Abstract
The active-site cysteines of DsbA, the periplasmic disulfide-bond-forming enzyme of Escherichia coli, are kept oxidized by the cytoplasmic membrane protein DsbB. DsbB, in turn, is oxidized by two kinds of quinones (ubiquinone for aerobic and menaquinone for anaerobic growth) in the electron-transport chain. We describe the isolation of dsbB missense mutations that change a highly conserved arginine residue at position 48 to histidine or cysteine. In these mutants, DsbB functions reasonably well aerobically but poorly anaerobically. Consistent with this conditional phenotype, purified R48H exhibits very low activity with menaquinone and an apparent Michaelis constant (Km) for ubiquinone seven times greater than that of the wild-type DsbB, while keeping an apparent Km for DsbA similar to that of wild-type enzyme. From these results, we propose that this highly conserved arginine residue of DsbB plays an important role in the catalysis of disulfide bond formation through its role in the interaction of DsbB with quinones.
Disulfide bonds contribute to the stability and activity of many proteins exported out of the cytoplasm (1). In Escherichia coli, disulfide bond formation in exported proteins is catalyzed by DsbA, a periplasmic protein containing a thioredoxin-like fold and a redox active site, Cys30-Pro31-His32-Cys33 (2–4). DsbA donates its Cys30-Cys33 disulfide to pairs of cysteines on target proteins (5, 6), thereby becoming reduced. The cytoplasmic membrane protein DsbB is responsible for the regeneration of oxidized DsbA (7–9). DsbB, in turn, is oxidized by components of the membrane electron-transport system (10, 11). Results suggest that in eukaryotes, PDI and EroI may be the analogues of bacterial DsbA and DsbB (12).
We recently showed that ubiquinone and menaquinone are used directly to reoxidize DsbB (13, 14). Aerobically, ubiquinone acts to reoxidize DsbB. The reduced ubiquinone then is reoxidized by terminal oxidases such as cytochrome bd and bo oxidases that then transfer electrons to oxygen (15). Anaerobically, menaquinone—which becomes the predominant quinone—replaces the function of ubiquinone in reoxidizing DsbB. Electrons are passed from the reduced menaquinone to final electron acceptors other than oxygen. This remarkable switching ability of DsbB allows the enzyme to work under both aerobic and anaerobic conditions (13).
The DsbB protein has four transmembrane segments and two periplasmic domains (16). Each periplasmic domain contains one pair of essential cysteines: Cys41 and Cys44 in the N-terminal periplasmic domain and Cys104 and Cys130 in the C-terminal periplasmic domain (16). Each pair of essential cysteines is disulfide-bonded in vivo. The analysis of mutants in these cysteines has contributed to our understanding of the function of these residues. The Cys104-Cys130 disulfide bond is thought to be the disulfide donor to DsbA (17, 18), whereas the Cys41-Cys44 disulfide bond is hypothesized to donate its disulfide bond to the Cys104 and Cys130 residues (10). Finally, the Cys41-X-X-Cys44 active site is maintained in the oxidized form, perhaps, by the membrane electron-transport system (10).
Many questions remain about the mechanism of DsbB action including how it interacts with DsbA, how electrons are transferred from the essential cysteines in the C-terminal periplasmic domain of DsbB to those of the N terminus, how and where quinones interact with DsbB, and how these different intra- and intermolecular reactions are coordinated.
One approach to these questions is to isolate and characterize mutations that retard a specific step of the enzyme reaction. Here, we report the isolation of mutants that have alterations of the Arg48 residue of DsbB. They are the first dsbB mutations in which amino acids other than the essential cysteines are altered. From the results obtained in vivo and in vitro, we propose that this amino acid residue is important for the catalysis of disulfide bond formation because of its role in the process of transfer of electrons from DsbB to quinones.
Materials and Methods
Bacterial Strains and Growth Conditions.
Strains are listed in Table 1. Cultures were grown at 30°C in NZ medium (19) or M63 minimal medium (20) supplemented with the appropriate antibiotics. When needed, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added (20 μg/ml). Aerobic or semiaerobic cultures were incubated in 30-ml glass tubes (17 mm in diameter) with either 5 ml or 20 ml, respectively, of M63 medium shaken at 90 rpm in a rotary shaker placed at a 30o angle. For anaerobic growth, cultures, grown aerobically at 30°C in M63 medium overnight, were diluted 1:250 into the same medium supplemented with 0.1% casamino acids (Difco)/0.1 mM CaCl2. Anaerobic growth was carried out in sealed jars (GasPak Anaerobic System; BBL Microbiology Systems) at 30°C without shaking for 12 h.
Table 1.
Strains and plasmids
| Strains and plasmids | Relevant genetic markers | Ref. |
|---|---|---|
| Strains | ||
| HK205 | RI89 fadR∷Tn10 | This work |
| HK207 | HK205 dsbB C41Y | This work |
| HK209 | HK205 dsbB R48C | This work |
| HK211 | HK205 dsbB R48H | This work |
| HK215 | HPT130 dsbB C41Y | This work |
| HK217 | HPT130 dsbB R48C | This work |
| HK219 | HPT130 dsbB R48H | This work |
| HK227 | HK205 dsbB∷Kan | This work |
| HK229 | HK205 dsbA∷Kan | This work |
| HPT57 | MC1000 phoA+phoR leu+ λ102(malF-lacZ 102, Ampr) | 20 |
| HPT130 | HPT57 fadR∷Tn10 | 20 |
| RI89 | MC1000 phoA+phoR−leu+ | 20 |
| Plasmids | ||
| placIqTet | laclq, Tetr | * |
| pHK503 | dsbB C8A, R48H, C48V, Ampr | This work |
| pHP18 | dsbA C33Y, Spcr | * |
Laboratory collection.
Mutagenesis and Screening of Mutants.
Mutagenesis of strain HPT57 was carried out by using either UV light (20) or N-methyl-N′-nitro-N-nitrosoguanidine (21) as mutagens. After the mutagenesis, cells were plated on M63 minimal plates supplemented with maltose and X-Gal, grown for two days at 30°C, and then kept at 4°C for several more days. Only faint blue colonies were collected. Linkage of mutations to dsbB was tested by P1 transduction as described (20). Cell lysates of these mutants were subjected to Western blotting, using anti-DsbB antibody (see below), to exclude mutants that produced DsbB less than one-tenth that of a wild-type strain. The dsbB gene of each mutant was amplified with two primers, DSBH1 (5′-TATTTTTGCTGCCTCCTGGTGG-3′) and DSBH2 (5′-CAACAATGGCAGATGAAGCGAG-3′), and subjected to sequence analysis.
Strain and Plasmid Constructions.
Bacterial strains were constructed by using P1 transduction. To construct a DsbB R48H overexpression plasmid, pHK503, a R48H substitution mutation was introduced into the dsbB gene of pWM76 (13) by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) with mutagenic primers DSBH7 (5′-GTGCTCTGTATTTATGAACACGTCGCGTTATTCGGCGTT-3′) and DSBH8 (5′-AACGCCGAATAACGCGACGTGTTCATAAATACAGAGCAC-3′). The entire sequence of the protein-coding region was verified. DsbB R48H, expressed from this plasmid, contains, on its C terminus, a six-residue histidine tag that allows its easy purification on a Ni-column (22).
Production and Purification of Antibodies to DsbB.
We prepared two kinds of anti-DsbB antibody: one raised against a synthetic peptide and one raised against purified DsbB. To prepare the peptide antibody, a synthetic peptide, with amino acid sequence corresponding to the C-terminal 14 aa of DsbB with cysteine at the N terminus, first was coupled with keyhole limpet hemocyanin and then injected into a rabbit. The whole-protein antibody was prepared by immunizing a rabbit with highly purified DsbB. Peptide synthesis, modification, and immunization were performed by Alpha Diagnostics (San Antonio, TX). Both sera were purified by affinity binding to poly(vinylidene difluoride) membrane-immobilized DsbB. We used the peptide antibody in routine analysis because nonspecific binding in Western blot, in general, was weaker than with the whole-protein antibody. We used the latter antibody only for detection of DsbA–DsbB hybrid, because a nonspecific band, recognized by the former one, prevented detection of this hybrid.
Determination of the in Vivo Redox State of DsbA, DsbB, and β-Lactamase.
The redox state of proteins was visualized by alkylating the free cysteine residues of proteins with 4-acetamido-4′-maleimidylstilbene-2, 2′-disulfonic acid (AMS; Molecular Probes) as described (10). Alkylated proteins were separated by 12% SDS/PAGE without using any reducing agent and then were blotted to nitrocellulose (for Bla and DsbA) or poly(vinylidene difluoride) (for DsbB) membranes. Washing, incubation, and detection of the blot followed the ECL Western blotting kit protocol (Amersham Pharmacia Biotech). Anti-β-lactamase (5-Prime → 3-Prime), anti-DsbA (8), anti-DsbB (raised against the synthetic peptide), and anti-DsbB (raised against the purified DsbB) antibodies were used at 1:4,000, 1:3,000, 1:1,000, and 1:500, respectively.
Production and Purification of DsbB R48H.
E. coli DH5 (α) carrying both placIqtet and pHK503 were grown in NZ medium. Expression of DsbB R48H was induced by the addition of isopropyl-β-thiogalactopyranoside to 10 μM at A600 = 0.5 and growth was continued for 4 h. The membrane fraction was purified and solubilized with 1% n-dodecyl-β-d-maltoside (DM). DsbB R48H then was bound to a Ni-nitrilotriacetic acid column (Qiagen, Chatsworth, CA) equilibrated with 50 mM sodium phosphate/300 mM NaCl/0.02% DM, pH 8.0. The column was washed with 50 mM sodium phosphate (pH 8.0)/300 mM NaCl/0.06 mg/ml lipids (E. coli total lipid extract; Avanti Polar Lipids)/0.02% DM, and then again with the same buffer with 50 mM imidazole. The lipid addition was required to prevent the aggregation of this mutant protein. DsbB R48H was eluted with a linear gradient from 50 mM to 0.5 M imidazole. Peak fractions were pooled and loaded to a hydroxyapatite column (Bio-Rad) equilibrated with 50 mM sodium phosphate (pH 6.2)/100 mM NaCl/0.1 mg/ml lipids/0.1% DM. The column was washed with the same buffer and then with a linear gradient from the same buffer to 500 mM sodium phosphate (pH 6.2)/0.1% DM/0.1 mg/ml lipids. DsbB R48H then was eluted with 750 mM sodium phosphate (pH 6.2)/0.1% DM/0.1 mg/ml lipids. This mutant protein was further purified by using, again, the Ni-nitrilotriacetic acid column. The buffer of the sample was then changed to 10 mM Hepes (pH 7.5)/300 mM NaCl using PD-10 Sephadex columns (Amersham Pharmacia Biotech). DsbB R48H was stored at −70°C. DsbB R48H obtained was >95% pure as judged by Coomassie-stained gels. The protein concentration was determined with Protein Assay Kit (Sigma). The final preparation was devoid of any oxidases that interfere with DsbB assay, because DsbB R48H showed no detectable activity to oxidize DsbA when assayed in the absence of quinones or terminal oxidases like cytochrome bo oxidase.
Activity Assays.
Reduced DsbA looses its fluorescence as it is oxidized. Unless otherwise stated, we followed DsbB activity by measuring this fluorescence decrease with fluorescence spectroscopy at 30°C (13). In later trials, we found that we can follow DsbB activity more easily by measuring reduction of ubiquinone with a spectrophotometer (14), because the reduction of ubiquinone causes a strong decrease in its absorbance at 275 nm. The assay was performed at 30°C in a reaction mixture containing 50 mM sodium phosphate (pH 6.0), 300 mM NaCl, 0.5 mM EDTA, 0.1% DM, ubiquinone-1 (coenzyme Q1; Sigma), DsbB, and the reduced form of DsbA that was prepared as described (13). The rates (nM ubiquinone-1 per nM DsbB per sec) were derived from the initial linear absorbance decrease by using an extinction coefficient of 12.25 mM−.
Results
New dsbB Mutants.
We have previously isolated dsbA and dsbB mutations by using a chromosomally encoded MalF-LacZ fusion that expresses β-galactosidase activity when there is a defect in disulfide bond formation (5). However, because selection for Lac+ colonies with this fusion strain yielded only dsbA or dsbB mutations with strong defects (nonsense or essential cysteine mutants) (8), we decided to use a screen that would allow us to detect mutant colonies with weaker defects (20). On X-Gal plates, such mutants should show a paler blue color than null mutants do. Employing this strategy, we isolated 169 independent mutants linked to dsbB after mutagenesis of strain HTP57 with either UV light or N-methyl-N′-nitro-N-nitrosoguanidine.
Most of the dsbB mutations resulted in significantly reduced amounts of DsbB protein (data not shown). However, five produced amounts of DsbB comparable to the wild type. Sequencing of the five mutant dsbB genes revealed two different mutations altering the same amino acid residue of DsbB: R48C (two isolates) and R48H (three isolates).
This arginine residue is located close to the Cys41-X-X-Cys44 motif and is predicted to be at the periplasmic end of the putative second transmembrane segment of DsbB (Fig. 1A). Similarity searches against the databases maintained at the National Center for Biotechnology Information revealed that all 20 DsbB homologues contain this arginine. It is the only amino acid residue, besides the essential cysteines, that is invariant (Fig. 1B shows nine homologues).
Figure 1.
(A) The position of mutations on a DsbB topological model (16). The positions of the four essential cysteines and the two missense mutations are indicated by bold and solid circles, respectively. (B) Sequence alignments of DsbB homologues. The source organisms are: Ec, E. coli; Sf, Shigella flexneri; Va, Vibrio alginolyticus; Hi, Hemophilus influenzae; Ea, Enterobacter aminigenus; Bc, Burkholderia cepacia; Pa, Pseudomonas aeruginosa; Rp, Rickettsia prowazekii; Cj, Campylobacter jejuni; and Bs, Bacillus subtilis. Black highlighting, completely conserved amino acid residue; boldface, amino acid residues identical in more than 60% but less than 100% of the sequences compared; ∗, the position of Arg48.
Center Blue Phenotype of the Mutants.
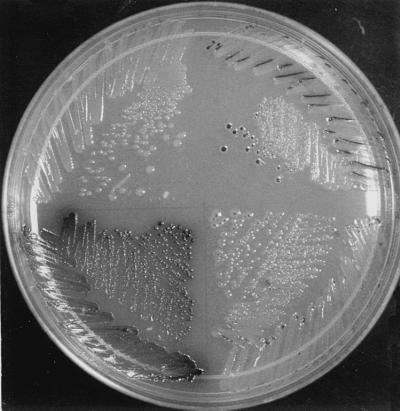
We moved these mutations to other strain backgrounds by P1 transduction. We noticed that the five R48 mutants share a striking feature distinguishing them from the other 164 faint blue mutants. Blue color on X-Gal plates was observed only in the center of large colonies or in cells growing in cuts in the surface of the agar medium inadvertently made by the edge of the toothpicks used for streaking in these mutants (as very small dots in HK217) (Fig. 2).
Figure 2.
Different patterns of β-galactosidase expression in dsbB mutants carrying malF-lacZ102 fusion. Cells of HK219 (dsbB R48H, Upper Right), HK217 (dsbB R48C, Lower Right), HK215 (dsbB C41Y, Lower Left), and HPT130 (wild type, Upper Left), were streaked on a minimal maltose X-Gal plate and incubated at 30°C for 5 days. Cells having defects in disulfide bond formation are stained with blue color (shown as black on this photograph).
Disulfide Bond Formation Is Defective in the Mutants Under Anaerobic but Not Aerobic Conditions.
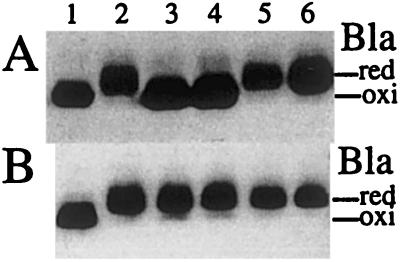
Because both the centers of E. coli-growing colonies (23) and the cuts in the agar likely represent low-oxygen conditions, we suspected that the observed defect in disulfide bond formation occurs under oxygen limitation in these mutants. To test this possibility, the mutants were cultured anaerobically with GasPak and the effectiveness of disulfide bond formation was assessed by examining the ratio of oxidized to reduced forms of β-lactamase (24). To separate both forms of this protein by SDS/PAGE, cellular proteins were alkylated with AMS, which reacts only with free thiols. This modification retards the mobility of the reduced form of proteins on gels (10). Although β-lactamase produced from the wild-type strain was mostly oxidized, whether it was grown aerobically or anaerobically, that of a dsbB null strain was reduced, confirming previous results (13) that dsbB is responsible for disulfide bond formation under both aerobic and anaerobic conditions (Fig. 3, lanes 1 and 5). When grown aerobically, the DsbB activity of the dsbB R48C and R48H mutants allowed oxidation of β-lactamase (Fig. 3A, lanes 3 and 4). However, the same mutants showed strong defects in β-lactamase oxidation under anaerobic growth conditions (Fig. 3B, lanes 3 and 4). These results indicate that the disulfide-bond-formation pathway is at least reasonably functional aerobically, but severely defective anaerobically in these mutants.
Figure 3.
Disulfide bond formation is impaired in the dsbB R48C and R48H mutants under anaerobic conditions but not under aerobic conditions. Strains HK205 (wild type; lane 1), HK207 (dsbB C41Y; lane 2), HK209 (dsbB R48C; lane 3), HK211 (dsbB R48H; lane 4), HK227 (dsbB∷Kan; lane 5), and HK229 (dsbA∷Kan; lane 6) were transformed with pUC18 carrying bla, and were grown aerobically (A) or anaerobically (B) at 30°C. Portions of exponentially growing cultures were harvested and alkylated with AMS. The AMS-modified proteins were separated by native SDS/PAGE and subjected to Western blotting with anti-β-lactamase antibody to detect oxidized (oxi) and reduced (red) β-lactamase.
DsbB R48C and R48H Mutant Proteins Are in Their Oxidized Form Aerobically, but Are Reduced Under Low Oxygen Conditions.
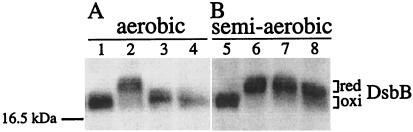
To further confirm the effect of oxygen limitation on the function of the mutant DsbB proteins, we determined the oxidation state of both DsbA and DsbB in mutant and wild-type backgrounds. We limited the oxygen supply of cells in these experiments by growing them in the same manner as an aerobic culture except that a four-times greater volume of M63 medium was put in our standard culture tube. We call this a semiaerobic culture. In the wild-type cells grown aerobically (Fig. 4, lane 1) or semiaerobically (Fig. 4, lane 5), DsbB antibody detected a band at an apparent molecular mass of 17 kDa representing DsbB with its four essential cysteines joined in two disulfide bonds (10). Under aerobic growth conditions, both dsbB R48C and R48H mutants also accumulated the oxidized form of DsbB (Fig. 4, lanes 3 and 4). However, in the semiaerobic cultures, these mutant proteins were reduced (Fig. 4, lanes 7 and 8). We observed the same effect when both mutants were grown under strongly anaerobic conditions using GasPak (see below).
Figure 4.
Effect of oxygen limitation on the redox state of mutant DsbB. Strains HK205 (wild type; lanes 1 and 5), HK207 (dsbB C41Y; lanes 2 and 6), HK209 (dsbB R48C; lanes 3 and 7), and HK211 (dsbB R48H; lanes 4 and 8) were grown in a test tube aerobically in 5 ml (A) or semiaerobically in 20 ml (B) of M63 minimal medium. Proteins of exponentially growing cells were AMS-alkylated, separated by native SDS/PAGE, and subjected to Western blotting with anti-DsbB antibody to detect the oxidized (oxi) and the reduced (red) forms of DsbB. The position of a molecular mass standard is indicated on the left. Note that wild-type DsbB contains two additional nonessential cysteine residues that remain in the sulfhydryl state and are subject to alkylation with AMS. As a reference, the previously isolated dsbB C41Y mutant, which renders DsbB fully reduced, was included in lanes 2 and 6. The increase in the apparent molecular mass, as compared with the wild-type strain, agrees well with the difference in the number of free cysteine residues; 2 for wild-type and 5 for the C41Y mutant enzyme. Note also the difference in the migration of DsbBs. R48C has one additional cysteine over the wild type and R48H, thus it migrates slower than they do.
To determine the effect of oxygen limitation on the redox states of DsbA, AMS-alkylated lysates of cells grown anaerobically were probed with anti-DsbA antibody. In normal cells DsbA is oxidized and the protein migrates as a band at 21 kDa (Fig. 5, lane 1). However, in the absence of functional dsbB, DsbA is reduced and subject to alkylation by AMS (Fig. 5, lanes 2 and 5). Aerobically, the dsbB R48C and R48H mutant cells accumulated about one-third of DsbA in the oxidized form (Fig. 5, lanes 3 and 4), apparently a sufficient amount for efficient disulfide bond formation in vivo (Fig. 3). When these mutants were grown semiaerobically or anaerobically, they accumulated all of the DsbA in the reduced form (data not shown). These results clearly show that the functioning of DsbB in these mutants is highly sensitive to oxygen limitation.
Figure 5.
Accumulation of a mixed disulfide between DsbA and DsbB in dsbB R48 mutants under aerobic conditions. Proteins of cells growing exponentially in M63 medium were AMS-alkylated, separated by native SDS/PAGE, and subjected to Western blotting with anti-DsbA serum (lanes 1–8) or purified DsbB antibody (lanes 9–16). HK205 (wild type; lanes 1, 7, 9, and 15), HK207 (dsbB C41Y; lanes 2 and 10), HK209 (dsbB R48C; lanes 3 and 11), HK211 (dsbB R48H; lanes 4 and 12), HK227 (dsbB∷Kan; lanes 5, 8, 13, and 16), and HK229 (dsbA∷Kan; lanes 6 and 14). Cells contain plasmid pHP18 carrying dsbA C33Y in lanes 7, 8, 15, and 16. ∗, DsbA–DsbB complex; oxi, the oxidized form of DsbA; red, the reduced form of DsbA; arrowhead, DsbA C33Y; arrows, a nonspecific crossreacting band. Positions of molecular mass standards are indicated on the left. The migration of mixed-disulfide complexes differs slightly reflecting differences in the number of free cysteine residues in each complex: DsbA–DsbBR48C (+1), DsbA–DsbBR48H (0), DsbAC33Y–DsbB (−1).
Enhanced Accumulation of a DsbA–DsbB Complex in dsbB R48C and R48H Mutants Grown Aerobically.
The use of antibody to DsbA revealed a band with an apparent molecular mass of about 37 kDa in the AMS-treated lysate of both dsbB R48C and R48H mutants grown aerobically (Fig. 5, lanes 3 and 4, indicated by asterisks). This band in the R48C mutant migrates slightly slower than that in the R48H mutant by about 0.5 kDa. These two bands also were recognized by anti-DsbB antibody (Fig. 5, lanes 11 and 12). They disappeared when samples were treated with reducing agent before electrophoresis (data not shown). Thus, they represent a mixed disulfide between DsbA and DsbB. For comparison, a mixed disulfide formed between DsbB and mutant DsbA that lacks Cys33 is shown in Fig. 5, lanes 7 and 15. When the dsbB R48C and R48H mutants were grown semiaerobically or anaerobically, this DsbA–DsbB complex was not detected (data not shown). Instead, these cells accumulate DsbA and DsbB in their reduced forms (Fig. 4 and data not shown). Thus, these mutants accumulate DsbA–DsbB complex only aerobically where disulfide bond formation in these cells is still active. DsbB-catalyzed DsbA oxidation will proceed via the DsbA–DsbB complex, because hybrid formation is considered to be essential for the disulfide-transfer reaction (17, 25). The enhanced accumulation of the disulfide-bonded hybrid in these mutants suggests that the mutation has resulted in a defect in a step required for the resolution of the mixed disulfide.
R48H Mutant Exhibits Very Low Activity with Menaquinone in Vitro.
To analyze the function of this arginine residue biochemically, the R48H mutant protein was purified and subjected to DsbB activity assays by using a fluorescence method (13). Under the assay conditions, the R48H mutant protein used decyl-ubiquinone, a ubiquinone analogue, to oxidize reduced DsbA at a rate approximately one-eighth of that of wild-type enzyme (Table 2). The same mutant showed almost no activity with menadione, a menaquinone analogue, less than 0.4% of the wild-type enzyme with decyl-ubiquinone. Thus, these in vitro results are consistent with the in vivo studies showing poor anaerobic function of DsbB R48H.
Table 2.
DsbB activities of R48H with different quinones
| Quinones | DsbB activity, nM
DsbA per nM DsbB per sec
|
|
|---|---|---|
| Wild type | R48H | |
| Decyl-ubiquinone | 6.2 | 0.8 |
| Menadione | 1.1 | <0.02 |
Rates of DsbA oxidation in the presence of DsbB (1 nM) for wild type, and of DsbB (12 nM) for R48H. DsbA was at 10 μM, while the concentration of the quinones was 20 μM.
DsbB R48H Mutant Has Defects in Its Interaction with Ubiquinone but Not with Reduced DsbA.
To further investigate the role of this R48 residue of DsbB, we examined the kinetic parameters of the mutant enzyme for two substrates: ubiquinone and reduced DsbA. To determine the parameters, the concentration of one of the two substrates was fixed (either 60 μM for ubiquinone-1 or 20 μM for the reduced DsbA), and the initial velocities of ubiquinone-1 reduction were measured at 30°C with different concentrations of the other substrate. The assay results were then fitted into Michaelis–Menten equations (Fig. 6). Apparent maximum velocities for the R48H protein obtained from both experiments were similar and were about five times less than the maximal velocity obtained for the wild-type enzyme (7.8 nmol DsbA per nmol DsbB per sec; ref. 14; and M.B. and J.C.A.B., unpublished data). The apparent Km of the mutant for ubiquinone-1 was 7.1 μM, which is 7-fold higher than the value (1 μM) obtained for the wild-type enzyme (M.B. and J.C.A.B., unpublished data). In contrast, the apparent Km of the mutant for DsbA was 3.3 μM, very close to that of the wild-type enzyme (3.5 μM; ref. 14 and M.B. and J.C.A.B., unpublished data). Thus, the alteration of Arg48 significantly reduced the affinity of the enzyme for ubiquinone without affecting its apparent Km for DsbA.
Figure 6.
Determination of apparent Km values of R48H for DsbA (A) and ubiquinone-1 (B). The amount of DsbB was 6.2 nM. The concentration of ubiquinone-1 was held at 60 μM in A and that of reduced DsbA was held at 20 μM in B. Initial velocities were obtained form the decrease of the absorbance of ubiquinone-1 on reduction. Each plot shows a fit of the data obtained to hyperbolic form of the Michaelis–Menten equation. The fits yield a Km = 3.3 μM for DsbA and a kcat = 1.6 sec− in A and a Km = 7.1 μM for ubiquinone-1 and a kcat = 1.7 sec− in B.
Discussion
DsbB is unique as an enzyme in that it uses the oxidizing power of quinones to form disulfides. The novelty of this reaction in combination with the role of DsbB as an essential component of a biologically important cellular process, protein disulfide bond formation, provides significant incentives to study the structure and function of this enzyme. We show here that an in vivo hunt for mutations of dsbB allows us to specify the role of a highly conserved amino acid residue, Arg48. To isolate these mutations, we used a modification of a previous genetic approach that had yielded only mutations altering one of the four essential cysteines of DsbB. A switch from a genetic selection to a genetic screen allowed us to detect mutations with much weaker effects on DsbB activity (20).
The dsbB mutants we obtained share a striking conditional phenotype. They carry out disulfide bond formation reasonably well aerobically but are almost completely defective anaerobically, being unable to maintain DsbA in the oxidized state. These results show that the functioning of the mutant DsbB is highly sensitive to oxygen limitation. Because changes in aerobiosis greatly alter the composition and the redox states of the E. coli quinone pool (ubiquinone-8, menaquinone-8, and demethylmenaquinone-8) (26, 27), the results suggest that the function of these DsbB mutants depends on the content and redox state of the quinone species available.
We showed that purified R48H exhibits an apparent Km for ubiquinone-1 seven times greater than that of the wild-type enzyme and almost no activity with menaquinone. In contrast to the effect of R48H on the interactions with quinones, the apparent Km of the protein for the reduced form of DsbA was comparable to that of the wild-type enzyme. Thus, the R48 residue seems to be specifically involved in the interaction with quinones but not with DsbA. We were unable to measure the precise apparent Km for menadione, because the activity of the mutant enzyme was too low to measure it under our assay conditions. This low activity is likely attributable to very low affinity of the mutant enzyme for menadione, which would explain the in vivo properties of R48H. The sensitivity of the mutant DsbB to oxygen limitation could be a result of the decreased ratio of oxidized to reduced forms of quinones (27) and the shift to menaquinone-8 as the predominant quinone species (26). Thus, all results are consistent with our proposal that Arg48 is an important component of the quinone binding site.
It has been suggested that the oxidization of DsbB by the respiratory chain (10, 13) takes place through the Cys41-X-X-Cys44 motif of DsbB (10). E. coli quinones, which contain long isoprenyl chains, are lipid-soluble molecules. Arg48 is located at the periplasmic end of the putative second transmembrane domain of DsbB and close to the active site, Cys41-X-X-Cys44 (Fig. 1), well positioned to mediate the interaction of quinones with this site.
The apparent maximal velocities obtained for R48H were about one-fifth that of the wild-type enzyme, suggesting that Arg48 may have some catalytic role. Alternatively, Arg48 may have a structural role and its alteration may render some portion of the enzyme inactive. However, the fact that the apparent Km for DsbA is not significantly altered by the R48H mutation strongly suggests that this mutation does not induce global unfolding of DsbB.
The accumulation of a substantial amount of DsbA–DsbB complex has been reported in two cases. First, this accumulation takes place in strains in which Cys33 of DsbA has been replaced with another amino acid. We and others have interpreted this accumulation to indicate that the Cys30 residue of DsbA reacts with Cys104 of DsbB as an intermediate in the DsbA oxidation reaction, and that this intermediate cannot be resolved because of the absence of Cys33 of DsbA (17, 18, 25). Second, the hybrid DsbA–DsbB is found in strains deficient in the respiratory chain (11). In this case, DsbB was reduced before the formation of the DsbA–DsbB complex and this binary complex formation preceded the reduction of the bulk of DsbA (12). From these results, it was speculated that this hybrid forms by a reverse reaction: The Cys104 of the reduced DsbB attacked the oxidized form of DsbA to make the hybrid (10). In this paper, we report that the accumulation of substantial amounts of the DsbA–DsbB hybrid occurs when the dsbB R48C and R48H mutants are grown aerobically but not anaerobically. Although further study is necessary to establish that the DsbA–DsbB hybrid is a true intermediate in the DsbB-catalyzed DsbA oxidation reaction, two results presented here support that inference. (i) The formation of the DsbA–DsbB hybrid was observed when protein disulfide bond formation is fairly efficient in the dsbB R48C and R48H mutants grown aerobically but not anaerobically. These findings differ from previous reports in which the hybrid was detected under conditions where protein disulfide bond formation was severely defective (11, 17, 18, 25). (ii) DsbB was found in the oxidized form in cells accumulating the DsbA–DsbB hybrid. This finding apparently excludes the possibility of hybrid formation by a reverse reaction (the Cys104 of the reduced DsbB attacks the oxidized form of DsbA to form the hybrid) as speculated for the hybrid formed when the respiratory chain is deficient (10).
In wild-type cells grown aerobically, we do not detect the DsbA–DsbB hybrid unless both DsbA and DsbB are overexpressed from plasmids (17). The resolution of this hybrid is very rapid in normal cells. Why then do the dsbB R48C and R48H mutants accumulate substantial amount of the hybrid under aerobic conditions? Because the purified R48H exhibits an apparent Km for ubiquinone-1 seven times greater than that in the wild-type cells, it is possible that the formation of a complex with quinone is a rate-limiting step for the mutant enzymes in vivo. The simplest explanation for the DsbA–DsbB hybrid accumulation in such mutants, then, is that quinone binding to DsbB is required for the resolution of the DsbA–DsbB hybrid. Although this model is attractive to us, further experimentation is necessary to clearly address this important issue related to the mechanism of the enzyme reaction.
Finally, our results show the advantages of devising various in vivo genetic approaches to study such problems. We obtained mutations affecting the enzymatic reaction of DsbB by using the malF-lacZ fusion to screen for weaker dsbB mutants, by maintaining expression of dsbB in single copy on the chromosome, and by the ability to observe informative phenotypes on the screening media. This approach revealed mutations affecting a specific enzymatic step: interaction with quinones. A further hunt for mutants using the same approach may yield mutations in other amino acid residues important for the interaction of DsbB with quinones.
Acknowledgments
We thank Hajime Tokuda for helpful advice. In addition, we would like to thank the other members of both the Bardwell and the Beckwith laboratories for suggestions and discussions. This work was supported by grants from the National Institute of Health (GM57039-02 to J.C.A.B. and GM41883 to J.B.). J.C.A.B. is a Pew Scholar. J.B. is an American Cancer Society Research Professor.
Abbreviations
- X-Gal
5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside
- AMS
4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- DM
n-dodecyl-β-d-maltoside
References
- 1.Gilbert H F. J Biol Chem. 1997;272:29399–29402. doi: 10.1074/jbc.272.47.29399. [DOI] [PubMed] [Google Scholar]
- 2.Debarbieux L, Beckwith J. Cell. 1999;99:117–119. doi: 10.1016/s0092-8674(00)81642-6. [DOI] [PubMed] [Google Scholar]
- 3.Frishman D. Biochem Biophys Res Commun. 1996;219:686–689. doi: 10.1006/bbrc.1996.0295. [DOI] [PubMed] [Google Scholar]
- 4.Martin J L, Bardwell J C, Kuriyan J. Nature (London) 1993;365:464–468. doi: 10.1038/365464a0. [DOI] [PubMed] [Google Scholar]
- 5.Bardwell J C, McGovern K, Beckwith J. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 6.Zapun A, Bardwell J C, Creighton T E. Biochemistry. 1993;32:5083–5092. doi: 10.1021/bi00070a016. [DOI] [PubMed] [Google Scholar]
- 7.Kishigami S, Akiyama Y, Ito K. FEBS Lett. 1995;364:55–58. doi: 10.1016/0014-5793(95)00354-c. [DOI] [PubMed] [Google Scholar]
- 8.Bardwell J C, Lee J O, Jander G, Martin N, Belin D, Beckwith J. Proc Natl Acad Sci USA. 1993;90:1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Missiakas D, Georgopoulos C, Raina S. Proc Natl Acad Sci USA. 1993;90:7084–7088. doi: 10.1073/pnas.90.15.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi T, Ito K. EMBO J. 1999;18:1192–1198. doi: 10.1093/emboj/18.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Proc Natl Acad Sci USA. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frand A R, Kaiser C A. Mol Cell. 1999;4:469–477. doi: 10.1016/s1097-2765(00)80198-7. [DOI] [PubMed] [Google Scholar]
- 13.Bader M, Muse W, Ballou D P, Gassner C, Bardwell J C. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 14.Bader W M, Xie T, Yu C-A, Bardwell C A J. J Biol Chem. 2000;275:26082–26088. doi: 10.1074/jbc.M003850200. [DOI] [PubMed] [Google Scholar]
- 15.Puustinen A, Finel M, Haltia T, Gennis R B, Wikstrom M. Biochemistry. 1991;30:3936–3942. doi: 10.1021/bi00230a019. [DOI] [PubMed] [Google Scholar]
- 16.Jander G, Martin N L, Beckwith J. EMBO J. 1994;13:5121–5127. doi: 10.1002/j.1460-2075.1994.tb06841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilhot C, Jander G, Martin N L, Beckwith J. Proc Natl Acad Sci USA. 1995;92:9895–9899. doi: 10.1073/pnas.92.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishigami S, Ito K. Genes Cells. 1996;1:210–208. doi: 10.1046/j.1365-2443.1996.d01-233.x. [DOI] [PubMed] [Google Scholar]
- 19.Debarbieux L, Beckwith J. J Bacteriol. 2000;182:723–727. doi: 10.1128/jb.182.3.723-727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian H, Boyd D, Beckwith J. Proc Natl Acad Sci USA. 2000;97:4730–4735. doi: 10.1073/pnas.090087297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 22.Bader M, Muse W, Zander T, Bardwell J. J Biol Chem. 1998;273:10302–10307. doi: 10.1074/jbc.273.17.10302. [DOI] [PubMed] [Google Scholar]
- 23.Peters A C, Wimpenny J W, Coombs J P. J Gen Microbiol. 1987;133:1257–1263. doi: 10.1099/00221287-133-5-1257. [DOI] [PubMed] [Google Scholar]
- 24.Walker K W, Gilbert H F. J Biol Chem. 1994;269:28487–28493. [PubMed] [Google Scholar]
- 25.Kishigami S, Kanaya E, Kikuchi M, Ito K. J Biol Chem. 1995;270:17072–17074. doi: 10.1074/jbc.270.29.17072. [DOI] [PubMed] [Google Scholar]
- 26.Shestopalov A I, Bogachev A V, Murtazina R A, Viryasov M B, Skulachev V P. FEBS Lett. 1997;404:272–274. doi: 10.1016/s0014-5793(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 27.Wallace B J, Young I G. Biochim Biophys Acta. 1977;461:84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]