Abstract
Stromal interaction molecule 1 (STIM1) is a recently discovered membrane-spanning protein thought to sense lumenal Ca2+ in sarco(endo)plasmic reticulum (SR/ER) and transduce activation of Ca2+-permeable store-operated channels (SOC) in plasmalemma in response to SR/ER Ca2+ depletion. To evaluate the role of STIM1 and a closely related protein, STIM2, in Ca2+ signaling of rat distal pulmonary arterial smooth muscle cells (PASMC) during hypoxia, we used fluorescent microscopy and the Ca2+-sensitive dye, fura 2, to measure basal intracellular Ca2+ concentration ([Ca2+]i), store-operated Ca2+ entry (SOCE), and increases in [Ca2+]i caused by acute hypoxia (4% O2) or depolarization (60 mmol/l KCl) in cells treated with small interfering RNA targeted to STIM1 (siSTIM1) or STIM2 (siSTIM2). As determined by real-time quantitative PCR analysis (qPCR), STIM1 mRNA was 200-fold more abundant than STIM2 in untreated control PASMC. siSTIM1 and siSTIM2 caused specific and significant knockdown of both mRNA measured by qPCR and protein measured by Western blotting. siSTIM1 markedly inhibited SOCE and abolished the sustained [Ca2+]i response to hypoxia but did not alter the initial transient [Ca2+]i response to hypoxia, the [Ca2+]i response to depolarization, or basal [Ca2+]i. The only effect of siSTIM2 was a smaller inhibition of SOCE. We conclude that STIM1 was quantitatively more important than STIM2 in activation of SOC in rat distal PASMC and that the increase in [Ca2+]i induced by acute hypoxia in these cells required SR Ca2+ release and STIM1-dependent activation of SOC.
Keywords: STIM2, TRPC1, hypoxic pulmonary vasoconstriction, calcium signaling, vascular smooth muscle, KCl
hypoxic pulmonary vasoconstriction (HPV) is triggered by an increase in intracellular Ca2+ concentration ([Ca2+]i) in pulmonary arterial smooth muscle cells (PASMC) (31, 43, 44, 46, 52, 54). This increase may be due to PASMC depolarization and secondary influx of Ca2+ through sarcolemmal L-type voltage-operated Ca2+ channels (VOCC) (6, 12, 31, 54). However, hypoxia may also cause release of Ca2+ from sarcoplasmic reticulum (SR) (15, 29, 44, 52, 55, 63), leading to activation of sarcolemmal Ca2+-permeable store-operated channels (SOC), store-operated Ca2+ entry (SOCE) in PASMC (29, 54), and HPV in isolated lungs (57). Which of these possibilities is correct remains controversial.
Recent studies in a wide variety of cell types, including smooth muscle (8, 18, 36, 51), have shown that SOCE requires a 90-kDa transmembrane Ca2+-binding protein known as stromal interaction molecule 1 (STIM1) (9, 13, 17, 42). STIM1 is found in sarco(endo)plasmic reticulum (SR/ER) and plasma membrane and functions as both the sensor of Ca2+ within the SR/ER lumen and the transducer of SOC activation in response to SR/ER Ca2+ depletion. These functions are accomplished when a decrease in SR/ER Ca2+ causes dissociation of Ca2+ from an EF-hand motif in the NH2-terminal region of STIM1 located within the SR/ER lumen, translocation of STIM1 proteins to form oligomeric collections termed “puncta” in portions of the SR/ER membrane close to SOC in plasma membrane, and interactions of STIM1 with SOC and/or associated regulatory proteins that lead to channel activation and SOCE (13, 59, 62). Indeed, it has been proposed that fulfillment of these functions by STIM1 should define SOC (58).
Closely related to STIM1 is STIM2, a 105-kDa protein detected in SR/ER but not plasma membrane, which has 61% structural homology with STIM1, including an EF-hand domain, a protein-protein interaction site known as the sterile α-motif domain, and a coiled-coils membrane spanning region within an ezrin/radixin/moesin (ERM) domain (9). The function of STIM2 is not as well understood as that of STIM1. Some studies indicate that STIM2 has little or no effect on SOCE (22, 30, 36, 51), whereas others suggest that STIM2 may inhibit STIM1 (50), act to maintain basal cytoplasmic and SR/ER luminal Ca2+ (4), or regulate store-dependent and -independent activation of SOC (35). Although STIM2 was detected in airway and coronary arterial smooth muscle (36, 51), expression in PASMC has not been reported.
In a previous study of distal and proximal pulmonary arteries (24), we found that STIM1 expression, SOCE, and [Ca2+]i responses to hypoxia but not KCl were greater in myocytes from distal arteries, which are thought to be the major locus of HPV (45, 47). In this study, we examined STIM2 expression and used RNA interference to evaluate the roles of STIM1 and STIM2 in SOCE and [Ca2+]i responses to acute hypoxia in distal PASMC.
METHODS
Isolation and culture of rat distal PASMC.
Animal protocols were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. PASMC were harvested from distal (>4th generation) intrapulmonary arteries of anesthetized (pentobarbital, 65 mg/kg ip) male Wistar rats (300–500 g body wt) and cultured for 3–4 days in smooth muscle growth media (SMGM-2; Clonetics, Walkersville, MD) in an atmosphere of 5% CO2-95% air at 37°C, as previously described (53).
RNA interference.
Small interfering RNA (siRNA) targeted to STIM1 (siSTIM1) and STIM2 (siSTIM2) were designed and synthesized by Dharmacon (accession numbers XM_341896 and NM_001105750, respectively; siGENOME SMARTpool; Dharmacon, Lafayette, CO). After 6-h exposure to 25 nmol/l siSTIM1, siSTIM2, or nontargeting siRNA (siNT) using transfection vehicle (GeneSilencer; Genlantis, San Diego, CA) at 1.0 μl/ml in smooth muscle basal media (SMBM; Clonetics, Walkersville, MD), PASMC were exposed for 20 h to SMBM containing 5% FBS, followed by 24 h in SMBM containing 0.3% serum to stop cell growth. PASMC treated with transfection vehicle alone and untreated PASMC from the same isolate served as additional controls.
To assess cytotoxicity of these treatments, we stained PASMC from 3 isolates with 1 μM calcein AM and 5 μM ethidium homodimer-1 (EthD-1) for 30 min at room temperature after washing with Dulbecco's phosphate-buffered saline. Calcein AM enters live cells, where it is deesterfied and retained as calcein, which is highly fluorescent. EthD-1 is excluded from live cells but enters dead or damaged cells, where binding to nucleic acids markedly increases its fluorescence. For each coverslip, we measured fluorescence at 517 nm after excitation at 488 nm (calcein) and at 617 nm after excitation at 514 nm (EthD-1) in ≥500 cells in randomly selected nonoverlapping fields using a laser-scanning confocal fluorescence microscope (LSM-510; Carl Zeiss, Thornwood, NY) equipped with a ×40 objective (Zeiss Plan-Neofluar; numerical aperture 1.3). The percentage of dead or damaged cells was calculated as 100 times the ratio of cells positive for EthD-1 to the sum of cells positive for calcein or EthD-1.
mRNA measurement by real-time quantitative PCR.
To assess the effectiveness and specificity of RNA interference, we measured intracellular concentrations of STIM1, STIM2, and canonical transient receptor potential protein 1 (TRPC1) mRNA relative to β-actin mRNA by real-time quantitative PCR analysis (qPCR). Total RNA was extracted from PASMC using a kit (RNeasy; Qiagen, Valencia, CA). After removal of DNA contamination (RNase-free DNase; Qiagen), reverse transcription was performed with a cDNA synthesis kit (iScript; Bio-Rad, Hercules, CA) in a 20-μl reaction mixture containing 250 ng of total RNA. cDNA was quantified using QuantiTect SYBR Green PCR Master Mix (Qiagen) and a real-time PCR detection system (iCycler iQ; Bio-Rad) using the following conditions: 95°C for 15 min followed by 45 cycles, each cycle consisting of 15 s at 94°C, 20 s at 57.5°C, and 20 s at 72°C. The 25-μl qPCR reaction mixture contained cDNA template from 6.25-ng RNA and forward and reverse primers (300 nmol/l). Primer sequences for rat STIM1, STIM2, TRPC1, and β-actin were designed using Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) as follows, where S is sense, and AS is antisense: STIM1-S (5′-ATGCCAATGGTGATGTGGAT-3′), STIM1-AS (5′-CCATGGAAGGTGCTGTGTTT-3′), STIM2-S (5′-TCCATTGGTCCCGTTGTTAT-3′), STIM2-AS (5′-CGCAAAGCATCTTCATTTCA-3′), TRPC1-S (5′-AGCCTCTTGACAAACGAGGA-3′), TRPC1-AS (5′-ACCTGACATCTGTCCGAACC-3′), β-actin-S (5′-ACGGTCAGGTCATCACTATC-3′), and β-actin-AS (5′-TGCCACAGGATTCCATACC-3′). Identities of qPCR products were confirmed by: 1) a single peak in the melting curve performed after cDNA amplification; 2) a single band of the expected size resolved by agarose gel electrophoresis; and 3) the correct DNA sequence. Melting curves were performed at 95°C for 1 min and 55°C for 1 min, followed by 80 increments of 0.5°C at 10-s intervals. Real-time qPCR detection threshold cycle values were generated by iCycler iQ software. The concentration of each transcript relative to β-actin was calculated according to the Pfaffl (38) method, using amplification efficiencies determined for each gene from 5-point serial dilutions of cDNA samples.
Protein measurement by Western blotting.
PASMC samples were lysed in Laemmli sample buffer containing 62.5 mmol/l Tris·HCl (pH 6.8), 2% SDS, 10% glycerol, 5% protease inhibitor cocktail, 1 mmol/l EDTA, and 200 μM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride and homogenized by sonication. Total protein concentration in the homogenates was determined by the bicinchoninic acid protein assay (Pierce, Rockford, IL) using bovine serum albumin as a standard. Homogenates were denatured by adding dithiothreitol to 150 mmol/l and heating at 95°C for 3 min. Homogenate proteins were resolved by 10% SDS-PAGE calibrated with prestained protein molecular weight markers (Precision Plus; Bio-Rad). Separated proteins were transferred to polyvinylidene difluoride membranes (pore size 0.45 μm; Bio-Rad). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween 20, blotted with a monoclonal antibody specific for STIM1 or affinity-purified polyclonal antibodies specific for STIM2 or α-actin. The membranes were then washed for 15 min three times and incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG for 1 h. Bound antibodies were detected using an enhanced chemiluminescence system (ECL; GE Healthcare, Piscataway, NJ).
Measurement of [Ca2+]i by fura 2 fluorescence.
As previously described (53), coverslips with myocytes were incubated with 5 μM fura 2-AM (Invitrogen, Carlsbad, CA) for 60 min at 37°C in 5% CO2-95% air, mounted in a closed chamber clamped in a heated platform (PH-2; Warner Instrument, Hamden, CT) on an inverted microscope (TSE 100 Ellipse; Nikon, Melville, NY), and perfused at 1.0 ml/min with Krebs-Ringer bicarbonate solution (KRBS), which contained (in mmol/l) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 0.57 MgSO4, 1.18 KH2PO4, 25 NaHCO3, and 10 glucose. Perfusate was equilibrated with 16% O2-5% CO2 in heated reservoirs and led to the chamber through stainless steel tubing. Chamber temperature was maintained at 37°C with a heat exchanger and heater controller (models SF-28 and TC-344B, respectively; Warner Instrument).
After 10 min of perfusion to remove extracellular dye, [Ca2+]i was determined at 12- to 30-s intervals from the ratio of fura 2 fluorescence emitted at 510 nm after excitation at 340 nm to that after excitation at 380 nm (F340/F380) measured using a xenon arc lamp, interference filters, electronic shutter, ×20 fluorescence objective, and a cooled charge-coupled device imaging camera. Data from 20 to 30 cells were collected online with InCyte software (Intracellular Imaging, Cincinnati, OH). [Ca2+]i was estimated from F340/F380 measured in vitro in calibration solutions with Ca2+ of 0–1,350 nmol/l (Invitrogen).
Measurement of SOCE.
PASMC were perfused for at least 10 min with Ca2+-free KRBS containing 0.5 mmol/l EGTA to chelate residual Ca2+, 5 μM nifedipine (Sigma Chemical, St. Louis, MO) to prevent calcium entry through L-type VOCC, and 10 μM cyclopiazonic acid (CPA; Sigma Chemical) to deplete SR Ca2+ stores. As in previous studies (53, 54), we then assessed SOCE in the continued presence of EGTA, nifedipine, and CPA by measuring: 1) the peak increase in [Ca2+]i caused by restoration of extracellular Ca2+ to 2.5 mmol/l; or 2) the rate at which fura 2 fluorescence excited at 360 nm (F360) was quenched by 200 μM Mn2+. Quenching was quantified as the change in F360 (ΔF360) measured from 5 to 15 min (i.e., during a 10-min exposure to Mn2+) and expressed as a percentage of F360 at 5 min.
Measurement of [Ca2+]i responses to hypoxia and KCl.
After a 5-min normoxic control period, PASMC were perfused with 1) KRBS equilibrated with 4% O2-5% CO2 for 15 min, or 2) KRBS in which KCl was increased to 60 mmol/l and NaCl decreased to 62.7 mmol/l for 10 min, followed by a 10-min recovery period during which the cells were perfused with normal KRBS equilibrated with 16% O2-5% CO2. [Ca2+]i was measured throughout these experiments by fura 2 fluorescence, as described above.
Materials and drugs.
Unless specified, all reagents were obtained from Sigma Chemical. Calcein AM and EthD-1 (LIVE/DEAD Viability/Cytotoxicity Kit) and fura 2-AM were obtained from Invitrogen. The latter was prepared daily as a 2.5 mmol/l stock solution in 20% Pluronic F-127 in dimethyl sulfoxide (Invitrogen). Stock solutions (30 mmol/l) of CPA and nifedipine were made in dimethyl sulfoxide. STIM1 and STIM2 antibodies were obtained from BD Biosciences (Franklin Lakes, NJ) and Prosci (Poway, CA), respectively.
Statistical analysis.
Data are expressed as means ± SE. n Is the number of experiments, which is the same as the number of animals providing PASMC. Statistical analyses were performed using analysis of variance. When significant F ratios were obtained in these analyses, Fisher's protected least significant difference was used for pairwise comparisons of means. Differences were considered significant when P < 0.05.
RESULTS
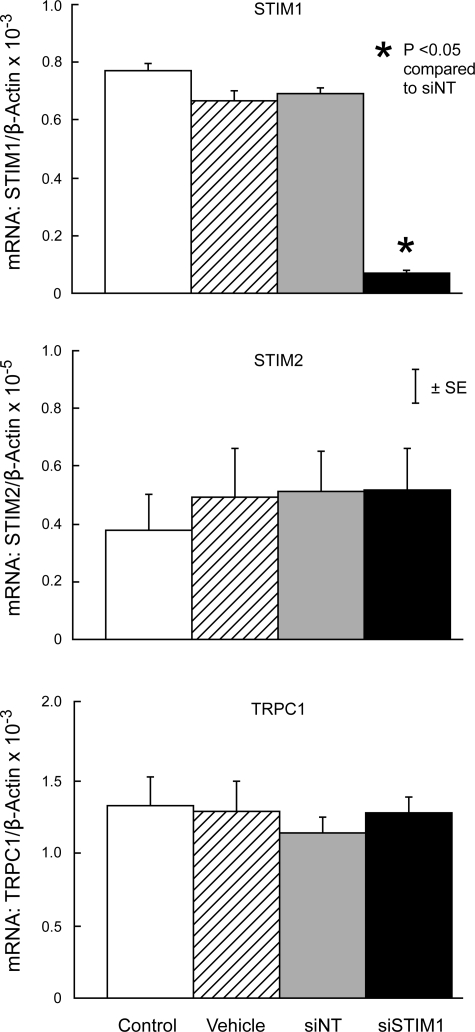
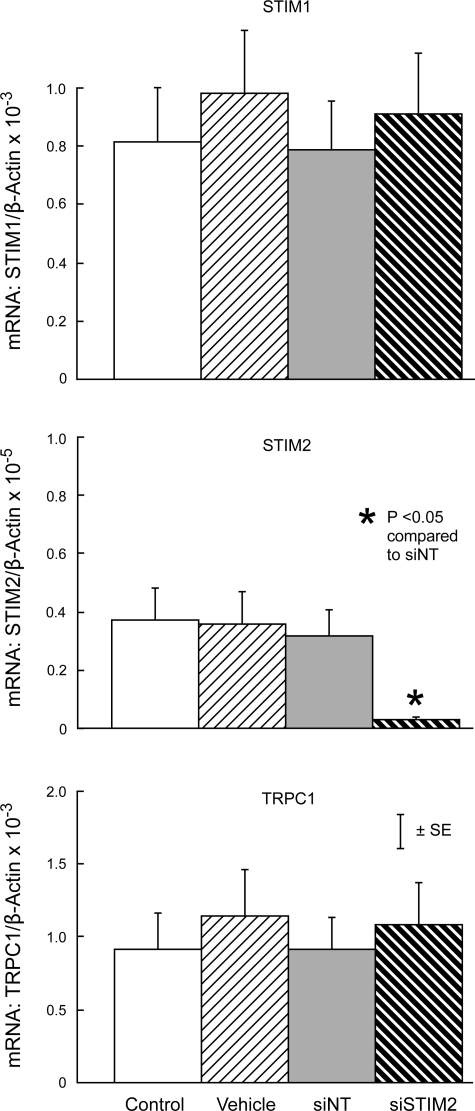
In untreated cells, STIM1 mRNA was ∼200-fold more abundant than STIM2 mRNA (Figs. 1 and 2). Expression of STIM1, STIM2, or TRPC1 mRNA in PASMC treated with siNT was not different from expression in untreated control cells or cells treated with transfection vehicle alone (Figs. 1 and 2). In contrast, siSTIM1 reduced STIM1 mRNA expression by 90.2 ± 1.1% compared with siNT (P < 0.0001) without altering expression of STIM2 or TRPC1 (Fig. 1). Similarly, siSTIM2 reduced STIM2 mRNA expression by 89.6 ± 1.4% (P < 0.01) without altering expression of STIM1 or TRPC1 (Fig. 2).
Fig. 1.
Expression of stromal interaction molecule 1 (STIM1; top), STIM2 (middle), and canonical transient receptor potential protein 1 (TRPC1; bottom) mRNA relative to β-actin mRNA measured by real-time quantitative PCR analysis (qPCR) in untreated control pulmonary arterial smooth muscle cells (PASMC) (Control) and PASMC treated with transfection vehicle alone (Vehicle), nontargeted small interfering RNA (siNT), or siRNA targeted to STIM1 (siSTIM1). n = 3 in each group.
Fig. 2.
Expression of STIM1 (top), STIM2 (middle), and TRPC1 (bottom) mRNA relative to β-actin mRNA measured by real-time qPCR in untreated control PASMC (Control) and PASMC treated with transfection vehicle alone (Vehicle), siNT, or siRNA targeted to STIM2 (siSTIM2). n = 4 in each group.
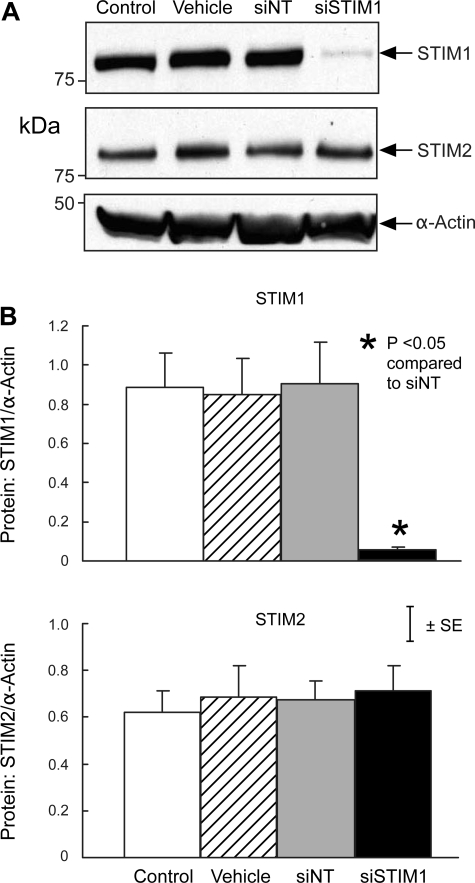
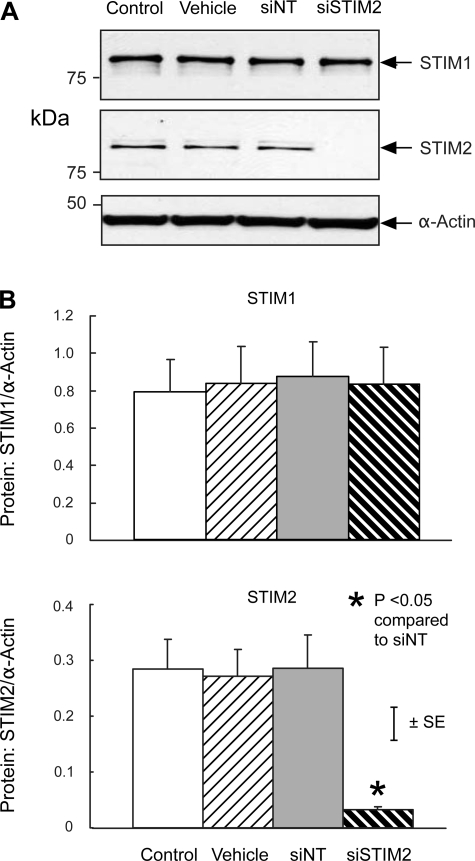
Consistent with these qPCR results, protein expression of STIM1 and STIM2 in PASMC treated with siNT was not different from that in untreated control cells or cells treated with transfection vehicle alone (Figs. 3 and 4). In contrast, siSTIM1 reduced STIM1 protein expression by 92.6 ± 3.3% compared with siNT (P < 0.01) without altering expression of STIM2 (Fig. 3), whereas siSTIM2 reduced STIM2 protein expression by 88.7 ± 0.7% (P < 0.005) without altering expression of STIM1 (Fig. 4).
Fig. 3.
A: Western blot showing expression of STIM1, STIM2, and α-actin protein in untreated control PASMC (Control) and PASMC treated with transfection vehicle alone (Vehicle), siNT, or siSTIM1. B: mean ratios of STIM1 (top) and STIM2 (bottom) protein relative to α-actin protein measured by Western blotting in Control, Vehicle, siNT, and siSTIM1 PASMC. n = 3 in each group.
Fig. 4.
A: Western blot showing expression of STIM1, STIM2, and α-actin protein in untreated control PASMC (Control) and PASMC treated with transfection vehicle alone (Vehicle), siNT, or siSTIM2. B: mean ratios of STIM1 (top) and STIM2 (bottom) protein relative to α-actin protein measured by Western blotting in Control, Vehicle, siNT, and siSTIM2 PASMC. n = 4 in each group.
Overall, the percentage of dead or damaged PASMC in these experiments averaged 2.8 ± 0.3. This percentage did not differ among untreated control PASMC or PASMC treated with transfection vehicle, siNT, siSTIM1, or siSTIM2 (P > 0.2).
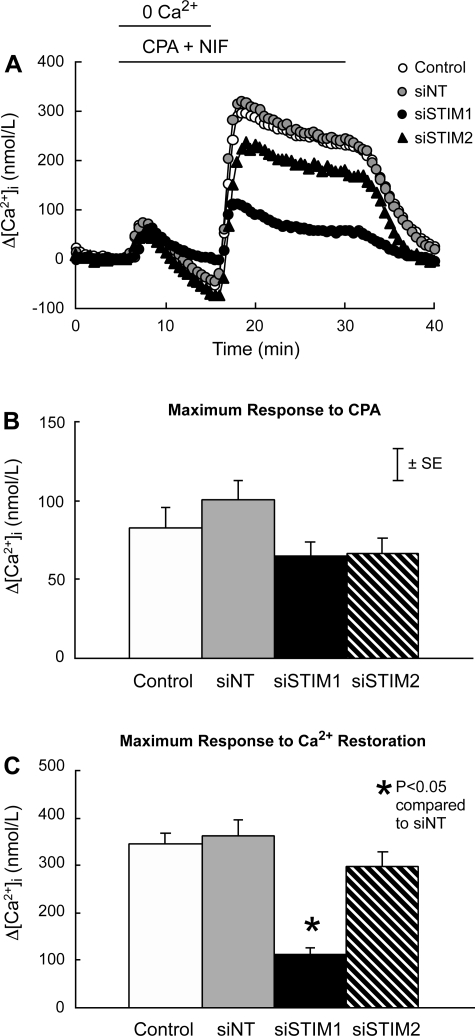
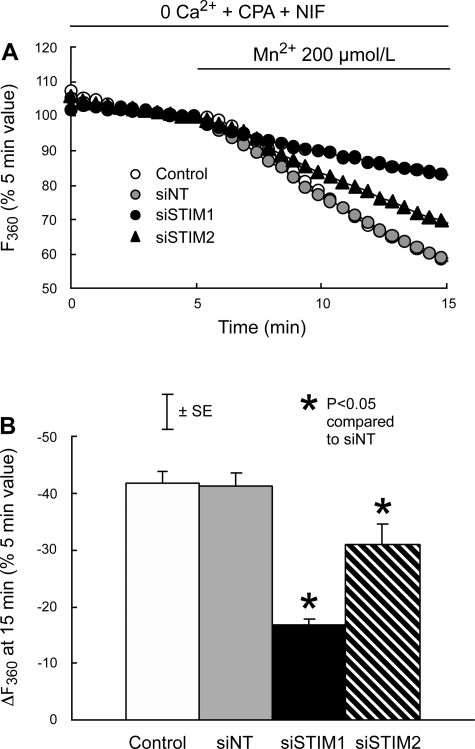
In the absence of extracellular Ca2+ and presence of nifedipine, CPA caused a transient increase in [Ca2+]i that averaged 82 ± 7 nmol/l and did not differ among untreated control PASMC and PASMC treated with siNT, siSTIM1, or siSTIM2 (Fig. 5, A and B). Subsequent restoration of extracellular Ca2+ to 2.5 mmol/l in the continued presence of CPA and nifedipine caused larger and more sustained increases in [Ca2+]i. In cells treated with siNT, this response peaked at 361 ± 35 nmol/l and was not different in untreated control PASMC (346 ± 21 nmol/l; P > 0.6) or cells treated with siSTIM2 (297 ± 30 nmol/l; P > 0.1); however, it was reduced to 111 ± 16 nmol/l (P < 0.0001) in PASMC treated with siSTIM1 (Fig. 5, A and C). Somewhat different results were obtained when SOCE was measured as Mn2+ quenching (Fig. 6). In cells treated with siNT, quenching averaged −41.4 ± 2.3% after exposure to Mn2+ for 10 min. Quenching was not different in untreated control PASMC (−41.9 ± 2.0%; P > 0.8) but was reduced in PASMC treated with either siSTIM1 (−16.8 ± 1.0%; P < 0.0001) or siSTIM2 (−31.0 ± 3.6%; P < 0.008). The inhibitory effect of siSTIM2 on Mn2+ quenching was significantly smaller than that of siSTIM1 (P < 0.003).
Fig. 5.
A: effects of cyclopiazonic acid (CPA; 10 μmol/l) and restoration of extracellular Ca2+ to 2.5 mmol/l on the time course of the mean change in intracellular Ca2+ concentration (Δ[Ca2+]i) from its average value at 3–5 min in PASMC perfused with Ca2+-free Krebs-Ringer bicarbonate solution (KRBS) containing nifedipine (NIF; 5 μmol/l). PASMC were untreated (Control; n = 6) or treated with siNT (n = 7), siSTIM1 (n = 4), or siSTIM2 (n = 4). B: mean maximum Δ[Ca2+]i measured as its peak increase between 5 and 10 min from its average value at 3–5 min in response to CPA in Control, siNT, siSTIM1, and siSTIM2 PASMC. C: mean maximum Δ[Ca2+]i measured as its peak increase between 15 and 30 min from its average value at 13–15 min in response to restoration of extracellular Ca2+ in Control, siNT, siSTIM1, and siSTIM2 PASMC.
Fig. 6.
A: effects of 200 μmol/l MnCl2 on the time course of mean fura 2 fluorescence at 510 nm after excitation at 360 nm (F360), expressed as a percentage of its value at 5 min in PASMC perfused with Ca2+-free KRBS containing 10 μmol/l CPA and 5 μmol/l NIF. PASMC were untreated (Control; n = 8) or treated with siNT (n = 8), siSTIM1 (n = 4), or siSTIM2 (n = 6). B: quenching of fura 2 fluorescence in each group measured as the mean change in F360 (ΔF360) during the 10-min exposure to Mn2+ (5–15 min) and expressed as a percentage of F360 at the beginning of the exposure (5 min).
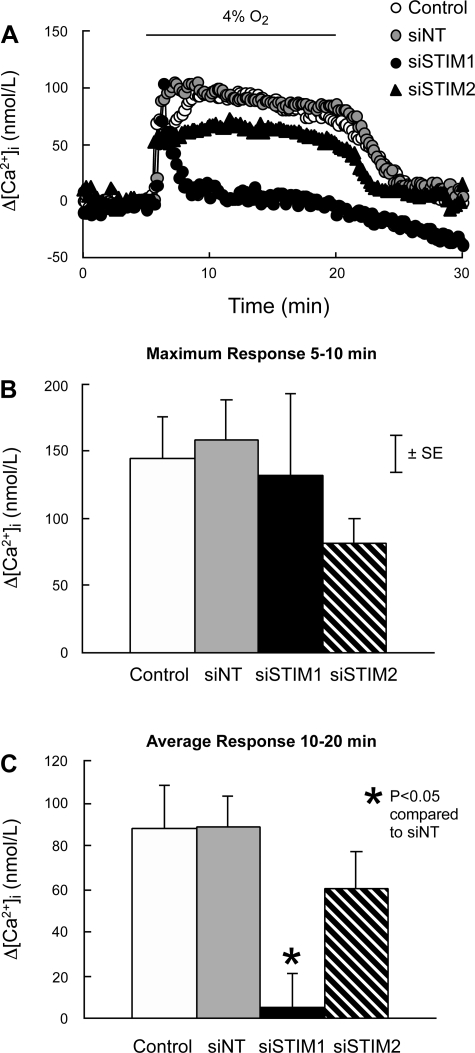
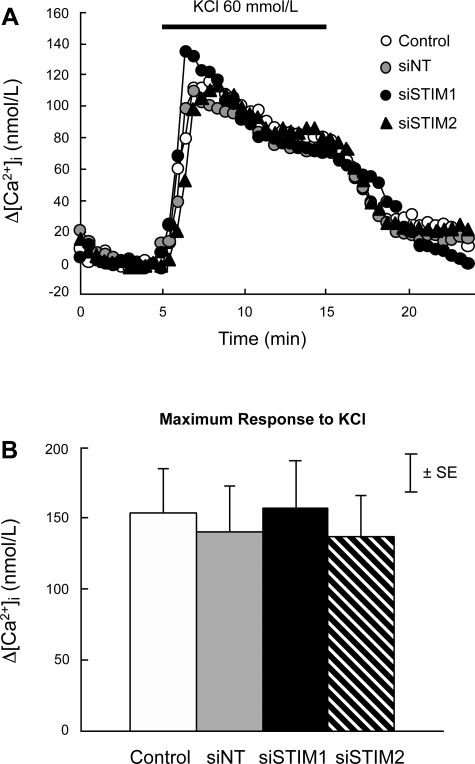
Baseline [Ca2+]i in PASMC perfused with normal KRBS averaged 141 ± 8 nmol/l and did not differ among groups (P > 0.7). In PASMC treated with siNT, hypoxia (4% O2) caused a rapid initial increase in [Ca2+]i followed by a slight decline to an elevated plateau (Fig. 7A). In these cells, the maximum increase in [Ca2+]i measured during the first 5 min of hypoxia and the mean increase in [Ca2+]i measured during 5–15 min of hypoxia averaged 158 ± 29.6 and 89 ± 14.6 nmol/l, respectively. Neither value was different from measurements in cells treated with siNT or siSTIM2 (Fig. 7, B and C). In PASMC treated with siSTIM1, the initial increase in [Ca2+]i was again not different (132 ± 60.8 nmol/l; P > 0.6); however, the sustained increase in [Ca2+]i was abolished (5.3 ± 15.5 nmol/l; P < 0.002). In contrast, the increase in [Ca2+]i caused by 60 mmol/l KCl (147 ± 15 nmol/l) was the same in all groups (Fig. 8).
Fig. 7.
A: effects of hypoxia (4% O2) on the time course of Δ[Ca2+]i from its average value at 3–5 min in PASMC perfused with normal KRBS. PASMC were untreated (Control; n = 9) or treated with siNT (n = 11), siSTIM1 (n = 7), or siSTIM2 (n = 5). B: average maximum Δ[Ca2+]i measured between 5 and 10 min in response to hypoxia in Control, siNT, siSTIM1, and siSTIM2 PASMC. C: mean Δ[Ca2+]i measured from 10 to 20 min in response to hypoxia in Control, siNT, siSTIM1, and siSTIM2 PASMC.
Fig. 8.
A: effects of KCl (60 mmol/l) on the time course of Δ[Ca2+]i from its value at 5 min (Δ[Ca2+]i) in PASMC perfused with normal KRBS. PASMC were untreated (Control; n = 8) or treated with siNT (n = 7), siSTIM1 (n = 5), or siSTIM2 (n = 5). B: average maximum Δ[Ca2+]i measured between 5 and 10 min in response to KCl in Control, siNT, siSTIM1, and siSTIM2 PASMC.
DISCUSSION
Knockdown of STIM1 or STIM2 expression by RNA interference in distal PASMC was highly specific and quantitatively significant for both mRNA and protein (Figs. 1–4). Furthermore, RNA interference was not cytotoxic, as indicated by calcein/EthD-1 staining and unaltered [Ca2+]i responses to KCl (Fig. 8).
STIM1 knockdown markedly inhibited SOCE, whether measured as the peak [Ca2+]i response to restoration of extracellular Ca2+ (Fig. 5, A and C) or quenching of fura 2 fluorescence by Mn2+ (Fig. 6). These findings are consistent with results in other smooth muscles, including murine aorta (8) and human airway (36), coronary artery (51), and saphenous vein (18), and confirm that STIM1 contributes significantly to SOCE in smooth muscle, as it does in other cells (9, 13, 17, 42, 58, 59, 62). It is unlikely that STIM1 knockdown inhibited SOCE by impairing CPA-induced depletion of SR Ca2+, since SR Ca2+ release, as measured by the transient increase in [Ca2+]i caused by CPA during Ca2+-free perfusion (Fig. 5, A and B), was not changed by siRNA treatment. More likely, inhibition was due to impaired sensing of SR Ca2+ depletion and transduction of SOC activation occurring as a result of decreased concentration of STIM1 protein (Fig. 3). As noted above, STIM1 has been documented to serve both functions in a wide variety of cells.
STIM1 may activate SOC by interacting with Orai and TRPC proteins (1, 2, 16, 19–21, 37, 56, 58). Orai1 is thought to be the pore-forming subunit of the Ca2+ release activated Ca2+ (CRAC) channel, the classic SOC found in hematopoietic cells, which exhibits high Ca2+-selectivity, low unitary conductance, and inward rectification (10, 13, 34, 41, 42). Orai1 appears to be widely expressed among cell types, but many of these did not exhibit CRAC channels. Nevertheless, SOCE was documented and found to be dependent on Orai1, suggesting that Orai1 regulates SOC other than CRAC channels (32, 37). Orai1 and its homologs, Orai2 and Orai3, have not yet been identified in PASMC. As an example of TRPC proteins, TRPC1 is thought to be a component of SOC found in smooth muscle and other cells, which have relatively low Ca2+ selectivity and high unitary conductance (34). In PASMC, TRPC1 was the most abundant of seven known TRPC isoforms at the mRNA level and about equal in abundance to STIM1 (Figs. 1 and 2; Ref. 53), with which it may interact to trigger SOCE (2, 14, 18, 23, 32, 58, 60). Further investigation will be necessary to determine how STIM1 interacts with Orai and TRPC proteins to regulate SOCE during hypoxia in PASMC.
The early increase in [Ca2+]i caused by hypoxia in PASMC is likely due to release of Ca2+ from SR (15, 29, 44, 52, 55, 63); therefore, persistence of this early hypoxic response in PASMC treated with siSTIM1 (Fig. 7) suggests that STIM1 knockdown did not alter hypoxia-induced release and depletion of SR Ca2+. STIM1 knockdown did, however, abolish the sustained increase in [Ca2+]i caused by hypoxia (Fig. 7) without altering the [Ca2+]i response to KCl (Fig. 8). In the absence of STIM1, SR Ca2+ depletion should be neither sensed nor signaled; therefore, SOC should not be activated. Since the sustained hypoxic response is due to Ca2+ influx (7, 44, 54), and store-operated (Figs. 5 and 6) but not voltage-operated Ca2+ entry (VOCE; Fig. 8) required STIM1, we conclude that the sustained [Ca2+]i response to hypoxia required activation of SOC.
In our (53–55) previous studies of distal PASMC, nifedipine, an antagonist of L-type Ca2+ channels, inhibited VOCE and [Ca2+]i responses to both hypoxia and KCl but did not block SOCE. Conversely, the SOC antagonists, SKF-96365, Ni2+, and La3+, inhibited SOCE and [Ca2+]i responses to hypoxia but not [Ca2+]i responses to KCl (53, 54). These effects are the same as those of STIM1 knockdown shown in Figs. 5–8. Together, these findings indicate that in distal PASMC hypoxia first caused SR Ca2+ release and activation of SOC followed by activation of L-type VOCC and VOCE, which made a quantitatively significant contribution to the sustained increase in [Ca2+]i.
Hypoxia-induced SR Ca2+ release and SOC activation could trigger VOCE in PASMC by shifting the current-voltage relation of L-type VOCC to lower potentials (11) and/or causing membrane depolarization (25, 31, 40, 61). The former was reported in rabbit distal PASMC (11); however, this observation remains unconfirmed and its underlying mechanism unknown. The latter could result directly from activation of SOC, which have reversal potentials near 0 mV and can be permeable to Na+ as well as Ca2+ (3, 28, 33, 49), or indirectly from Ca2+-dependent inhibition of voltage-dependent K+ channels (39) or activation of Ca2+-dependent Cl− channels (5). More investigation is needed to evaluate these possibilities.
A possible caveat to our conclusions about STIM1 is provided by data demonstrating that activation of arachidonic acid-regulated Ca2+ (ARC) channels, which have biophysical properties similar to CRAC channels, required STIM1 localized to plasma membrane but not store depletion, Ca2+ binding to the EF-hand region of STIM1, or translocation of STIM1 from endoplasmic reticulum to plasma membrane (26, 27, 48). These results suggest that STIM1 may regulate Ca2+ signaling pathways other than SOCE. The relevance of these results to PASMC is unclear, since ARC channels have been identified in pancreatic and parotid acinar cells and several cell lines but not smooth muscle.
In contrast to STIM1, knockdown of STIM2 did not significantly alter the [Ca2+]i response to restoration of extracellular Ca2+ in PASMC perfused with Ca2+-free KRBS containing CPA and nifedipine (Fig. 5). These results are consistent with findings in human airway and coronary arterial smooth muscle (36, 51) and suggest that STIM2 did not contribute to activation of SOC by CPA; however, this possibility is not supported by the Mn2+ quenching data (Fig. 6), which indicates that siSTIM2 caused a decrease in SOCE smaller than that caused by siSTIM1. This difference between the Ca2+ restoration and Mn2+ quenching experiments could be related to efflux of Ca2+ by sarcolemmal Ca2+ ATPase and/or uptake of Ca2+ by organelles other than SR in the Ca2+ restoration experiments. Such efflux and uptake could prevent or reduce a difference in [Ca2+]i due to a small difference in Ca2+ influx and thereby obscure an inhibitory effect of siSTIM2 on SOCE as measured by the [Ca2+]i response to Ca2+ restoration. This may also explain why STIM2 knockdown decreased Mn2+ quenching by ∼25% but did not significantly alter the sustained [Ca2+]i response to hypoxia (Fig. 7). Alternatively, STIM2 may not have been involved in hypoxic activation of SOC.
In other cell types, STIM2 has been found to have a smaller effect than STIM1 on SOCE (22, 30) or even to inhibit activation of SOCE by STIM1 (50). Because STIM2 appears to sense SR Ca2+ at higher concentrations than STIM1, it has been proposed that STIM2 regulates SOC activity under resting conditions to control basal [Ca2+]i (4). This did not appear to be true of PASMC, since basal [Ca2+]i in PASMC treated with siSTIM2 was not different from that in control PASMC or PASMC treated with siNT. The lack of effect of STIM2 knockdown on basal [Ca2+]i and the [Ca2+]i response to hypoxia, as well as its relatively small effect on Mn2+ quenching, could be due to a lower abundance of STIM2 relative to STIM1, as suggested by the qPCR data (Figs. 1 and 2).
In conclusion, STIM1 was quantitatively more important than STIM2 in activation of SOC in rat distal PASMC. Moreover, in the context of previous results (29, 54, 57), our data indicate that the increase in [Ca2+]i induced by acute hypoxia was initiated by SR Ca2+ release, followed by STIM1-dependent activation of SOC and SOCE-dependent activation of VOCE. The molecular identity of these SOC, how they are activated by STIM proteins, and how SOCE leads to VOCE remain to be determined.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-75113 (J. T. Sylvester) and HL-67191 (L. A. Shimoda), an American Heart Association Scientist Development Grant (J. Wang), a National Institutes of Health Independent Scientist Award (HL-079981; J. Wang), and an American Lung Association of Maryland Research Grant (J. Wang).
REFERENCES
- 1.Albert AP, Saleh SN, Peppiatt-Wildman CM, Large WA. Multiple activation mechanisms of store-operated TRPC channels in smooth muscle cells. J Physiol 583: 25–36, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambudkar IS, Ong HL, Liu X, Bandyopadhyay B, Cheng KT. TRPC1: the link between functionally distinct store-operated calcium channels. Cell Calcium 42: 213–223, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bae YM, Park MK, Lee SH, Ho WK, Earm YE. Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit. J Physiol 514: 747–758, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131: 1327–1339, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapp LH, Turner JL, Kozlowski RZ. Ca2+-activated Cl− currents in pulmonary arterial myocytes. Am J Physiol Heart Circ Physiol 270: H1577–H1584, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM. Acute hypoxia causes membrane depolarization and calcium influx in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 266: L469–L475, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Cornfield DN, Stevens T, McMurtry IF, Abman SH, Rodman DM. Acute hypoxia increases cytosolic calcium in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 265: L53–L56, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich A, Kalwa H, Storch U, Mederos YS, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflügers Arch 455: 465–477, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Dziadek MA, Johnstone LS. Biochemical properties and cellular localisation of STIM proteins. Cell Calcium 42: 123–132, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Franco-Obregon A, Lopez-Barneo J. Differential oxygen sensitivity of calcium channels in rabbit smooth muscle cells of conduit and resistance pulmonary arteries. J Physiol 491: 511–518, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harder DR, Madden JA, Dawson C. Hypoxic induction of Ca2+-dependent action potentials in small pulmonary arteries of the cat. J Appl Physiol 59: 1389–1393, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42: 173–182, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol 8: 1003–1010, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Kang TM, Park MK, Uhm DY. Characterization of hypoxia-induced [Ca2+]i rise in rabbit pulmonary arterial smooth muscle cells. Life Sci 70: 2321–2333, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kim MS, Zeng W, Yuan J, Shin DM, Worley P, Muallem S. Native store-operated Ca2+ influx requires the channel function of Orai1 and TRPC1. J Biol Chem, 2009. [DOI] [PMC free article] [PubMed]
- 17.Lewis RS The molecular choreography of a store-operated calcium channel. Nature 446: 284–287, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Sukumar P, Milligan CJ, Kumar B, Ma ZY, Munsch CM, Jiang LH, Porter KE, Beech DJ. Interactions, functions, and independence of plasma membrane STIM1 and TRPC1 in vascular smooth muscle cells. Circ Res 103: e97–e104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA 105: 2895–2900, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA 104: 4682–4687, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA 106: 3202–3206, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem 281: 28254–28264, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madden JA, Dawson CA, Harder DR. Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol 59: 113–118, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J Biol Chem 276: 35676–35683, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol 579: 703–715, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res 89: 923–929, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ng LC, Wilson SM, Hume JR. Mobilization of SR stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 563: 409–419, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 9: 432–443, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olschewski A, Hong Z, Nelson DP, Weir EK. Graded response of K+ current, membrane potential, and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 283: L1143–L1150, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem 282: 9105–9116, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parekh AB, Penner R. Store depletion and calcium influx. Physiol Rev 77: 901–930, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Parvez S, Beck A, Peinelt C, Soboloff J, Lis A, Monteilh-Zoller M, Gill DL, Fleig A, Penner R. STIM2 protein mediates distinct store-dependent and store-independent modes of CRAC channel activation. FASEB J 22: 752–761, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res 7: 119, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol 38: 744–749, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfaffl MW A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed]
- 39.Post JM, Gelband CH, Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res 77: 131–139, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol Cell Physiol 262: C882–C890, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Putney JW Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 42: 103–110, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robertson TP, Aaronson PI, Ward JP. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol Heart Circ Physiol 268: H301–H307, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Salvaterra CG, Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L323–L328, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Schwenke DO, Pearson JT, Umetani K, Kangawa K, Shirai M. Imaging of the pulmonary circulation in the closed-chest rat using synchrotron radiation microangiography. J Appl Physiol 102: 787–793, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Sham JS, Crenshaw BR, Deng LH, Shimoda LA, Sylvester JT. Effects of hypoxia in porcine pulmonary arterial myocytes: roles of KV channel and endothelin-1. Am J Physiol Lung Cell Mol Physiol 279: L262–L272, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Shirai M, Sada K, Ninomiya I. Effects of regional alveolar hypoxia and hypercapnia on small pulmonary vessels in cats. J Appl Physiol 61: 440–448, 1986. [DOI] [PubMed] [Google Scholar]
- 48.Shuttleworth TJ, Thompson JL, Mignen O. STIM1 and the noncapacitative ARC channels. Cell Calcium 42: 183–191, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol 140: 97–106, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol 16: 1465–1470, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, Nobori K, Iijima T, Ito H. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun 361: 934–940, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Vadula MS, Kleinman JG, Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol Lung Cell Mol Physiol 265: L591–L597, 1993. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Weigand LA, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Deng X, Hewavitharana T, Soboloff J, Gill DL. STIM, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin Exp Pharmacol Physiol 35: 1127–1133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium 42: 205–211, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 174: 803–813, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol 9: 636–645, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L116–L123, 1993. [DOI] [PubMed] [Google Scholar]
- 62.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia- but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125: 427–440, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]