Abstract
Hyperoxia disrupts postnatal lung development in part through inducing inflammation. To determine the contribution of leukocyte-derived reactive oxygen species, we exposed newborn wild-type and NADPH oxidase p47phox subunit null (p47phox−/−) mice to air or acute hyperoxia (95% O2) for up to 11 days. Hyperoxia-induced pulmonary neutrophil influx was similar in wild-type and p47−/− mice at postnatal days (P) 7 and 11. Macrophages were decreased in wild-type hyperoxia-exposed mice compared with p47phox−/− mice at P11. Hyperoxia impaired type II alveolar epithelial cell and bronchiolar epithelial cell proliferation, but depression of type II cell proliferation was significantly less in p47−/− mice at P3 and P7, when inflammation was minimal. We found reciprocal results for the expression of the cell cycle inhibitor p21cip/waf in type II cells, which was induced in 95% O2-exposed wild-type mice, but significantly less in p47phox−/− littermates at P7. Despite partial preservation of type II cell proliferation, deletion of p47phox did not prevent the major adverse effects of hyperoxia on alveolar development estimated by morphometry at P11, but hyperoxia impairment of elastin deposition at alveolar septal crests was significantly worse in wild-type vs. p47phox−/− mice at P11. Since we found that p47phox is expressed in a subset of alveolar epithelial cells, its deletion may protect postnatal type II alveolar epithelial proliferation from hyperoxia through effects on epithelial as well as phagocyte-generated superoxide.
Keywords: bronchopulmonary dysplasia, reactive oxygen species, neutrophil cytosolic factor-1, p47phox
postnatal lung development is disrupted by oxidative stress, particularly in the very immature lung (9). Disrupted alveolar development is a hallmark of modern day bronchopulmonary dysplasia (BPD) that develops in premature newborns requiring oxygen therapy and mechanical ventilation (17). Hyperoxia-impaired postnatal alveolar development in newborn rats is opposed by blocking neutrophil influx (3, 32) that contributes to DNA damage (6), protein nitration, and lipid peroxidation (18). Selective attenuation of maladaptive inflammatory mechanisms that contribute to oxidative stress during a critical window of postnatal lung development could represent a therapeutic approach towards preventing BPD.
Release of superoxide by leukocytes via the respiratory burst can lead to downstream generation of reactive oxygen intermediates, •OH, H2O2, peroxyl radicals, etc., that can damage proteins, lipids, and nucleic acids (12). Protecting against superoxide through overexpression of extracellular superoxide dismutase (SOD3) in hyperoxia-exposed newborn mice preserved alveolar type II epithelial proliferation (5) and protected postnatal lung development (1).
We therefore sought to determine whether or not neutrophil-derived superoxide generation is a major contributor to hyperoxia-impaired postnatal alveolar development. Leukocytes generate superoxide through NADPH oxidase, which requires the p47phox organizer protein (also known as neutrophil cytosolic factor-1, NCF-1) for transmembrane localization and cytochrome activation (28). Mice lacking p47phox have deficient leukocyte superoxide generation (16). Since p47phox is also expressed in non-phagocytic cells in the lung, determination of the leukocyte-derived superoxide contribution would ideally have been accomplished through adoptive transfer of bone marrow from p47phox null mice into marrow-ablated mice, but this is not feasible in newborn mice.
As a first approximation, we therefore exposed p47phox null (p47phox−/−) or wild-type C57 black 6 (C57BL/6) newborn mice to hyperoxia, beginning at birth, for 3, 7, or 11 days, traversing the transition from saccular to alveolar stages of lung development. We found that p47phox−/− mice were significantly protected against hyperoxia-induced impairment of alveolar type II proliferation at postnatal days (P) 3 and 7, as well as against impairment of septal crest elastin deposition, but not against impairments of alveolar number and surface area. The effects on type II cell proliferation were most prominent before significant lung inflammation developed. Hyperoxia also induced p47phox expression in some type II cells as well as Clara cells at P7. The endogenous superoxide effects on type II epithelial cells at an early stage of postnatal hyperoxia exposure are not exclusively mediated via phagocytes, but possibly through alveolar and bronchiolar expressed p47phox.
METHODS
Reagents
Bromodeoxyuridine (BrdU) and anti-BrdU were from Zymed (South San Francisco, CA). Polyclonal rabbit anti-SP-C clone C-19, rabbit anti-p47phox clone SC-7660, and blocking peptide (SC-7660P) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-CC10 (also known as Clara cell secretory protein and Sctgb1a1) was obtained from Upstate Cell Signaling Solutions (Lake Placid, NY). Monoclonal anti-mouse p21cip/waf (clone sx-118) was from BD Biosciences (San Jose, CA). Polyclonal rabbit anti-β-actin was from Abcam (Cambridge, MA). Anti-α-actin-Cy3 was from Sigma (St. Louis, MO). Anti-elastin (RA-75) was from Elastin Products (Owensville, MO). Secondary antibodies, chromogens, and detection reagents were from Vector (Burlingame, CA) except where otherwise noted. Custom primers were from Integrated DNA Technologies (Coralville, IA). Other reagents are from Sigma except where noted.
Hyperoxia and Air Exposures
Animal experimental procedures were approved by the Institutional Animal Care and Use Committee. The neonatal hyperoxia exposure regimen has been previously described in detail (1). The p47phox−/− mice were a generous gift of Dr. Steven Holland (NIH, Bethesda, MD) and have been described previously (16). All exposures were conducted in mice that were further backcrossed >5 generations with C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). Newborn mice delivered spontaneously to time-mated pregnant dams (heterozygote crosses) were exposed with dams to air or 95% O2, beginning on the day of birth for up to 11 days as previously described in detail (1). Survival and body weight measurements were from three litters/treatment group. Nursing dams were alternated daily between air- and hyperoxia-exposed litters. At postnatal days 3, 7, and 11 (P3-P11), air- and hyperoxia-exposed groups were injected 6 h before euthanasia with 10 mg/kg BrdU subcutaneously according to the manufacturer's directions (Zymed). Pups were killed with 150 mg/kg pentobarbital sodium intraperitoneally, and lungs were inflation-fixed with 10% phosphate-buffered formalin at 25 cmH2O pressure for 30 min, immersed overnight in fixative at 4°C, and then paraffin embedded and sectioned at 4–6 μm. Genotyping was performed by PCR analysis of tail snips using three primer sequences: A, ACATCACAGGCCCCATCATCCTCC; B, GGAGAGCCCCCTTTCTCTCCCTCA; and C, CAACGTCGAGCACAGCTGCGCAAG.
Duplicate PCR reactions were run with primer pairs A/B and A/C, with wild-type mice yielding a 600-bp amplicon with A/B and p47phox−/− mice yielding an 800-bp amplicon with A/C, under previously described conditions (16).
p47phox Expression
Paraffin sections from 4–5 pups/treatment group in wild-type pups at P3, P7, and P11 were dewaxed, rehydrated, and detected with 10 ng/ml polyclonal rabbit anti-p47phox, followed by secondary goat anti-rabbit Alexa Fluor 546 1:500. Sections were extensively washed and detected with anti-pro-SP-C (1:300) or anti-CC10 (1:300), which were detected with goat anti-rabbit Alexa Fluor 488 (1:500) and counterstained with 0.2 μM 4′-6-diamidino-2-phenylindole (DAPI), and mounted with FluorSave (Calbiochem). Images were obtained with a Zeiss LSM 510 META laser confocal microscope as previously described in detail (20). Colocalization was determined using merged images. Sections from hyperoxia-exposed wild-type and p47phox−/− mice (P11) were incubated with anti-p47phox followed by peroxidase-conjugated anti-rabbit, diaminobenzidine, and counterstaining with hematoxylin as assay control.
Bronchoalveolar Lavage Cytology and Tissue Myeloperoxidase
At P7 and P11, animals were euthanized with pentobarbital sodium, and bronchoalveolar lavage (BAL) was performed after perfusing the pulmonary artery with 0.9% NaCl and 1 mM EDTA, pH 7.3, as previously described (1). Four 0.5-ml lavages per animal using the same buffer were pooled, and cell counts were done with a hemacytometer. Differential cell counts were determined after modified Wright-Giemsa stain. Myeloperoxidase activity was detected in perfused and lavaged lungs from P7 and P11 mice in each treatment group, as previously described (3).
MIP-2 in BAL and Whole Lung
MIP-2 was detected in undiluted BAL fluid (BALF) from 5 pups/group and in protein extracted (180 μg/well) from lung homogenates in 6 pups/group using a commercial ELISA (R&D Systems, Minneapolis, MN) as we have described in detail (10).
BrdU Uptake in Type II Alveolar and Bronchiolar Epithelium: P3-P11
Type II alveolar epithelium.
We examined the proliferation of alveolar type II epithelium at P3, P7, and P11 because this spans the transition from the saccular to alveolar stages of postnatal lung development in the mouse. Sections from air- and hyperoxia-exposed BrdU-injected pups, wild-type, and p47phox−/− were first immunostained with rabbit anti-mouse pro-SP-C and then with anti-BrdU, as we have previously described in detail (5). Sections were examined under oil immersion at ×1,000 magnification. A minimum of 500 pro-SP-C positive cells per animal were counted, 6–8 animals/treatment group. Type II cell BrdU labeling index = number of BrdU/SP-C colocalizing cells ÷ total number of pro-SP-C-positive cells.
Bronchiolar Epithelium
BrdU histochemical staining and analysis was performed as previously described in detail (5). Four non-overlapping randomly chosen ×400 fields from two random lung sections/pup in 6 pups/treatment group were examined by an observer masked to treatment condition. Immunolabeled nuclei in epithelial cells were counted, along with unlabeled hematoxylin-counterstained nuclei. Labeling indices were calculated for BrdU in bronchiolar epithelial cells in each treatment group.
Expression of p21cip/waf by Immunoblot and Immunofluorescence
Protein concentration was measured in whole lung homogenates from perfused lavaged newborn mice in each treatment group at P7 by the Bradford method (Bio-Rad, Hercules, CA). Precast 15% acrylamide gels (ReadyGel, Bio-Rad) were loaded with 100 μg/lane of total lung homogenate protein as described (5). Gels were electroblotted onto PVDF, blocked for 1 h (SuperBlock; Pierce-Endogen, Rockford, IL), incubated overnight in anti-p21cip/waf (20 ng/ml, Pharmingen) diluted in blocking buffer with 0.05% detergent (SurfactAmps, Pierce-Endogen), followed by detection with 1:20,000 goat anti-rabbit peroxidase and enhanced chemiluminescence (SuperSignal, Pierce-Endogen) and exposure to film (CL-Xposure, Pierce-Endogen). Blots detected with anti-p21cip/waf were reprobed with anti-β-actin (32 ng/ml). We detected p21cip/waf in type II cells by colocalization of p21cip/waf with anti-pro-SP-C. Random sections from 5 pups/treatment group were dewaxed, treated with antigen retrieval as described above, blocked, and detected with biotinylated 2.5 μg/ml anti-p21cip/waf followed by 4 μg/ml streptavidin-Alexa Fluor 568 (Invitrogen, Carlsbad, CA). The sections were washed, blocked with 3% goat serum in PBS, incubated with 2.8 μg/ml anti-pro-SP-C (Chemicon, Temecula, CA) overnight at 4°C, incubated with biotinylated anti-rabbit IgG at 1:500 dilution, followed by incubation with 2 μg/ml avidin Alexa Fluor 488. The sections were washed, finally incubated with 30 ng/ml DAPI to stain nuclei, and mounted in aqueous media. In all of the immunohistochemistry studies, some sections were processed with omission of the primary antibody to control for nonspecific labeling. Sections were imaged by laser confocal microscopy or by using a fluorescence microscope, appropriate filters, and a cooled-chip digital camera equipped with image analysis software (Metamorph; Molecular Devices, West Chester, PA) as previously described (5). Random fields were chosen from six sections per animal, in five pups/treatment group, with a minimum of 500 pro-SP-C-positive cells per animal counted. Pro-SP-C-positive cells were categorized as p21cip/waf positive or negative, to obtain a labeling index for p21cip/waf as described above. Digital images were obtained under conditions of identical illumination and exposure times.
Morphometric Evaluation of Alveolar Development
Alveolar development was quantified by stereologic morphometry. At the end of 11 days of air or 95% O2 exposure, wild-type and p47phox−/− pups were euthanized, inflation-fixed at 25 cmH2O at 4°C, and then fixed overnight by immersion in phosphate-buffered 4% paraformaldehyde. Three random sections were obtained from the center third of the left lung in five animals per treatment group. Sections were stained with Hart's elastin and counterstained with malachite green according to the manufacturer's directions (Sigma) for maximum contrast, and digitally imaged on a Nikon E400 microscope at ×200 magnification using an Olympus DP11 digital camera. Three random, non-overlapping images per section were color thresholded using image analysis software (Metamorph version 6; Molecular Dynamics, West Chester, PA) to create binary black and white images. Images were overlain with an array of test points to obtain alveolar volume density (estimates alveolar number) from the intersection of points with alveolar tissue divided by points within the alveolar parenchyma; similarly, an array of test lines of known length were digitally overlain, and the number of line intercepts of septal tissue per test line length were automatically counted to obtain alveolar surface density (estimates alveolar surface area) as we have previously described in detail (4).
Alveolar Septal Crest Development
Alveolar septal crest development was qualitatively assessed by staining random sections from five pups in each treatment group at P11 with Hart's elastin as previously described (32). Additional sections were dewaxed, rehydrated, treated with 6 M guanidine HCl, 50 mM dithiothreitol, 20 mM Tris·HCl, pH 8.0, for 15 min, washed in 20 mM Tris·HCl, pH 8.0, and incubated in the dark with 100 mM iodoacetamide for 15 min to retrieve the elastin antigen. Sections were blocked in 3% normal goat serum in PBS + 0.1% Triton X-100. They were then sequentially immunolabeled with mouse monoclonal anti-smooth muscle α-actin-Cy3 (Sigma) 1:400 and polyclonal anti-rat lung elastin 1:1,000 overnight at 4°C in blocking buffer, followed by detection with goat anti-rat Alexa Fluor 488. Images were obtained by laser confocal microscopy to colocalize septal elastin and α-smooth muscle actin.
Quantification of septal crest density was measured as previously described in detail (32). In brief, elastin-stained alveolar septal tips were counted in Hart's elastin-stained sections selected as described above. The number of septal tips in each image was divided by the alveolar volume density for each image to account for potentially confounding effects of variable inflation.
Statistical Analysis
Treatment group differences were identified by ANOVA and post hoc analyses of significance done using Tukey-Kramer. Survival was compared using Kaplan-Meier analysis. Significance was accepted at P < 0.05, and analyses were performed using SPSS software version v.14 (SPSS, Chicago. IL).
RESULTS
Effect of Hyperoxia and Postnatal Age on p47phox Expression
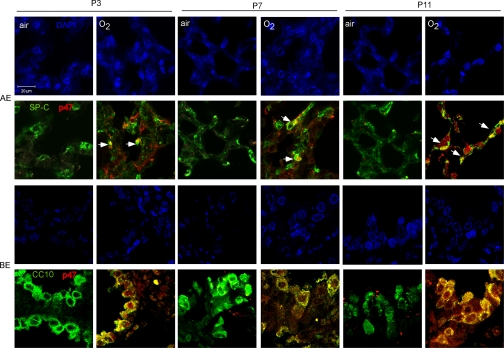
Labeling of p47phox in alveolar and bronchiolar epithelial cells was increased by hyperoxia at P3, P7, and P11 (Fig. 1). Only a subset of pro-SP-C-labeled cells demonstrated colocalization with p47phox. In contrast, we found that p47phox labeling was rare in CC10-positive cells in air-exposed pups, but nearly universal in hyperoxia-exposed pups, P3-P7. CC10-negative bronchiolar epithelium did not demonstrate significant p47phox signal. As expected, we found that p47phox also labeled leukocytes and endothelium, and failed to label cells in p47phox−/− mice (see online data supplement at the AJP-Lung web site).
Fig. 1.
Effect of air or 95% O2 on p47phox (red) expression colocalization with SP-C (green) in alveolar epithelium (AE) and CC10 (green) in bronchiolar epithelium (BE) in wild-type mice at P3, P7, and P11. Yellow indicates colocalization of p47phox with SP-C in a subset (arrows) of pro-SP-C-labeled cells and in all CC10-labled cells, with accompanying panels depicting the corresponding DAPI images. Bar = 20 μm.
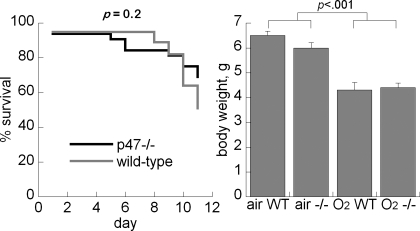
Effect of p47phox Deletion on Hyperoxia-Impaired Postnatal Survival
Hyperoxia-impaired postnatal survival in both wild-type (20/39, 51%) and p47phox−/− mice (22/32, 69%) was measured at P11, but the differences were not statistically significant (P = 0.2). Mortality in wild-type pups was predominantly at days 7–11 (Fig. 2). Body weights were significantly decreased in the hyperoxia-exposed animals at P7 and P11, but there were no body weight differences between wild-type and p47phox−/− pups in either treatment group (Fig. 2).
Fig. 2.
Effect of air or 95% O2 on survival (left) and body weight (right) at P11, wild-type (WT) vs. p47phox−/−. Data are + SE, n = 20–25/group.
Effect of p47phox Deletion on Hyperoxia-Exposed Mouse Lung Inflammation
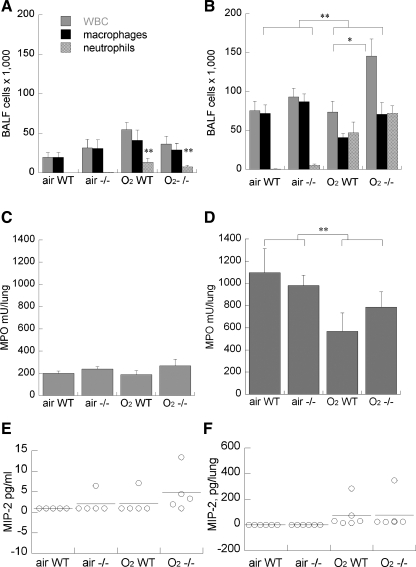
We found that hyperoxia significantly induced airway neutrophils measured by BALF cytology at P7, which further increased by P11, shown in Fig. 3. Total BALF leukocyte counts and differential counts were unaffected by genotype at P7. By P11, the airway leukocyte counts had increased in all groups, but were significantly greater in hyperoxia-exposed mice, more in p47phox−/− vs. wild-type littermates. There were no significant differences in BALF cell differential counts between wild-type and p47phox−/− mice in air or hyperoxia-exposed groups. In contrast with the BALF leukocyte measurements, 95% O2 exposure did not increase whole lung myeloperoxidase accumulation at either P7 or P11. There was a nonsignificant trend towards increased MIP-2 in BALF from hyperoxia-exposed p47phox−/− mice, but no effect of p47phox deletion on MIP-2 in whole lung (Fig. 3).
Fig. 3.
Effect of air or 95% O2 on bronchoalveolar lavage fluid (BALF) leukocytes (A and B) and whole lung myeloperoxidase (C and D) at P7 (A and C) and P11(B and D) in WT vs. p47phox−/− littermates. Data are means of 8–10/group + SE, *P < 0.05 vs. WT, **P < 0.05 air vs. 95% O2. E and F: effect of 95% O2 or air on BALF and whole lung MIP-2 at P11, WT vs. p47phox−/−, bar = mean of 5–6/group.
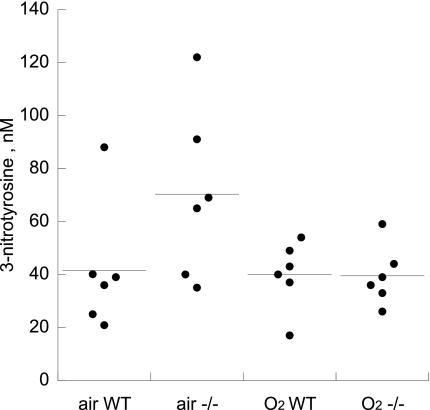
Effect of p47phox Deletion on 3-Nitrotyrosine in Whole Lung
There was no significant induction of 3-nitrotyrosine in whole lung homogenates, and no significant effect of p47phox deletion in air- or hyperoxia-exposed pups at P11 (Fig. 4).
Fig. 4.
Effect of air or 95% O2 on whole lung 3-nitrotyrosine at P11, WT vs. p47phox−/−. Bar = mean of 5/group.
Effect of p47phox Deletion on Hyperoxia-Impaired Type II Epithelial Proliferation
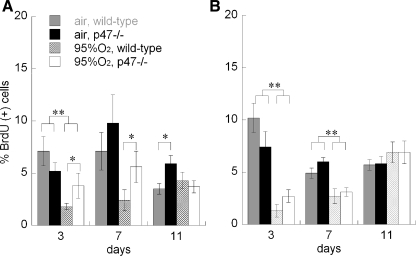
We found that hyperoxia impaired type II (SP-C labeled) alveolar epithelial proliferation (BrdU uptake) at P3 and P7, but not at P11, in wild-type mice (Fig. 5). In contrast, type II cell proliferation indices in hyperoxia-exposed p47phox−/− mice were significantly greater than in wild-type littermates at P3 and P7, but not at P11. Air-exposed p47phox−/− mice at P11 also had a higher type II cell proliferation index than in the air-exposed wild-type mice, but were not significantly different at P3 and P7. Hyperoxia impaired bronchiolar epithelial proliferation at P3 and P7, but not at P11. There was no significant effect of p47phox deletion on bronchiolar epithelial proliferation in any condition.
Fig. 5.
Effect of air or 95% O2 on proliferation (BrdU labeling index) in type II alveolar (A; pro-SP-C-positive) or bronchiolar epithelial cells (B) at P3, P7, and P11, WT vs. p47phox−/−. Data are means of 6–8/group, ± SE, *P < 0.05 vs. WT, **P < 0.05 95% O2 vs. air, for the indicated comparisons.
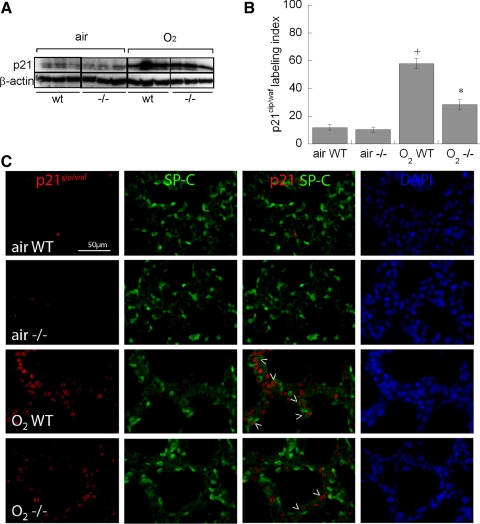
Effect of p47phox Deletion on p21cip/waf Expression in Hyperoxia-Exposed Newborn Mouse Lung
Hyperoxia-induced DNA damage-associated cell cycle/proliferation arrest is regulated by p21cip/waf, which induces cell cycle arrest at the G1 checkpoint. We measured p21cip/waf expression in whole lung homogenates by immunoblot and found that hyperoxia induced p21cip/waf expression in both wild-type and p47phox−/− mice at P7 (Fig. 6A) with no effect on whole lung β-actin. We performed colocalization immunohistochemical studies of p21cip/waf and SP-C at P7, when type II cells demonstrated the greatest impairments in proliferation in the hyperoxia-exposed groups. Hyperoxia treatment led to a fourfold induction of p21cip/waf in type II (SP-C positive) cells in wild-type pups at P7, but only a twofold induction in p47phox−/− littermates (Fig. 6B). Expression of p21cip/waf was predominantly nuclear, especially in the hyperoxia-exposed groups (Fig. 6C).
Fig. 6.
Effect of hyperoxia on p21cip/waf expression and colocalization with pro-SP-C. A: effect of air or 95% O2 on p21cip/waf expression in whole lung homogenates detected by Western blotting in WT vs. p47phox−/− littermates. B: labeling index of p21cip/waf positive cells in type II (pro-SP-C-positive) cells. Data are means of >500 pro-SP-C-positive cells counted in 5 pups/treatment group ± SE, +P < 0.05 vs. air WT, *P < 0.05 vs. O2 WT. C: representative confocal immunofluorescence from each treatment group at P7. Arrowheads indicate p21cip/waf colocalization with pro-SP-C-positive cells.
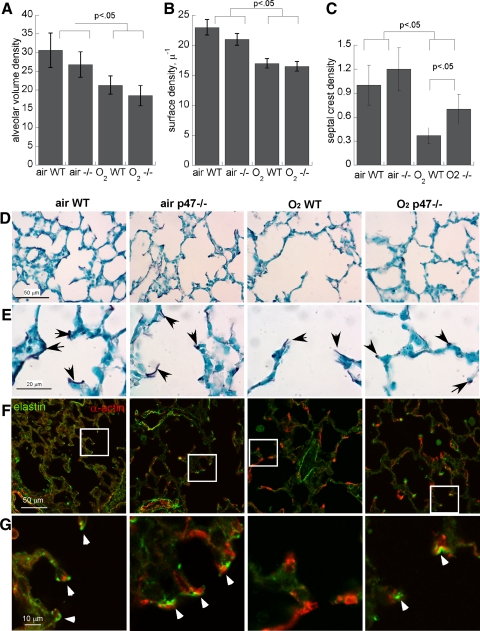
Effect of p47phox Deletion on Hyperoxia-Impaired Alveolar Development at P11
Hyperoxia impaired alveolar volume density and alveolar surface density in both wild-type and p47phox−/− pups measured at P11 (Fig. 7), but with no statistically significant difference between genotypes. We examined elastin staining in each treatment group and found that hyperoxia-exposed wild-type, but not p47phox−/−, mice displayed abnormal, frayed-appearing elastin labeling in alveolar secondary crest septal tips at P11. We found that elastin and α-smooth muscle actin typically colocalized in alveolar septae in air-exposed animals (wild-type and p47phox−/−), but in hyperoxia-exposed wild-type pups, α-actin-elastin colocalization was infrequent. This effect was less pronounced in hyperoxia-exposed p47phox−/− pups, which had significantly more elastin-stained septal crests (normalized to alveolar volume density, Fig. 7).
Fig. 7.
Effect of p47phox deletion on hyperoxia-impaired lung development at P11. A: alveolar volume density; B: alveolar surface density; C: septal crest density, means of 5/group ± SE. D: Hart's elastin stain at low magnification. Insets are shown in E at higher magnification; arrows denote septal tips. F: Colocalization of elastin (green) and α-actin (red) at septal tips at low and high (G) magnification; arrowheads denote colocalization.
DISCUSSION
Although our aim was to determine the contribution of phagocyte-derived ROS to hyperoxia-impaired postnatal lung development, we found that deletion of p47phox prevented hyperoxia-impaired type II epithelial proliferation at P3 and P7, before significant hyperoxia-induced inflammation had occurred. Since we also found that p47phox is expressed in alveolar type II cells during the first postnatal week, it may be that ROS generated in alveolar epithelium may have a greater role in the observed effects on epithelial proliferation.
Hyperoxia effects on type II cell proliferation in wild-type and p47phox−/− pups were accompanied by reciprocal effects on the cell cycle inhibitor p21cip/waf: hyperoxia induced nuclear p21cip/waf expression in type II cells in wild-type pups, but significantly less so in p47phox−/− littermates. We found that p21cip/waf was primarily nuclear, consistent with its induction by DNA damage in type II cells (14, 29). This is consistent with our earlier observation that type II cell-targeted SOD3 overexpression prevented hyperoxia-induced DNA damage and preserved type II proliferation (5).
Elastin and α-smooth muscle actin staining at alveolar septal tips was disrupted by hyperoxia exposure in wild-type, but not in p47phox−/−, mice at P11, with quantitatively fewer elastin-stained septal crests. Disruption of alveolar septal elastin deposition has previously been described in animal models of BPD (7, 21) and in hyperoxia-exposed newborn rodents (11, 15). The hyperoxia-exposed p47phox−/− pups had preservation of the compact elastin staining at septal tips, rather than the frayed appearance noted in other models of BPD and in their wild-type littermates at P11. Smooth muscle α-actin normally colocalizes with elastin at alveolar crest septal tips (8). We did not quantify effects on septal actin labeling. Since elastin and α-actin localization was preserved in hyperoxia-exposed p47phox−/− mice, we conclude that p47phox-dependent generation of superoxide or downstream reactants contribute to the hyperoxia-induced impairments of postnatal secondary crest development.
Deletion of p47phox did not completely protect postnatal alveolar development from hyperoxia assessed by measurements of alveolar volume and surface densities. Beneficial effects of p47phox deletion may have been offset in part by the increased leukocyte recruitment measured in BALF from P11 in p47phox−/− mice. However, we did not find corresponding increases in whole lung MPO or in the neutrophil chemokine MIP-2 in the p47phox−/− group exposed to hyperoxia. The effects of p47phox deletion on inflammation vary depending on the model system. Inflammation is reduced in response to bleomycin in adult p47phox−/− mice (19), but increased in p47phox−/− adult mice in response to acid aspiration (24) and tobacco smoke(30), possibly through effects on counterinflammatory pathways. We found no effect of p47phox deletion on hyperoxia-induced pulmonary leukocyte accumulation at P7, when the effects on type II proliferation were most prominent.
Although our aim was to eliminate phagocyte-generated ROS in this hyperoxia-exposure model, deletion of p47phox would be expected to affect superoxide generation in other lung cells in which its expression has been previously determined, including smooth muscle, endothelium, and fibroblasts, in addition to cells of myeloid origin (see Ref. 26 for review). We did not observe any abnormalities of alveolar development in p47phox−/− air-exposed pups despite a marginally higher type II cell proliferation rate at P11, so it seems unlikely that p47phox-generated ROS are indispensable to normal lung development under basal conditions.
On the other hand, we found that p47phox was also expressed in some bronchiolar and alveolar epithelial cells at P3-P11, and that expression was increased in hyperoxia-exposed pups. To our knowledge, p47phox has not been previously described in Clara cells or in type II pneumocytes, although NADPH-oxidase-like activity has been described in type II cells (23, 27). This expression was only prominent in hyperoxia-exposed pups and only in subsets of type II cells. ROS effects generated by extra-myeloid p47phox are likely to be spatially restricted, with effects partly governed by spatial proximity of antioxidants like SOD3, also expressed in alveolar and bronchiolar epithelium. Others have reported subsets of type II cells that appear to have varying vulnerability to oxidative stress during the immediate postnatal period, which correlates with later susceptibility to pulmonary infection (22). We speculate that expression of p47phox may indicate a subset of type II epithelial cells with increased vulnerability to oxidative stress, which we did not directly address.
Superoxide from sources besides p47phox may contribute to hyperoxia-impaired postnatal alveolar development. Hyperoxia can generate superoxide and other reactive oxygen intermediates in the absence of leukocytes (13), particularly through mitochondrial production (25). Non-phagocytic oxidases expressed in lung (NOX 1-4, DUOX1) are capable of generating superoxide (see Ref. 26 for review). The total contribution of superoxide from these nonleukocyte NOX sources is likely to be low but may be important in particular microenvironments.
Other mechanisms like protease-mediated damage may be more significant contributors to neutrophil-mediated impairments of postnatal alveolar development. The matrix of postnatal hyperoxia-exposed alveoli could be vulnerable to proteolytic enzyme release by recruited leukocytes, regardless of superoxide production, providing that local pH and redox conditions permit protease activity. Some studies suggest that proteases and/or protease/antiprotease imbalance may play a role in the pathogenesis of BPD, particularly in its more severe forms (2, 15, 31). The respective roles of proteases and/or superoxide in hyperoxia-impaired postnatal lung development will likely depend on the magnitude of the inflammatory response, and on the ability of the host to induce both antioxidants and antiproteases in the appropriate compartments.
In summary, NADPH oxidase (p47phox) is expressed in bronchiolar and alveolar epithelium in hyperoxia-exposed newborn mice, as well as in leukocytes. Deletion of p47phox results in partial preservation of postnatal type II alveolar but not bronchiolar epithelial proliferation in hyperoxia-exposed mice at P3 and P7, when inflammation was minimal, but when p47phox expression in alveolar and bronchiolar epithelium was increased. Deletion of p47phox was not sufficient to completely protect against hyperoxia-impaired alveolar development, despite salutary effects on alveolar septal crest elastin expression. We conclude that endogenous superoxide generation via NADPH oxidase, possibly in a subset of type II cells, mediates hyperoxia-impairment of type II cell proliferation in the immediate postnatal period, but that other sources of ROS as well as proteases may play more important roles in hyperoxia-impaired postnatal alveolar development.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant R01-HL-067021 and the Jean and George Brumley Neonatal-Perinatal Research Institute.
Supplementary Material
Acknowledgments
We thank Dr. Steven Holland for providing the p47phox−/− mice, Mary Whorton for expert technical assistance, and Dr. Tim Oliver (Dept. of Cell Biology) for assistance with confocal microscopy.
REFERENCES
- 1.Ahmed MN, Suliman HB, Folz RJ, Nozik-Grayck E, Golson ML, Mason SN, Auten RL. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am J Respir Crit Care Med 167: 400–405, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med 173: 318–326, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auten RL, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am J Physiol Lung Cell Mol Physiol 281: L336–L344, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 176: 291–299, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L32–L40, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auten RL, Whorton MH, Nicholas Mason S. Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Biol 26: 391–397, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bland RD, Albertine KH, Pierce RA, Starcher BC, Carlton DP. Impaired alveolar development and abnormal lung elastin in preterm lambs with chronic lung injury: potential benefits of retinol treatment. Biol Neonate 84: 101–102, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol 36: 32–42, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coalson J Neonatal chronic lung disease in extremely premature baboons. Am J Respir Crit Care Med 160: 1333–1346, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Deng H, Mason SN, Auten RL Jr. Lung inflammation in hyperoxia can be prevented by antichemokine treatment in newborn rats. Am J Respir Crit Care Med 162: 2316–2323, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Frank L, McLaughlin GE. Protection against acute and chronic hyperoxic inhibition of neonatal rat lung development with the 21-aminosteroid drug U74389F. Pediatr Res 33: 632–638, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Frank L, Sosenko IR. Oxidants and antioxidants: what role do they play in chronic lung disease? In: Chronic Lung Disease in Early Infancy, edited by Bland RD and Coalson J. New York: Marcel Dekker, 2000, p. 257–284.
- 13.Freeman BA, Panus PC, Matalon S, Buckley BJ, Baker RR. Oxidant injury to the alveolar epithelium: biochemical and pharmacologic studies. Res Rep Health Eff Inst 1–30; discussion 31–39, 1993. [PubMed]
- 14.Gehen SC, Vitiello PF, Bambara RA, Keng PC, O'Reilly MA. Downregulation of PCNA potentiates p21-mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol 292: L716–L724, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hirakawa H, Pierce RA, Bingol-Karakoc G, Karaaslan C, Weng M, Shi GP, Saad A, Weber E, Mariani TJ, Starcher B, Shapiro SD, Cataltepe S. Cathepsin S deficiency confers protection from neonatal hyperoxia-induced lung injury. Am J Respir Crit Care Med 176: 778–785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med 182: 751–758, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobe A The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Liao L, Ning Q, Li Y, Wang W, Wang A, Wei W, Liu X, Auten RL, Tanswell AK, Luo X. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res 60: 299–303, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 6: 11, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall HE, Potts EN, Kelleher ZT, Stamler JS, Foster WM, Auten RL. Protection from LPS-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med. In press. [DOI] [PMC free article] [PubMed] [Research Misconduct Found]
- 21.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, Crapo JD, Grubb PH, Shaul PW. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 288: L450–L459, 2005. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 177: 1103–1110, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabat R, Guthmann F, Rustow B. Formation of reactive oxygen species in lung alveolar cells: effect of vitamin E deficiency. Lung 186: 115–122, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Segal BH, Davidson BA, Hutson AD, Russo TA, Holm BA, Mullan B, Habitzruther M, Holland SM, Knight PR 3rd. Acid aspiration-induced lung inflammation and injury are exacerbated in NADPH oxidase-deficient mice. Am J Physiol Lung Cell Mol Physiol 292: L760–L768, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Turrens JF, Freeman BA, Levitt JG, Crapo JD. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys 217: 401–410, 1982. [DOI] [PubMed] [Google Scholar]
- 26.van der Vliet A NADPH oxidases in lung biology and pathology: host defense enzymes and more. Free Radic Biol Med 44: 938–955, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Klaveren RJ, Roelant C, Boogaerts M, Demedts M, Nemery B. Involvement of an NAD(P)H oxidase-like enzyme in superoxide anion and hydrogen peroxide generation by rat type II. Thorax 52: 465–471, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vignais PV The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci 59: 1428–1459, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Morris GF. p53-mediated regulation of proliferating cell nuclear antigen expression in cells exposed to ionizing radiation. Mol Cell Biol 19: 12–20, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 172: 1222–1237, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'Donnell E, Cataltepe S. SERPINB1 up-regulation is associated with in vivo complex formation with neutrophil elastase and cathepsin G in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 291: L619–L627, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, Tanswell AK. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 170: 1188–1196, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.