Abstract
Fibroblasts from patients with pulmonary fibrosis express higher levels of the receptor for urokinase, and the extent of fibrosis in some animal models exhibits a dependence on the urokinase receptor. Recent observations have identified the urokinase receptor as a trans-interacting receptor with consequences on signaling and cell responses that vary depending on its interacting partner, the relative levels of expression, and the state of cellular transformation. We undertook this study to define the urokinase-type plasminogen activator cellular receptor (u-PAR)-integrin interactions and to determine the functional consequences of such interactions on normal human lung fibroblast attachment and migration. u-PAR colocalizes in lammelipodia/filopodia with relevant integrins that mediate fibroblast attachment and spreading on the provisional matrix proteins vitronectin, fibronectin, and collagens. Inhibitory antibody studies have revealed that human lung fibroblasts utilize αvβ5 to attach to vitronectin, predominantly α5β1 (and αvβ3) to attach to fibronectin, and α1β1, α2β1, and α3β1 to attach to collagen. Blocking studies with α-integrin subunit decoy peptides and u-PAR neutralizing antibodies indicate that u-PAR modulates the integrin-mediated attachment to purified provisional matrix proteins, to anti-integrin antibodies, or to fibroproliferative lesions from fibrotic lungs. Furthermore, these decoy peptides blunt fibroblast spreading and migration. We show that u-PAR can interact with multiple α-integrins but with a preference for α3. Taken together, these data demonstrate that u-PAR may interact with multiple integrins in normal human lung fibroblasts thereby promoting attachment, spreading, and migration. Modulation of fibroblast invasion would be expected to lead to amelioration of fibroproliferative diseases of the lung.
Keywords: pulmonary, fibrosis, plasmin
studies in vitro and in transgenic animals have demonstrated that the fibrinolytic system has protean effects on numerous disease processes including acute and chronic inflammatory disorders, cancer invasion and metastasis, angiogenesis, and fibroproliferative disorders of the vasculature, lung, kidney, and eye (3, 4, 11, 16, 22, 23, 27, 29, 37, 55). These disorders have the common feature of a complex, yet coordinated movement of cells and the biochemical and structural remodeling of the extracellular matrix. The molecular interactions and cell biological processes that mediate the effects of the fibrinolytic system on cell-matrix interactions are active areas of investigation.
Protease-protease inhibitor mechanisms of the urokinase/plasmin system that relate to intravascular or alveolar fibrin turnover are deranged in pulmonary fibrosis but are clearly only a part of the relevant biology (38). The receptor for urokinase is a 50-kDa, cell surface glycoprotein receptor that binds urokinase with high affinity (Kd = 2 nM) and that is linked to the plasma membrane via a glycosylphosphatidyl inositol side chain (5, 8, 9, 44). This receptor can interact in trans with other cell surface receptors including integrins, EGFR, and the G protein-coupled receptor (FPXLR1; Refs. 32, 46, 67). Interaction of urokinase-type plasminogen activator cellular receptor (u-PAR) with the extracellular domain of integrins is demonstrable by coimmunoprecipitation and colocalization (12, 65, 67). u-PAR also exhibits specific, saturable binding to immobilized integrins (12, 65, 67). Further, these interactions modulate several biological processes including adhesion, migration, phagocytosis of fibrinogen, intracellular signaling, and tumor dormancy in a cell type-specific manner (2, 10, 42, 50, 67).
Integrins are a large family of heterodimeric transmembrane receptors (composed of an α- and a β-subunit) that typically cluster in the cell membrane upon integrin receptor recognition of its ligand in the extracellular matrix (7, 18). Integrin clustering leads to the assembly of a signaling complex that is also an adhesion structure (known as a focal contact or focal adhesion) and is localized to the submembranous region at the base of the cell. These adhesion structures/signaling complexes regulate cell adhesion, spreading, migration, survival, and proliferation through modulation of the actin cytoskeleton. Other signaling molecules, such as kinases, cytoskeletal proteins, and adaptor/scaffolding proteins, are recruited to and become concentrated at these structures (7, 18). Regulation of integrin signaling occurs at multiple levels and includes the specific heterodimeric (αβ) subunit pairing, the association of the integrin with other cell membrane molecules (i.e., u-PAR or tetraspans), the available divalent cations, the cell type, and the environmental context (7, 18, 24, 52, 67). The integrin β-subunit cytoplasmic tail is necessary for most integrin signaling events due to its association with specific cytoskeletal and signaling molecules, and the integrin α-subunit can also modulate integrin signaling, for example, through its extracellular W4 domain association with u-PAR (65, 72).
Integrins have been shown to play a role in the fibroproliferative response in the lung. For example, integrins αvβ6 and αvβ8 activate TGFβ, a potent profibrotic growth factor (25, 33, 36, 45, 47). Furthermore, proliferation and migration of idiopathic pulmonary fibrosis fibroblasts, in response to integrin ligation, are aberrantly regulated through reductions in the activity of the tumor suppressor phosphatase and tensin homologue protein (70, 71). In addition, integrin-initiated signaling is vital to the trans-differentiation of fibroblasts to myofibroblasts and for the epithelial-mesenchymal transformation (15, 28, 60). The importance of u-PAR modulation of integrin function in fibroblasts to pulmonary fibrosis has yet to be explored.
In the case of mononuclear cells, it is known that their activation and transvascular emigration of mononuclear cells (neutrophils and monocytes) into the pulmonary interstitium are a urokinase receptor-dependent process in several inflammatory states, including those of infectious etiology, chemokine-driven (KC), and fibrogenic stimuli (bleomycin-induced lung injury; Refs. 1, 20–22, 53, 54, 57, 63). Recently, renal podocyte cell u-PAR has been shown to play a role in the renal dysfunction due to podocyte foot process effacement and proteinuria seen after endotoxin administration (64). In the specific case of fibroblasts, invasion, and remodeling of a fibrinogen, the vitronectin and fibronectin-rich provisional matrix within the pulmonary alveolus is a fundamental feature of the normal and pathologic repair process after lung injury (8a, 58, 59). We and others (48, 56) have shown that fibroblasts derived from patients with fibrotic lungs exhibit a promigratory phenotype in vitro and exhibit upregulation of u-PAR. However, the role and mechanisms by which the urokinase receptor mediates migration of fibroblasts have not been fully elucidated. We sought to characterize and determine the mechanism of the effect of u-PAR on human lung fibroblast attachment, spreading, and migration that are critical to the fibrotic process.
MATERIALS AND METHODS
Materials.
Eagle's modified minimum essential medium (EMEM) and DMEM were purchased from Mediatech (Herndon, VA). Gelatin Sepharose 4B, horseradish peroxidase (HRP)-conjugated secondary antibodies, and ECL Western blot reagents were from Amersham Pharmacia Biotech (Piscataway, NJ). Human vitronectin, fibronectin (from human plasma), and collagen (type I; from calf skin) were from Molecular Innovations (Southfield, MI), Boehringer Mannheim (Indianapolis, IN), and ICN Biomedicals (Irvine, CA), respectively. Fibronectin-depleted human fibrinogen was from Enzyme Research Laboratories (South Bend, IN). Protein G-agarose was from Pierce (Rockford, IL). Fluorochrome-conjugated secondary antibodies were from Molecular Probes (Eugene, OR). Mouse MoAb and rabbit polyclonal anti-human u-PAR antibodies were from American Diagnostica (Greenwich, CT). Mouse MoAb anti-human integrins β1 (AB1965 and P4C10), αvβ3 (LM609), αvβ5 (P1F6), α3 (P1B5), α5 (SAM-1), and rabbit polyclonal anti-integrin β1 (AB1952) were from Chemicon International (Temecula, CA). Mouse MoAb anti-human integrins α2 (Clone A2-IIE10) and β1 (Clone DE9) were from Upstate Biotechnology (Lake Placid, NY). Mouse MoAb anti-integrin αv (clone L230) was from the University of Alabama at Birmingham Hybridoma Core Facility and was purified in Dr. Gladson's laboratory. Mouse MoAb anti-vinculin antibody (M0520) was from American Qualex Antibodies (San Clemente, CA). Peptides homologous to the β-propeller repeat regions of the extracellular domains of the α3-integrin chain (AA 273–289) α325 PRHRHMGAVFLLSQEAG, the scrambled control peptide S-325 HQLPGAHRGVEARFSML, the α5-integrin chain (AA 294–310) PKGNLTYGYVTILNGSD, and the αv-integrin chains (AA 274–290) PRAARTLGMVYLYDGKN were provided by Dr. H. Chapman, Jr. or were purchased from Anaspec (San Jose, CA; Ref. 66).
Flow cytometry analysis of cell surface expressed proteins.
CCD-19 Lu normal adult human lung fibroblasts (ATCC; passages 4–9) were maintained and propagated as described (41). Washed, resuspended fibroblasts (4 × 105 cells in 300 μl of HBSS/1% BSA) were incubated with primary antibodies (10 μg/ml IgG or 1:100 dilution of ascites) at 4°C for 45 min. followed by incubation with fluorochrome (488 or 594 nm)-conjugated rabbit anti-mouse IgG (20 μg/ml) at 4°C for 30 min. in the dark. Flow cytometric analysis was performed on EPICS XL-MCL flow cytometer (Beckman-Coulter, Miami, FL).
Cell surface labeling of integrins.
Cell surface integrins were characterized using biotinylated surface molecules followed by immunoprecipitation as previously published (19). In brief, surface labeling of the cells was accomplished by treatment of 0.5 mg/ml of sulfosuccinimidyl-6-biotinamido hexanoate (sulfo-NHS-LC-biotin) for 30 min at 22°C. Cells were lysed (1% DOC/0.5% SDS/1% Triton X-100 in TBS), and equivalent amounts of protein lysates were immunoprecipitated with Sepharose bead-bound-integrin-specific antibodies and then subjected to SDS-PAGE under nonreducing conditions followed by transfer to Immobilon membranes. Surface-labeled immunoprecipitates were detected by reaction with streptavidin-HRP (10 pg/ml) and ECL kit. The pairings of the integrin subunits are based on the known association of the α- and β-subunits, the known relative migration of the subunits, and the fluorescence-activated cell sorting (FACS) analysis results.
Western blot analysis of u-PAR and integrins.
Solubilized proteins were separated by SDS-PAGE and then immunoblotted on Immobilon-P membranes as described previously (40). The membranes were blocked (TBS-Tween-20/5% BSA; room temperature for 1 h) and incubated with either rabbit polyclonal anti-integrin β1 or u-PAR antibodies (4°C overnight) followed by HRP-conjugated donkey anti-rabbit IgG (1:7, 500 dilution; room temperature 1 h). Specific protein bands were detected using ECL Western blot reagents on Kodak BioMax films.
Colocalization of u-PAR and integrins by immunofluorescence.
Fibroblasts were plated on human vitronectin (5 μg/ml)-, fibronectin (10 μg/ml)-, or collagen (10 μg/ml)-coated glass coverslips or chamber wells slides and were allowed to attach and spread (0.5–6 h) in attachment assay buffer. The attached cells were fixed with 3% paraformaldehyde in some cases, permeabilized (0.15% Triton X-100/PBS, room temperature for 1 min), and blocked (10% horse serum, 10 min at room temperature). For double antigen colocalization, cells were first incubated with the first mouse primary antibody (1 h at room temperature) and secondary Alexa Fluor 488-conjugated goat anti-mouse antibody (20 μg/ml; 1 h at room temperature) pair followed by the second primary/secondary pair. The cell nuclei were stained with Hoechst (33258; 20 μg/ml for 2 min at room temperature) and mounted (90% glycerol/PBS/0.01% p-phenylenediamine). Immunofluorescence was viewed under Leica inverted microscope, and digital image processing was performed using a Retiga EX camera, Compix software, and Adobe Photoshop.
Cell attachment and spreading assay.
Human lung fibroblasts were suspended in attachment assay buffer [10 mM HEPES, 140 mM NaCl, 5.4 mM KCl, 5.6 mM d-glucose, and 1% BSA, pH 7.4 (100 μM MnCl2, 1 mM MgCl2, ± 1 mM CaCl2)] and seeded (in quadruplicate) on either matrix protein-coated [human vitronectin (5 μg/ml), human fibronectin (10 μg/ml), collagen (10 μg/ml), or fibrinogen (100 μg/ml)], or anti-integrin antibody-coated (10 μg/ml)/1% BSA blocked 96-well plates as described previously (43). Preliminary experiments indicated that the inclusion of 1 mM CaCl2 in the attachment assay buffer had no effect on fibroblast attachment to vitronectin. Cells were allowed to attach for 15–30 min at 37°C, and nonadherent cells were washed off in 37°C PBS. Ovalbumin (5 μg/ml)-coated wells served as the negative control. Attached cells were counted. Wells were coated by either or the indicated concentrations of these proteins. Attachment inhibition studies were performed by incubation of the fibroblasts (either human lung fibroblasts or primary isolates of murine lung fibroblasts from u-PAR null mice) with antibodies and/or the indicated peptides (for 20 min at room temperature at the indicated concentrations) before their seeding on matrix protein-coated wells. Attachment to integrin antibodies was performed in wells precoated with Goat-anti-mouse IgG and the indicated anti-integrin mouse monoclonal function-blocking IgG or irrelevant mouse monoclonal IgG, as described previously (43). Attachment was calculated as the ratio of the cell number noted in the presence of equimolar α325/serum-containing medium (SCM), after subtracting the number of cells present in the IgG control well. Fibroblast spreading was assessed by allowing the cells to attach (20 min at 37°C) and either scrambled (S-325; 75 μM), active peptide (α325; 75 μM), or vehicle (DMSO) was added. Photomicrographs were taken as described above, and the individual cell area was measured for at least 200 cells/condition using Compix software.
Cell attachment to murine lung tissue.
All animal protocols were approved by the University of Alabama at Birmingham animal resources program (Institutional Animal Care and Use Committee). C57Bl/6 (8- to 12-wk-old females) mice were instilled intratracheally with bleomycin sulfate (4 U/kg). Mice were euthanized 21 days later, and the lungs were perfused with 5 ml cold PBS through the right ventricle and then instilled with optimum cutting temperature (OCT) compound via the airway, embedded in OCT, and frozen. Normal human lung fibroblasts (19 LU) were labeled with the fluorescent dye PKH-36 (Sigma) according to the manufacturer's instructions with minor modifications. Frozen lung tissue sections (5 μm) were thawed and blocked (PBS/1% BSA, 2 h), and the labeled fibroblasts (19 LU) were allowed to adhere for 45 min (37°C, 5% CO2). The tissue sections with cells were exhaustively washed to remove nonadherent cells. Cell number and tissue area were determined in photomicrographs (phase and fluorescent) from randomly selected areas of lung exhibiting either normal histology or fibroproliferative lesions using Compix software. In all cases, a minimum of 200 cells was counted per determination.
In vitro migration assay.
Fibroblasts were seeded at confluent density (2 × 105 cells/2 cm2 growth area) and allowed to attach overnight in 1–10% SCM. The confluent monolayers were then washed with serum-free medium and made quiescent (0.4% SCM for 48 h). A denuded area (wound) was created using a 200-μl pipette tip. Wounded cell monolayers were carefully washed, and the media were replaced with EMEM with or without the indicated agonists or inhibitor reagents. Digital pictures of the wounds were taken at 0.5 h (time 0) and indicated intervals later, and areas devoid of cells, “wounds,” were measured using Compix software. The effects of peptides on the wound healing process were assessed by adding the active (α325) or scrambled (S-325) peptides, or vehicle control (DMSO) to the medium, after the wound. Fresh medium/peptides were replenished every 96 h when necessary.
Statistical analysis.
All data are means ± SD unless otherwise indicated. Continuous variables from more than two groups were compared by means of an ANOVA with a post-hoc analysis (Dunnett's test or Student-Newman-Keuls). Significance was accepted at the P ≤ 0.05 level.
RESULTS
Human lung fibroblasts express the urokinase receptor and multiple integrin receptors.
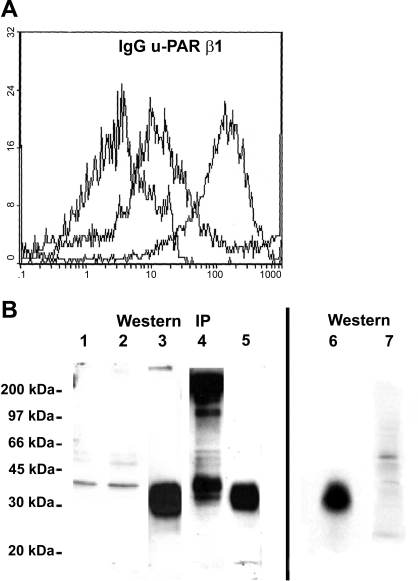
To investigate the possible involvement of u-PAR and integrin receptors in fibroblast attachment and migration during the repair of injured lung, we first characterized the cell surface expression of these integrin proteins in fibroblasts derived from normal human lung. Surface expression of u-PAR was detected, albeit at a low level, by flow cytometric analysis (FACS), by immunobloting of fibroblast lysates, and by ELISA (Fig. 1). Fibroblast cell lysates contained 2,433 pg u-PAR/106 cells. By FACS analysis, the cells demonstrated a significantly weaker signal for u-PAR than for integrin β1-chain. Cell surface labeling followed by immunoprecipitation with anti-integrin specific antibodies demonstrated the expression of αvβ3-, αvβ5-, and multiple β1-containing integrins (Fig. 2A). Bands at 95 and 125 kDa and in the αv immunoprecipitate suggest that αv (150 kDa) is pairing with β3/β5 and β1, respectively (Fig. 2A). Bands at 150 and 220 kDa in the β1-immunoprecipitate suggest that β1 (125 kDa) is pairing with αv-integrin and at least α1-integrin subunits, respectively (Fig. 2A). FACS analysis further revealed the surface expression of α1-, α2-, α3-, α5-, αvβ3-, αvβ5-, and β1-integrins (Fig. 2B), as summarized in Table 1.
Fig. 1.
Human lung fibroblasts express urokinase-type plasminogen activator cellular receptor (u-PAR) and integrin β1. A: flow cytometry detection of cell surface expression of u-PAR, integrin β1, and IgG controls on human lung fibroblasts were performed using specific primary antibodies. Cells express u-PAR and integrin β1 with fluorescence intensities of 5 and 50 times higher, respectively, compared with IgG control. B: Western blotting and immunoprecipitation analysis of cell lysates. Shown are the following: fibroblasts attached to vitronectin (20 min) lysed in either Triton X-100 lysis buffer (lane 1) or RIPA buffer (lane 2); human su-PAR (10 ng) control (different lanes from same gel; lanes 3, 5, and 6); human u-PAR from fibroblast lysates immunoprecipitated by a mixture of 2 monoclonal antibodies (American Diagnostica No. 3936 and 3937; lane 4) and detected by a polyclonal antibody against u-PAR (399R); and TX-100 lysates from 293 epithelial cells that do not express u-PAR (lane 7). Both Western and immunoprecipitated (IP) u-PAR migrated at 42 kDa.
Fig. 2.
Human lung fibroblasts express multiple integrins that recognize provisional matrix binding proteins. A: immunoprecipitation analysis of cell surface integrins in fibroblasts. Cells were surface labeled with biotin and immunoprecipitated using integrin-specific antibodies as indicated. Telative migration of the individual integrin subunits is indicated at left. Expression of integrins αvβ5 (lane 2) and αvβ3 (lane 3) was detected using heterodimeric-specific integrin antibodies. In the αv-immunoprecipitate (lane 4), bands at 160, 120, and 100 kDa were detected consistent with the αv-, β1-, and β3/β5-subunits, respectively, indicating αv pairs with β1 and β3/β5 in these cells. In the anti-β1-immunoprecipitate (lane 5), bands at 220, 160, and 120 kDa were detected, consistent with expression of the α1-, αv-, and β1-subunits, respectively, indicating expression of α1β1 and αvβ1. No expression of α8- or α9-integrins was detected (lanes 6 and 7). B: flow cytometry detection of cell surface integrin expression in fibroblasts. Cell surface expression of integrins were detected by monoclonal antibodies against integrins u-PAR (No. 3936 American Diagnostica), α1 (Mab 1973; Chemicon), α2 (clone A2-IIE10), α3 (clone P1B5), α5 (clone SAM-1), β1 (clone P4C10), αvβ3 (clone LM609), and αvβ5 (clone P1F6), and Mac-1 (Clone ICRF-44), respectively.
Table 1.
Integrin expression profile in human lung fibroblasts
| Integrins Present | Integrins Absent |
|---|---|
| aVβ1 | α8β1 |
| αVβ3 | α9β1 |
| αVβ5 | |
| α1β1 | |
| α2β1 | |
| α3β1 | |
| α5β1 |
Human lung fibroblasts attach strongly to vitronectin, fibronectin, and collagen but weakly to fibrinogen.
To characterize the attachment of human lung fibroblasts to provisional alveolar matrix proteins, we first assessed their time and concentration dependencies. Fibroblasts attached rapidly to vitronectin-, fibronectin-, and collagen-coated microplate wells, reaching near-maximal attachment within 15 min (Fig. 3A). In sharp contrast, the attachment to fibrinogen was slower and less complete, reaching a maximal attachment of 68.2 ± 4.9% (mean ± SD; n = 4) of that seen with vitronectin after 60 min. Furthermore, the concentration dependence of attachment to vitronectin, fibronectin, and collagen (at 30 min) was similar (EC50 ≈ 100 ng/ml), while the fibrinogen attachment (at 60 min) concentration curve was shifted to the right by more than two orders of magnitude (EC50 ≈ 7,000 ng/ml; Fig. 3B). As these data indicate that fibroblast attachment to vitronectin, fibronectin, and collagen is stronger than that to fibrinogen, we focused on defining the mechanisms of attachment to vitronectin, fibronectin, and collagen.
Fig. 3.
Time-dependent and concentration-dependent attachment of fibroblasts to extracellular matrix proteins. A: time dependency of fibroblast attachment. Fibroblasts (10 × 103 cells) attached to vitronectin (VN; 5 μg/ml), fibronectin (FN; 10 μg/ml), collagen (CL; 10 μg/ml), or fibrinogen (FGN; 10 μg/ml) in 96-well plates for 30 min in attachment assay buffer. After being washed at indicated times, remaining attached cells were counted. Value of cell attachment to fibrinogen at 60 min was 58.2 ± 4.9% (means ± SE; n = 4) of that to vitronectin at same time point. Results are expressed as percentage of maximal attachment to same matrix protein at 60 min. Values are means ± SE for n = 4. B: concentration dependency of fibroblast attachment. Cell attachment was assayed at either 20 min (for vitronectin, collagen, and fibronectin) or 60 min (for fibrinogen). Results are expressed as percentage of attachment to vitronectin at 20 min. Values are means ± SE for n = 4.
Multiple integrin subunits colocalize with u-PAR in cell membrane protrusions during fibroblast spreading.
To dissect potential interactions between u-PAR and integrin receptors during cell attachment and spreading, double label immunofluorescence analysis was performed on early phase spreading fibroblasts. This analysis reveals that u-PAR and the integrins αv-, α3-, and α5 (and β1, not shown)-subunits colocalize within filopodia/lamellipodia during the initial, but not later, phases of spreading cells (Fig. 4A). These data reveal a spatial and temporal interaction of u-PAR with several integrins in the protrusive structures in spreading fibroblasts.
Fig. 4.
Colocalization of relevant integrins with u-PAR in filopodia/lamellipodia of spreading fibroblasts. Fibroblasts were attached to indicated matrix-protein-coated glass coverslips for 15–30 min in attachment assay buffer. Cells were fixed (3% paraformaldehyde), blocked with (10% horse serum in HBSS), and then immunostained for u-PAR (green, Alexa Fluor 488-conjugated goat anti-mouse IgG secondary) and for indicated integrins (red, Alexa Fluor 594 secondary). Photomicrographs were performed (×1,000 original magnification), and basal 1-μm Z stack images were selected and merged. Yellow color indicates colocalization. Bottom right: immunofluorescent staining of u-PAR (red) and α5-integrin (green) in spread cells on fibronectin. α5-Integrin localizes to focal adhesions.
Attachment of fibroblasts to vitronectin, fibronectin, and collagen is dependent on u-PAR-integrin interactions.
To determine the integrins that mediate fibroblast attachment and the significance of u-PAR-integrin interactions in fibroblast attachment, we examined the attachment of the fibroblasts to matrix proteins present in normal and injured lungs interstitium (i.e., fibronectin, vitronectin, and collagen). To ferret out the relative importance of integrins and u-PAR-integrins interactions, attachment assays were performed in the presence or absence of function-blocking antibodies to individual integrins, function-blocking antibodies to u-PAR, and peptides that have been documented to selectively inhibit u-PAR-integrin interactions (52,67, 72). Function-blocking antibody studies revealed that attachment of fibroblasts to vitronectin was dependent on integrin αvβ5 but not α3 (Fig. 5A) and that attachment to fibronectin was largely dependent on α5β1 but not α3 (Fig. 5A). Integrin αvβ3 played a minor role in fibroblast attachment to fibronectin. Attachment to collagen was dependent on integrins α1β1, α2β1, and α3β1 but not αvβ3 (Fig. 5A).
Fig. 5.
Determination of molecules that mediate fibroblast attachment to vitronectin, fibronectin, and collagen. A: specific integrins that mediate fibroblast attachment. Fibroblasts were incubated with function-blocking antibodies against indicated integrins or serotype-matched IgGs at room temperature for 20 min with gentle rocking. Cell attachment to either vitronectin (5 μg/ml)-, fibronectin (10 μg/ml)-, or collagen (10 μg/ml)-coated tissue culture plastic microplate wells (15–30 min, 37°C, 5% CO2). Results are expressed as percent attachment of IgG-pretreated cells. Values are means ± SD for n = 4/condition. *P < 0.05, compared with IgG control. B: role of u-PAR and u-PAR-integrin interactions in fibroblast attachment. Cells were incubated with indicated reagents (IgG control or u-PAR antibodies) or integrin-homologous peptides (α325) or scrambled peptides [serum-containing medium (SCM)], and cell attachment was measured as described above. Results are expressed as percent attachment of either IgG- or vehicle (DMSO)-treated cells. Values are mean ± SD for n = 4/condition. *P < 0.05, compared with IgG or vehicle control. C: lack of effect of integrin-homologous peptides (α325) on lung fibroblasts that are devoid of u-PAR. Murine lung fibroblast from u-PAR knockout mice were incubated with integrin-homologous peptides (α325) or scrambled peptides (SCM) and assayed for their effects on attachment to vitronectin, fibronectin, and collagen, as in B. Values are means ± SD for n ≥ 4/condition.
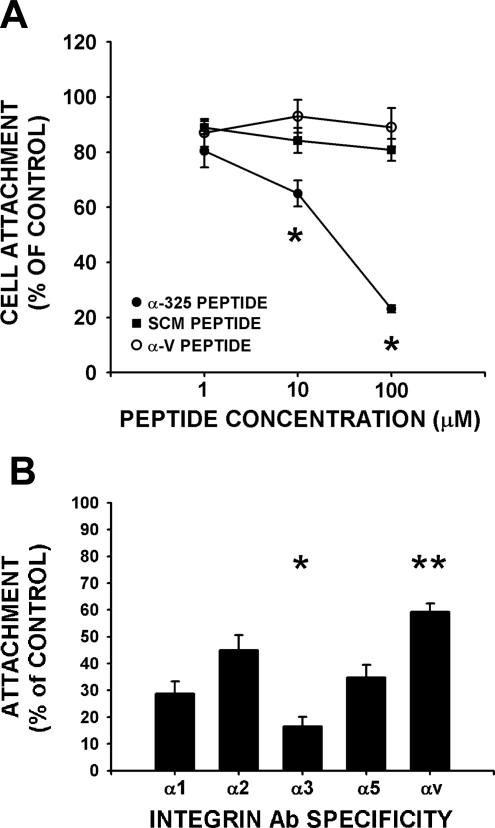
Blocking studies of u-PAR function with anti-u-PAR antibody, previously shown to inhibit u-PAR function, demonstrate that u-PAR plays a direct role in attachment to vitronectin, as published in other cell types, whereas u-PAR played no direct role in cell attachment to fibronectin and collagen (Fig. 5B). To determine the involvement of u-PAR-integrin interactions in the cell attachment process, we studied the effect of a previously characterized 17-mer peptide homologous to the putative u-PAR binding site on the W4 extracellular region of integrin α-chains (52, 67, 72). This peptide (α325) inhibited fibroblast attachment to vitronectin, fibronectin, and collagen (Fig. 5B). The α325-peptide inhibited attachment to all matrix ligands (vitronectin shown) in a concentration-dependent manner (EC50 approximately = 25 μM) with a maximal inhibition of 78 ± 3%. Furthermore, this inhibition was sequence specific as a scrambled version of the 17-mer peptide (SCM) or the corresponding αv-integrin homologous peptide at the same concentration had no effect on cell attachment to all three matrix proteins (Fig. 6A). This latter result is notable in light of the fact that αvβ5 is the predominant integrin involved in fibroblast attachment to vitronectin. The results in Figs. 5 and 6 indicate that fibroblasts attach to vitronectin through 1) integrin ligation by vitronectin, 2) u-PAR-integrin interactions, and 3) likely direct u-PAR ligation to vitronectin. In contrast, u-PAR-integrin interactions are uniquely important for fibroblast attachment to fibronectin and collagen, as u-PAR antibody blocking studies do not demonstrate a role for direct u-PAR ligation in attachment to fibronectin and collagen (Fig. 5B, grey and open bars). To demonstrate that α325 had no direct effect on integrin function or other confounding effects, we examined the effect of the α325-peptide of attachment of cells lacking u-PAR (primary isolates of lung fibroblasts from mice genetically devoid of u-PAR). The number of attached u-PAR-devoid fibroblasts to vitronectin, fibronectin, or collagen in the presence of the α325-peptide was 99 ± 6, 89.5 ± 19, and 115 ± 27% (n = 4/condition, all P = NS) of an equivalent concentration (10 μM) of the scrambled peptide, respectively (Fig. 5C). Thus the peptide had no effect on attachment in the u-PAR null fibroblasts. The data regarding cell attachment, when taken together with the immunofluorescence colocalization data, indicate that u-PAR interacts with and modulates the attachment function of several different integrins, specifically, integrin αvβ5 in cells attaching to vitronectin, integrin α5β1 in cells attaching to fibronectin, and one or more integrins (i.e., α1β1, α2β1, or α3β1) in cells that are attaching to collagen.
Fig. 6.
Preference of u-PAR association with α-integrins. A: specificity of integrin α-chain homologous peptides. Lung fibroblasts were preincubated with indicated concentrations of the peptide homologous regions of either α3- or αv-integrins, or a scrambled peptide control (SCM), and tested for attachment to vitronectin. Data are plotted as cells attached compared with vehicle alone (DMSO; n ≥ 4/condition). *P < 0.05, compared with SCM peptide by ANOVA. B: α3-integrin chain peptide effects on attachment to individual α-chain integrin antibodies. Lung fibroblasts were preincubated with 30 μM α325 or SCM control peptides and then allowed to attach to indicated integrin α-chain-specific antibodies. Data are plotted relative to attachment after incubation with S-325 to each integrin α-chain antibody (n ≥ 5/condition). *P < 0.05, greater inhibition compared with other integrin antibodies; **P < 0.05, less inhibition compared with other integrin antibodies.
u-PAR exhibits preference for functional association with the α3β1-integrin.
As human lung fibroblasts utilize multiple and unique integrins to attach to the individual provisional matrix proteins, we analyzed the effect of the inhibitory peptide (α325) on u-PAR-integrin interactions in fibroblast attachment to specific immobilized anti-integin antibodies. The α325-peptide maximally inhibited attachment to immobilized monoclonal anti-integrin α3β1 (Fig. 6B). This observation is not a consequence of differential expression of integrins as FACS analysis reveals roughly similar cell surface expression levels of α1-, α2-, α3-, α5-, and αv-integrins (Fig. 2B). The relative order of inhibition of attachment to immobilized monoclonal anti-integrin antibodies using the α325-integrin peptide was integrins α3β1 > α5β1, α2β1, α1β1 > αv (Fig. 6B; P < 0.05 by ANOVA). Concordantly, a preference for u-PAR association with integrin α3β1 was noted compared with other integrins, as the αv-homologous peptide was ineffective as an inhibitor of fibroblast attachment to any matrix ligand (Fig. 6A). This is notable given the requirement for integrin αvβ5 in fibroblast attachment to vitronectin and for α5β1 for fibroblast attachment to fibronectin. The α5-integrin homologous peptide inhibited attachment to vitronectin and fibronectin in an intermediate manner but had no effect on fibroblast attachment to collagen (data not shown). In summary, the α325-peptide inhibited attachment to all matrix ligands and to all anti-integrin antibodies, despite the lack of α3β1-integrin dependence of attachment to vitronectin or fibronectin. Our data, taken together with prior work demonstrating that the α3-homologous peptide directly inhibits binding of soluble u-PAR to immobilized integrins, suggest that the α325-peptide has more favorable kinetics of association with u-PAR than the αv- or α5-homologous peptides.
Fibroblast spreading depends on u-PAR-integrin interactions.
Cell spreading requires a spatial-temporal coordinated action of integrin ligation to matrix, as well as integrin clustering, integrin-mediated intracellular signaling, and cytoskeletal reorganization. Initially, human lung fibroblasts spread circumferentially on purified vitronectin, fibronectin, or collagen, reaching a t1/2max for spreading at 42, 28, and 32 min after plating, respectively (data not shown). To study the effect of interactions of u-PAR with integrins on cell spreading, the effect of either α325-peptide (75 μM), scrambled peptide (S-325; 75 μM), or vehicle alone (equivalent; DMSO) on cell area was measured in fibroblasts plated and spreading on matrix. Incubation of matrix-attached fibroblasts with the α325-peptide (75 μM) reduced the maximal extent of cell spreading by 85 ± 8% (P < 0.05 by repeated measures ANOVA) compared with scrambled peptide, DMSO vehicle, or no additives (Fig. 7A). Furthermore, the poorly spread fibroblasts incubated with α325-peptide exhibited a lower density of actin stress fiber than that of the controls (Fig. 7B). In contrast, cell spreading of control, u-PAR-devoid fibroblasts on collagen, fibronectin, and vitronectin in the presence of the α325-peptide was 93 ± 17, 92 ± 23, and 104 ± 9% (n = 100 cells/condition, all P = NS) that of an equivalent concentration of the scrambled peptide, respectively. Thus the peptide had no effect on spreading in the u-PAR null fibroblasts.
Fig. 7.
Inhibition of fibroblast spreading by peptide α3. Fibroblasts attached to vitronectin-coated plates as described above. Scrambled (S-325) or active peptides (α325, 75 μM) were added and the cells were observed for an additional 140 min. Photomicrographs were taken at indicated times thereafter and cell area was measured using Compix software (>100 cells/time point/condition). A: cell area measurement. Values are means ± SD for all cells in at least 2 different pictures. *P < 0.05, compared with scrambled peptide (SCM) by repeated measures ANOVA. B: representative photomicrograph (at 160 min). Fibroblasts were incubated with indicated reagents for 160 min, fixed, and stained with phalliodin (green) and Hoescht (blue).
In vitro wound closure of fibroblast monolayers is dependent on u-PAR-integrin interactions.
To determine whether u-PAR-integrin interactions are important to fibroblast migration, the effect of peptide α325 on fibroblast migration was investigated using an in vitro monolayer “wound closure” assay. As this is a several day assay, fibroblast migration in this system occurs on a complex matrix derived from both the initial serum and that synthesized by the fibroblast themselves. Nonetheless, such migration is highly dependent on fibronectin-α5β1-integrin interaction (92 ± 7% inhibition with α5-integrin blocking Abs; P < 0.05) and less so on collagen-integrin α3β1 interaction (42 ± 9% inhibition; P < 0.05) and vitronectin-integrin αv (35 ± 11% inhibition; P < 0.05) function (Fig. 8A). Wound closure was delayed (time to 1/2 maximum closure, 53 vs. 38 h) and less complete (by 30 ± 6%; P < 0.001 by repeated measures ANOVA) upon incubation of the monolayer with the α325-peptide (100 μM) compared with the scrambled peptide (Fig. 8, B and D). Furthermore, u-PAR-integrin interactions can modulate wound closure, even in the presence of the multiple promotility factors in serum (Fig. 8C). In contrast, the scrambled peptide (SCM) did not affect wound healing in human lung fibroblasts. The α325-peptide wound healing was 101 ± 21% (n = 4/condition, P = NS) of that of an equivalent concentration of the scrambled peptide in fibroblasts that lack u-PAR. Thus the peptide had no effect on migration in the u-PAR null fibroblasts. Similar conclusions are reached if wound closure is measured as migrating cell number or migrating cell area (data not shown). Our observations are not a consequence of peptide effects on proliferation, as serum-induced DNA synthesis (bromodeoxyuridine incorporation) was not different among fibroblasts incubated with α325-peptide, scrambled peptide (SCM), or vehicle (DMSO) (data not shown) and it occurred in the absence of proliferation-requiring serum. Taken together, these data indicate that u-PAR modulates the integrin-mediated cell functions of attachment and that integrin-initiated signaling drives cell spreading and migration.
Fig. 8.
Inhibition of fibroblast wound healing by α3-integrin homologous peptide. Confluent fibroblast monolayers were quiescent in EMEM medium containing 0.4% FBS for 48 h and wounded by pipette tip. Cell monolayers were then fed with EMEM medium containing either 0, 0.4, or 10% FBS with or without indicated function-blocking integrin antibodies or with or without α325- or S-325 peptides. Photomicrographs of same wound area were taken at 15 min post-“wounding” (time 0) and every 6–12 h thereafter. Wound areas were quantified using Compix software and compared with that noted at time 0. A: α5β1 is the predominant integrin involved in fibroblast wound closure assay. The indicated function-blocking integrin antibodies (10 μg/ml) were added immediately after wounding. Data are plotted as means ± SD percent wound area closed relative to time 0. Control IgG (10 μg/ml) wounds underwent 57 ± 4% closure at 24 h. *,+P < 0.05 either decreased or increased compared with IgG by ANOVA (n > 4/condition). B: inhibition of fibroblast wound healing by α3-peptide. Peptide α3 (α325) and scrambled peptide (S-325) control (SCM) were added after the “wound.” Data are plotted as means ± SD wound area as percentage of initial value over time. *P < 0.05, compared with SCM peptide by repeates measures ANOVA (n ≥ 4/condition). C: inhibition of fibroblast wound healing by α3 peptide is independent of serum. Peptide α3 and scrambled peptide control (SCM) were added after the “wound” in EMEM medium containing indicated concentrations of serum. Results are expressed as means ± SD (n = 6/condition) of wound area remaining (at 72 h) relative to maximal closing at the same time. *P < 0.05, compared with SCM peptide under the same conditions by ANOVA (n ≥ 4/condition). D: representative photomicrographs of inhibitory effect of peptide wound closure. As above, with serum-free EMEM, conditions indicated on each photomicrograph.
Fibroblasts preferentially attach to fibroproliferative lesions.
To extend the physiological relevance to pulmonary fibrosis, we evaluated the capacity of the fibroblasts to adhere to either normal areas or fibroproliferative lesions from a well-established murine model of experimentally induced pulmonary fibrosis. Fibroblasts preferentially attached to fibroproliferative lesional areas by fivefold, compared with normal areas of lung, measuring absolute numbers, and approximately threefold (2.7 ± 0.3; P < 0.001) when measured as the number of cells per unit area of lung tissue (Fig. 9A –C). Furthermore, attachment of fibroblasts to lung tissue was reduced by 35 ± 7% in the presence of α325-peptide (10 μM) compared with the density of fibroblasts attached in the presence of the scrambled peptide (10 μM; Fig. 9D). Taken together these data suggest that u-PAR-intergrin interactions mediate fibroblast attachment to remodeling lung lesional matrix, indicating the physiological relevance of our observations.
Fig. 9.
Preferential attachment of fibroblasts to fibroproliferative lesions of fibrotic lungs. Lungs were harvested from mice 21 days after intravenous instillation of bleomycin, inflated, embedded with optimum cutting temperature compound, and frozen. Tissue sections were blocked and incubated with labeled human lung fibroblasts for 45 min (37°C in 5% CO2). Slides were exhaustively washed, and attached cells were photographed (×10 original magnification) under phase contrast and fluorescent conditions. Cell number was counted and tissue area was measured using Compix software. A: fibroblast attachment to normal lung areas. Representative dark field/fluorescent photomicrograph (×10 original magnification). Fibroblasts are red. B: fibroblast attachment to fibroproliferative lesional areas. Representative dark field/fluorescent photomicrograph (×10 original magnification). Fibroblasts are red. C: quantitative comparison of cell attachment to normal and lesional areas. Data are reported as cell number per 166 mm2 lung tissue. P value as indicated, by t-test. (n ≥ 4 in duplicate/condition). D: effect of α325-peptide on fibroblast attachment to fibroproliferative lesions. *P < 0.05, compared with no peptide value; +P < 0.05 compared with scrambled peptide (SCM) value (n ≥ 4/condition in duplicate).
DISCUSSION
The key findings in this study are that u-PAR mediates human lung fibroblast attachment to vitronectin, fibronectin, and collagen through a functional modulation of different integrins and, further, that this interaction extends its influence to cell spreading, to stress fiber formation on a purified matrix proteins, and to closure of an in vitro wound on a complex, serum-derived/fibroblast-synthesized matrix. The direct relevance to lung disease was demonstrable through preferential adhesion of normal lung fibroblasts to areas of fibroproliferative lesions from experimentally induced fibrotic lungs. The association of u-PAR and integrins was demonstrable spatiotemporally using immunofluorescence in which we show co-concentration in the lamellipodia/filopodia of early spreading fibroblasts. The physiological relevance of this association was further supported by the ability of the integrin α-chain homologous peptide to inhibit adhesion, spreading, and migration on multiple purified matrices and to inhibit attachment to experimentally induced fibrotic lung tissue.
The similar kinetics and concentration dependency of attachment of fibroblasts to vitronectin, fibronectin, and collagen and their inhibition by a u-PAR-integrin peptide demonstrate promiscuity of u-PAR-integrin interactions. While promiscuous, we detected a clear preference of u-PAR modulation of integrin function for integrins α3 > α5 > αv, despite similar integrin expression levels. First, the α3-integrin-homologous peptide inhibited attachment to multiple immobilized anti-integrin-antibodies and the inhibitory effects were greater on fibroblast attachment to immobilized antibodies directed toward intregins α3 > α5 > αv. Second, the α3-integrin chain homologous peptide, but not the αv-chain-derived peptide, partially blocked αvβ5-mediated attachment to vitronectin, α5β1-mediated attachment to fibronectin, and α1-, α2-, and α3-integrin-mediated attachment to collagen. Taken together, these data are most consistent with a model whereby the α3-peptide binds to u-PAR integrin-association site with high affinity and thereby competes with u-PAR binding to other integrins. Further support comes from the greater sequence homology (60 vs. 40%) of the α3-sequence-derived peptide than the αv sequence-derived peptide to the potent αM-sequence-derived peptide (52, 66). Complementary findings (66) of other investigators in cell free systems further support this model as soluble u-PAR bound to immobilized α3β1 in a saturable manner that could not be competed by α5- or αv-integrin homologous peptides (500-fold molar excess), and soluble u-PAR binds to immobilized α3β1 with higher affinity than to α5β1.
The physiological importance of u-PAR-integrin interactions is further demonstrable in that they play a dominant regulatory role in fibroblast spreading, they blunt α5β1-dependent monolayer wound closure, and they inhibit fibroblast attachment to fibroproliferative lesional areas of fibrotic lung. These matrices are more representative of those seen in vivo during tissue repair. Our interest in studying these interactions in native lung fibroblasts under normal/disease-relevant conditions precluded the use of alternate methods, such as transfection technologies designed to alter the u-PAR and integrin levels or structure. We and others (17, 66) have demonstrated the capacity and specificity of the peptides in both solid phase, solution phase, and cell-based systems. Nonetheless, we cautiously interpret these findings using the peptide to suggest that u-PAR-integrin interactions mediate the cell adhesive and migratory processes necessary for normal tissue repair after injury and may potentially be manipulated to blunt fibrotic reactions in vivo.
Prior work in cells that express high levels of u-PAR, either de novo in neoplastic cells or experimentally induced, demonstrates that putative u-PAR-interacting partners, physiological consequences on cell physiology, and activation of intracellular signaling pathways vary depending on the levels of u-PAR and with the cell type (13, 32, 46, 51, 65). These studies also show that u-PAR will bind the somatomedin B (SMB) domain of vitronectin directly (Kd < 20 nM), an interaction that is enhanced upon u-PAR ligation with u-PA (49, 69). Despite this direct u-PAR-vitronectin (SMB domain) interaction, RGD-dependent integrin binding to vitronectin did contribute to weak cell adhesion to vitronectin, and this two-step process was required for downstream changes in cell morphology (lamellipodial formation) in u-PAR overexpressing renal epithelial cells (34). We similarly found that the u-PAR antibody was capable on blocking adhesion selectively to vitronectin, thereby demonstrating the importance of u-PAR direct mediation of adhesion to vitronectin. However, u-PAR mutants with combined substitution of amino acids in the published integrin binding sites in u-PAR were functionally similar to wild-type u-PAR, suggesting that u-PAR integrin interactions are not required for vitronectin adhesion, signaling, and actin cytoskeletal remodeling (34). Similarly, u-PAR overexpression in murine embryo fibroblasts has shown that u-PAR can induce protrusions and motility, as well as enhance nondirectional motility on vitronectin in a Rac-dependent manner but in a u-PA-independent manner (30). This phenotypic change, while involving integrins, was surprisingly independent of integrin-vitronectin ligation (30). These studies have led to important mechanistic insights into u-PAR biology; however, u-PAR interactions and physiology may be dramatically different in native cells that express u-PAR at a low level. Our data showing a reduction in fibroblast attachment to vitronectin with neutralizing antibodies to either u-PAR or integrin αvβ5 documents the cooperative role of u-PAR and αvβ5 and extends these findings to the low level u-PAR expressing normal human lung fibroblasts. Further, the ability of the α-chain integrin peptide to reduce adhesion to vitronectin and to immobilized anti-integrin αvβ5 monoclonal antibody suggests that u-PAR-integrin interactions have physiological consequences, even in cells expressing low levels of u-PAR.
In support of the importance of integrin u-PAR interactions to cell adhesion/motility dynamics is the hard evidence that 1) mutagenesis studies have mapped the u-PAR-interacting site in integrins [W4 BC loop outside the laminin-5 binding region in α3 (AA 224–232 or Ser 227) in the β1-chain of α5β1], and the areas of u-PAR critical for its interaction with integrins [domain 2 of u-PAR (AA 130–142) of u-PAR for αvβ3 and α5β1 and domain 3 (AA 240–248) for interaction with α5β1 (13, 14, 65, 66, 72)]; 2) su-PAR binding to immobilized integrins and u-PAR-integrin coimmunoprecipitates from u-PAR expressing cell lysates can be blocked by integrin chain peptides (65, 66, 68, 72); and 3) even in u-PAR overexpressing 293 cells, cell morphologic changes, lamellipodial formation, and signal transduction (ERK 1/2 activation) was dependent on integrins (34).
u-PAR has been shown to directly bind integrin α5β1 in HT-1080 fibrosarcoma cells, or in purified protein systems (mapped to AA 224–232 in the β1-chain), thereby stabilizing integrin α5β1 in a unique conformation. This conformational change modulates the α5β1-function, such that it becomes an efficient mediator of RGD-independent cell attachment to fibronectin (attachment to fibronectin type III repeats 12–14) and enhances fibronectin matrix assembly, while not affecting the RGD integrin site-dependent binding to fibronectin (type III repeats 9–11; Ref. 65). The level of u-PAR expression was again critical in that low u-PAR expressing cancer cells (MCF-7 and T47D) exhibited only RGD-dependent attachment to fibronectin (65). Similarly, colon carcinoma cell proliferation, ERK activation, and u-PAR-integrin α5β1 interaction were dependent on the level of u-PAR expression (2). The peptide blocking studies in our lung fibroblasts demonstrate that u-PAR interaction with integrin α5β1 decreases cell adhesion to fibronectin and to immobilized anti-integrin α5β1 antibody. We would suggest that u-PAR interacts with integrin α5β1 in a manner that alters its RGD-dependent binding to the fibronectin type III repeat 9–11 (i.e., different from that observed in cells expressing high levels of u-PAR). These data further support that u-PAR modulates cell processes in a manner dependent on its expression level. Thus the use of cells that have endogenous low levels of u-PAR supports the determination of mechanisms that do not depend on vast molar excesses of interacting components and thereby demonstrates interactions that have physiological relevance to fibroproliferative disorders. The use of a single, unaltered, nontransformed, fibroblast cell strain is a strength, as we have fully characterized the expression levels and physiology of the relevant receptors. However, further testing, in future work, of several fibroblast cell lines with varying receptor levels will surmount the limitations and support greater generalizibility of our results.
The consequences of u-PAR on fibroblast biological events important to the fibroproliferative process extend beyond adhesion and migration. First, u-PAR expression and function is upregulated in fibroblasts from patients with idiopathic pulmonary fibrosis (48). u-PAR can regulate fibronectin matrix fibrillar organization on the cell surface (35). As fibronectin has been shown to guide collagen fibrillar deposition, then u-PAR could regulate dysfunctional fibrillar collagen organization in fibrotic tissue (31, 62). u-PAR has also been shown to modulate TGFβ-induced myofibroblast differentiation (6). u-PAR can regulate plasminogen-induced apoptosis of fibroblasts through enhancement of cell surface plasminogen activation upon ligation with u-PA, an effect blunted by TGFβ-mediated induction of PAI-1 (26). We have shown that repletion of glutathione, a potent antioxidant, in TGFβ-treated fibroblasts restores the TGFβ-induced collagen degradation defect (61) and, furthermore, that the effect of glutathione on overall collagen degradation in TGFβ-treated fibroblasts is a consequence of regulation of plasminogen activation (61).
In conclusion, u-PAR-integrin interactions mediate attachment to vitronectin, fibronectin, and collagen through different integrins in low u-PAR expressing, human lung fibroblasts. Furthermore, this interaction extends its influence to cell spreading, stress fiber formation, closure of an in vitro wound, and attachment to the physiologically relevant matrix from fibrotic lungs. These observations suggest that u-PAR-integrin interactions mediate the cell adhesive and migratory processes necessary for normal tissue repair after injury and may be manipulated to control fibrotic reactions in the lung and other organs.
GRANTS
We thank Janice Buffett for secretarial assistance.
Acknowledgments
This work was supported by grants from the Veterans Administration MERIT Review (to M. A. Olman), the National Heart, Lung, and Blood Institute Grants HL-58655 (to M. A. Olman) HL-44712 (to H. A. Chapman, Jr.), and a grant from the American Heart Association (to Q. Ding).
REFERENCES
- 1.Abraham E, Gyetko MR, Kuhn K, Arcaroli J, Strassheim D, Park JS, Shetty S, Idell S. Urokinase-type plasminogen activator potentiates lipopolysaccharide-induced neutrophil activation. J Immunol 170: 5644–5651, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol 147: 89–104, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet P, Loskutoff D, Collen D, Carmeliet G, Foidart JM, Noel A. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol 152: 777–784, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, Skobe M, Fusenig NE, Carmeliet P, Collen D, Foidart JM. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 4: 923–928, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Behrendt N, Renne E, Ploug M, Petri T, Lober D, Nielsen LS, Schleuning WD, Blasi F, Appella E, Dano K. The human receptor for urokinase plasminogen activator. J Biol Chem 265: 6453–6460, 1990. [PubMed] [Google Scholar]
- 6.Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor clevage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell 18: 2716–2727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol 213: 565–573, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Besser D, Verde P, Nagamine Y, Blasi F. Signal transduction and the u-PA/u-PAR system. Fibrinolysis 10: 215–237, 1996. [Google Scholar]
- 8a.Bitterman P Pathogenesis of fibrosis after lung injury. Am J Med 92: 139S–143S, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Blasi F The urokinase receptor. A cell surface, regulated chemokine. APMIS 107: 96–101, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Blasi F, Carmeliet P. UPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol 3: 932–943, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Moons L, Lijnen R, Baes M, Lemaitre V, Tipping P, Drew A, Eeckhout Y, Shapiro S, Lupu F, Collen D. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet 17: 439–444, 1997.9398846 [Google Scholar]
- 12.Chapman HA Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol 9: 714–724, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Chaurasia P, Aguirre-Ghiso JA, Liang OD, Gardsvoll H, Ploug M, Ossowski L. A region in urokinase plasminogen receptor domain III controlling a functional association with alpha5beta1 integrin and tumor growth. J Biol Chem 281: 14852–14863, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem 280: 24792–24803, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Ding Q, Gladson CL, Wu H, Hayasaka H, Olman MA. Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem 283: 26839–26849, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest 1: 232–237, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh S, Johnson JJ, Sen R, Mukhopadhyay S, Liu S, Zhang F, Wei Y, Chapman HA, Stack MS. Functional relevance of urinary-type plasminogen activator receptor-alpha3beta1 integrin association in proteinase regulatory pathways. J Biol Chem 281: 13021–13029, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Giancotti FG, Ruoslahti E. Integrin signaling. Science 285: 1028–1032, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Gladson CL, Stewart JE, Olman MA, Chang PL, Schnapp LM, Grammer JR, Benveniste EN. Attachment of primary neonatal rat astrocytes to vitronectin is mediated by integrins alphavbeta5 and alpha8beta1: modulation by the type 1 plasminogen activator inhibitor. Neurosci Lett 283: 157–161, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Gyetko MR, Chen GH, McDonald RA, Goodman R, Huffnagle GB, Wilkinson CC, Fuller JA, Toews GB. Urokinase is required for the pulmonary inflammatory response to Cryptococcus neoformans. J Clin Invest 97: 1818–1826, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyetko MR, Sud S, Kendall T, Fuller JA, Newstead MW, Standiford TJ. Urokinase receptor-deficient mice have impaired neutrophil recruitment in response to pulmonary pseudomonas aeruginosa infection. J Immunol 165: 1513–1519, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Gyetko MR, Todd RF, Wilkinson CC, Sitrin RG. The urokinase receptor is required for human monocyte chemotaxis in vitro. J Clin Invest 93: 1380–1387, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heiligenhaus A, Steinmetz B, Lapuente R, Krallmann P, Althaus C, Steinkamp WK, Dick B. Recombinant tissue plasminogen activator in cases with fibrin formation after cataract surgery: a prospective randomised multicentre study. Br J Ophthalmol 82: 810–815, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemler ME Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol 19: 397–422, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Horan GS, Wood S, Ona V, Li dJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O'Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 177: 56–65, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol 38: 78–87, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaikita K, Fogo AB, Ma L, Schoenhard JA, Brown NJ, Vaughan DE. Plasminogen activator inhibitor-1 deficiency prevents hypertension and vascular fibrosis in response to long-term nitric oxide synthase inhibition. Circulation 104: 839–844, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitching AR, Holdsworth SR, Ploplis VA, Plow EF, Collen D, Carmeliet P, Tipping PG. Plasminogen and plasminogen activators protect against renal injury in crescentic glomerulonephritis. J Exp Med 185: 963–968, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjoller L, Hall A. Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol 152: 1145–1157, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Van Den Diepstraten C, D'Souza SJ, Chan BM, Pickering JG. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol 163: 1045–1056, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cells 1: 445–457, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Munger JS, Steadele M, Busald C, Tellier M, Schnapp LM. Integrin alpha8beta1 mediates adhesion to LAP-TGFbeta1. J Cell Sci 15: 4641–4648, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol 177: 927–939, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaghan E, Gueorguiev V, Wilkins-Port C, McKeown-Longo PJ. The receptor for urokinase-type plasminogen activator regulates fibronectin matrix assembly in human skin fibroblasts. J Biol Chem 279: 1400–1407, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Oda T, Jung YO, Kim HS, Cai X, Lopez-Guisa JM, Ikeda Y, Eddy AA. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int 60: 587–596, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Olman MA Mechanisms of fibroproliferation in acute lung injury. In: Acute Respiratory Distress Syndrome, edited by Matthay MA, Lenfant C. New York: Marcel Dekker, 2003, p. 313–354.
- 40.Olman MA, Simmons WL, Pollman DJ, Loftis AY, Bini A, Miller EJ, Fuller GM, Rivera K. Polymerization of fibrinogen in murine bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 271: L519–L526, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Olman MA, White KE, Ware L, Simmons WL, Pugin J, Benveniste EN, Matthay MA, Zhu S. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1beta-induced IL-6 expression. J Immunol 172: 2668–2677, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr Opin Cell Biol 12: 613–620, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Pijuan-Thompson V, Gladson CL. Ligation of integrin alphavbeta1 is required for integrin alphavbeta3 internalization of vitronectin. J Biol Chem 272: 2736–2743, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Ploug M, Ronne E, Behrendt N, Jensen AL, Blasi F, Dano K. Cellular receptor for urokinase plasminogen activator. J Biol Chem 266: 1926–1933, 1991. [PubMed] [Google Scholar]
- 45.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME, Violette SM, Grant KS, Colarossi C, Formenti SC, Munger JS. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Crit Care 177: 82–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1 LXA4R. Proc Natl Acad Sci USA 99: 1359–1364, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheppard D Roles of alphav integrins in vascular biology and pulmonary pathology. Curr Opin Cell Biol 16: 552–557, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Shetty S, Kumar A, Johnson AR, Pueblitz S, Holiday D, Raghu G, Idell S. Differential expression of the urokinase receptor in fibroblasts from normal and fibrotic human lungs. Am J Respir Cell Mol Biol 15: 78–87, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Sidenius N, Andolfo A, Fesce R, Blasi F. Urokinase regulates vitronectin binding in vivo and in vitro by controlling urokinase receptor oligomerization. J Biol Chem 277: 27982–27990, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Simon DI, Ezratty AM, Francis S, Rennke H, Loscalzo J. Fibrin(ogen) is internalized and degraded by activated human monocytiod cells via mac-1 (CD11b/CD18): A non-plasmin fibrinolytic pathway. Blood 82: 2414–2422, 1993. [PubMed] [Google Scholar]
- 51.Simon DI, Rao NK, Xu H, Majdic O, Ronne E, Kobzik L, Chapman HA. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood 88: 3185–3194, 1996. [PubMed] [Google Scholar]
- 52.Simon DI, Wei Y, Zhang L, Rao NK, Xu H, Chen Z, Liu Q, Rosenberg S, Chapman HA. Identification of a urokinase receptor-integrin interaction site. Promiscuous regulator of integrin function. J Biol Chem 275: 10228–10234, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Sitrin RG, Johnson DR, Pan PM, Harsh DM, Huang J, Petty HR, Blackwood RA. Lipid raft compartmentalization of urokinase receptor signaling in human neutrophils. Am J Respir Cell Mol Biol 30: 233–241, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Sitrin RG, Pan PM, Blackwood RA, Huang J, Petty HR. Cutting edge: evidence for a signaling partnership between urokinase receptors (CD87) and L-selectin (CD62L) in human polymorphonuclear neutrophils. J Immunol 166: 4822–4825, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Stefansson S, Petitclerc E, Wong MK, McMahan GA, Brooks PC, Lawrence DA. Inhibition of angiogenesis in vivo by plasminogen activator inhibitor-1. J Biol Chem 276: 8135–8141, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Suganuma H, Sata A, Tamura R, Chida K. Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 50: 984–989, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol 157: 177–187, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GSV, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, Luster AD. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol 31: 395–404, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chung CY, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14: 45–54, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278: 12384–12389, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Vayalil PK, Olman M, Murphy-Ullrich JE, Postlethwait EM, Liu RM. Glutathione restores collagen degradation in TGF-beta-treated fibroblasts by blocking plasminogen activator inhibitor-1 expression and activating plasminogen. Am J Respir Cell Mol Biol 289: L937–L945, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha11beta1 and alpha2beta1. J Biol Chem 277: 37377–37381, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Waltz DA, Fujita RM, Yang X, Natkin L, Zhuo S, Gerard CJ, Rosenberg S, Chapman HA. Nonproteolytic role for the urokinase receptor in cellular migration in vivo. Am J Respir Cell Mol Biol 22: 316–322, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Wei Y, Czekay RP, Robillard L, Kugler MC, Zhang F, Kim KK, Xiong JP, Humphries MJ, Chapman HA. Regulation of alpha5beta1 integrin conformation and function by urokinase receptor binding. J Cell Biol 168: 501–511, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell 12: 2975–2986, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA. Regulation of integrin function by the urokinase receptor. Science 273: 1551–1555, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, Chapman HA. Urokinase receptors are required for alpha5beta1 integrin-mediated signaling in tumor cells. J Biol Chem 282: 3929–3939, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem 269: 32380–32388, 1994. [PubMed] [Google Scholar]
- 70.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 168: 436–442, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med 205: 1659–1672, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang F, Tom CC, Kugler MC, Ching TT, Kreidberg JA, Wei Y, Chapman HA. Distinct ligand binding sites in integrin alpha3beta1 regulate matrix adhesion and cell-cell contact. J Cell Biol 163: 177–188, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]