Abstract
Angiotensin-converting enzyme 2 (ACE2) is a terminal carboxypeptidase and the receptor for the SARS and NL63 coronaviruses (CoV). Loss of ACE2 function is implicated in severe acute respiratory syndrome (SARS) pathogenesis, but little is known about ACE2 biogenesis and activity in the airways. We report that ACE2 is shed from human airway epithelia, a site of SARS-CoV infection. The regulation of ACE2 release was investigated in polarized human airway epithelia. Constitutive generation of soluble ACE2 was inhibited by DPC 333, implicating a disintegrin and metalloprotease 17 (ADAM17). Phorbol ester, ionomycin, endotoxin, and IL-1β and TNFα acutely induced ACE2 release, further supporting that ADAM17 and ADAM10 regulate ACE2 cleavage. Soluble ACE2 was enzymatically active and partially inhibited virus entry into target cells. We determined that the ACE2 cleavage site resides between amino acid 716 and the putative transmembrane domain starting at amino acid 741. To reveal structural determinants underlying ACE2 release, several mutant and chimeric ACE2 proteins were engineered. Neither the juxtamembrane stalk region, transmembrane domain, nor the cytosolic domain was needed for constitutive ACE2 release. Interestingly, a point mutation in the ACE2 ectodomain, L584A, markedly attenuated shedding. The resultant ACE2-L584A mutant trafficked to the cell membrane and facilitated SARS-CoV entry into target cells, suggesting that the ACE2 ectodomain regulates its release and that residue L584 might be part of a putative sheddase “recognition motif.” Thus ACE2 must be cell associated to serve as a CoV receptor and soluble ACE2 might play a role in modifying inflammatory processes at the airway mucosal surface.
Keywords: a disintegrin and metalloprotease 17, severe acute respiratory syndrome, coronavirus
severe acute respiratory syndrome (SARS) emerged as a regional and global health threat in 2002–2003 resulting in ∼8,000 cases and 800 deaths. The causative agent was identified as a novel human coronavirus (SARS-CoV; Refs. 8, 33, 51). Studies (13, 47) of patients with SARS demonstrated that the respiratory tract is a major site of SARS-CoV infection and disease-associated morbidity. In 2003, angiotensin-converting enzyme 2 (ACE2) was discovered as a receptor for the virus (39). ACE2 is expressed in vascular endothelia, lung, renal and cardiovascular tissues, testes, and epithelia of the small intestine (9, 17, 18). It is a terminal carboxypeptidase that cleaves a single residue from ANG II, generating ANG-(1-7) (10, 32, 60). In addition, ACE2 cleaves the terminal residues from several other bioactive peptides, including neurotensin, dynorphin A (1–13), apelin-13, and des-Arg bradykinin (9, 60). As a type I transmembrane protein, ACE2 is comprised of a short cytoplasmic domain, a transmembrane domain, and a large ectodomain (57). A region of the ACE2 ectodomain that includes the first α-helix and lysine 353 and proximal residues of the N-terminus of β-sheet 5 interacts with high affinity with the receptor-binding domain of the SARS-CoV S glycoprotein (40).
Previous studies (30) indicate that the susceptibility of human airway epithelia to infection by SARS-CoV correlates with the state of cell differentiation and ACE2 expression and localization. ACE2 is predominantly expressed on the apical surface of well-differentiated airway epithelia, especially ciliated cells (30, 56). Since a virus must attach to and enter cells before it can replicate, surface expression of ACE2 and the state of cell differentiation may directly influence SARS-CoV disease pathogenesis (30). Loss of pulmonary ACE2 function has been hypothesized to play an independent role in the acute lung injury associated with acid aspiration, sepsis, and SARS. This hypothesis, which remains controversial, suggests that ACE2 has a protective role in settings of acute lung injury. Imai et al. (27) observed in murine ARDS models that loss of ACE2 expression resulted in enhanced vascular permeability, increased lung edema, neutrophil accumulation, and worsened lung function. More importantly, treatment with catalytically active recombinant ACE2 protein improved the symptoms of acute lung injury in wild-type mice, as well as in ACE2 knockout mice, suggesting ACE2 plays a protective role in acute lung injury settings (27). Kuba et al. (34) further speculated that ACE2 and other components of the renin-angiotensin system may play a central role in controlling the severity of acute lung failure once a disease process, such as SARS, has started (34). Additional data indicate that SARS-CoV S protein or SARS virus infection directly downregulates pulmonary ACE2 expression. Together these studies indicate that loss of ACE2 catalytic function perturbs the pulmonary renin-angiotensin system, enhancing inflammation and vascular permeability. In addition to loss of ACE2 cleavage of renin-angiotensin system components, the failure to inactivate other ACE2 targets such as bradykinin metabolites and other vasoactive peptides might also contribute to SARS lung disease.
The surface abundance of many type I and type II transmembrane proteins may decrease in response to disease states (23, 26). The mechanisms for such reductions in cell surface abundance include decreased transcription and translation, increased protein internalization, and ectodomain shedding (20, 44). Lambert et al. (36) reported that a disintegrin and metalloprotease 17 [ADAM17; also known as TNFα cleavage enzyme (TACE)] is responsible for ACE2 shedding in ACE2-transfected HEK293 cells and endogenously ACE2 expressing Huh7 cells. In contrast, Allinson et al. (1) demonstrated that the ADAM10 and ADAM17 sheddases do not cleave the related protein ACE from the surface of HEK293 cells. Ectodomain shedding is important in regulating protein expression and function, and changes from membrane bound to soluble forms may be required for protein function. For example, the soluble TNFα receptor can alleviate cell and tissue damage caused by binding of this proinflammatory cytokine to the membrane bound form of the receptor (14, 43, 45). Furthermore, Gomez et al. (15) note that bacteria are physiological activators of TACE expression in human airway epithelia, initiating TACE-dependent release of IL-6 receptor-α and providing a mechanism to regulate inflammatory signaling in airway epithelia.
Given the central role of ACE2 in the pathogenesis of SARS-CoV infection and the observation that ACE2 is shed, we sought to investigate the biology of ACE2 release from the surface of human airway epithelia. We found that ACE2 is constitutively released from the apical cell surface, that sACE2 retains its enzymatic activity, and that sACE2 release is acutely enhanced by specific stimuli. Through studies of a series of modified and chimeric ACE2 proteins, we show that the cell-associated form of the protein is required for SARS-CoV infection and that sACE2 has modest inhibitory effects on viral infection efficiency. We further show that sACE2 is generated by proteolytic cleavage of membrane-associated ACE2 within the ectodomain region near its predicted transmembrane sequence. Interestingly, sACE2 release was prevented by the introduction of a single amino acid substitution in the ACE2 extracellular domain (L584A), suggesting this residue may reside within a putative recognition motif for the ACE2 sheddase. Both wild-type ACE2 and the ACE2 L584A mutant supported productive infection with the SARS-CoV, indicating that the generation of sACE2 is not required for the protein to function as a coronavirus receptor.
MATERIALS AND METHODS
Reagents
Unless indicated otherwise, reagent grade chemicals were obtained from Sigma Chemical (St. Louis, MO).
Cell Culture
Polarized epithelial cell culture.
Primary airway epithelia were isolated from human donor trachea or bronchi and grown at the air-liquid interface on collagen-coated polycarbonate filters as described previously (31). All preparations used were well differentiated (>2 wk old; resistance >1,000 Ω × cm2). This study was approved by the Institutional Review Board at the University of Iowa. Calu-3 cells were first cultured in EMEM supplemented with 10% FCS, 1% NEAA, 1% sodium pyruvate, 1% l-glutamine, 1% penicillin and streptomycin, and 0.15% NaHCO3. The cell cultures were maintained at 37°C in 5% CO2. When 100% confluent under submerged culture condition, the cells were transferred to collagen-coated polycarbonate filters as described previously (31) to generate polarized cells. The culture medium is the same as primary human airway epithelia (31).
Cell lines.
HEK293 cells and HeLa cells were obtained from ATCC. 17C-1 cells were a gift of Dr. Stanley Perlman (Department of Microbiology, University of Iowa). All cells were maintained at 37°C in 5% CO2. Culture media were as follows: 293 cells: 10% FBS and 1% penicillin-streptomycin in DMEM; HeLa cells: 10% FBS, 1% penicillin-streptomycin, and 1% NEAA in MEM; and 17C-1 cells: 5% FCS, 5% tryptose phosphate broth, and 1% penicillin-streptomycin in DMEM.
Immunoblot analysis.
Cells were lysed in 0.1% Triton X-100 in PBS, and total protein was determined using the BCA method (Pierce, Rockford, IL). Protein was separated by SDS-PAGE on precast 15% Tris·HCl gels (Bio-Rad, Hercules, CA) under reducing conditions and transferred to PVDF membranes. Goat anti-ACE2 primary polyclonal antibody (No. AF933; R&D Systems, Minneapolis, MN) and a HRP-conjugated mouse anti-goat secondary antibody were used to detect ACE2. In some experiments, an antibody that recognizes the C-terminal domain of ACE2 was used (Cat. No. SC17720, goat anti-ACE2 C-terminus; Santa Cruz Biotechnology). A human CD4 antibody (MAB3791; R&D Systems) and goat-anti mouse IgG (HRP conjugated) secondary were used to detect the CD4 protein. The HuT78 cell (gift from J. Houtman, Department of Microbiology, University of Iowa) was used as positive control for CD4 protein. Reactive proteins were visualized using SuperSignal chemiluminescent substrate (Pierce).
Indirect ACE2 ELISA.
To generate a standard curve, recombinant human ACE2 (R&D Systems) was coated onto 96-well immunoplates (Nunc, Rochester, NY) at concentrations of 2 ng/ml and seven consecutive twofold dilutions. The experimental samples (1:10 dilution) were coated onto the remainder of wells on the same immunoplate, and the plate was incubated at 37°C for 2 h or at 4°C overnight. The wells were washed with PBS Tween solution three times, and the goat anti-ACE2 polyclonal antibody (1:100) was added to each well and incubated at 37°C for 1 h, followed by three PBS Tween washes. A secondary rabbit anti-goat-antibody (HRP conjugated, 1:1,000 dilution) was added to the wells for an additional 1 h incubation at 37°C. After three washes, substrate reagent (R&D Systems) was added to wells at room temperature for 20 min, followed by addition of 2 N H2SO4 to stop the color reaction. Absorbance readings at 450 nM were made using a Biomax plate reader, and the ACE2 concentration was extrapolated from the standard curve.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was carried out in normal volunteers as described previously (42). Briefly, lavage was performed with five 10-ml aliquots of warmed normal saline at two to three separate sites. The first aliquot for each site was discarded. Lavage fluid was filtered through gauze and then centrifuged at 228 g for 5 min. Supernatants were stored at −80°C until gel electrophoresis. BAL fluid collected in this way is estimated to contain ∼1 ml of airway surface liquid for every 100 ml of recovered saline. For SDS-PAGE, equal volumes of this material (25 μl) were loaded for each sample. BAL samples were obtained using protocols approved by the Institutional Review Board of the University of Iowa.
Catalytic activity of ACE2.
ACE2 activity was determined in the cell washes collected by measuring the conversion of ANG II to ANG-(1-7) as previously described (55). Radiolabeled 125I-ANG II (2 × 106 cpm) was added to the cell washes for 1–2 h at 37°C containing a mixture of peptidase inhibitors to prevent the metabolism of either ANG II or ANG-(1-7) by other peptidases. At 1 and 2 h, 200 μl of the reaction vessel contents were mixed with 1% phosphoric acid to stop the reaction. The mixture was centrifuged (∼10,000 g, 1 min) and placed on ice until HPLC analysis. The radiolabeled products were separated by HPLC using a Waters NovaPak C18 narrow bore column (2 × 150 mm) and an inline gamma γ-detector to monitor the amount of radiolabel (55) as previously described. PMA (10 ng/ml) was added to the cells to stimulate sheddase activity and the release of ACE2 into the media. In some samples, the ACE2-specific inhibitor MLN was added to evaluate whether the conversion of ANG II to ANG-(1-7) was ACE2 dependent.
Inhibition and stimulation of ACE2 release.
For ADAM17 and ADAM10 inhibition studies, either the ADAM17 inhibitor DPC 333 ((2R)-2-[(3R)-3-amino-3{4-[2-methyl-4-quinolinyl) methoxy] phenyl}-2-oxopyrrolidinyl]-N-hydroxy-4-methylpentanamide)) (52), a gift from Bristol-Meyers Squibb) at 1.5 nM final concentration in Opti-MEM with or without PMA (10 ng/ml) or the ADAM10 inhibitor GI254023 (gift from Dr. Andreas Ludwig, Institute for Pharmacology and Toxicology, RWTH Aachen University, Aachen, Germany; Ref. 41) at 5 μM final concentration in Opti-MEM with or without 2.5 μM ionomycin was added to apical surface of the cells, and the apical liquid was collected after 3 h (for constitutive sACE2 release) and 1 h (for induced sACE2 release) for sACE2 ELISA assay. Before reagents were applied, cells were washed three times with PBS to remove accumulated sACE2. To induce sACE2 release airway epithelia were stimulated by applying the following reagents to the basal medium or to both cell surfaces: IL-1β (100 ng/ml), TNFα (100 ng/ml), PMA (10 ng/ml), ionomycin (2.5 μM), and LOS:MD-2 complex (1 ng/ml apical application only; Ref. 28). All reagents are diluted with Opti-MEM (Invitrogen) for apical application or diluted into culture medium for basolateral application. A total of 100 μl of Opti-MEM with or without reagents was added to cells and at each stimulation time point. sACE2 was recovered from cells by rinses with Opti-MEM, and concentrations were determined by ELISA.
Preparation of SARS S protein-pseudotyped lentivirus and infection of cells.
The SARS-CoV S protein cDNA (Urbani strain, “S-H2” as described in Ref. 39) was used to pseudotype feline immunodeficiency virus (FIV) expressing a nuclear targeted β-galactosidase using previously described methods (61). The virus was concentrated 250-fold by centrifugation and titered on HT1080 cells, obtaining titers of ∼2 × 105–4 × 106 transducing units/ml. Well-differentiated human airway epithelia were transduced with the pseudotyped FIV by applying 100 μl of solution to the apical surface of cells. After a 1 h incubation at 37° C, the virus was removed and cells incubated at 37° C for 2 days.
β-Galactosidase activity assays.
The Galacto-light chemiluminescent reporter assay (Tropix, Bedford, MA) was used to quantify β-galactosidase activity following the manufacturer's protocol. Relative light units were quantified using a luminometer (Monolight 3010; Pharmingen, San Diego, CA). To control for the efficiency of transfection for each ACE2 construct, a pGL3 plasmid expressing luciferase under the control of the SV40 promoter (Promega, Madison, WI) was cotransfected with the ACE2 cDNA vectors. β-Galactosidase activity was then normalized to relative luciferase activity for each experimental condition.
Surface biotinylation.
HEK293 cells cotransfected with plasmids expressing either wild-type ACE2, ACE2-L584A, and CAR receptor expressing vector were treated with 1 mg/ml N-hydroxysulfosuccinimidobiotin (Pierce) in PBS for 30 min at 25°C. The cells were washed and then incubated with 100 mM glycine in PBS for 20 min at 25°C to quench unreacted biotin. Total cell protein lysates were prepared by sonication in lysis buffer, and biotinylated proteins were precipitated using neutravidin covalently linked to immobilized diaminodipropylamine (Pierce). Biotinylated proteins were released in 8% SDS-containing loading buffer, boiled, and underwent immunoblot analysis for ACE2. P65 and CAR proteins were detected as control intracellular and membrane protein markers, respectively (59) using rabbit polyclonal anti-CAR antibody (a gift of J. Zabner, Department of Internal Medicine, University of Iowa) and an anti-P65 antibody (Santa Cruz).
Purification of sACE2 and identification of cleavage sites.
sACE2 was collected from Calu-3 cell cultures by washing the apical surface with PBS. The pooled washes were quantified by the BCA method (Pierce) and deglycosylated using N-glycanase PNGase F following the manufacturer's instructions (Glyko, San Leandro, CA). Deglycosylated samples were divided into two aliquots and sACE2 was isolated by parallel two-dimensional electrophoresis using isoelectric focusing in the pH 3–10 range followed by SDS-PAGE in 4–15% polyacrylamide gradient gels (Bio-Rad). One gel was transferred to a PVDF membrane and used for immunoblotting to identify the gel location of ACE2. The companion gel was stained with Coomassie blue, and the location of ACE2 was identified and cut from the gel. The sample then underwent in gel tryptic digestion and after extraction underwent fragment mapping using linear ion trap mass spectrometry (Thermo, Waltham, MA). Analysis of fragments was performed using the Mascot search engine (Matrix Science, Boston, MA).
Generation of Chimeric, Mutant, and Tagged ACE2 Proteins
Mutants.
All primers used to generate mutants were designed using the Quick-change Program (Stratagene) to substitute targeted residues with alanine by PCR using the human ACE2 cDNA in the pcDNA3.1 vector as a template. To generate the cytoplasmic tail deletion construct, a stop codon was introduced at position amino acid 763 of ACE2 (termed “ACE2-ΔCyto”; see Fig. 6A). The ACE2 stalk region deletion was generated by two-step PCR reactions with ACE2 cDNA as template for first round PCR and with two PCR products from the first round amplification as templates for the second round PCR reaction (termed “ACE2-del681-740”; see Fig. 6A). For first round PCR, one pair of primers consisted of the following: forward: 5′-AAGAAGGGTACCATGTCAAGCTCTTCCTGGCT-3′, and reverse: 5′-CCAAAAACAATCAGCCATATGGAGATTCTTGGTTTCAAAT-3′. A second pair of primers consisted of the following: forward: 5′-ATTTGAAACCAAGAATCTCCATATGGCTGATTGTTTTTGG-3′, and reverse: 5′-AAGAAGCGGCCGCCTAAAAGGAGGTCTGAACATCATCAGT-3′. The second round of PCR was conducted using the following PCR primers: forward: 5′-AAGAAGGGTACCATGTCAAGCTCTTCCTGGCTCC-3′, and reverse: 5′-AAGAAGCGGCCGCCTAAAAGGAGGTCTGAACATCATCAGT-3′. The PCR products were cloned into the pADV5 vector (2) with Kpn I and Not I restriction sites at either the 5′- or 3′-end.
Chimeras.
All ACE2 chimeras were made using the two-step PCR strategy as described above. The chimeric proteins contained portions of human ACE2 and portions of human CD4 (ACE2-CD4/TM-Cyto, ACE2-CD4/Ecto1, and ACE2-CD4/Ecto2; see Fig. 6A) or human β-defensin-2 (ACE2-SEC; see Fig. 4A). Human β-defensin-2 or human CD4 cDNAs were used as templates to generate the respective relevant fragments by PCR.
Immunohistochemistry.
17C-1 cells grown on chamber slides were transfected with plasmid cDNAs expressing either wild-type ACE2 or the ACE2-L584A mutant using Lipofectamine 2000. Two days later, the cells were rinsed with PBS and wild-type SARS-CoV (Urbani strain) was applied to the cells under BSL3 containment conditions. One hour after SARS-CoV application, cells were washed twice with PBS and fixed with 100% methanol for 1 h at −20°C. Fixed cells were next incubated with mouse anti-SARS-CoV N protein monoclonal antibody (FITC conjugated, gift of J. Nicholls, University of Hong Kong, Hong Kong) for detection of SARS-CoV protein. Immunostaining was visualized by confocal microscopy.
Statistical analysis.
Unless otherwise noted, all numerical data are presented as means ± SE. Statistical analysis was performed using a two-tailed, unpaired Student's t-test using Microsoft Excel software.
RESULTS
ACE2 Release Occurs at Sites of SARS-CoV Infection In Vitro and In Vivo
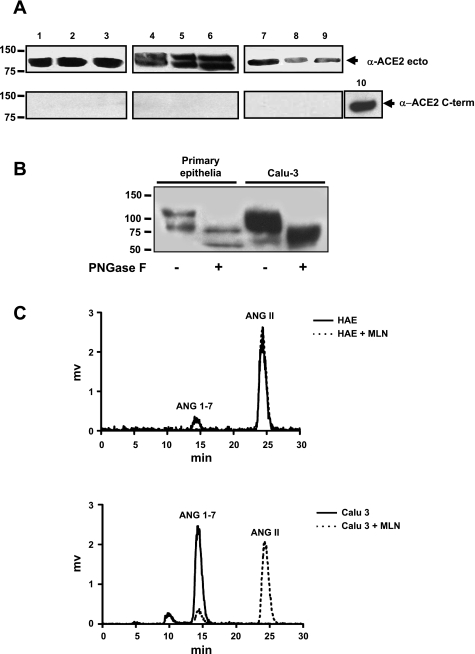
We previously showed that ACE2 is expressed predominantly at the apical surface of human airway epithelia, facilitating SARS-CoV entry (29). To determine whether ACE2 is also released from airway epithelia, we evaluated ACE2 shedding in well-differentiated primary cultures and the Calu-3 cell line. As shown in Fig. 1A, sACE2 was readily detected in apical washes of both cell types by immunoblot (lanes 1–6). Two prominent immunoreactive bands were noted in primary epithelia (∼120 and 95 kDa, lanes 4–6), suggestive of different glycoforms and/or sACE2 forms. We next asked whether ACE2 release occurs in the airways in vivo. Human BAL from normal subjects was similarly analyzed, and an immunoreactive band consistent with sACE2 was detected (Fig. 1A, lanes 7–9). The results indicate that at sites of SARS-CoV infection in the human airways, ACE2 is expressed and released into airway surface liquid. When the sACE2 recovered from primary airway epithelia was deglycosylated with N-glycanase PNGase F, the bands of ∼120 and 95 kDa were reduced to ∼85 and 70 kDa (Fig. 1B). For Calu-3 cells, the broad ∼120-kDa band was reduced to ∼85 kDa by PNGase F treatment. The estimated concentrations of sACE2 were determined by ELISA. Of note, under the same culture conditions and time intervals, much more sACE2 was generated and released by Calu-3 cells (63 ± 18 pg/cell; n = 3) than by primary epithelia (0.35 ± 0.3 pg/cell; n = 3). Cell-associated ACE2 was also higher in Calu-3 cells (21.8 ± 13.5 pg/cell; n = 3) than in primary epithelia (0.5 ± 0.3 pg/cell; n = 3).
Fig. 1.
Soluble angiotensin-converting enzyme 2 (sACE2) protein is detected in apical secretions of polarized epithelial cells and in human bronchoalveolar lavage fluid (BAL) and is enzymatically active. A, top: immunoblot for sACE2 using antibody that recognizes the extracellular portion of protein. Samples include secretions from Calu-3 cells (lanes 1–3), primary epithelia (3 different donor samples; lanes 4–6), and bronchoalveolar lavage (3 different donor samples; lanes 7, 8, and 9). When loaded on SDS-PAGE gel for electrophoresis, Calu-3 cell washes were diluted 10-fold in PBS, and BAL samples were concentrated 10-fold. A, bottom: immunoblot for ACE2 using an antibody against a C-terminal protein epitope. Lane 10 is a positive control consisting of cell lysate from 293 cells transfected with ACE2 expression plasmid. B: sACE2 recovered from primary airway epithelia or Calu-3 cells was deglycosylated with N-glycanase PNGase F; bands of ∼120 kD were reduced to ∼85 kDa. C: chromatograms from sACE2 enzymatic activity assay. Surface washes from human airway epithelia (HAE) or Calu-3 cells converted ANG II to ANG-(1-7). Addition of the ACE2-specific antagonist MLN into the reaction markedly inhibited the conversion.
sACE2 Is Enzymatically Active
To date, the known functions of ACE2 include terminal carboxypeptidase activity and its role as a receptor for the SARS and NL63 coronaviruses. Several peptides may be modified by ACE2 including ANG II, neurotensin, and des-Arg(9) bradykinin. Thus sACE2 may play a role in modifying peptides in airway surface liquid involved in processes such as inflammation. We next asked whether enzymatic activity is preserved after ACE2 is released from the cell surface. Apical secretions from primary cultures of airway epithelia or Calu-3 cells were coincubated with ANG II to assess the conversion of ANG II to ANG-(1-7). As shown in Fig. 1C, the secretions from both cell types converted ANG II to ANG-(1-7) and the converting activity was diminished when the ACE2-specific inhibitor MLN was added, indicating ACE2-dependent conversion. Because Calu-3 cells produce sACE2 in greater abundance than primary epithelia, the ACE2-dependent ANG II conversion was greater in Calu-3 cell secretions than in samples from primary epithelia. These findings indicate that the enzymatic properties of ACE2 are retained after its release from the cell surface.
Effect of sACE2 on SARS-CoV Infection
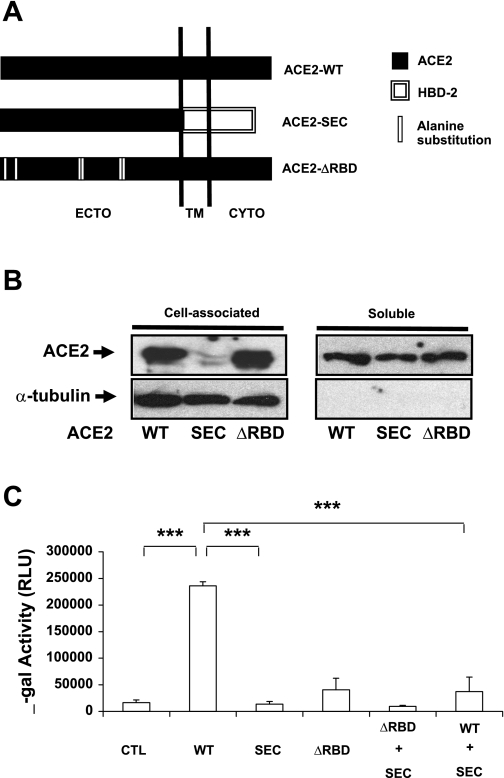
Since the ectodomain of ACE2 is released from epithelia, it may serve as a soluble viral receptor (38). To directly test the hypothesis that sACE2 inhibits SARS-CoV infection in airway epithelia, a series of modified ACE2 proteins were constructed (Fig. 2A). One, termed ACE2-SEC, contained the entire ACE2 ectodomain (residues 1-740) fused to the secretion signal peptide of the human β-defensin 2 gene. This construct allowed the ectodomain to be continuously released from cells (Fig. 2B). A second construct, termed ACE2-ΔRBD, contained alanine substitution mutations at residues 31, 41, 82–84, 353, 355, and 357. These residues are critical components of the ACE2-RBD that interacts with the SARS-CoV S protein (38). As shown in Fig. 2B, ACE2-ΔRBD was also released from cells. When SARS S protein pseudovirions were applied to HEK cells transfected with wild-type ACE2, the expected increase in β-galactosidase reporter gene expression was observed in contrast to ACE2 null cells (Fig. 2C). As anticipated, HEK293 cells expressing ACE2-SEC or ACE2-ΔRBD supported little transduction by SARS S pseudovirions. When ACE2-SEC and ACE2-ΔRBD were cotransfected into HEK293 cells, the transduction efficiency of S protein pseudotyped virions failed to increase. More interestingly, cotransfecting ACE2-SEC with ACE2 in HEK cells significantly reduced the S protein pseudovirus transduction efficiency compared with HEK cells transfected with wild-type ACE2 (Fig. 2C), consistent with sACE2 competition for S protein binding.
Fig. 2.
sACE2 acts as receptor binding SARS-CoV glycoprotein S pseudotyped FIV virus and blocks virus infection of target cells. A: schematic representation of chimeric and alanine substitution mutant of human ACE2. ACE2-SEC comprises the full ACE2 ectodomain (residues 1-740) and the human β-defensin-2 peptide (hBD2, residues 1-45); ACE2-ΔRBD contains full-length ACE2 residues but has alanine substitutions at residues 31, 41, 82–84, 353, 355, and 357. B: immunoblot immunodetection of cell-associated and sACE2 from HEK293 cells transfected with indicated expression vectors. C: β-galactosidase activity indicates SARS-CoV S protein pseudotyped FIV transduction efficiency in transfected cells. HEK293 cells were transfected with expression vectors. Forty eight hours later S protein pseudotyped FIV was added for 4 h and cells incubated at 37°C for 48 h, followed by measurement of β-galactosidase activity (normalized to luciferase transfection control). Data are means ± SE for triplicate samples in each group.
Regulation of ACE2 Release In Vitro
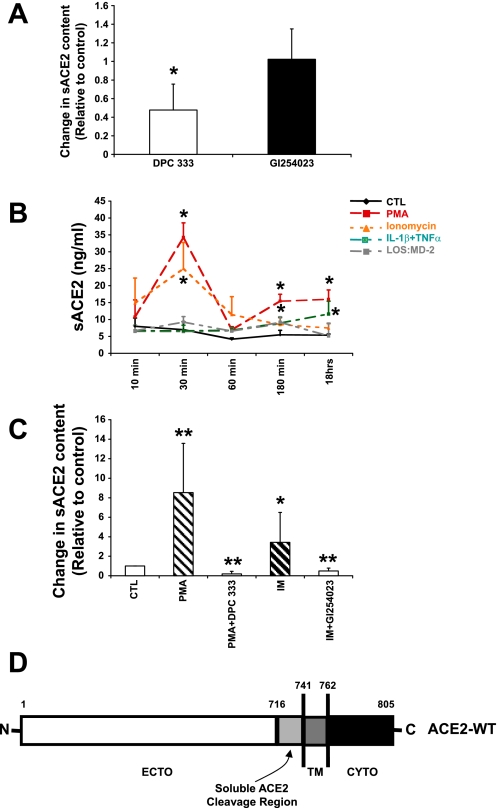
ADAM17 is implicated in the proteolytic cleavage of ACE2 in nonairway cell types (36). We asked whether chemical inhibitors of this sheddase reduced the constitutive release of sACE2 from polarized primary human airway epithelia. As shown in Fig. 3A, the ADAM17 inhibitor DPC 333 significantly reduced constitutive sACE2 generation, while the ADAM10 inhibitor GI254023 did not. To further investigate the regulation of ACE2 release from human airway epithelia, cells were treated with either PMA or ionomycin and a time course of ACE2 release was performed. At time intervals of 10 min to 18 h, apical secretions were collected from epithelia and sACE2 abundance was determined by ELISA. As shown in Fig. 3B, PMA, a stimulant of ADAM17 activity (36), increased ACE2 release at concentrations of 10 ng/ml in a time-dependent manner. sACE2 release peaked 30 min after stimulation and had decreased by 60 min after PMA treatment. sACE2 shedding remained stable between 180 min and 18 h after PMA stimulation. Ionomycin, an ADAM10 agonist (54), produced a similar increase in ACE2 release. The ionomycin-induced shedding peaked 30 min after stimulation and decreased by 60 min. Unlike PMA, ionomycin-induced sACE2 shedding gradually declined between 180 min and 18 h after stimulation. These data implicate both ADAM17 and ADAM10 in the release of ACE2 from airway epithelia. Also, as shown in Fig. 3B, the proinflammatory cytokines IL-1β and TNFα induced sACE2 release with maximum induction after 18 h of stimulation. Endotoxin complexed with the MD-2 protein (indicated as LOS:MD-2; Ref. 28), a TLR4 ligand, also moderately induced sACE2 release (Fig. 3B). These results demonstrate that ACE2 release from epithelia is constitutive and inducible. We next asked whether PMA- or ionomycin-stimulated sACE2 release was inhibited by DPC 333 or GI254023, respectively. As shown in Fig. 3C, after exposure to the indicated experimental conditions for 60 min, sACE2 was recovered from well-differentiated epithelial cells and the abundance was determined by ELISA. The findings demonstrate that PMA-induced ACE2 release is significantly blocked by the ADAM17 inhibitor, while ionomycin-stimulated ACE2 release was significantly decreased by the ADAM10 inhibitor.
Fig. 3.
Induction of sACE2 release from air-liquid interface cultures of airway epithelia. A: a disintegrin and metalloprotease 17 (ADAM17) is responsible for constitutive ACE2 shedding in primary human airway epithelial cells. The ADAM17 inhibitor DPC 333 at 1.5 nM concentration inhibited about 50% ACE2 shedding in airway epithelia (P < 0.05, compared with control group; means ± SE; n = 3). The ADAM10 inhibitor GI254023 (5 μM) did not significantly change constitutive ACE2 shedding. B: induction of sACE2 release from primary epithelia. Indicated stimuli were added to either the apical surface or both apical and basolateral sides of polarized cells and apical solution was collected at the indicated experimental time points for ACE2 ELISA assay. LOS:MD-2 is a TLR4 ligand. Data are means ± SE from 3 human donor samples. *P < 0.05, compared with the control group by Student's t-test. C: ADAM17 inhibitor DPC 333 (1.5 nM) blocks PMA-stimulated sACE2 release; the ADAM10 inhibitor GI254023 (5 μM) blocks ionomycin (IM) stimulated sACE2 release. Primary epithelia were treated with indicated conditions for 60 min and sACE2 recovered from apical surface and abundance determined by ELISA. Data are means ± SE from 6 human donor samples. *P < 0.05 and **P < 0.01, compared with the control group by Student's t-test. D: ACE2 cleavage site. Diagrammatic representation of protein sequence data. The identity of the C-terminal most peptide fragment indicates region where cleavage occurs.
Identification of sACE2 Cleavage Site
Determination of the site of ACE2 cleavage may provide insights into the sheddase responsible for protein release and allows a basis for comparison to the properties of the related protein ACE. To map the site of ACE2 cleavage by airway epithelia, apical washes from Calu-3 cells were deglycosylated and sACE2 was isolated and identified using two-dimensional gel electrophoresis. Samples then underwent tryptic digestion and linear ion trap mass spectrometry, followed by fragment analysis. The isolated fragment that was the most C-terminal and closest to the putative transmembrane domain contained ACE2 amino acids 711–716, with other fragments identified at more N-terminal locations. These results confirm that sACE2 contains the ectodomain and suggest the cleavage site is located between amino acids 716 and 741 (see Fig. 3D). These results are similar and extend other studies (16, 36) that have examined ACE2 cleavage in nonairway tissues and cells.
Structural Determinants of ACE2 Shedding
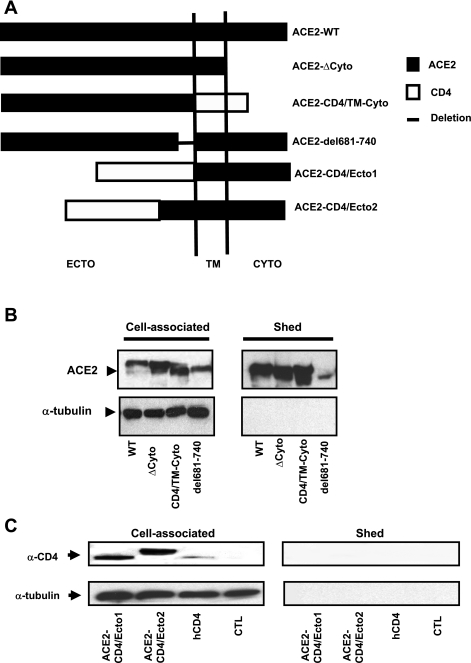
We presume that for ACE2 to serve as a coronavirus receptor it must remain cell associated. While the shedding of ACE2 appears to occur at the protein's juxtamembrane domain, it is possible that other domains of the protein influence this process, either by recruiting specific proteases to the site(s) of cleavage or by directly enhancing or inhibiting the dynamics of release. To characterize the structural determinants of ACE2 release, we used several strategies. First, a stop codon was introduced at the C-terminal end of the predicted transmembrane domain, removing the intracellular domain (ACE2-ΔCyto; Fig. 4A). Expression of this construct in HEK cells was associated with abundant sACE2 release (Fig. 4B), indicating that the ACE2 cytoplasmic domain is not required for sheddase activity. A second chimeric protein, termed ACE2-CD4/TM-Cyto (Fig. 4A), contained the ACE2 ectodomain fused to the transmembrane and intracellular domains of the human CD4, a protein that is not cleaved by sheddases. As shown in Fig. 4B, expression of ACE2-CD4/TM-Cyto in HEK cells caused no reduction in ectodomain release, demonstrating that the ACE2 transmembrane and intracellular domains are not required for protein release. To further confirm this observation, another chimeric protein containing the entire ectodomain of human CD4 was fused with the transmembrane and intracellular domains of ACE2 (ACE2-CD4/Ecto1; Fig. 4A). This construct exhibited no ectodomain shedding (Fig. 4C), again suggesting that the transmembrane and intracellular domains are not required for human ACE2 ectodomain shedding.
Fig. 4.
Structural determinants of sACE2 release. A: schematic representation of constructs. ACE2-ΔCyto comprises full-length ACE2 ecto and transmembrane domain (residues from 1-762); ACE2-CD4/TM-Cyto comprises ACE2 ectodomain (residues 1-740) and transmembrane, cytosolic domain of human CD4 (residues 397-458); ACE2-del681-740 contains full length ACE2 with deletion of residues between 681 and 740; ACE2-CD4/Ecto1 is constituted by CD4 ectodomain (residues 1-396) and transmembrane and cytosolic domain of ACE2 (residues 741-805); and ACE2-CD4/Ecto2 is composed of CD4 ectodomain (1-396) and ACE2 residues 621-850. B: Western blot detection of cell-associated and sACE2. Each lane was loaded with 5 μg total protein. C: ACE2 ectodomain sequence contributes to sACE2 generation. Immunoblot detection of cell-associated and soluble ectodomain using an anti-CD4 antibody. Each lane was loaded with 5 μg total protein. A human CD4 expression contruct (hCD4) served as a positive control.
The ACE2 juxtamembrane domain, or stalk region, presumably contains a sheddase cleavage site (Fig. 3D). To determine whether the primary sequence of this region is an important determinant in ACE2 shedding, we next deleted the first 60 amino acid residues of the predicted ACE2 ectodomain from the extracellular side (residues 681-740). This construct, termed ACE2-del681-740 (Fig. 4A), also conferred ACE2 shedding (Fig. 4B), suggesting that this juxtamembrane stalk region of ACE2 is not the major determinant of shedding. We constructed another chimeric protein, ACE2-CD4/Ecto2 (Fig. 4A), comprised of the N-terminal nonsheddable human CD4 ectodomain fused to the stalk, transmembrane domain, and 120 amino acid residues of the ACE2 ectodomain. ACE2-CD4/Ecto2 showed no shedding despite the inclusion of 120 AA of the ACE2 stalk region (Fig. 4C), demonstrating again that the juxtamembrane domain of ACE2 is not a primary determinant of its ectodomain shedding. We assessed the relative release of sACE2 for the wild-type ACE2, ΔCyto, CD4/TM-Cyto, and Del681-740 by ELISA. When expressed as a percentage of total (% sACE2/total ACE2), the ratios were similar: wild-type ACE2 (32%), ΔCyto (48.5%), CD4/TM-Cyto (32%), and Del681-740 (21.5%) (n = 3 for all conditions).
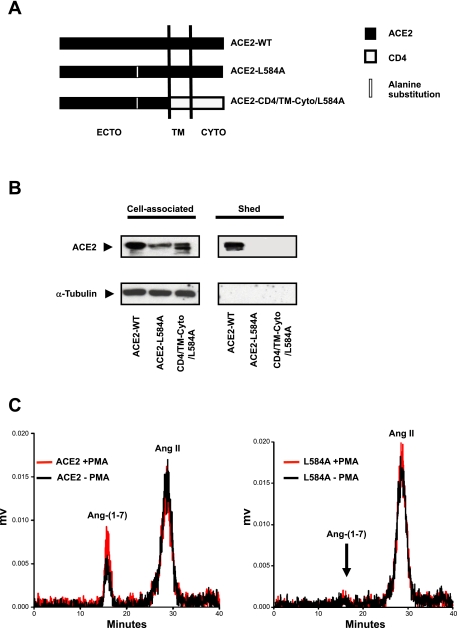
ACE2-L584A Mutant Attenuates Ectodomain Shedding
These data indicate that neither the cytoplasmic, transmembrane, nor juxtamembrane domains of ACE2 are determinants of constitutive shedding. The results further suggest that a site(s) or region(s) within the ACE2 ectodomain, at least 120 residues distal to the transmembrane domain, determines ACE2 release. The site(s) or region(s) might comprise a posited protease “recognition motif.” Since ADAM17 is implicated as a major protease for constitutive and PMA-induced ACE2 shedding and ADAM17 tends to cleave proteins near lipophilic or basic amino acids (37), we hypothesized that the ADAM17 recognition motif in ACE2 shares similar characteristics. To test this hypothesis, we mutated basic and lipophilic residues within the ACE2 ectodomain residing within 120 amino acids distal to the predicted transmembrane domain. By substituting alanine for several residues within this region, we identified a single mutation (ACE2-L584A) that prevented ACE2 release (Fig. 5A). Several additional point mutations near this site, including N580A, V581A, R582A, P583A, and V604A, had no effect on sACE2 release (data not shown). This result suggests an important role for L584 in the ACE2 ectodomain shedding process. The importance of this residue was further confirmed by introducing the L584A mutation into a second chimeric protein ACE2-CD4/TM-Cyto (termed ACE2-CD4/TM-Cyto/L584A; Fig. 5A). The L584A mutation conferred loss of shedding to both proteins (Fig. 5B). In contrast to wild-type ACE2, culture medium from ACE2-L584A-transfected cells lost ACE2 enzymatic activity and failed to convert ANG II to ANG-(1-7), providing further evidence that this mutant protein is not released from cells (Fig. 5C).
Fig. 5.
ACE2 mutant ACE2-L584A attenuates sACE2 release from cells. A: diagram of ACE2-L584A ACE2 mutants and chimeric ACE2-CD4/TM-Cyto (see Fig. 5A). B: immunodetection of cell-associated and sACE2. Results indicated that mutants of ACE2 (ACE2-L584A) and ACE2-CD4/TM-Cyto (ACE2-CD4/TM-Cyto/L584A) were not released from respective transfected HEK293 cells. C: chromatographs of ANG II to ANG (1–7) conversion by cell culture media collected from cells expressing either wild-type ACE2 or ACE2-L584A protein. Left: ACE2 transfected HEK293 cell culture medium efficiently converted ANG II to ANG (1–7) and PMA further enhanced the conversion efficiency. Right: culture medium from ACE2-L584A mutant shows no conversion of ANG II to ANG (1–7). PMA-stimulated ACE2-L584A transfected cell culture medium also showed no converting activity, consistent with an absence of shedding.
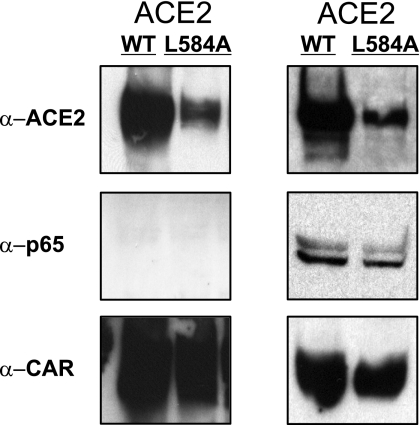
To test the possibility that the ACE2-L584A mutation altered ACE2 trafficking to the cell surface, we cotransfected HEK cells with wild-type and ACE2-L584A mutant cDNAs followed by surface biotinylation and immunoprecipitation of surface proteins with streptavidin and immunoblotting for ACE2. As shown in Fig. 6, ACE2-L584A and wild-type ACE2 colocalize in transfected cells. We conclude that the ACE2-L584A protein is displayed on the cell surface but not released by sheddase activity. We also asked whether ACE2-L584A retained the enzymatic activity of wild-type ACE2. Membrane preparations from HEK cells transfected with ACE2-L584A were used to detect ACE2 enzymatic activity. ANG II was added to the culture medium, and then the medium was subjected to HPLC analysis as described in Materials and Methods. We found thatACE2-L584A retained its enzymatic activity and converted ANG II to ANG-(1-7) (not shown).
Fig. 6.
Wild-type ACE2 and ACE2-L584A proteins are present at cell membrane. Left: HEK293 cells were cotransfected with wild-type ACE2 and a CAR receptor vector or ACE2-L584A mutant and a CAR receptor vector; 48 h after transfection, the cell surface was biotinylated and immobilized with neutravidin for immunoblot. The CAR protein serves as a positive control for a cell surface protein and MAPK P65 is a control for an intracellular protein. Right: immunodetection of same proteins as at left in the whole cell lysates of the samples at left.
Finally, we asked whether the nonshedding ACE2-L584A protein supported transduction of cells by SARS S protein pseudotyped FIV virions or wild-type SARS-CoV. As shown in Fig. 7A, cells expressing either the ACE2-L584A mutant or wild-type ACE2 were transduced by S protein pseudotyped FIV at similar efficiencies. In 17C-1 cells expressing either wild-type ACE2 or ACE2-L584A, we observed similar infection efficiencies as shown by immunostaining for the viral N protein (Fig. 7B) or by viral egress (Fig. 7C). Thus the membrane-associated form of ACE2 serves as a SARS-CoV receptor in vitro, and shedding is not required for infection to occur.
Fig. 7.
ACE2-L584A supports SARS-CoV entry. A: ACE2-L584A supports SARS-CoV S protein pseudotyped FIV transduction with efficiency similar to wild-type ACE2. ACE2-L584A and ACE2 ectodomain vectors (ACE2-SEC; see Fig. 2) were cotransfected into HEK293 cells. S protein pseudotyped FIV transduced cells coexpressing ACE2 and ACE2-SEC poorly, as assessed by β-galactosidase activity (normalized to luciferase transfection control as described in Materials and Methods). B: 17C-1 cells were transfected with plasmids expressing either wild-type ACE2 or ACE2-L584A. Next, cells were infected with SARS-CoV. Immunolocalization of SARS-CoV N protein (green) reveals similar levels of immunostaining. C: SARS-CoV titers of supernatants harvested from HEK cells transfected with wild-type ACE2 or ACE2-L584A. Data are means of 3 replicates.
DISCUSSION
The present studies are the first to show that ACE2 is released from human airway epithelial cells, an important initial site of infection in SARS. sACE2 retains its terminal carboxypeptidase activity in airway surface liquid, as well as its ability to bind the virus. The release of sACE2 from epithelia was both constitutive and inducible, regulated by sheddases and inflammatory stimuli. Through a series of experiments including the generation of a sheddase-resistant form of ACE2, we show that ACE2 must be cell attached to function as a SARS-CoV receptor.
While previous studies (9, 10) demonstrated soluble forms of ACE2 in human and murine tissues and fluids, no studies have directly examined the airways. It is likely that more than one sheddase generates sACE2 at the airway surface. Our findings with sheddase inhibitors and stimuli implicate both ADAM17 and ADAM10 in this process. Lambert et al. (36) concluded that ADAM17 is responsible for constitutive and PMA-induced ACE2 shedding from stably transfected HEK-ACE2 cells and endogenously expressing Huh7 cells. Furthermore, a recent report (35) showed that calmodulin inhibitors increase ACE2 shedding in HEK cells, suggesting that a calcium signaling pathway is involved in ACE2 release. PMA may also decrease the half-life of surface ADAM17, as ADAM17 was internalized in response to PMA stimulation (7). This could explain our observation that PMA-induced ACE2 shedding was transient and declined 30 min after stimulation. Our finding that ionomycin rapidly induced ACE2 shedding in human airway epithelial cells suggests a role for intracellular calcium signaling. ADAM10 is involved in ionomycin-induced shedding of CX3CL1 and CXCL16 from leukocytes and EGFR-ligand shedding from fibroblasts (24, 25). The rapid induction of ACE2 release by both PMA and ionomycin is consistent with enhanced sheddase activity rather than transcriptional regulation.
Endotoxin activates the shedding of receptors in several cell types (22, 48, 49, 62), a property attributed to modulation of ADAM17 activity (22). The increases in sACE2 after cytokine stimulation were not due to increased ACE2 mRNA transcription because quantitative PCR showed no ACE2 mRNA induction after IL-1β and TNFα stimulation (data not shown). Wyble et al. (64) reported that IL-1β and TNFα induce E-selectin shedding and participate in the later inflammatory response. Our observations that endotoxin and the proinflammatory cytokines IL-1β and TNFα all enhanced ACE2 shedding from airway epithelia led us to speculate that sACE2 generation may have host defense functions.
Ectodomain shedding influences the biological activity of several proteins. For example, shedding of heparin-binding EGF-like growth factor results in its activation, and the released fragment binds to EGF receptors and stimulates cell proliferation (19, 21). In contrast, the membrane-bound forms of Kit ligand (12) and ephrins, the latter representing the ligands of Eph receptor tyrosine kinases, are fully functional, while soluble forms generated by ectodomain shedding have little or no biological activity (12). Our studies of sACE2 released from airway epithelia revealed two activities for the protein. sACE2 from primary human airway epithelia and Calu-3 cells retained its enzymatic activity and converted ANG II to ANG-(1-7). This indicates that sACE2, like other structurally and functionally diverse proteins including adhesion molecules, cytokines, growth factors, and their receptors (11, 20, 50), is released from epithelial cells and into airway surface liquid in a biologically active form. In addition to its soluble carboxypeptidase activity, sACE2 retains its coronavirus receptor properties.
Like ACE2, the human ACE protein also undergoes ectodomain shedding and the cleavage site was identified between Arg1203 and Ser1204, 27 residues on the extracellular side of the transmembrane domain (63). Sadhukhan et al. (53) reported that rabbit pulmonary and testis ACE undergoes ectodomain shedding at two sites, one major site is between Arg663 and Ser664 and a minor site is between Arg673 and Val674, which are 27 and 17 residues away from the transmembrane domain, respectively. In the present study, we localized the region of ACE2 cleavage between amino acids 716 and 741, near the predicted transmembrane domain.
The structural determinants of proteins that are shed from cell surfaces are diverse. For example, the cytoplasmic domains of pro-TGF-α and the 80-kDa TNFα receptor are required for the shedding of these two molecules (3, 6), whereas Kit ligand, IL-6 receptor, β-APP, and ACE are shed to the same extent as the wild-type molecules, even with the deletion of their cytoplasmic domains (5, 46, 53). Our results indicate that ACE2 shares features of the second group of shed proteins and that its cytoplasmic domain is not critical for its constitutive ectodomain shedding. Even the complete substitution of the ACE2 transmembrane and cytoplasmic domains with that of the nonsheddable CD4 did not prevent ectodomain shedding. This observation is similar to results reported by Sadhukhan et al. (53) for ACET. Recently, Haga et al. (16) reported that the cytosolic domain of human ACE2 is important for SARS S protein-induced ACE2 shedding. In that study, expression of an ACE2 cDNA truncated by deletion of the 32 most C-terminal amino acids resulted in diminished ACE2 shedding when expressed in VeroE6 cells under resting or SARS S protein pseudovirion stimulated conditions. In contrast, when we deleted the 44 most C-terminal amino acids from ACE2 and expressed the construct in HEK 293 cells, we observed no change in constitutive shedding, suggesting no involvement of the cytosolic domain in the regulation of the basal release of sACE2. These disparate findings may reflect the cell types selected for protein expression or subtle differences in the ACE2 cDNAs expressed. Our ACE2 deletion mutant, ACE2-del681-740, with 60 residues removed from the proximal ectodomain, retained ectodomain shedding, indicating that for both ACE2 and ACE (53) ectodomain shedding is not determined by the residues around its juxtamembrane stalk region. This supposition is further supported by the finding that the chimeric protein ACE2-CD4/Ecto2, containing 120 residues of the ACE2 proximal ectodomain, failed to cause CD4 ectodomain release.
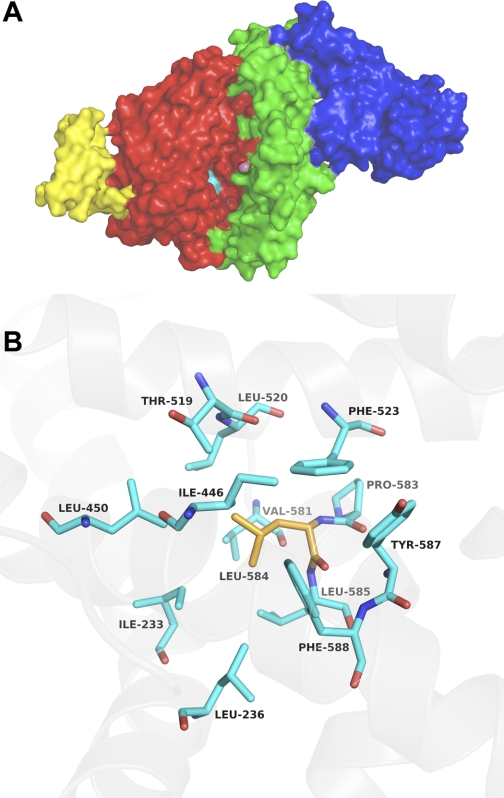
An important finding in current study is the identification of a single mutation, L584A, that prevented shedding of sACE2. Sheddase “recognition motifs” are speculated to reside in distal portions of the ectodomain of released proteins, and these elements are determined by the higher order structure of the shed protein. Therefore, they are not readily predicted from the primary protein sequence (4, 53). The human ACE2 crystal structure revealed it contains 20 α-helical segments (58). In the L584A mutant, a hydrophobic leucine was replaced by alanine at the end of 20th helix (38). The ACE2 structure models in Fig. 8 demonstrate the relationships between the site of the L584A mutation near the catalytic domain, the receptor binding domain, and the bound SARS S protein. We speculate that this mutation modifies the tertiary structure of ACE2 by interrupting interactions among neighboring hydrophobic residues (Fig. 8B). Such a change in protein structure may interfere with sheddase interactions, thereby altering ectodomain cleavage. We show that the ACE2-L584A protein present at the cell surface retained its virus binding properties but was not shed, indicating that L584 plays a role in dictating ACE2 ectodomain shedding. Carboxypeptidase activity assays of cell-associated ACE2-L584A indicate that its activity is preserved. While the SARS-CoV S protein may require few ACE2 molecules resident on the cell surface to facilitate virus binding and entry, we observed no difference in susceptibility to SARS-CoV infection in cells expressing either wild-type ACE2 or ACE2-L584A.
Fig. 8.
A: surface representation of ACE2 protein (pdb id 1R42) with the 2 catalytic subdomains in red (103–289, 398–416, 431–615) and green (19–102, 290–397, 417–430) and the catalytic zinc (purple sphere). In yellow is the partial collectrin domain of ACE2 from the X-ray structure (pdb id 1R42) and in blue is the SARS CoV spike receptor-binding domain (from pdb id 2AJF) bound to ACE2. Loss of shedding by the L584A ACE2 mutant suggests that this residue and the hydrophobic core it is part of (shown in cyan) may be a putative sheddase recognition motif. This hydrophobic core is close to the hinge axis of the catalytic subdomains and its destabilization could affect catalysis by interfering with the hinge motion. This mutation is, however, far away from the SARS-CoV spike receptor-binding domain's binding site and unlikely to directly affect its binding. B: close-up view of the hydrophobic core (cyan) around L584 (orange).
In summary, the release of ACE2 from the apical surface of human airway epithelia into airway surface liquid is a dynamic, regulated process. While sACE2 retains enzymatic activity, its native substrates in the airways are yet to be identified. ACE2 must be cell associated to serve as a coronaviruses receptor, and we speculate that sACE2 might play a role in modifying inflammatory processes at the airway mucosal surface.
GRANTS
We thank Stanley Perlman for critical comments on the manuscript. We thank Jian Shao for technical advice and assistance. We thank Yalan Li at the University of Iowa Proteomics Core Facility and Curtis Wilkerson at the Michigan State University Mass Spectrometry Facility for expert assistance in protein analysis. We thank Lois D. Lehman-McKeeman at Bristol-Myers Squibb and Dr. Andreas Ludwig, IZKF Research Group, for providing the chemical inhibitors of ADAM17 and ADAM10, respectively.
Acknowledgments
We acknowledge the support of National Institutes of Health Grants PO1 AI-060699 (to P.B. McCray, Jr., and D.C. Look) and PO1 HL-051952 (to M. C. Chappell). We acknowledge the support of the Cell Culture and Gene Transfer Vector Cores, partially supported by the Center for Gene Therapy for Cystic Fibrosis (National Institutes of Health Grant P30 DK-54759) and the Cystic Fibrosis Foundation, for preparing airway epithelial cultures and adenoviral vectors.
REFERENCES
- 1.Allinson TM, Parkin ET, Condon TP, Schwager SL, Sturrock ED, Turner AJ, Hooper NM. The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur J Biochem 271: 2539–2547, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 7: 1034–1038, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bosenberg MW, Pandiella A, Massagué J. The cytoplasmic carboxy-terminal amino acid specifies cleavage of membrane TGF alpha into soluble growth factor. Cell 71: 1157–1165, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Engel P, Tedder TF. Structural requirements regulate endoproteolytic release of the l-selectin (CD62L) adhesion receptor from the cell surface of leukocytes. J Exp Med 182: 519–530, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng HJ, Flanagan JG. Transmembrane kit ligand cleavage does not require a signal in the cytoplasmic domain and occurs at a site dependent on spacing from the membrane. Mol Biol Cell 5: 943–953, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowe PD, VanArsdale TL, Goodwin RG, Ware CF. Specific induction of 80-kDa tumor necrosis factor receptor shedding in T lymphocytes involves the cytoplasmic domain and phosphorylation. J Immunol 151: 6882–6890, 1993. [PubMed] [Google Scholar]
- 7.Doedens JR, Black RA. Stimulation-induced down-regulation of tumor necrosis factor-alpha converting enzyme. J Biol Chem 275: 14598–14607, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, Chan KP, Lam TH, Tse LY, Tsang T, Liu SH, Kong JH, Lau EM, Ferguson NM, Anderson RM. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361: 1761–1766, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–9, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol 35: 1043–1053, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Botran R Soluble cytokine receptors: basic immunology and clinical applications. Crit Rev Clin Lab Sci 36: 165–224, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell 64: 1025–1035, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Franks TJ, Chong PY, Chui P, Galvin JR, Lourens RM, Reid AH, Selbs E, McEvoy CP, Hayden CD, Fukuoka J, Taubenberger JK, Travis WD. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol 34: 743–748, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 370: 555–557, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Gomez MI, Sokol SH, Muir AB, Soong G, Bastien J, Prince AS. Bacterial induction of TNF-alpha converting enzyme expression and IL-6 receptor alpha shedding regulates airway inflammatory signaling. J Immunol 175: 1930–1936, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Haga S, Yamamoto N, Nakai-Murakami C, Osawa Y, Tokunaga K, Sata T, Yamamoto N, Sasazuki T, Ishizaka Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci USA 105: 7809–7814, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532: 107–110, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem 269: 20060–20066, 1994. [PubMed] [Google Scholar]
- 20.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol 64: 135–146, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251: 936–939, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, Givan AL, Wallace PK, Yeager MP, Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol 72: 711–717, 2002. [PubMed] [Google Scholar]
- 23.Hooper NM, Karran EH, Turner AJ. Membrane protein secretase. Biochem J 321: 265–279, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel CP. Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol Biol Cell 18: 176–188, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, Winter U, Paliga K, Reiss K, Saftig P, Weber C, Ludwig A. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol 178: 8064–8072, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Huovila AP, Turner AJ, Pelto-Huikko M, Kärkkäinen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci 30: 413–422, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB Jr. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol 287: L428–L437, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Jia HP, Look DC, Hickey M, Shi L, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. Infection of human airway epithelia by SARS coronavirus is associated with ACE2 expression and localization. Adv Exp Med Biol 581: 479–484, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karp PH An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 115–137, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature 375: 146–148, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953–1966, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert DW, Clarke NE, Hooper NM, Turner AJ. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett 582: 385–390, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambert MH, Blackburn RK, Seaton TD, Kassel DB, Kinder DS, Leesnitzer MA, Bickett DM, Warner JR, Andersen MW, Badiang JG, Cowan DJ, Gaul MD, Petrov KG, Rabinowitz MH, Wiethe RW, Becherer JD, McDougald DL, Musso DL, Andrews RC, Moss ML. Substrate specificity and novel selective inhibitors of TNF-alpha converting enzyme (TACE) from two-dimensional substrate mapping. Comb Chem High Throughput Screen 8: 327–339, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309: 1864–1868, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, Wong SK, Huang IC, Xu K, Vasilieva N, Murakami A, He Y, Marasco WA, Guan Y, Choe H, Farzan M. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 24: 1634–1643, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig A, Hundhausen C, Lambert MH, Broadway N, Andrews RC, Bickett DM, Leesnitzer MA, Becherer JD. Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules. Comb Chem High Throughput Screen 8: 161–171, 2005. [DOI] [PubMed] [Google Scholar]
- 42.McCray PB Wang G, Kline JN, Zabner J, Chada S, Jolly DJ, Chang SM, and Davidson BL. Alveolar macrophages inhibit retrovirus-mediated gene transfer to airway epithelia. Hum Gene Ther 8: 1087–1093, 1997. [DOI] [PubMed] [Google Scholar]
- 43.McGeehan GM, Becherer JD, Bast RC, Boyer CM, Champion B, Connolly KM, Conway JG, Furdon P, Karp S, Kidao S, McElroy AB, Nichols J, Pryzwansky KM, Schoenen F, Sekut L, Truesdale A, Verghese M, Warner J, Ways JP. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature 370: 558–561, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Meissner U, Blum H, Schnare M, Röllinghoff M, Gessner A. A soluble form of the murine common gamma chain is present at high concentrations in vivo and suppresses cytokine signaling. Blood 97: 183–191, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS, Torrance DS, Otten-Evans C, Greenstreet T, Weerawarna K. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 370: 218–220, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Müllberg J, Oberthür W, Lottspeich F, Mehl E, Dittrich E, Graeve L, Heinrich PC, Rose-John S. The soluble human IL-6 receptor. Mutational characterization of the proteolytic cleavage site. J Immunol 152: 4958–4968, 1994. [PubMed] [Google Scholar]
- 47.Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, Yan KW, Chan KH, Tsang NC, Guan Y, Yuen KY, Peiris JS. Lung pathology of fatal severe acute respiratory syndrome. Lancet 361: 1773–1778, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedron T, Girard R, Chaby R. TLR4-dependent lipopolysaccharide-induced shedding of tumor necrosis factor receptors in mouse bone marrow granulocytes. J Biol Chem 278: 20555–20564, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Penton-Rol G, Orlando S, Polentarutti N, Bernasconi S, Muzio M, Introna M, Mantovani A. Bacterial lipopolysaccharide causes rapid shedding, followed by inhibition of mRNA expression, of the IL-1 type II receptor, with concomitant up-regulation of the type I receptor and induction of incompletely spliced transcripts. J Immunol 162: 2931–2938, 1999. [PubMed] [Google Scholar]
- 50.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ. Identification of severe acute respiratory syndrome in Canada. N Engl J Med 348: 1995–2005, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Qian M, Bai SA, Brogdon B, Wu JT, Liu RQ, Covington MB, Vaddi K, Newton RC, Fossler MJ, Garner CE, Deng Y, Maduskuie T, Trzaskos J, Duan JJ, Decicco CP, Christ DD. Pharmacokinetics and pharmacodynamics of DPC 333 ((2R)-2-((3R)-3-amino-3{4-[2-methyl-4-quinolinyl) methoxy] phenyl}-2-oxopyrrolidinyl)-N-hydroxy-4-methylpentanamide)), a potent and selective inhibitor of tumor necrosis factor alpha-converting enzyme in rodents, dogs, chimpanzees, and humans. Drug Metab Dispos 35: 1916–1925, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Sadhukhan RSG, Ramchandran R, Sen I. The distal ectodomain of angiotensin-converting enzyme regulates its cleavage-secretion from the cell surface. Proc Natl Acad Sci U S A 95: 138–143, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scholz F, Schulte A, Adamski F, Hundhausen C, Mittag J, Schwarz A, Kruse ML, Proksch E, Ludwig A. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. J Invest Dermatol 127: 1444–1455, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Sims Baric RS AC, Yount B, Burkett SE, Collins PL, Pickles RJ. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J Virol 79: 15511–15524, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 27: 33238–33243, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 279: 17996–18007, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422: 322–326, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277: 14838–14843, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Wang G, Slepushkin VA, Zabner J, Keshavjee S, Johnston JC, Sauter SL, Jolly DJ, Dubensky T, Davidson BL, McCray PB Jr. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest 104: R49–R56, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver LK, Hintz-Goldstein KA, Pioli PA, Wardwell K, Qureshi N, Vogel SN, Guyre PM. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol 80: 26–35, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Woodman ZL, Oppong SY, Cook S, Hooper NM, Schwager SL, Brandt WF, Ehlers MR, Sturrock ED. Shedding of somatic angiotensin-converting enzyme (ACE) is inefficient compared with testis ACE despite cleavage at identical stalk sites. Biochem J 347: 711–718, 2000. [PMC free article] [PubMed] [Google Scholar]
- 64.Wyble CW, Hynes KL, Kuchibhotla J, Marcus BC, Hallahan D, Gewertz BL. TNF-alpha and IL-1 upregulate membrane-bound and soluble E-selectin through a common pathway. J Surg Res 73: 107–112, 1997. [DOI] [PubMed] [Google Scholar]