in the current study; Refs. 21, 39). The nutritional state and the nature of the subject population may have accounted for the discrepancies seen in the literature. Consistent across all our studies is that we report data for rest on 3-h postprandial women, while the exercise glucose flux rates are on subjects 4 h after a standardized breakfast. Previous studies measuring glucose flux in postmenopausal women have been conducted in overnight fasted conditions (36, 37). Thus an unexpected result is that we may have uncovered a previously unrecognized effect of menopause on the ability to control glycemia after eating (42).
Previously, others (17, 31) used orally and vascularly delivered tracers to assess contributions of endogenous hepatic glucose production and exogenous (dietary) glucose to the overall glucose Ra. Prolonged (<3 h) elevations in glucose Ra and insulin following an oral glucose challenge are taken as measures of insulin resistance. Viewed in this context, the elevated rates of glucose Ra and plasma insulin seen in postmenopausal compared with younger women may be interpreted to reflect a subtle form of insulin resistance attributable to estrogen lack. Previous studies have demonstrated that estradiol causes a decrease in glucose flux (7, 35) and estradiol in combination with progesterone has shown a similar trend toward a decrease glucose flux (14). Taking these observations into account, the decrease in 17β-estradiol and progesterone at menopause may be a contributing factor to the elevations in resting glucose flux rates and insulin levels we observed in 3-h postprandial women. These findings indicate that in apparently healthy postmenopausal women, the static measurements of glucose and insulin concentrations may be insufficient to reveal the true extent of the metabolic disturbances that occur at menopause and underlines the importance of using dynamic measurements of glucose metabolism.
Glucose flux during exercise.
Our finding of increased blood glucose flux rates in postmenopausal women during exercise compared with rest, but a training-induced decrease glucose flux at the same absolute, but not relative intensity after endurance training is consistent in direction with the results of other studies on young men (3, 8, 10, 20, 28, 30, 33), young women (8, 21), and elderly men and women (37).
Endurance training of postmenopausal women resulted in a profound increase in glucose MCR during exercise; abundant data exist to explain such a training effect on glucose clearance. Endurance training has been shown to significantly increase muscle GLUT-4 content (13, 25) and hexokinase activity, decrease intramuscular glucose-6-phosphate concentration (28), and improve insulin action (12) during exercise. Although we did not directly measure muscle net glucose uptake, previous studies on young men have demonstrated that endurance training results in a decrease in the net working muscle glucose uptake (3, 34). Furthermore, in a previous report we showed that the training-induced decrease in muscle glucose uptake at a given power output explains the decrease in whole body glucose uptake (3).
The present finding of a 35% increase in the glucose clearance rate during the same relative intensity compared with pretraining contrasts with the % increase seen previously in younger women undergoing a similar training and trial protocol (21). Furthermore, the 50% increase in glucose MCR during RLT compared with ABT differs from the 31% increase seen previously in younger women (21). The relatively greater effect of training on glucose clearance in postmenopausal compared with younger women during RLT needs to be understood from standpoints of the MCR computation (=Rd/[glucose]). As noted above, for a given exercise intensity, training decreased glucose Rd in both young and postmenopausal women, but the decrease was greater in younger women. However, the MCR contrast between cohorts was affected most by differences in blood glucose response. In young women there were no differences in the glucose concentration between rest and exercise or among exercise trials (21). However, while glucose concentrations were stable in postmenopausal women during rest and exercise, compared with rest blood glucose concentration fell during exercise, especially during RLT. Hence, the apparently greater rise in glucose MCR in postmenopausal compared with younger women may be compensatory for a lesser ability to match glucose supply and demand during times of metabolic stress.
Endocrine training response.
Responses of insulin and glucoregulatory hormones in postmenopausal women to exercise and exercise training were largely predictable from studies on younger men and women (11, 15, 21, 22, 30, 33, 40), which is, insulin fell during exercise, while counter-regulatory hormones rose or tended to rise (Table 1). As well, training tended to lessen the extent of counter-regulatory responses during ABT. However, the dampened responses of glucoregulatory hormones during ABT was significant for glucagon and epinephrine, but not other hormones in postmenopausal women. At present we have no explanation for the apparent lack of a training effect on glucoregulatory hormones in postmenopausal women (Table 1). For example, although epinephrine levels were lower after training during the ABT trial, a higher insulin concentration was not observed. The lack of response of insulin to exercise after training may be attributed to an age-related decreased sensitivity of β-cells to the inhibitory effects of epinephrine on insulin secretion (9).
Our finding of unaltered resting insulin concentrations in resting subjects after endurance training differs from the results of some studies (19, 29). One possible explanation for this finding is that a larger decrease in fat mass may have been needed to cause a significant decline in the resting insulin concentrations in these subjects. For example, a study found that 6 mo of intense endurance exercise in older men (61–82 yr) resulted in a significant decrease in body weight, body fat percentage, fasting insulin levels, and an improvement in insulin sensitivity (29). Furthermore, the insulin levels at the start of the intervention may also be a factor. Frank et al. (19) found that a 12-mo moderate-intensity training intervention in overweight/obese, sedentary postmenopausal women (60.7 ± 6.7 yr) resulted in a significant decrease in the fasting insulin concentration. However, in that study the postmenopausal women lost weight and had higher initial fasting insulin concentrations (18.4 μU/ml) compared with the postmenopausal women in our study (10 μU/ml). Because the postmenopausal women in our study already had normal insulin levels at the beginning of the training intervention it may have made it more difficult to induce a decrease in the insulin concentrations and may explain why we did not see an improvement after the training intervention.
Training-induced alterations in lactate concentration.
Among the most striking findings in our study was that of the effect of endurance training on circulating lactate (Fig. 2). Lactate is considered to be the most important gluconeogenic precursor, and therefore the reduction in gluconeogenic precursor supply could contribute to a decrease in hepatic gluconeogenesis and a reduction in glucose Ra. Because we did not use a lactate tracer, at present our ability to interpret the finding is limited. In previous studies on female rats, Donovan and Brooks (16) found that training lowered lactate during exercise mainly by increasing clearance. Similarly, in studies on young men following very similar exercise testing and training protocols as employed in the present investigation, Bergman et al. (2) also found that training had a major effect on decreasing circulating lactate by increasing lactate clearance during exercise. Furthermore, they were able to attribute part of the decrease in circulating lactate to increased GNG during exercise (4).
SUMMARY AND CONCLUSIONS
The main findings of this study were that, in important respects, postmenopausal women adapt to endurance training much like younger women and men. As estimated from tracer-measured glucose rate of appearance, hepatic glucose production rises during exercise and a stable, albeit slightly lesser, glucose concentration can be maintained during 60 min of moderate intensity exercise. As in younger individuals after training, during exercise of a given moderate intensity (ABT in this experiment) glucose disposal decreases as does overall carbohydrate oxidation rate. Again as seen previously in younger individuals, insulin falls during exercise while counter-regulatory hormone levels rise; and, to a limited extent, changes in glucoregulatory hormone levels are dampened after training during ABT. Compared with rates in younger women, 3-h resting postprandial glucose flux is higher in postmenopausal women, perhaps suggesting nascent gasteroparesis and postprandial insulin resistance. Still, 12 wk of supervised endurance training resulted in a similar decrease in glucose flux as seen previously in younger women during a given exercise task. Given lower blood glucose, but similar insulin levels and glucose disposal rates in exercising young and postmenopausal women, our results indicate high capacities for glucose clearance in postmenopausal women during exercise after endurance training. Results of this study indicate that despite the changes in the hormonal milieu and metabolic changes that occur at menopause, postmenopausal women have similar training adaptations as that seen in younger populations. Furthermore, subclinical signs of developing insulin resistance are not apparent during exercise in endurance-trained postmenopausal women.
GRANTS
This work was supported by National Institutes of Health Grant R01-AR-42906 to G. A. Brooks.
Acknowledgments
We thank the subjects for their participation and compliance with training and experimental procedures and for their good cheer. They are truly amazing and accomplished people. We also thank T. Mau, P. Nguyen, T. Nguyen, M. Patella, E. Mayeda, B. Martinelli, and N. Wortham for their technical support and assistance and C. Chang, S. Dixit, A. Luke, and H. Masket for providing medical coverage during exercise stress testing.
REFERENCES
- 1.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription (7th ed.). New York: Lippincott Williams & Wilkins, 2006.
- 2.Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol 87: 1684–1696, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bergman BC, Butterfield GE, Wolfel EE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Muscle net glucose uptake and glucose kinetics after endurance training in men. Am J Physiol Endocrinol Metab 277: E81–E92, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 278: E244–E251, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept, brief review. J Appl Physiol 76: 2253–2261, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Brooks GA, Butte NF, Rand WM, Flatt JP, Caballero B. Chronicle of the Institute of Medicine physical activity recommendation: how a physical activity recommendation came to be among dietary recommendations. Am J Clin Nutr 79: 921S–930S, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Carter S, McKenzie S, Mourtzakis M, Mahoney DJ, Tarnopolsky MA. Short-term 17β-estradiol decreases glucose Ra but not whole body metabolism during endurance exercise. J Appl Physiol 90: 139–146, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 280: E898–E907, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab 284: E7–E12, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol 68: 990–996, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Coggan AR, Swanson SC, Mendenhall LA, Habash DL, Kien CL. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Am J Physiol Endocrinol Metab 268: E375–E383, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Dela F, Mikines KJ, Von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol Endocrinol Metab 263: E1134–E1143, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Dela F, Handerg A, Mikines KJ, Vinten J, Galbo H. GLUT 4 and insulin receptor binding and kinase activity in trained human muscle. J Physiol 469: 615–624, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'eon TM, Sharoff C, Chipkin SR, Grow D, Ruby BC, Braun B. Regulation of exercise carbohydrate metabolism by estrogen and progesterone in women. Am J Physiol Endocrinol Metab 283: E1046–E1055, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Deuster PA, Chrousos GP, Luger A, DeBolt JE, Bernier LL, Trostmann UH, Kyle SB, Montgomery LC, Loriaux DL. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism 38: 141–148, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol Endocrinol Metab 244: E83–E92, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E, Bjorkman O, Reichard GA Jr, Pilo A, Olsson M, Wahren J, DeFronzo RA. The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes 34: 580–588, 1985. [DOI] [PubMed] [Google Scholar]
- 18.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in V̇o2max. J Appl Physiol 65: 1147–1151, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, Irwin ML, Rudolph RE, Rajan KB, Stanczyk F, Bowen D, Weigle DS, Potter JD, McTiernan A. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res 13: 615–625, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Friedlander AL, Casazza GA, Horning MA, Huie MJ, Brooks GA. Training-induced alterations of glucose flux in men. J Appl Physiol 82: 1360–1369, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini MF, Trimmer JK, Brooks GA. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J Appl Physiol 85: 1175–1186, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Gyntelberg F, Rennie MJ, Hickson RC, Holloszy JO. Effect of training on the response of plasma glucagon to exercise. J Appl Physiol 43: 302–305, 1977. [DOI] [PubMed] [Google Scholar]
- 23.Henderson GC, Fattor JA, Horning MA, Faghihnia N, Johnson ML, Luke-Zeitoun M, Brooks GA. Glucoregulation is more precise in women than in men during postexercise recovery. Am J Clin Nutr 87: 1686–1694, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Horning MA, Friedlander AL, Casazza GA, Huie MA, Brooks GA. Arterial vs. “arterialized” sampling sites do not change isotopic enrichment using [6,6-d-glucose] and [1,1,2,3,3-d-glycerol] (Abstract). FASEB J 12: A854, 1998. [Google Scholar]
- 25.Houmard JA, Egan PC, Neufer PD, Friedman JE, Wheeler WS, Israel RG, Dohm GL. Elevated skeletal muscle glucose transporter levels in exercise-trained middle-aged men. Am J Physiol Endocrinol Metab 261: E437–E443, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: National Academies Press, 2002. [DOI] [PubMed]
- 27.Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, Yukawa M, Aiello E, Potter JD, McTiernan A. Effect of exercise on total and intra-abdominal body fat in postmenopausal women. A randomized controlled trial. JAMA 289: 323–330, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Jansson E, Kaijser L. Substrate utilization and enzymes in skeletal muscle of extremely endurance-trained men. J Appl Physiol 62: 999–1005, 1987. [DOI] [PubMed] [Google Scholar]
- 29.Kahn SE, Larson VG, Beard JC, Cain KC, Felingham GW, Schwartz RS, Veith RS, Stratton JR, Cerqueira MD, Abrass IB. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol Endocrinol Metab 258: E937–E943, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall LA, Swanson SC, Habash DL, Coggan AR. Ten days of exercise training reduces glucose production and utilization during moderate-intensity exercise. Am J Physiol Endocrinol Metab 266: E136–E143, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 326: 22–29, 1992. [DOI] [PubMed] [Google Scholar]
- 32.National Cholesterol Education Program. Expert Panel Executive Summary of the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol 81: 2182–2191, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Richter EA, Jensen P, Kiens B, Kristiansen S. Sarcolemmal glucose transport and GLUT-4 translocation during exercise are diminished by endurance training. Am J Physiol Endocrinol Metab 274: E89–E95, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Ruby BC, Robergs RA, Waters DL, Burge M, Mermier C, Stolarczyk L. Effects of estradiol on substrate turnover during exercise in amenorrheic females. Med Sci Sports Exerc 29: 1160–1169, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Sial S, Coggan AR, Carroll R, Goodwin J, Klein S. Fat and carbohydrate metabolism during exercise in elderly and young subjects. Am J Physiol Endocrinol Metab 271: E983–E989, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Sial S, Coggan AR, Hickner RC, Klein S. Training-induced alterations in fat and carbohydrate metabolism during exercise in elderly subjects. Am J Physiol Endocrinol Metab 274: E785–E790, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Steele R Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959. [DOI] [PubMed] [Google Scholar]
- 39.Suh SH, Casazza GA, Horning MA, Miller BF, Brooks GA. Luteal and follicular glucose fluxes during rest and exercise in 3-h postabsorptive women. J Appl Physiol 93: 42–50, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Winder WW, Hickson RC, Hagberg JM, Ehsani AA, McLane JA. Training-induced changes in hormonal and metabolic responses to submaximal exercise. J Appl Physiol 46: 766–771, 1979. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe RR Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992.
- 42.Zarins ZA, Wallis GA, Faghihnia N, Johnson ML, Fattor JA, Horning MA, Brooks GA. Effects of endurance training on cardiorespiratory fitness and substrate partitioning in postmenopausal women. Metabolism. In press. [DOI] [PMC free article] [PubMed]