Abstract
Cigarette smoke (CS) induces a rapid, sustained upregulation of ceramide production in human bronchial epithelial cells, leading to increased apoptosis. Using loss-of-function and overexpression analyses, we show that neutral sphingomyelinase 2 (nSMase2) is required for CS-mediated ceramide generation and apoptosis. Glutathione (GSH), a crucial antioxidant in lung defense, blocks nSMase2 activity and thus inhibits apoptosis triggered by CS. We found that the exposure to CS, as with exposure to H2O2, results in increased nSMase2 activation leading to ceramide generation and therefore increased apoptosis. Interestingly, exposure of cells to GSH abolishes nSMase2 activation caused by CS and leads to a decrease in CS-induced apoptosis. This suggests that the effects of CS oxidants on nSMase2 are counteracted by GSH. Our data support a model where CS induces nSMase2 activation thereby increasing membrane-sphingomyelin hydrolysis to ceramide. In turn, elevated ceramide enhances airway epithelial cell death, which causes bronchial and alveolar destruction and lung injury in pulmonary diseases.
Keywords: oxidative stress, airway epithelial cells, lung injury
our previous studies have demonstrated that oxidative stress induced by H2O2 causes activation of an endogenous membrane neutral sphingomyelinase (nSMase) to stimulate ceramide accumulation and apoptosis (5, 6). Furthermore, we found that in human airway epithelial (HAE) cells, nSMase2 is the SMase that is activated by H2O2, leading to the increase in ceramide levels and apoptosis, which are quenched by GSH (14). To further investigate the physiological relevance of oxidant-induced activation of nSMase2, we focused our studies on cigarette smoke (CS).
Each puff of cigarette smoke (CS) contains ∼5,000 toxic compounds, with 1015 free radicals in the gas phase and 1018 free radicals per gram of tar, and includes potent oxidants such as hydrogen peroxide, hydroxyl anion, and organic radicals (18, 37). These toxic compounds contribute to the adverse effects of CS on lung epithelial cells, playing a key role not only in chronic obstructive pulmonary diseases (COPD) but also in other pulmonary diseases such as ARDS, asthma, and interstitial pulmonary fibrosis (29, 30, 32, 34). Moreover, as argued by Spurzem and Rennard as early as 2005 (34), smoke can also alter lung repair responses. Inhibition of repair (or enhanced apoptosis) may lead to tissue destruction that typifies emphysema, whereas abnormal repair can direct peribronchial fibrosis that causes airflow constraint in small airways. Therefore, early intervention blocking these destructive processes instigated by CS may represent better targets for treatments.
Recent studies provide a very strong case for apoptosis having a major role in lung injury in several pulmonary diseases (39). Therefore, elevated loss of cells by augmented apoptosis is expected to be involved in, or perhaps even to initiate, the overall tissue destruction normally believed responsible for lung injury (32, 34).
Extensive evidence has been presented to date describing roles of reactive oxidants in the pathogenesis of pulmonary diseases such as ARDS, COPD, and asthma. Despite differences in the site and specific characteristics of the inflammatory responses in each of these diseases, they are all characterized by an oxidant/antioxidant imbalance (18, 30). The variation in an individual's ability to regulate antioxidant defense mechanisms, such as the level of GSH in response to CS, may in part explain why only 15–20% of smokers develop COPD (33).
GSH is involved in one of the fundamental antioxidant defense mechanisms protecting against oxidant-induced lung injury. GSH is a strong nucleophile and often inactivates electrophilic reactive compounds by either direct nonenzymatic conjugation or by inflammatory and anti-inflammatory agents (30).
Although there is a clear link between reactive oxidants and epithelial injury (22), the cellular and molecular mechanisms leading to epithelial dysfunction are still poorly understood. The pathogenesis of emphysema has historically been attributed to the protease-antiprotease imbalance resulting from chronic lung inflammation (37). However, the concept of inflammation as the initiator of the cascade of events in lung destruction in diseases such as COPD may be questioned. Instead, inflammation could represent the result of a long-standing destructive process in the lung and might by itself be a source of additional injury.
Ceramide is well established as a bioactive molecule implicated in processes such as the cellular response to stress, cell growth, and apoptosis (10), and sphingomyelin hydrolysis by sphingomyelinases (SMases) has emerged as a key pathway of stress-induced ceramide generation. There are five types of SMase categorized according to their optimum pH and metal ion dependence: acid lysosomal SMase, Zn2+-dependent secreted form of acidic SMase (aSMase), neutral Mg2+-dependent SMase, neutral Mg2+-independent SMase, and alkaline SMase (21).
Several recent studies implicate sphingomyelin hydrolysis in acute lung injury (29) and pulmonary edema (4). However, multiple questions still emerge, and additional characterization of the ceramide pathway that leads to lung injury is still needed. Moreover, reports regarding septic patients and their increase in ceramide levels suggest that the ceramide pathway has a pivotal role in contributing through its proapoptotic activity to multiple organ dysfunction syndrome (3). Activation of the destructive processes of apoptosis and their mediators such as ceramide may represent potential targets for therapies.
Progress has recently evolved in the understanding of the underlying mechanisms of these destructive lung processes. Petrache et al. (29) reported that ceramide, a second messenger lipid, may be a critical mediator of endothelial and alveolar cell apoptosis in the VEGF mouse model of COPD. The VEGF blockade model was mainly manifested via endothelial cells. In addition, a recent report demonstrated that superoxide dismutase protected against apoptosis and alveolar enlargement induced by ceramide (28), and Teichgraber et al. (35) and Hamai et al. (9) have recently reported that ceramide accumulation mediates inflammation, cell death, and infection susceptibility in cystic fibrosis (CF). Therefore, extending our cellular and molecular studies to better understand the mechanism(s) involved in ceramide generation in lung injury may lead to the discovery of new targets that could play a critical role in the epithelial and alveolar injury and destruction taking place in the incurable COPD lung disease.
Given that CS is a predominant cause of COPD, animal models using smoke exposure would appear to be very useful for investigation, but it is only relatively recently that such models have been created (38). Chronically exposing animals to CS has provided some insight into strain differences among mice as well as into the disease pathogenesis (8, 19, 20). Here, our studies demonstrate for the first time that exposure to CS leads to ceramide generation and excessive apoptosis induction in airway epithelial cells through the specific activation of nSMase2, which can be quenched by pretreatment with GSH. Therefore, we suggest that nSMase2, a member of the ceramide-generating machinery, could present a novel target in the prevention of CS-induced apoptosis in lung epithelial cells, the initiation step of epithelial/alveoli injury in COPD.
MATERIALS AND METHODS
Cell culture and transfections.
Human bronchial epithelial cells (HBE1) were used in all experiments and cultured as previously described (2, 31). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2. HBE1 cells were grown in serum-free medium supplemented with insulin (5 μg/ml), transferrin (5 μg/ml), EGF (5 ng/ml), dexamethasone (0.1 μM), cholera toxin (20 ng/ml), and bovine hypothalamus extract (15 μg/ml). Transient transfections using pFLAG-nSMase2 were performed using Lipofectamine Transfection Reagent (Invitrogen) according to the manufacturer's protocol. Transfected cells were treated 48 h posttransfection with 250 μM H2O2 or 10 mM GSH. For nSMase2 silencing, HBE1 cells were transiently transfected using Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer's instructions. Treatments of nSMase2-silenced cells were carried out 24 h posttransfection with nSMase2 small interfering RNA (siRNA).
CS exposure.
The lids were removed from tissue culture dishes, and human bronchial (HBE1) cells were placed in a vacuum oven with a chamber volume of 0.45 cu ft, and the temperature was set at 37°C. A vacuum was generated (∼25 in Hg), and smoke from one cigarette (Univ. of Kentucky 2R4F) was drawn into the chamber by bleeding the chamber (a decrease of ∼5 in Hg over ∼30 s) through a short piece of tubing holding the cigarette. The chamber atmosphere was then lowered to 2.5 in Hg for the remainder of the exposure time.
H2O2 quantification.
Measurement of H2O2 generated by smoke from different doses of cigarettes was performed by the method of Thurman et al. (36). Briefly, DMEM (Invitrogen) containing 25 mM glucose and no phenol red was incubated with smoke from a cigarette. At the indicated times, 1-ml aliquots of medium were collected, and 1 ml of 50% wt/vol trichloroacetic acid was added to each aliquot. The samples were centrifuged at 500 g for 10 min, and 0.2 ml of 10 mM ferrous ammonium sulfate and 0.1 ml of 2.5 M sodium thiocyanate (both from ICN Pharmaceuticals, Costa Mesa, CA) were added to the supernatant. Absorbance of the ferrithiocyanate complex was measured using a GENios Multi-Detection Reader (Tecan, Männedorf, Switzerland) at 480 nm and compared with standard curves obtained from dilutions of a standard H2O2 solution.
Ceramide measurement.
Ceramide was quantified by the diacylglycerol (DAG) kinase assay as previously described (2). Briefly, cells were extracted with methanol:chloroform:1 N HCl (100:100:1, vol/vol/vol). The lipids in the organic phase were dried under vacuum, resuspended in 100 μl of reaction mixture containing [γ-32P]ATP, and incubated at room temperature for 1 h. The reactions were terminated by extraction of lipids with 1 ml of methanol-chloroform-1 N HCl, 170 μl of buffered saline solution, and 30 μl of 0.1 M EDTA. The lower organic phase was dried under vacuum, and the lipids were resolved by thin-layer chromatography on silica gel 60 plates (Whatman) using a solvent of chloroform-methanol-acetic acid (76:18:6, vol/vol/vol). 32P-labeled ceramide-1-phosphate was detected by a phosphoscreen and analyzed by a phosphorimager machine. All treatments for ceramide analysis were done in duplicate; each experiment was repeated two or more times.
Apoptosis assessment.
Cells were pulsed for 1 h with 250 μM H2O2 or with smoke from one cigarette. After 24 h, both nonadherent and adherent cells were collected and analyzed using BD ApoAlert kit (BD Biosciences, Palo Alto, CA) as described (12, 31). The cells that were collected were incubated in binding buffer with Annexin V (FITC-conjugated) and propidium iodide (PI) for 15 min at RT to detect for early apoptosis. The cells were analyzed by flow cytometry using the FITC signal detector (FL1) and the PI detector (FL2) in a FAC-Scan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cells negative for both annexin V and PI staining were live cells; annexin V-positive- and PI-negative-stained cells are early apoptotic cells; annexin V- and PI-positive-stained cells are necrotic and/or late apoptotic cells; and PI-positive and annexin V-negative stained cells are necrotic cells.
Sphingomyelinase assays.
The enzyme activity of nSMase was determined as described (13, 27). For determining nSMase activity, an enzyme preparation (100 μg of protein) in 20 mM Tris·HCl, pH 7.4, was mixed with [14C]sphingomyelin (10 nmol/100,000 dpm) in 0.1% Triton X-100 in 100 mM Tris·HCl, pH 7.4, containing 10 mM MgCl2, 10 mM DTT, 10 nM phosphatidylserine, and 1 mg/ml BSA. For aSMase activity, the pH was adjusted to 4.5. The incubation time was 1 h at 37°C. The reaction was terminated by the addition of 1 ml of chloroform:methanol:HCl (100:100:1) followed by 0.3 ml of 1 N HCl. After phase separation, the upper phase was removed and the radioactivity was determined by liquid scintillation counting.
IHC ceramide-staining.
Detection of ceramide by IHC was performed with anti-ceramide MAb (MID 15B4) followed by Alexa Fluor 594 goat anti-mouse antibody (red); nuclei were DAPI-stained (blue).
RESULTS
CS smoke upregulates ceramide generation in a dose- and time-dependent manner.
Since more than 90% of patients with COPD are smokers (33), we wanted to find out if ceramide is involved in this lung injury by leading to enhanced levels of apoptosis in the lung epithelium (5–7, 14, 31). Our previous studies demonstrated that ceramide levels increased in lung epithelial cells when exposed to oxidative stress in the form of H2O2. Given that we previously also demonstrated that ceramide accumulation precedes caspase-3 cleavage during apoptosis (31), we proceeded to determine whether there were any changes in apoptosis levels after CS exposures that were linked to ceramide generation.
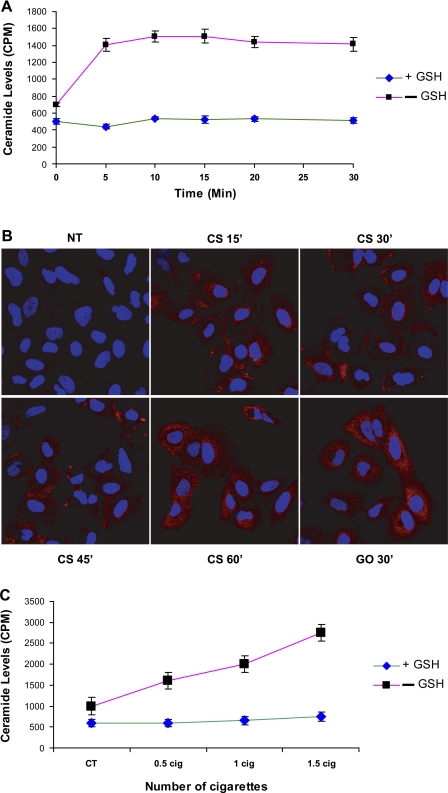
We were first interested in determining the effects of CS on ceramide generation. Using HBE1 cells (papilloma virus-immortalized human bronchial epithelial cells), we showed that exposures of these cells to CS over the indicated time points or doses result in an increase in ceramide levels in a time- (Fig. 1, A and B) and dose- (Fig. 1C) dependent manner. As we have shown before with H2O2, CS is capable of inducing significant increases in ceramide levels in exposed cells compared with nontreated cells or compared with cells treated with CS in the presence of GSH.
Fig. 1.
Ceramide generation is upregulated in HBE1 cells exposed to cigarette smoke (CS), and the increased ceramide levels are abolished in the presence of glutathione (GSH). HBE1 cells were exposed to different time points of CS (Kentucky 2R4F reference cigarette) (A and B) and different doses of CS for 30 min (C). A and C: cellular lipids were extracted and assayed for ceramide by the DAG assay. The reaction products were analyzed by TLC and quantified using a phosphor imager. B: IHC is presented with anti-ceramide antibody (red staining) as described in materials and methods.
CS and glucose oxidase generate comparable levels of H2O2 and apoptosis.
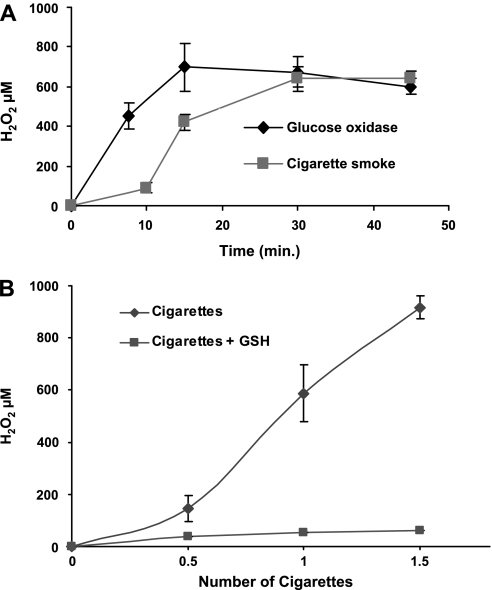
Since ceramide increase is induced by both CS and H2O2 generated by glucose oxidase (GO) (11, 14), we wanted to determine the amount of H2O2 generated by CS, compare it to H2O2 generated by GO (Fig. 2), and then also determine whether apoptosis is induced in these lung epithelial cells in a manner that is dependent on H2O2 production (Fig. 3). First, cell culture medium was exposed to smoke from one cigarette or incubated with 1 U/ml GO at 37°C for the indicated time points (Fig. 2A), and the amount of H2O2 was determined as described in materials and methods. Aliquots of medium were taken at the end of each exposure for H2O2 determination. As shown, at 30 min, both GO and CS produce ∼600–700 μM H2O2 (Fig. 2A) as determined by the absorption of the red ferrithiocyanate complex formed in the presence of peroxides (11, 36). Although the levels of H2O2 vary between experiments, the measurements have been performed numerous times with each experiment performed in triplicate, and the overriding outcome is that H2O2 measured from both GO and CS at the 30- to 60-min time points is consistently comparable (600–800 μM H2O2). In addition to demonstrating dose dependence of H2O2 generation on the number of cigarettes used (Fig. 2B), we could totally “quench” H2O2 generation in the presence of 10 mM GSH (Fig. 2B).
Fig. 2.
CS and glucose oxidase (GO) generate comparable levels of H2O2 in a time- (A) and dose-dependent (B) manner, quenched by GSH. Cells were pretreated with 10 mM GSH for 1 h before exposure to the indicated doses of CS or GO. One-milliliter aliquots of exposed medium were taken for H2O2 determination. H2O2 levels were measured in DMEM without phenol red as described in materials and methods, after no treatment or exposure to 1 U/ml GO or smoke from 1 cigarette at 37°C for the indicated time points (A) or for different amounts of smoke from different numbers of cigarettes for 30 min (B).
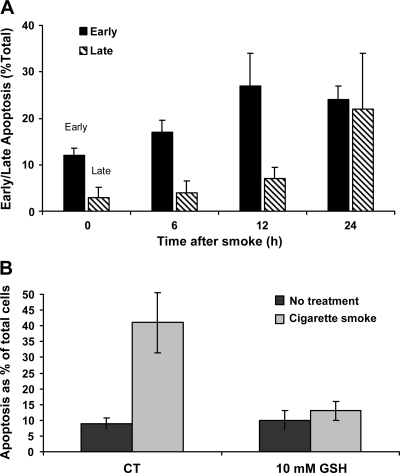
Fig. 3.
Apoptosis induction is upregulated in HBE1 cells exposed to CS. A: HBE1 cells were exposed to smoke from 1 cigarette for 30 min. Immediately after exposure, the medium was replaced with fresh medium, and the cells were incubated for the different time points. Apoptosis was assessed by FACS analysis after the cells were stained with annexin V-FITC and propidium iodide (PI). B: GSH protects the cells against CS-induced apoptosis. HBE1 cells were treated or not treated with 10 mM GSH for 1 h before CS exposure, pulsed with smoke from 1 cigarette for 30 min. Immediately the media were replaced with fresh media (with or without 10 mM GSH). Twelve hours after smoke exposure, cells were stained with annexin V-FITC and PI and were evaluated by FACS analysis for early apoptosis.
Next, we set up experiments to find out whether H2O2 generated by CS causes apoptosis in the exposed HBE1 cells. Cells were exposed to one cigarette for 30 min, washed with PBS, and incubated with fresh medium for different time points up to 24 h followed by annexin V and PI staining and flow cytometry for apoptosis assessment (Fig. 3A). As shown, ∼60% of the cells were still viable 12 h after the exposure to smoke from one cigarette for 30 min, whereas only 40% stayed viable after 24 h. At the same time, we could detect a slow progression of cells from an “early” apoptotic state to the “late” stage, in which apoptotic cells are also “contaminated” with early necrotic cells. While after 12 h of the exposure ∼30% of the cells demonstrated early apoptosis and 8% late apoptosis, after 24 h, the distribution between the early and late apoptosis cells was changed to almost 30% each. As shown, there is an unequivocal shift of cells to the late apoptosis stage, which tends to be a mixture of apoptotic cells indistinguishable from necrotic cells (31). Therefore, the subsequent quantitative analyses of apoptosis were carried out at 12-h time points after exposure to CS (usually with 1 cigarette for 30 min). As clearly demonstrated, this is a time point in which the number of cells undergoing defined early apoptosis is maximized.
To determine the specificity of apoptosis induction by H2O2 generated from CS, cells were pretreated with 10 mM GSH for 1 h followed by treatment with smoke from one cigarette for 30 min. Aliquots of cell culture medium were taken from the dishes of exposed cells for measurement of H2O2. Then, the cells were also collected for FACS analysis with annexin V. As shown, not only does H2O2 generated by CS completely vanish in the presence of GSH (Fig. 2B), but there is a concurrent inhibition of CS-induced apoptosis of HBE1 cells in the presence of GSH (Fig. 3B).
CS exposure results in upregulation of nSMase activity in lung epithelial cells.
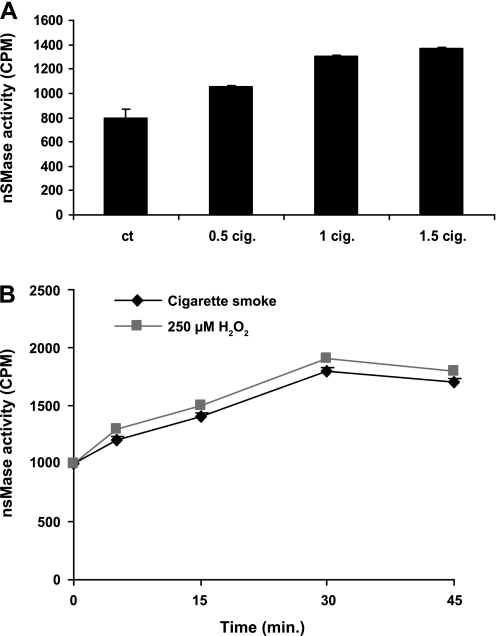
We have shown before (2, 5, 14, 31) that exposure of HAE cells to H2O2-induced oxidative stress generated an increase in ceramide levels via activation of a neutral SMase and sphingomyelin hydrolysis. Since CS generates an amount of H2O2 that is comparable to the levels of H2O2 exposure in our previous studies (2, 5, 14, 31), we wanted to see if the pattern of nSMase activation would be similar between the two treatments. Figure 4A shows that exposures of HBE1 cells to CS enhance nSMase activity in a dose-dependent manner. Moreover, the kinetics of nSMase activation by CS is similar to that of H2O2 exposure (Fig. 4B).
Fig. 4.
Neutral sphingomyelinase (nSMase) activity is upregulated in bronchial epithelial cells exposed to either CS or H2O2 in a dose- and time-response manner. A: nSMase activity after 30 min of exposure of HBE1 cells to various doses of CS. B: nSMase activity after exposure to smoke from 1 cigarette or to 250 μM H2O2 for the indicated time points.
CS specifically upregulates nSMase2 activity in lung epithelial cells.
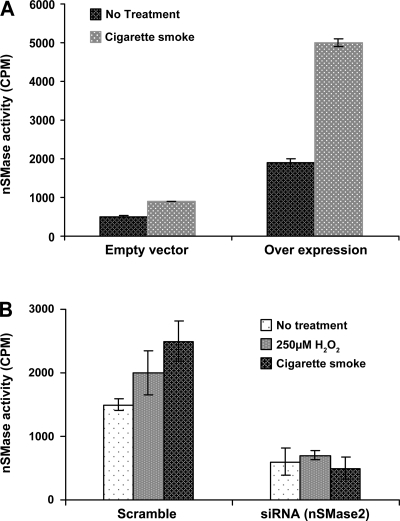
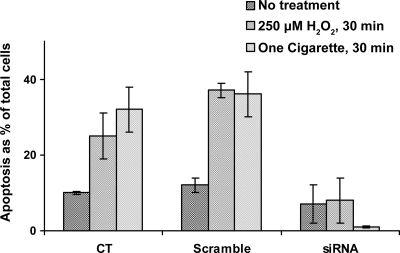
Based on our recent findings (14), nSMase2 is the SMase that is modulated by H2O2. Therefore, HBE1 cells were transiently transfected with pFLAG-nSMase2 or with siRNA for nSMase2 and exposed to CS 48 h posttransfection. Cells that were overexpressing nSMase2 and exposed to CS showed a high increase in enzyme activity (Fig. 5A), whereas cells in which nSMase2 was silenced by siRNA showed no increase in nSMase activity in response to CS (Fig. 5B). Most importantly, HBE1 cells in which nSMase2 was silenced with siRNA transfection and were then exposed to a pulse of CS (1 cigarette for 30 min) or to a pulse of H2O2 (250 μM for 30 min) did not induce apoptosis 12 h later compared with cells that were transfected with a scrambled siRNA control (Fig. 6). This suggests that nSMase2 is an essential component for the induction of apoptosis in airway epithelial cells that are exposed to CS.
Fig. 5.
Loss or gain of nSMase2 function demonstrates that this is the specific nSMase activated by CS to induce apoptosis. A: overexpression: HBE1 cells transfected with pFLAG-nSMase2 were exposed to smoke from 1 cigarette for 30 min, and nSMase activity was measured. B: silencing: cells transfected with siRNA for nSMase2 were exposed to smoke from 1 cigarette for 30 min, and nSMase activity was measured.
Fig. 6.
siRNA silencing of nSMase2 causes loss of apoptosis induction by CS or H2O2. HBE1 cells transfected with scrambled siRNA or siRNA for nSMase2 were exposed to either 250 μM H2O2 or to smoke from 1 cigarette for 30 min. Twelve hours after H2O2 or smoke exposure, cells were stained with annexin V-FITC and PI and were evaluated by FACS analysis for early apoptosis.
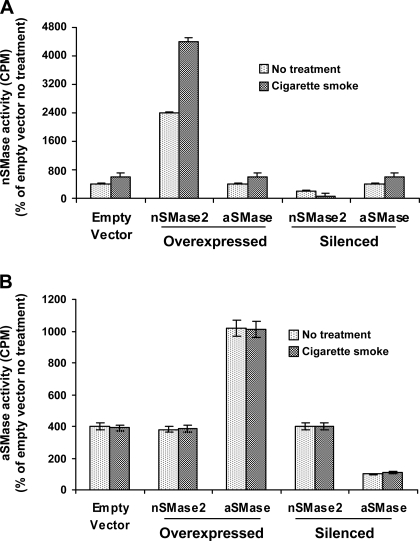
These results were further substantiated by testing the effects of CS on aSMase in HBE1 cells. Previously, we demonstrated that treatments with 75 μM H2O2 induced a 2.5-fold activation of nSMase activity within 15 min, but not of aSMase activity (2). Here we demonstrate that when testing for nSMase activity, silencing of nSMase2 eliminates the response to CS exposure, whereas overexpression of nSMase2 doubles the response (Fig. 7A). At the same time, Fig. 7B demonstrates that when aSMase activity was tested, silencing or overexpression of either aSMase or nSMase had no effect on the activity of aSMase in response to CS exposure, further supporting the observation that nSMase2 is the specific target for CS modulation.
Fig. 7.
Acidic SMase (aSMase) is not modulated by CS. HBE1 cells in which nSMase2 or aSMase are either overexpressed or silenced were exposed to smoke from 1 cigarette for 30 min. A: nSMase activity was measured immediately following smoke exposure. aSMase overexpression or silencing had no effect on nSMase activity. Only exposure to CS had an effect on nSMase2 activity. B: aSMase activity was measured immediately following smoke exposure. aSMase activity was not modulated by CS.
Activation of nSMase2 by CS is inhibited by GSH.
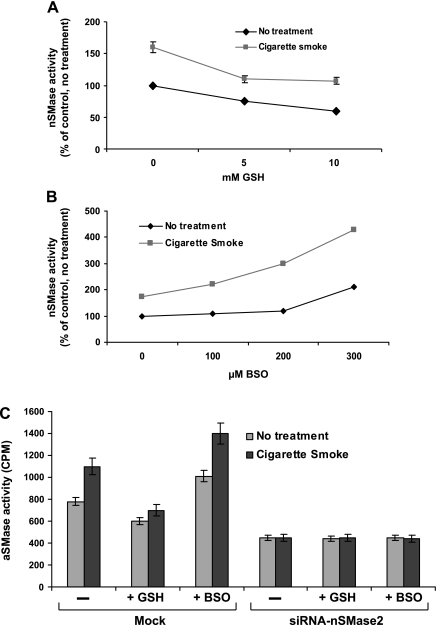
Studies in the past showed that the thiol antioxidant GSH is critical to lung cellular antioxidant defenses, particularly in protection from oxidant injury (15). Previously, we demonstrated that GSH inhibits nSMase activity in HAE cells (12). However, at that time we did not know the identity of the specific member of the nSMase family that could be stimulated by oxidative stress. Then, we showed that nSMase2 was the specific SMase that is modulated by H2O2 and by GSH (14). Our current data provide additional support to these earlier results by demonstrating that GSH as a pretreatment to CS exposure prevents the activation of nSMase2 (by CS) and keeps the enzyme activity at the same level as nontreated (Fig. 8A). Additionally, depletion of intracellular GSH with l-buthionine-(S, R)-sulfoximine (BSO) resulted in a dose-dependent increase in nSMase2 activity, which was higher after CS exposure (Fig. 8B). However, when nSMase2 was silenced by siRNA, both addition and depletion of GSH followed by exposure to CS did not change nSMase activity (Fig. 8C), reinforcing that nSMase2 is indeed the enzyme that is modulated by GSH and by CS.
Fig. 8.
GSH quenches the specific activation of nSMase2 by CS. HBE1 cells were transfected with pFLAG-nSMase2 and were treated. A: GSH was added for 1 h followed by exposure to smoke from 1 cigarette for 30 min. B: GSH was depleted using BSO for 24 h. Then, the cells were exposed to smoke from 1 cigarette for 30 min. C: cells transiently transfected with siRNA to nSMase2 were exposed to 250 μM BSO for 24 h followed by exposure to smoke from 1 cigarette for 30 min. Another set of cells were treated with 10 mM GSH for 1 h followed by exposure to smoke from 1 cigarette for 30 min.
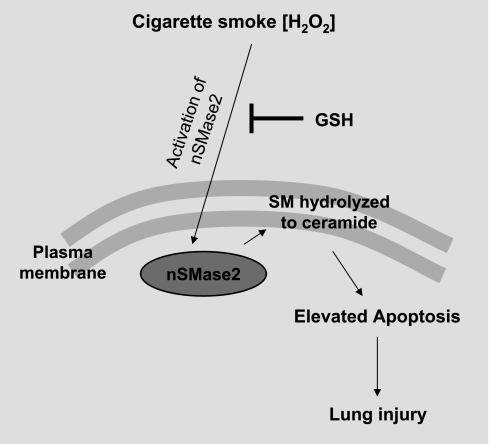
Together, these data support our suggestion that exposure of human bronchial epithelial cells to CS causes the activation of nSMase2, which is critical in elevating ceramide generation and thus has a key role in elevating cellular apoptosis and epithelium injury under CS exposure (Fig. 9). Pretreatment with GSH quenches nSMase2 activation and thereby protects the lung from injury initiated by ceramide generation under CS exposure.
Fig. 9.
Schematic model of nSMase2 modulation by CS. CS modulates nSMase2 by upregulating its activity. This leads to enhanced ceramide generation via hydrolysis of sphingomyelin (SM) and thus pathological apoptosis in airway epithelial cells, which causes lung injury. GSH blocks this effect of CS.
DISCUSSION
Previously, we found that exposure of HAE cells to exogenously generated H2O2 activates nSMase2 to induce apoptosis in HAE cells (14). In addition, we demonstrated that GSH inhibits ceramide production in HAE cells and that depletion of intracellular GSH induces elevation of ceramide levels and apoptosis (12, 17). We then showed that nSMase2 is the specific target that is activated by H2O2 in HAE cells and therefore is essential for their oxidative stress-induced apoptosis (14).
CS contains more than 1014 free radicals/oxidants per puff, but H2O2 is known to be a major component of the gas phase of CS (26). Therefore, our present studies were undertaken to determine whether CS has a similar effect to that of H2O2 on nSMAse2 activation and its ability to induce apoptosis.
As we previously demonstrated (11), we now show again that CS generates an amount of H2O2 that is comparable to the amount generated by GO, which was used in our previous studies (11). We presently show that not only can CS activate nSMase2 but that it also generates ceramide in a dose- and time-dependent manner. Moreover, we present new data demonstrating that very little H2O2 can be detected and that SMase2 cannot be activated in cell culture medium exposed to CS or to GO in the presence of GSH, an antioxidant that specifically targets H2O2 and organic hydroperoxides.
In the United States alone, there are ∼4–5 million people with emphysema, a characteristic lung-destructive process that is part of the spectrum of chronic obstructive lung diseases induced by CS. Unfortunately, once the destruction of the epithelium and alveoli has begun, the damage is irreversible, and there are no effective treatments for this disease. The understanding of the underlying mechanisms of the initiation of these destructive lung processes has evolved significantly in the past few years. However, a major challenge remains in that the specific molecular targets involved in this irreversibly fatal pathology developed with cigarette smoking are still unknown.
It has been reported that ceramide, a second messenger lipid, is a critical mediator of alveolar cell apoptosis in an experimental VEGF mouse model of COPD (29). At the same time, ceramide is considered a lipid-signaling molecule in cellular pathways that lead to enhanced cell apoptosis (10). Because recent studies show that enhanced apoptosis induction may be part of lung injury processes, it is possible that ceramide is also involved in the pathological processes of emphysema induced by cigarette smoking. But, still, the downstream consequences and the direct injury of alveolar cells by components of CS and the machinery of ceramide induction are poorly understood.
If indeed ceramide is a critical pathogenic element in the development of lung injury in emphysema, then, it is important to elucidate the exact cellular mechanism of ceramide generation under CS exposure. Therefore, the component of the ceramide-generating machinery that is specifically activated by CS exposure could be an important target for pharmacological intervention in COPD, a disease developed with smoking that has no available therapies.
In the present study, we demonstrate that CS and H2O2 elicit the same apoptotic response in HBE1 cells. We found by using loss-of-function analysis that nSMase2 is the SMase that is modulated by CS.
Recent studies in transgenic mice exposed to ceramide analogs suggested that aSMase could be affected by superoxide dismutase (28). However, as shown before with H2O2 (14), we demonstrate here unequivocally that aSMase is not affected by CS. It is only and specifically nSMase2 that is modulated by CS and by H2O2.
Under CS, nSMase2 is activated, leading to an increase in ceramide levels and subsequent apoptosis. We and others (11, 25, 40) found that CS exposure generates H2O2 in cell culture media in a dose-dependent manner. Since the activation of nSMase2 by CS and the subsequent induction of HBE1 cell apoptosis were also abrogated by GSH, we suggest that the H2O2 component of CS is responsible for the observed effects on nSMase2 and apoptosis.
GSH is critical to lung cellular antioxidant defenses (12, 16). It is a ubiquitous, essential tripeptide (l-γ-glutamyl-l-cysteinyl-glycine) containing a sulfhydryl group that enables it to be a key intracellular reducing agent, thus providing a fundamental antioxidant defense mechanism in oxidant-induced lung injury (23). Indeed, when cells were pretreated with 10 mM GSH before CS exposure, we found inhibition of nSMase2 activity and apoptosis, suggesting that nSMase2 is a specific target modulated by CS and GSH to induce or inhibit apoptosis, respectively. Therefore, nSMase2 may act as a redox sensor (2, 12), changing its activation in response to the oxidant/antioxidant balance. When HBE1 cells are treated with CS or H2O2, nSMase2 is activated, whereas when cells are treated with GSH, the activation is quenched.
In addition, and as was previously explained (11), although catalase inhibits H2O2 more specifically, we found that it was inhibited by CS (data not shown), which was also observed by Mendes-Alvarez et al. (24). Therefore, this paper makes no argument that H2O2 is the only component of CS to cause the activation of the nSMase2. Rather, we are using our previous knowledge of the effects of H2O2 on the nSMase2 to draw parallels to the effects of CS on this target and suggest that CS-generated H2O2 has a major role in the activation of nSMase2 and its apoptosis induction. Whether other hydroperoxides species have a role in activating nSMase2 remains to be established.
In summary, chronic cigarette smoking accounts for 95% of cases of emphysema, and it is known that the imbalance between oxidants and antioxidants is involved in the development of pulmonary emphysema (1). Here we show that nSMase2 is activated by the reactive oxygen species (specifically H2O2) component of CS, thereby increasing ceramide generation via hydrolysis of sphingomyelin, and thus pathological apoptosis in airway epithelial cells that may cause lung injury. GSH blocks this effect of CS. Therefore, with such a potential critical role in lung epithelial cell apoptosis, nSMase2 and the ceramide-generating machinery may be attractive targets for the prevention of the airway destruction that is the hallmark of CS-induced emphysema.
GRANTS
This work was supported by grants from the Tobacco-Related Disease Research Program (13FT-0126 to E. Khan, 15FT-0041 to M. Levy, and 17RT-0131 to T. Goldkorn) and National Heart, Lung, and Blood Institute Grants HL-71871 and HL-66189 (to T. Goldkorn).
REFERENCES
- 1.Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 118: 617–621, 1978. [DOI] [PubMed] [Google Scholar]
- 2.Chan C, Goldkorn T. Ceramide path in human lung cell death. Am J Respir Cell Mol Biol 22: 460–468, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Claus R, Bunck A, Bockmeyer C, Brunkhorst F, Losche W, Kinscherf R, Deigner H. Role of increased SMase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J 19: 1719–1721, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med 10: 155–160, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Goldkorn T, Balaban N, Shannon M, Chea V, Matsukuma K, Gilchrist D, Wang H, Chan C. H2O2 acts on cellular membranes to generate ceramide signaling and initiate apoptosis in tracheobronchial epithelial cells. J Cell Sci 111: 3209–3220, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Goldkorn T, Lavrentiadou S, Chan C, Rassooly R, van der Vliet A, Kawcak T. Glutathione regulation of ceramide pathway in lung epithelial cells. Am J Respir Crit Care Med 161: A242, 2000. [Google Scholar]
- 7.Goldkorn T, Ravid T, Khan EM. Life and death decisions: ceramide generation and EGF receptor trafficking are modulated by oxidative stress. Antioxid Redox Signal 7: 119–128, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Groneberg DA, Chung KF. Models of chronic obstructive pulmonary disease. Respir Res 5: 18, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamai H, Keyserman F, Quittell LM, Worgall TS. Defective CFTR increases synthesis and mass of sphingolipids that affect membrane composition and lipid signaling. J Lipid Res. In press. [DOI] [PMC free article] [PubMed]
- 10.Hannun YA, Obeid LM. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem 277: 25847–25850, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Khan EM, Lanir R, Danielson AR, Goldkorn T. EGF receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB J 22: 910–917, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavrentiadou SN, Chan C, Ravid T, Tsaba A, van der Vliet A, Rasooly R, Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by GSH. Am J Respir Cell Mol Biol 25: 676–684, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawler JF Jr, Yin M, Diehl AM, Roberts E, Chatterjee S. Tumor necrosis factor-alpha stimulates the maturation of sterol regulatory element binding protein-1 in human hepatocytes through the action of neutral sphingomyelinase. J Biol Chem 273: 5053–5059, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun 344: 900–905, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li XY, Donaldson K, Rahman I, MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med 149: 1518–1525, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Li XY, Rahman I, Donaldson K, MacNee W. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax 51: 465–471, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Andrieu-Abadie N, Levade T, Zhang P, Obeid LM, Hannun YA. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J Biol Chem 273: 11313–11320, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Macnee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: S58–S65, 1999. [DOI] [PubMed] [Google Scholar]
- 19.March TH, Bowen LE, Finch GL, Nikula KJ, Wayne BJ, Hobbs CH. Effects of strain and treatment with inhaled aII-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. COPD 2: 289–302, 2005. [PubMed] [Google Scholar]
- 20.March TH, Wilder JA, Esparza DC, Cossey PY, Blair LF, Herrera LK, McDonald JD, Campen MJ, Mauderly JL, Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci 92: 545–559, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol 82: 27–44, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Martin LD, Rochelle LG, Fischer BM, Krunkosky TM, Adler KB. Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur Respir J 10: 2139–2146, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Meister A, Anderson ME. Glutathione. Annu Rev Biochem 52: 711–760, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Mendes-Alvarez E, Soto-Otero R, Danchez I, Lopez-Rivadulla L. In vitro inhibition of catalase activity by cigarette smoke: relevance for oxidative stress. J Appl Toxicol 18: 443–448, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Church DF, Pryor WA. Quantitative analysis of the hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic Biol Med 7: 9–15, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama T, Kodama M, Nagata C. Generation of hydrogen peroxide and superoxide anion radical from cigarette smoke. Gann 75: 95–98, 1984. [PubMed] [Google Scholar]
- 27.Okazaki T, Bielawska A, Domae N, Bell RM, Hannun YA. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem 269: 4070–4077, 1994. [Published erratum. J Biol Chem 269: June 1994, p. 16518.] [PubMed] [Google Scholar]
- 28.Petrache I, Medler TR, Richter AT, Kamocki K, Chukwueke U, Zhen L, Gu Y, Adamowicz J, Schweitzer KS, Hubbard WC, Berdyshev EV, Lungarella G, Tuder RM. Superoxide dismutase protects against apoptosis and alveolar enlargement induced by ceramide. Am J Physiol Lung Cell Mol Physiol 295: L44–L53, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11: 491–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman I, Swarska E, Henry M, Stolk J, MacNee W. Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease? Thorax 55: 189–193, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravid T, Tsaba A, Gee P, Rasooly R, Medina EA, Goldkorn T. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol 284: L1082–L1092, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc 3: 703–708, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snider GL Chronic obstructive pulmonary disease: risk factors, pathophysiology and pathogenesis. Annu Rev Med 40: 411–429, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med 26: 142–153, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 14: 382–391, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Thurman RG, Ley HG, Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem 25: 420–430, 1972. [DOI] [PubMed] [Google Scholar]
- 37.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art. Cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc 3: 503–510, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295: L1–L15, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 87: 1047–1082, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Zang LY, Stone K, Pryor WA. Detection of free radicals in aqueous extracts of cigarette tar by electron spin resonance. Free Radic Biol Med 19: 161–167, 1995. [DOI] [PubMed] [Google Scholar]